Abstract
Aplysia motoneurons cocultured with a presynaptic sensory neuron exhibit homosynaptic depression when stimulated at low frequencies. A single bath application of serotonin (5HT) leads within seconds to facilitation of the depressed synapse. The facilitation is attributed to mobilization of neurotransmitter-containing vesicles from a feeding vesicle store to the depleted, readily releasable pool by protein kinase C (PKC). Here, we demonstrate that the calpain inhibitors, calpeptin, MG132, and ALLN, but not the proteasome inhibitors, lactacystin and clasto-lactacystin β-lactone, block 5HT-induced facilitation of depressed synapses. Likewise the 5HT-induced enhancement of spontaneous miniature potentials (mEPSPs) frequency of depressed synapses is significantly reduced by calpeptin. In contrast, neither the facilitation of nondepressed synapses nor the enhancement of their mEPSPs frequency is affected by the inhibitor. The data suggest that action potentials-induced calcium influx activate calpains. These, in turn, play a role in the refilling processes of the depleted, releasable vesicle store.
Chemical synapses formed by cultured Aplysia mechanosensory and motoneurons have proven to be an extremely useful model system to study mechanisms underlying different forms of short and long-term synaptic plasticity (for review, see Kandel 2001). These synapses undergo depression in response to repetitive stimuli delivered at low frequencies. The depressed synapse undergoes rapid facilitation in response to a single bath application of 10 μM 5HT (Hochner et al. 1986). Based on pharmacological experiments, it was suggested that the 5HT-induced facilitation of depressed synapse results from PKC activation (Braha et al. 1990; Ghirardi et al. 1992; Byrne and Kandel 1996; Manseau et al. 2001), which, in turn, induces the mobilization of neurotransmitter-containing vesicles from a nonreleasable pool to the depleted pool of readily releasable vesicles (Gingrich and Byrne 1985; Bailey and Chen 1988; Klein 1995; Byrne and Kandel 1996; Zhao and Klein 2002, 2004). Alternative explanations have also been considered; namely, that PKC activates voltage-gated calcium channels strategically located close to the release site, or that PKC act directly on the exocytotic machinery (Byrne and Kandel 1996; Kandel 2001; Zhao and Klein 2002).
Theoretical considerations led Gingrich and Byrne (1985) to suggest that the process of sensory-motor synaptic depression is partially opposed by a Ca2+-mediated facilitatory process, which may be described in terms of vesicles mobilization to a ready releasable status. This view was further supported by Eliot et al. (1994) and Bao et al. (1997) who argued that post-tetanic potentiation (PTP) in the synapse is expected to be mediated by a calcium-dependent enzymatic reaction. Nevertheless, the mechanisms that link alterations in the free intracellular calcium concentration ([Ca2+]i) and the refilling processes of the depleted release competent vesicle pool were not investigated.
Here, we examine the hypothesis that the calcium-activated proteinase—calpain (Sato and Kawashima 2001; Goll et al. 2003) is the linking molecular element between the elevation of the [Ca2+]i and vesicle refilling.
We report that the calpain inhibitors, calpeptin, MG132, and ALLN (Figueiredo-Pereira et al. 1994; Lee and Goldberg 1998), but not the proteasome inhibitors, lactacystin and clasto-lactacystin β-lactone, inhibit 5HT-induced facilitation of depressed synapses. Likewise, the 5HT-induced enhancement of spontaneous miniature excitatory postsynaptic potentials (mEPSPs) frequency of depressed synapses is significantly reduced by calpeptin. In contrast, neither the facilitation of nondepressed synapses nor the enhancement of its mEPSPs frequency is affected by the inhibitor.
Our results are consistent with the hypothesis that calcium influx during homosynaptic depression activates calpain. The active calpain in turn assists in the replenishing processes of the depleted vesicle stores, possibly by facilitating the mobilization of vesicles from a nonreleasable compartment to a readily releasable store. When calpain activation is inhibited, and the releasable vesicle store is depleted, 5HT application fails to induce synaptic dishabituation, as vesicle mobilization is impaired. The effect of calpain activation by calcium influx on vesicle mobilization is transient and lasts for minutes, since the structural integrity of the cleaved protein recovers after mild proteolysis.
Results
Calpain inhibitors accelerate the rate of synaptic depression and inhibit 5HT-induced facilitation of highly depressed synapses
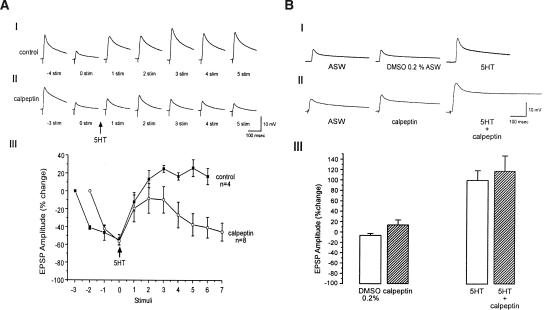
We began by examining the effects of calpeptin on the rate of synaptic depression and 5HT-induced facilitation. To that end, the excitatory post synaptic potentials (EPSPs) were depressed by 40 consecutive extracellular stimuli delivered at 0.05 Hz to the presynaptic sensory neuron in the absence and presence of 100 μM calpeptin.
In control experiments, carried out in the presence of the carrier solution, 0.2% DMSO ASW, following synaptic depression, 5HT application led to the recovery of the EPSP's amplitude to 91 ± 9.1% SEM (n = 6) of the initial level (Fig. 1A). In the presence of 100 μM calpeptin, the rate of synaptic depression was significantly accelerated and the degree of synaptic depression was significantly higher. Thus, while in control experiments, 40 stimuli depressed the EPSP amplitude from a mean value of 16.9 ± 4.7 mV to 18 ± 4.5% SEM of the initial level (n = 6) in the presence of calpeptin, the rate of depression was significantly faster and resulted in synaptic depression from a mean value of 11.9 ± 2.7mV to 6.8 ± 1.5% (F(39,390) = 1.971, P < 0.01; repeated measures one-way ANOVA, n = 6) (Fig. 1A).
Figure 1.
