Abstract
Microbial cells of Pseudomonas delafieldii were coated with magnetic Fe3O4 nanoparticles and then immobilized by external application of a magnetic field. Magnetic Fe3O4 nanoparticles were synthesized by a coprecipitation method followed by modification with ammonium oleate. The surface-modified Fe3O4 nanoparticles were monodispersed in an aqueous solution and did not precipitate in over 18 months. Using transmission electron microscopy (TEM), the average size of the magnetic particles was found to be in the range from 10 to 15 nm. TEM cross section analysis of the cells showed further that the Fe3O4 nanoparticles were for the most part strongly absorbed by the surfaces of the cells and coated the cells. The coated cells had distinct superparamagnetic properties. The magnetization (δs) was 8.39 emu · g−1. The coated cells not only had the same desulfurizing activity as free cells but could also be reused more than five times. Compared to cells immobilized on Celite, the cells coated with Fe3O4 nanoparticles had greater desulfurizing activity and operational stability.
Immobilized microbial cells are frequently used in bioconversions, biotransformation, and biosynthesis processes due to their better operational stability, easier separation from products for possible reuse, and satisfactory efficiency in catalysis compared to free cells (6, 12). Generally, entrapment and adsorption are preferred methods for cell immobilization. In entrapment, living cells are enclosed in a polymeric matrix which is porous enough to allow diffusion of substrates to the cells and of products away from the cells. The materials used for entrapment of cells are mainly natural polymers, such as alginate, carrageenan, gelatin, and chitosan. They may also be synthetic polymers such as polysaccharides, photo-cross-linkable resins, polyurethane, polyvinyl alcohol (1), polyacrylamide, and so on. Major drawbacks of an entrapment technique are diffusional limitations and steric hindrance, especially when diffusion of macromolecular substrates, such as starch and proteins, is involved. Mass transfer involved in diffusion of a substrate to a reaction site and in removal of inhibitory or toxic products from the environment may be impeded.
Cell immobilization by adsorption is currently gaining considerable importance because of a major advantage, namely, reducing or eliminating the mass transfer problems associated with the common entrapment methods. However, the adsorption technique is generally limited by biomass loading, strength of adhesion, biocatalytic activity, and operational stability. This is because immobilization by adsorption involves attachment of cells to the surface of an adsorbent like Celite. Adsorption is a simple physical process in which the forces involved in cell attachment are so weak that cells that are several micrometers across are not strongly adsorbed and are readily lost from the surface of the adsorbent.
In this study, a new technique was developed in which microbial cells were coated with magnetic nanoparticles by adsorption. The cells could be immobilized by an externally applied magnetic field. The nanoparticles were strongly adsorbed on the cell surfaces because of their high specific surface area and high surface energy. It was possible to concentrate the dispersed coated cells by application of a magnetic