The inhibition of calcium-dependent proteinases accelerates the rate of homosynaptic depression and inhibits 5HT-induced facilitation of depressed synapses. To examine the roles of proteinases in the cascade leading to homosynaptic depression and facilitation of highly depressed synapse by 5HT, we compared the rates of synaptic depression and 5-HT-induced facilitation in the presence and absence of proteinase inhibitors; (A) 100 μM calpeptin, (B) 100 μM MG132, (C) 100 μM ALLN, and (D) intracellularly injected lactacystin into the sensory neuron. Examples of EPSPs recorded during synaptic depression induced by 40 stimuli at 0.05 Hz and 5HT-induced facilitation (arrows) in control experiments are shown in aI, bI, cI, and dI, and the solid squares graph in aIII, bIII, cIII, and dIII. The traces shown in aI, bI, cI, and dI depict the first EPSP, the 40th EPSP, and the second EPSP after 5HT application (42 stim). The effects of protease inhibitors are depicted in aII, bII, cII, and dII and the mean values are shown in aIII, bIII, cIII, and dIII (○). For experiments A, B, and C, the preparations were bathed in the protease inhibitor containing ASW for 15 min before the onset of the experiment. The paired controls were incubated for 15 min in 0.2% DMSO ASW (the vehicle solution of the inhibitors). For the experiment in D, lactacystin was pressure injected into the presynaptic neuron (reaching an estimated concentration of 25 μM) 1 h prior to the experiment. Note that in the presence of (A) calpeptin and (B) MG132, the rate of synaptic depression is significantly accelerated with respect to the control (F(39,390) = 1.971, P < 0.01 repeated measures one-way ANOVA for calpeptin and F(39,312) = 2.063, P < 0.001 for MG132) and that the 5-HT-induced facilitation is significantly reduced (F(4,40) = 8.348, P < 0.001 repeated measures one-way ANOVA for calpeptin and F(4,32) = 5.909, P < 0.01 for MG132). The 100 μM ALLN (C) does not accelerate the rate of synaptic depression, but inhibits 5HT-induced synaptic facilitation. In contrast, the proteasome inhibitor lactacystin neither affected the rate of synaptic depression nor the facilitation induced by 5HT.
Application of 5HT in the presence of calpeptin-induced synaptic facilitation to 14 ± 4.8% of the initial level, a value significantly smaller than that of control experiments in which the 5HT-induced facilitation reached a mean value of 91 ± 9.1% SEM (F(4,40) = 8.348, P < 0.001; repeated measures one-way ANOVA; n = 6) (Fig. 1A).
To better evaluate whether the above observations are related to calpain inhibition rather than to other unknown side affects of calpeptin, we tested the effects of four additional protease inhibitors, MG132, ALLN, lactacystin, and clasto-lactacystin β-lactone. Whereas MG132 and ALLN are recognized as calpain inhibitors with some inhibitory effects on proteasomes (Figueiredo-Pereira et al. 1994; Lee and Goldberg 1998), lactacystin and clasto-lactacystin β-lactone are highly specific inhibitors of the proteasome (Omura et al. 1991; Fenteany et al. 1994, 1995; Dick et al. 1996). Following incubation of the preparation in 100 μM MG132 for 15 min, the mean amplitude of the first EPSP was 13.04 ± 3.5 mV, whereas in the control experiments of this series, it was 15.01 ± 3 mV. We found that as in calpeptin, in the presence of 100 μM MG132, the rate of the EPSP depression was significantly accelerated compared with the control experiments, from a mean of 13.04 ± 3.5 mV to 7.5 ± 0.6%, (F(39,312) = 2.063, P < 0.001 repeated measures one-way ANOVA). Furthermore, in the presence of MG132, the 5HT-induced facilitation is significantly reduced to 30 ± 8% of the initial level (n = 5, F(4,32) = 5.909, P < 0.01) compared with the control experiments (Fig. 1B).
In another series of experiments in which the effects of ALLN were studied, the mean EPSP amplitude of the control experiments was 15.7 ± 5.54 mV, whereas after 15 min in 100 μM ALLN, the mean amplitude was 17.7 ± 4.2 mV. Repeated stimulation as described for the calpeptin experiments revealed that 100 μM ALLN does not significantly accelerate the rate of EPSP depression (F(4,40) = 2.218, P > 0.05; ANOVA), yet it reduced the synaptic dishabituation induced by 5HT from 107 ± 14% SEM in control experiments to 47 ± 9% (n = 6, P < 0.05; t-test) (Fig. 1C). The mechanisms underlying the different action of the above described calpain inhibitors were not studied. Most likely, they could be attributed, however, to different affinities of the inhibitors to Aplysia calpain.
The proteasome inhibitor, lactacystin (intracellularly injected into presynaptic sensory neurons to reach an estimated final concentration of 25 μM, (Fig. 1D; n = 4) or 10 μM clasto-lactacystin β-lactone applied to the bathing solution (data not shown; n = 3) had no significant effect on the rates and degrees of synaptic depression or on the degree of 5HT-induced facilitation of the depressed synapses (Fig. 1D).
Collectively, these results suggest that calcium-activated protease, but not proteasome, plays a role in (1) the determination of the rate of synaptic depression and, (2) the cellular processes that lead to 5HT-induced synaptic facilitation of highly depressed synapses.
Effect of calpain inhibition on 5HT-induced synaptic facilitation of partially depressed synapses
We next examined the effect of the calpain inhibitor, calpeptin on 5HT-induced facilitation of moderately depressed synapses. To that end, the EPSP's amplitude was depressed to ∼45% of its initial level, following which, 5HT was applied in the absence (control) or presence of calpeptin (Fig. 2A). In the control experiments (0.2% DMSO ASW), a single bath application of 5HT led to an increase of the partially depressed EPSP's amplitude to 124 ± 3.8% of the control values (n = 4). In the presence of calpeptin, 5HT facilitated the partially depressed EPSP's amplitude to a lesser degree (91 ± 15% of the control, n = 8). It is important to note that, while in the control experiments the EPSP's amplitude remained relatively unchanged following 5HT-induced facilitation, in the presence of calpeptin, the EPSP's amplitude decayed within the following stimuli (Fig. 2A). Significant differences in the EPSP's amplitudes in the control experiments and in the presence of calpeptin become clear from the fourth stimuli after 5HT application (P < 0.05; t-test).
Figure 2.
Effect of calpeptin on 5HT-induced synaptic facilitation of moderately depressed and nondepressed synapses. Examples of 5HT-induced facilitation of moderately depressed synapses in controls (0.2% DMSO ASW) (AI) and preparations bathed for 15 min in 100μM calpeptin (AII). The figure shows representative traces of EPSP in the course of the experiments. The number shown under the EPSPs in AI and AII corresponds to the order of the EPSP's generation. To moderately depress the synaptic potentials to about 45% of the initial level, four stimuli at 0.05 Hz were given to the sensory neuron in control experiments. Since the rate of depression is faster in calpeptin, only three stimuli at 0.05 Hz were given to depress the synapses to about 45% of the initial level in calpeptin. The arrow (time 0) indicates 5HT application. In the control experiments, 5HT induces facilitation of the depressed EPSPs. In the presence of calpeptin, 5HT induces partial recovery, which then rapidly declines. The kinetics of synaptic depression and 5HT-induced facilitation in control (AIII, ▪) and in the presence of calpeptin (AIII, ○) are shown. Significant difference between control and calpeptin experiments becomes apparent from the fourth stimulus after 5HT application, (P < 0.05; t-test). (B) In rested synapses, calpeptin has no effect on 5HT-induced facilitation. BI and BII are examples of 5HT-evoked facilitation of rested synapses in the controls and in the neurons incubated in 100 μM calpeptin for 15 min, respectively. (BII) EPSP before (ASW), 15 min after calpeptin application (calpeptin) and 3 min following 5HT application (5HT + calpeptin). In control experiments, DMSO 0.2% was added instead of calpeptin (BI). (BIII) Summary of the short-term facilitation of rested synapses in the presence and absence of calpeptin. Each bar represents the mean percent change ±SEM of the EPSP amplitude with respect to the first EPSP. Note that facilitation produced by 5HT in rested synapses is not affected by calpeptin (P > 0.05; t-test).
Calpeptin has no significant effect on 5HT-induced facilitation of nondepressed synapses
Finally, we studied the effect of calpeptin on 5HT-induced facilitation of nondepressed synapses. The experiment was done as follows: the EPSP amplitude evoked by a single test stimulus to the sensory neuron was recorded. This was followed by calpeptin application. Fifteen minutes later, a second stimulus was delivered in the presence of calpeptin (Fig. 2B). In this series of experiments, the EPSP's amplitude measured after 15 min in 100μM calpeptin was higher by 14.5 ± 8.9% (SEM; n = 6) than the control EPSPs. 5HT was then applied, and 3 min later, a third stimulus was delivered. This led to an increase in EPSP's amplitude by 118 ± 28% (n = 6) over the initial control value. The control experiments were conducted using the same protocol, except for application of 0.2% DMSO ASW rather than calpeptin-containing solution. In these controls, a slight decrease of 6.3 ± 3.4% (n = 6) in the PSP amplitude was recorded. 5HT application doubled the EPSP amplitude (by 100 ± 18.6% of its initial level, n = 6) (Fig. 2B). Thus, calpeptin has no significant effect on 5HT-induced facilitation of nondepressed synapses (P > 0.05; t-test). These results are consistent with earlier reports demonstrating that calpain inhibitors have no significant effects on 5HT-induced facilitation of nondepressed Aplysia sensory motor synapses (Chain et al. 1999).
Calpain activity with respect to the PKC cascade of synaptic dishabituation
In a series of studies, it was suggested that 5HT-induced facilitation of depressed synapses involves the activation of PKC (Braha et al. 1990; Ghirardi et al. 1992; Byrne and Kandel 1996; Manseau et al. 2001). It was argued that the activation of PKC, in turn, leads to the mobilization of neurotransmitter-containing vesicles from a nonreleasable pool to a readily releasable store (Gingrich and Byrne 1985; Hochner et al. 1986; Bailey and Chen 1988; Braha et al. 1990; Dale and Kandel 1990). The experimental results described above are phenomenologically similar to those described for the PKC inhibitor, H7, by Braha et al. (1990) and Ghirardi et al. (1992). Namely, H7 does not block 5HT-induced facilitation of rested synapses, yet it blocks the facilitation of depressed synapses. These earlier studies also demonstrated that direct activation of PKC by phorbol dibutyrate-PDBu is sufficient to induce facilitation of depressed synapses (Braha et al. 1990; Ghirardi et al. 1992).
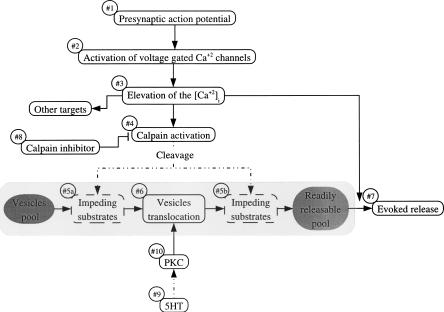
Thus, we began to explore the role of calpain action with respect to PKC within the cascade leading to 5HT-induced synaptic facilitation of depressed synapses. If activated calpain operates by cleavage of substrates that impede vesicle translocation from a reserve pool to a readily releasable pool, then the facilitation of depressed synapses by PDBu application would be inhibited in the presence of calpeptin (Fig. 5, below, and see Discussion). It should be noted that, in this case, the hypothetical vesicle translocation-impeding substrates could operate downstream (Fig. 5, #5a) or upstream (Fig. 5, #5b) with respect to PKC (Fig. 5, #10). Furthermore, it is expected that if the putative calpain substrates impede vesicle translocation, but do not block it completely, then PDBu application in the presence of calpeptin would lead to partial facilitation of depressed synapses by enhancing vesicle mobilization to some degree. Thus, the result would appear as if calpain and PKC operate in parallel.
Figure 5.
A model for the role of calpain in the sensory motor synapse. Presynaptic action potentials (#1) induce calcium influx through voltage-gated calcium channels (#2). The elevated [Ca2+]i (#3) activate calpain (#4), and in parallel, invokes neurotransmitter release (#7). The activated calpain cleaves substrates (#5a; #5b) that impede vesicle translocation from the reserve pool to a readily releasable pool of neurotransmitter (#6). The nature of the calpain substrate is not known. Nevertheless, based on the experimental results, the impeding substrates could be located upstream (#5a) or downstream (#5b) with respect to the vesicle-mobilization functions of PKC. When calpain activation is inhibited (#8), the impeding substrates are not cleaved, and as a consequence, the translocation of vesicles is slowed down, leading to an increased rate of homosynaptic depression. Furthermore, following homosynaptic depression, in the presence of calpeptin, 5HT application (#9) and PKC activation (#10) fail to mobilize vesicles, since vesicle translocation is impeded by the hypothetical uncleaved calpain substrate.
To gain some insight into these processes, we depressed the synaptic potential, and then applied 20 nM PDBu in the presence or absence of calpeptin (Fig. 3). In control experiments, PDBu induced significant synaptic facilitation from 22 to ±3% (3.7 ± 0.5 mV) to 90 ± 3.9% of the initial level (n = 5, Fig. 3, P < 0.001; paired t-test). In the presence of 100 μM calpeptin, PDBu induced a much smaller facilitation, from 9.2 ±1.8% after depression (1.5 ± 0.29 mV) to 22 ± 6% of the initial level (P > 0.05; paired t-test, n = 4). In view of the insignificant facilitatory effects of PDBu on depressed synapses in the presence of calpeptin, it is reasonable to assume that calpain and PKC exert their effect on the same cascade of events as described in Figure 5, below.
Figure 3.
Effect of PDBu on 5HT-evoked facilitation of depressed synapses. Facilitation of the depressed synapses by 20 nM PDBu (A) in control cells (0.2% DMSO) and (B) in cells incubated for 15 min in 100 μM calpeptin EPSPs before depression, after depression, and 1 min following 20 nM PDBu application (B) in the presence and (A) absence of calpeptin are shown. (C) Summary of the synaptic depression and PDBu (20 nM) induced facilitation in the presence (n = 4) and absence (n = 5) of calpeptin expressed as the mean percent change ±SEM in the amplitude of the EPSP compared with the EPSP before depression. PDBu-induced facilitation is greatly inhibited by calpeptin (P > 0.05; paired t-test).
The effect of calpeptin on spontaneous neurotransmitter release
As stated earlier, 5HT-induced facilitation following synaptic depression is thought to depend on the mobilization of synaptic vesicles from a nonreleasable store into a readily releasable store (Gingrich and Byrne 1985; Hochner et al. 1986; Bailey and Chen 1988; Dale and Kandel 1990; Zhao and Klein 2002). The availability of a releasable pool of vesicles is reflected by the mEPSP frequency (Dale and Kandel 1990) (but, see Eliot et al. 1994). Thus, we next examined the effects of calpeptin on 5HT-induced enhancement of spontaneous release.
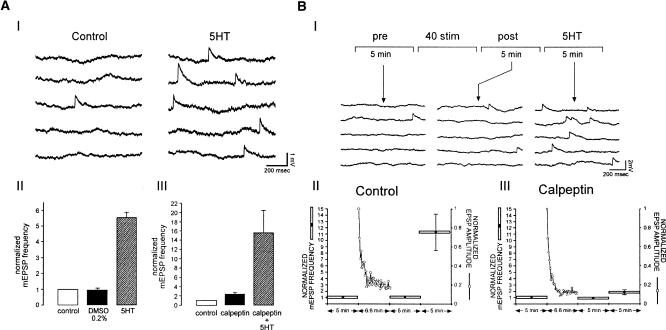
In control experiments, performed on nondepressed synapses, the mean mEPSP frequency was 0.091 ± 0.03 Hz. Bath application of 0.2% DMSO (the vehicle solution for calpeptin) did not induce any significant effects on mEPSP frequency (n = 4) (Fig. 4A). Application of 5HT led to a significant 5.57 ± 0.31-fold increase in mEPSP frequency (n = 6, P < 0.01, paired t-test) (Fig. 4A). Application of 100 μM calpeptin alone induced a 2.4 ± 0.37-fold insignificant increase in mEPSPs frequency (P > 0.05, paired t-test, n = 6) (Fig. 4A). This increase in mEPSP frequency is most likely related to a small (∼100 nM) increase in the free intraneuronal calcium concentration as revealed by fura-2 imaging (Ziv and Spira 1995; data not shown). In the presence of calpeptin bath, application of 5HT induced a significant 5.9-fold increase in mEPSP frequency with respect to the frequency measured with calpeptin alone (n = 6, P < 0.05; paired t-test) (Fig. 4A, III). These results demonstrate that calpeptin does not inhibit the effects of 5HT on mEPSP frequency in rested synapses.
Figure 4.
Effect of calpeptin on spontaneous release in rested and highly depressed synapses. The effects of calpeptin on spontaneous release of (A) rested and (B) highly depressed synapses were examined. (AI) In rested synapse, 10μM 5HT increases the frequency of spontaneous neurotransmitter release (compare control traces on the left to the traces on the right following 5HT application). (AII) Summary of the effects of 10 μM 5HT on the mean mEPSP frequency (n = 6). 5HT increases significantly the mEPSP frequency (right, P < 0.01; paired t-test). All values were normalized with respect to the control values. (AIII) Calpeptin alone enhances the mean rate of spontaneous release with respect to the control experiments, but this enhancement is not significant (n = 6, P > 0.05; paired t-test). In rested synapses, calpeptin does not inhibit 5HT-induced increases of the mEPSP frequency (right bar, n = 6. P < 0.05; paired t-test). (B) To evaluate the effect of calpeptin on spontaneous release of depressed synapses, we sampled the spontaneous release for 5 min before stimulation, after a train of 40 stimuli (at 0.1 Hz) and following the application of 10 μM 5HT. (BI) Examples of traces of spontaneous release recorded before stimulation (pre-), after stimulation (post-), and following 5HT application (5HT). Summary of mEPSP frequency measurements according to the protocol in BI. (BII) Controls (n = 6). (BIII) Cells incubated in 100 μM calpeptin for 15 min before the experiment (n = 6). Horizontal bars indicate the duration over which mEPSP frequency was sampled for each data point. mEPSP frequency is normalized with respect to the frequency in the control period of the experiment. The left y-axis shows normalized mEPSP frequencies. On the right, the ordinates indicate the normalized EPSP's amplitude. Note that in the control experiments, 5HT significantly increases the mEPSP frequency (P < 0.05; paired t-test). In contrast, the mEPSP frequency is only slightly increased in the presence of calpeptin (P > 0.05; paired t-test). Error bars represent SEM.
An insignificant decrease in the mean mEPSP amplitude from 1.025 to ±0.24 mV in control to 0.92 ± 0.26 mV was noted after calpeptin application (n = 3). This clearly indicates that calpeptin has no significant postsynaptic actions.
We next turned to examine whether calpeptin alters the effects of 5HT on mEPSP frequency in highly depressed synapses. In these experiments, we first sampled the mEPSP frequency for 5 min. Then, the synapse was depressed by 40 stimuli delivered at 0.1 Hz., and the mEPSP frequency was sampled once again for an additional 5 min. Thereafter, 5HT was applied to the bathing solution to reach a final concentration of 10 μM (Fig. 4B). In the control experiments, 5HT application resulted in a significant 11.29 ± 2.89-fold increase in mEPSP frequency (n = 6, P < 0.05; paired t-test). In the presence of calpeptin, 5HT application produced an insignificant (P > 0.05; paired t-test) 1.83 ± 0.4-fold enhancement of mEPSP frequency (n = 6) (Fig. 4B). These results demonstrated that calpains are involved in the 5HT-induced increase of spontaneous release in depressed synapses, but not in rested synapses.
Discussion
The main observation of the present study is that the calpain inhibitors, calpeptin, MG132, and ALLN, largely inhibit the 5HT-induced facilitation of depressed sensory-motor synapses. These observations are consistent with the interpretation that the activation of calcium-dependent protease is essential for synaptic vesicle mobilization from a nonreleasable pool into a readily releasable pool (Fig. 5).
The refilling of the releasable pool of neurotransmitter-containing vesicles in the sensory motor synapse appears to be a rate-limiting process. A single bath application of 5HT leads to significant facilitation of the synaptic potential of both depressed and rested synapse (Hochner et al. 1986). A series of experiments lead to the conclusion that the 5HT-induced facilitation of depressed synapses results from the activation of a calcium-independent PKC (Braha et al. 1990; Ghirardi et al 1992; Sossin et al. 1993; Byrne and Kandel 1996; Manseau et al. 2001), which, in turn, induces the mobilization of neurotransmitter-containing vesicles from the nonreleasable pool to the depleted pool of readily releasable vesicles (Gingrich and Byrne 1985; Bailey and Chen 1988; Klein 1995; Byrne and Kandel 1996; Zhao and Klein 2002, 2004) (Fig. 5, #9 and #10). It is important to note that the contribution of PKC to synaptic facilitation was always detected only after the depression of the synaptic potentials by repetitive stimuli. Thus, in the experimental paradigm used to examine the role of PKC, elevation of [Ca2+]i always precedes the activation of PKC.
The results presented here suggest that the activation of calpain by the incoming calcium underlies the refilling process. Since calpain inhibitors do not significantly alter evoked release of rested synapses, we conclude that the process of neurotransmitter release itself, including the exocytotic machinery, is not affected by calpain blockade. Calpains appear to regulate two processes, i.e., the rate of homosynaptic depression (Figs. 1A,B and 2A) and the facilitation of depressed synapses by 5HT (Figs. 1,2,4). Both processes can be interpreted as indications that activated calpains regulate vesicle mobilization from nonreleasable to releasable pools (Fig. 5, #5a and #5b).
Specificity of the proteinase inhibitors
Most of the arguments for the role of calpains in the sensory motor synapse rest on the specificity of the calpain inhibitors used. We have shown that calpeptin, a membrane-permeable cystein proteinase inhibitor (Tsujinaka et al. 1988) inhibits calcium-dependent proteolytic processes in cultured Aplysia neurons (Gitler and Spira 1998, 2002; Spira et al. 2003). In a preliminary study, MG132 and ALLN were also shown to inhibit the calcium-dependent protease in Aplysia neurons (Spira et al. 2001). While MG132 and ALLN are known to function as calpain and proteasome inhibitors (Figueiredo-Pereira et al. 1994; Lee and Goldberg 1998), calpeptin is only a weak proteasome inhibitor (Figueiredo-Pereira et al. 1994). Since we found that the proteasome inhibitors lactacystin and clasto-lactacystin β-lactone have no short-term effects on 5HT-induced facilitation of depressed synapses, we suggest that the described inhibition of 5HT-induced facilitation of depressed synapses is indeed related to inhibition of calpains.
Interestingly, the concept that synaptic depression is opposed by a Ca2+-mediated process that mobilizes vesicles was suggested 20-yr ago by Gingrich and Byrne (1985), and see also Klein et al. (1980). This view was further supported by Eliot et al. (1994) and Bao et al. (1997), who argued that post-potentiation in the sensory motor synapse is expected to be mediated by a calcium-activated molecule that exerts its effects long after the [Ca2+]i is down-regulated (see also Fisher et al. 1997). These observations are consistent with the proposed model that action potential-induced Ca2+ influx activates calpains, which, in turn, facilitate the mobilization of vesicles for minutes (see below).
Activation of calpain
Calpains are the only known proteolytic enzymes that depend on direct calcium binding (Perrin and Huttenlocher 2002; Goll et al. 2003). Following activation, calpains undergo autocatalytic processing, yielding a calcium-independent isoform (Goll et al. 2003). Thus, calpains can (1) integrate the number of spikes over time, as calpain activation depends on the [Ca2+]i; (2) once the calcium concentration reaches a level sufficient for calpain activation, the calpain undergoes an autoproteolytic process leading to the formation of a calcium-independent isoform, and thus, may serve as molecular memory.
During a train of action, potentials leading to synaptic depression, the [Ca2+]i is elevated in the sensory neuron neurites (Blumenfeld et al. 1990). We hypothesize that this, in turn, activates an Aplysia form of μ-calpain that facilitates vesicle mobilization from a nonreleasable to ready releasable domains (Fig. 5, #5a and #5b). When calpain activation is inhibited (Fig. 5, #8), the refilling rate of the depleted pool is reduced, leading to an enhanced depression rate of the evoked EPSP (Figs. 1 and 5, #5a), and the inhibition of 5HT-induced facilitation of evoked and spontaneous neurotransmitter release (Figs. 1,4,5, #5b).
Mechanisms by which calpain regulate the facilitation of depressed synapses
Several mechanisms have been proposed to account for the observation that 5HT induces facilitation of depressed synapses. These include mobilization of vesicles (Dale and Kandel 1990; Angers et al. 2002; Zhao and Klein 2002), increased calcium influx at the release site, or priming of vesicles that are already docked (Zhao and Klein 2002). While the molecular mechanisms underlying the facilitation are still not totally understood, most studies reveal that the activation of PKC by DAG is the key event leading to facilitation. Consistently, PKC inhibition by pharmacological reagents, such as H7, blocks the 5HT-induced facilitation of depressed synapses (Braha et al. 1990; Sacktor and Schwartz 1990; Ghirardi et al. 1992) and PDBu application is sufficient to induce the facilitation (Braha et al. 1990).
In that respect, it is important to note that Munc-13 is an additional phorbol ester receptor (Betz et al. 1998). It was shown that in some experiments in which facilitation of neurotransmitter release by phorbol esters was attributed to PKC activation, the facilitation was in fact related to the effect of phorbol esters on Munc13 (Rhee et al. 2002; Silinsky and Searl 2003). Nevertheless, we are not aware of studies showing that Munc13 or related proteins serve as calpain substrate.
The calpain substrate, spectrin (Bennett 1990; Bennett and Gilligan 1993) is considered as a submembrane skeletal component that may impede the refilling processes of depleted synapses. In neurons, spectrin is often preferentially localized to synapses (Bloch and Morrow 1989; Daniels 1990; Goodman et al. 1995; Kordeli 2000). Spectrin interacts with many of the synaptic components, and thus, could affect vesicle mobility. Indeed, it was suggested that spectrin cleavage might expose release sites to incoming vesicles (Gitler and Spira 1998; Sikorski et al. 2000; Zimmer et al. 2000). Interestingly, it was suggested by the laboratory of G. Lynch (Lynch and Baudry 1987) that calpain is involved in long-term potentiation of hippocampal neurons in slices. Inhibition of calpain reduced the incidence and magnitude of long-term potentiation. It was suggested that calpain proteolyses postsynaptic spectrin (Lynch and Baudry 1987). These observations were not followed up, and the mechanism of calpain action was not determined. Another potential site for calpain could be the anchorage of vesicles to the actin skeleton by synapsins (Landis et al. 1988; Goodman et al. 1995; Sikorski et al. 2000). Thus, both synapsin (Fig. 5, #5a) and spectrin (Fig. 5, #5b) could serve as impeding-substrates.
Other substrates including calcineurin, Ca2+/calmodulin-protein kinase, Ca2+ ATPase, and phospholipase C should be considered as a potential calpain substrate that might influence the mobilization of vesicles (for review, see Chan and Mattson 1999).
In conclusion, the data presented here are interpreted to suggest the following model: (1) action potentials delivered to the presynaptic sensory neurons elevate the [Ca2+]i (Fig. 5, #1–#3) and lead to neurotransmitter release (Fig. 5, #7). Repetitive stimulation of the sensory neuron leads to homosynaptic depression by depletion of the available neurotransmitter pool; (2) in parallel, the increased [Ca2+]i activates calpains (Fig. 5, #4); (3) calpain, in turn, cleaves substrates that impede the mobilization of vesicles from a nonreleasable into a readily releasable store of vesicles (Fig. 5, #5a or #5b). We propose that while PKC acts by facilitation of vesicle mobilization, calpain operates by removal of barriers that impede the translocation of the vesicles. Thus, when calpain is inhibited the rate of homosynaptic depression is accelerated and 5HT-induced synaptic dishabituation is inhibited (Fig. 1). The proposed model also accounts for the observations that in the presence of calpeptin, the partially depressed synapse undergoes only a transient 5HT-induced facilitation (Fig. 3). Thus, under these conditions, application of 5HT results in action potential broadening, which leads to increased calcium influx and increased neurotransmitter release from the readily releasable store. As a consequence, the available store is depleted. Since calpain is not activated in the presence of calpeptin, the mobilization of vesicles to the releasable pool is inhibited and the EPSP amplitude is further depressed. The same mechanism could account for the observation that in the presence of calpeptin, 5HT fails to increase the spontaneous miniature frequency following homosynaptic depression (Fig. 4).
We propose tentatively that the substrate for calpain action could be the untethering of vesicles from the cytoskeleton (Fig. 5, #5a, upstream to PKC) or proteolysis of spectrin that impede vesicles from reaching the release sites (Fig. 5, #5a, downstream to PKC). It is reasonable to assume that the formation of a calcium-independent isoform of calpain by an autocatalytic process may serve as short-term molecular memory that promotes vesicle mobilization and refilling of depleted stores. It should be noted, however, that calpain activity is limited by the presence of endogenous inhibitors such as calpastatin. Thus, the duration of calpain action is limited by endogenous inhibition and dilution, and opposed by spontaneous recovery and delivery of its substrates.
Materials and Methods
Cell cultures
Sensory neurons from the pleural ganglia of adult animals (60–80 g) were cocultured with either an L7, or an LFS motor neuron from the abdominal ganglia of juvenile (2–5 g), or adult animal, respectively, as described by Schacher and Proshansky (1983). Briefly, animals were anesthetized by injection of isotonic MgCl2 solution. The ganglia were isolated and incubated for 1.5–3 h in 1% protease (type IX, Sigma) at 34°C. The ganglia were then desheathed, and the cell bodies of their neurons with their long axons were pulled out with sharp micropipettes and placed on poly-L-lysine-coated (Sigma) glass-bottom culture dishes. The culture medium consisted of 10% filtered hemolymph from Aplysia faciata collected along the Mediterranean coast, and L-15 (Gibco-BRL) supplemented for marine species. Twenty-four hours after plating, dishes were transferred to an 18°C incubator. Experiments were performed 3–5 d after plating.
Electrophysiology
All recordings were performed at room temperature in artificial sea water (ASW) composed of NaCl 460 mM, KCL 10 mM, CaCl2 10 mM, MgCl2 55 mM, and HEPES [N-(2-hydroxyethyl)piperazine-N'-2ethanesulfonic acid, Sigma] 11 mM, adjusted to pH 7.6.
Sensory neurons were extracellularly stimulated. EPSPs were intracellularly recorded from the motor neurons with 7–9 MΩ sharp microelectrodes filled with 2 M KCl. EPSPs were recorded while holding the motor neurons transmembrane potential at approximately -70 mV. Intracellular stimulation was done by sharp microelectrodes (12–15 MΩ) filled with 2 M KCl.
Spontaneous miniature potentials were recorded in culture formed by a single sensory neuron in contact with a single LFS motor neuron. Throughout the experiments, the LFS motor neurons were hyperpolarized to -80 mV in current-clamp mode with microelectrodes filled with 2 M KCl (15–20 MΩ). Spontaneous miniature potentials were identified visually.
Drugs
Calpeptin, MG132, and ALLN (Calbiochem) were prepared as a 50-mM stock solution in DMSO (Sigma) and diluted to the final concentration in ASW just before the experiments. Clasto-lactacystin β-lactone (Calbiochem) and PDBu (Sigma) were prepared as 10-mM stock solutions in DMSO. Stock solution of 5-Hydroxytryptamine (Sigma) was prepared fresh on the same day of the experiment.
Loading of lactacystin (Calbiochem) into the sensory neurons was done by pressure injection of a 1-M KCl solution containing 5 mM lactacystin and 5 mM fura-2 (Molecular Probes). The intracellular concentrations of fura-2 were estimated by measuring the fura-2 fluorescent intensity within the main neurite of the sensory neuron by excitation at 360 nm. Using a fluorescent calibration curve of fura-2 intensity/fura-2 concentration, the final concentration of lactacystin was estimated (see Ziv and Spira 1995).
Statistical analysis
The effects of various treatments are presented as the percent change of EPSP's amplitude after treatment with respect to the initial EPSP's amplitude pretreatment. All of the data are presented as mean ±SEM. Statistical analysis was performed with the software package SPSS 10.0 (Genius Systems, Israel) for Windows using repeated measures one-way ANOVA in Figure 1, independent samples t-test, and the paired samples t-test in Figures 1C and 2, and 3 and 4, respectively.
Acknowledgments
This study was supported by a grant from the German Israel Foundation for scientific research and development (No. I-0598-162.01/98). Partial support was also provided by grants from the USA-Israeli Binational Science Research Foundation (No. 97-00297-1), the Israeli National Science Foundation (620/98), and the Charles E. Smith Family Laboratory for Collaborative Research in Psychobiology. M.E.S. is the Levi DeViali Professor in neurobiology.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.92105.
References
- Angers, A., Fioravante, D., Chin, J., Cleary, L.J., Bean, A.J., and Byrne, J.H. 2002. Serotonin stimulates phosphorylation of Aplysia synapsin and alters its subcellular distribution in sensory neurons. J. Neurosci. 22: 5412-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, C.H. and Chen, M. 1988. Morphological basis of short-term habituation in Aplysia. J. Neurosci. 8: 2452-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, J., Kandel, E.R., and Hawkins, R.D. 1997. Involvement of pre- and postsynaptic mechanisms in posttetanic potentiation at Aplysia synapses. Science. 275: 969-973. [DOI] [PubMed] [Google Scholar]
- Bennett, V. 1990. Spectrin: A structural mediator between diverse plasma membrane proteins and the cytoplasm. Curr. Opin. Cell. Biol. 2: 51-56. [DOI] [PubMed] [Google Scholar]
- Bennett, V. and Gilligan, D.M. 1993. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu. Rev. Cell. Biol. 9: 27-66. [DOI] [PubMed] [Google Scholar]
- Betz, A., Ashery, U., Rickmann, M., Augustin, I., Neher, E., Sudhof, T.C., Rettig, J., and Brose, N. 1998. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron 21: 123-136. [DOI] [PubMed] [Google Scholar]
- Bloch, R.J. and Morrow, J.S. 1989. An unusual β-spectrin associated with clustered acetylcholine receptors. J. Cell. Biol. 108: 481-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld, H., Spira, M.E., Kandel, E.R., and Siegelbaum, S.A. 1990. Facilitatory and inhibitory transmitters modulate calcium influx during action potentials in Aplysia sensory neurons. Neuron 5: 487-499. [DOI] [PubMed] [Google Scholar]
- Braha, O., Dale, N., Hochner, B., Klein, M., Abrams, T.W., and Kandel, E.R. 1990. Second messengers involved in the two processes of presynaptic facilitation that contribute to sensitization and dishabituation in Aplysia sensory neurons. Proc. Natl. Acad. Sci. 87: 2040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, J.H. and Kandel, E.R. 1996. Presynaptic facilitation revisited: State and time dependence. J. Neurosci. 16: 425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chain, D.G., Casadio, A., Schacher, S., Hegde, A.N., Valbrun, M., Yamamoto, N., Goldberg, A.L., Bartsch, D., Kandel, E.R., and Schwartz, J.H. 1999. Mechanisms for generating the autonomous cAMP-dependent protein kinase required for long-term facilitation in Aplysia. Neuron 22: 147-156. [DOI] [PubMed] [Google Scholar]
- Chan, S.L. and Mattson, M.P. 1999. Caspase and calpain substrates: Roles in synaptic plasticity and cell death. J. Neurosci. Res. 58: 167-190. [PubMed] [Google Scholar]
- Dale, N. and Kandel, E.R. 1990. Facilitatory and inhibitory transmitters modulate spontaneous transmitter release at cultured Aplysia sensorimotor synapses. J. Physiol. 421: 203-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, M.P. 1990. Localization of actin, β-spectrin, 43 × 10(3) Mr and 58 × 10(3) Mr proteins to receptor-enriched domains of newly formed acetylcholine receptor aggregates in isolated myotube membranes. J. Cell. Sci. 97: 615-626. [DOI] [PubMed] [Google Scholar]
- Dick, L.R., Cruikshank, A.A., Grenier, L., Melandri, F.D., Nunes, S.L., and Stein, R.L. 1996. Mechanistic studies on the inactivation of the proteasome by lactacystin: A central role for clasto-lactacystin β-lactone. J. Biol. Chem. 271: 7273-7276. [DOI] [PubMed] [Google Scholar]
- Eliot, L., Kandel, E.R., and Hawkins, R.D. 1994. Modulation of spontaneous transmitter release during depression and posttetanic potentiation of Aplysia sensory-motor neuron synapses isolated in culture. J. Neurosci. 14: 3280-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenteany, G., Standaert, R.F., Reichard, G.A., Corey, E.J., and Schreiber, S.L. 1994. A β-lactone related to lactacystin induces neurite outgrowth in a neuroblastoma cell line and inhibits cell cycle progression in an osteosarcoma cell line. Proc. Natl. Acad. Sci. 91: 3358-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenteany, G., Standaert, R.F., Lane, W.S., Choi, S., Corey, E.J., and Schreiber, S.L. 1995. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268: 726-731. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira, M.E., Banik, N., and Wilk, S. 1994. Comparison of the effect of calpain inhibitors on two extralysosomal proteinases: The multicatalytic proteinase complex and m-calpain. J. Neurochem. 62: 1989-1994. [DOI] [PubMed] [Google Scholar]
- Fisher, S.A., Fischer, T.M., and Carew, T.J. 1997. Multiple overlapping processes underlying short-term synaptic enhancement. Trends Neurosci. 20: 170-177. [DOI] [PubMed] [Google Scholar]
- Ghirardi, M., Braha, O., Hochner, B., Montarolo, P.G., Kandel, E.R., and Dale, N. 1992. Roles of PKA and PKC in facilitation of evoked and spontaneous transmitter release at depressed and nondepressed synapses in Aplysia sensory neurons. Neuron 9: 479-489. [DOI] [PubMed] [Google Scholar]
- Gingrich, K.J. and Byrne, J.H. 1985. Simulation of synaptic depression, post-tetanic potentiation, and presynaptic facilitation of synaptic potentials from sensory neurons mediating gill-withdrawal reflex in Aplysia. J. Neurophysiol. 53: 652-669. [DOI] [PubMed] [Google Scholar]
- Gitler, D. and Spira, M.E. 1998. Real time imaging of calcium-induced localized proteolytic activity after axotomy and its relation to growth cone formation. Neuron 20: 1123-1135. [DOI] [PubMed] [Google Scholar]
- ———. 2002. Short window of opportunity for calpain induced growth cone formation after axotomy of Aplysia neurons. J. Neurobiol. 52: 267-279. [DOI] [PubMed] [Google Scholar]
- Goll, D.E., Thompson, V.F., Li, H., Wei, W., and Cong, J. 2003. The calpain system. Physiol. Rev. 83: 731-801. [DOI] [PubMed] [Google Scholar]
- Goodman, S.R., Zimmer, W.E., Clark, M.B., Zagon, I.S., Barker, J.E., and Bloom, M.L. 1995. Brain spectrin: Of mice and men. Brain Res. Bull. 36: 593-606. [DOI] [PubMed] [Google Scholar]
- Hochner, B., Klein, M., Schacher, S., and Kandel, E.R. 1986. Additional component in the cellular mechanism of presynaptic facilitation contributes to behavioral dishabituation in Aplysia. Proc. Natl. Acad. Sci. 83: 8794-8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel, E.R. 2001. The molecular biology of memory storage: A dialogue between genes and synapses. Science 294: 1030-1038. [DOI] [PubMed] [Google Scholar]
- Klein, M. 1995. Modulation of ion currents and regulation of transmitter release in short-term synaptic plasticity: The rise and fall of the action potential. Invert. Neurosci. 1: 15-24. [DOI] [PubMed] [Google Scholar]
- Klein, M. and Kandel, E.R. 1980. Mechanism of calcium current modulation underlying presynaptic faciitation and behavioral sensitization in Aplysia, Proc. Natl. Acad. Sci. 77: 6912-6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordeli, E. 2000. The spectrin-based skeleton at the postsynaptic membrane of the neuromuscular junction. Microsc. Res. Tech. 49: 101-107. [DOI] [PubMed] [Google Scholar]
- Landis, D.M., Hall, A.K., Weinstein, L.A., and Reese, T.S. 1988. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron 1: 201-209. [DOI] [PubMed] [Google Scholar]
- Lee, D.H. and Goldberg, A.L. 1998. Proteasome inhibitors: Valuable new tools for cell biologists. Trends Cell Biol. 8: 397-403. [DOI] [PubMed] [Google Scholar]
- Lynch, G. and Baudry, M. 1987. Brain spectrin, calpain and long-term changes in synaptic efficacy. Brain Res. Bull. 18: 809-815. [DOI] [PubMed] [Google Scholar]
- Manseau, F., Fan, X., Hueftlein, T., Sossin, W., and Castellucci, V.F. 2001. Ca2+-independent protein kinase C Apl II mediates the serotonin-induced facilitation at depressed Aplysia sensorimotor synapses. J. Neurosci. 21: 1247-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura, S., Matsuzaki, K., Fujimoto, T., Kosuge, K., Furuya, T., Fujita, S., and Nakagawa, A. 1991. Structure of lactacystin, a new microbial metabolite which induces differentiation of neuroblastoma cells. J. Antibiot. (Tokyo) 44: 117-118. [DOI] [PubMed] [Google Scholar]
- Perrin, B.J. and Huttenlocher, A. 2002. Calpain. Int. J. Biochem. Cell. Biol. 34: 722-725. [DOI] [PubMed] [Google Scholar]
- Rhee, J.S., Betz, A., Pyott, S., Reim, K., Varoqueaux, F., Augustin, I., Hesse, D., Sudhof, T.C., Takahashi, M., Rosenmund, C., et al. 2002. β phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell 108: 121-133. [DOI] [PubMed] [Google Scholar]
- Sacktor, T.C. and Schwartz, J.H. 1990. Sensitizing stimuli cause translocation of protein kinase C in Aplysia sensory neurons. Proc. Natl. Acad. Sci. 87: 2036-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K. and Kawashima, S. 2001. Calpain function in the modulation of signal transduction molecules. Biol. Chem. 382: 743-751. [DOI] [PubMed] [Google Scholar]
- Schacher, S. and Proshansky, E. 1983. Neurite regeneration by Aplysia neurons in dissociated cell culture: Modulation by Aplysia hemolymph and the presence of the initial axonal segment. J. Neurosci. 3: 2403-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, A.F., Sangerman, J., Goodman, S.R., and Critz, S.D. 2000. Spectrin (βSpIIσ1) is an essential component of synaptic transmission. Brain Res. 852: 161-166. [DOI] [PubMed] [Google Scholar]
- Silinsky, E.M. and Searl, T.J. 2003. Phorbol esters and neurotransmitter release: More than just protein kinase C? Br. J. Pharmacol. 138: 1191-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin, W.S., Diaz-Arrastia, R., and Schwartz, J.H. 1993. Characterization of two isoforms of protein kinase C in the nervous system of Aplysia californica. J. Biol. Chem. 268: 5763-5768. [PubMed] [Google Scholar]
- Spira, M.E., Oren, R., Dormann, A., Ilouz, N., and Lev, S. 2001. Calcium, protease activation, and cytoskeleton remodeling underlie growth cone formation and neuronal regeneration. Cell. Mol. Neurobiol. 21: 591-604. [DOI] [PubMed] [Google Scholar]
- Spira, M.E., Oren, R., Dormann, A., and Gitler, D. 2003. Critical calpain-dependent ultrastructural alterations underlie the transformation of an axonal segment into a growth cone after axotomy of cultured Aplysia neurons. J. Comp. Neurol. 457: 293-312. [DOI] [PubMed] [Google Scholar]
- Tsujinaka, T., Kajiwara, Y., Kambayashi, J., Sakon, M., Higuchi, N., Tanaka, T., and Mori, T. 1988. Synthesis of a new cell penetrating calpain inhibitor (calpeptin). Biochem. Biophys. Res. Commun. 153: 1201-1208. [DOI] [PubMed] [Google Scholar]
- Zhao, Y. and Klein, M. 2002. Modulation of the readily releasable pool of transmitter and of excitation-secretion coupling by activity and by serotonin at Aplysia sensorimotor synapses in culture. J. Neurosci. 22: 10671-10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2004. Changes in the readily releasable pool of transmitter and in efficacy of release induced by high-frequency firing at Aplysia sensorimotor synapses in culture. J. Neurophysiol. 91: 1500-1509. [DOI] [PubMed] [Google Scholar]
- Zimmer, W.E., Zhao, Y., Sikorski, A.F., Critz, S.D., Sangerman, J., Elferink, L.A., Xu, X.S., and Goodman, S.R. 2000. The domain of brain β-spectrin responsible for synaptic vesicle association is essential for synaptic transmission. Brain Res. 881: 18-27. [DOI] [PubMed] [Google Scholar]
- Ziv, N.E. and Spira, M.E. 1995. Axotomy induces a transient and localized elevation of the free intracellular calcium concentration to the millimolar range. J. Neurophysiol. 74: 2625-2637. [DOI] [PubMed] [Google Scholar]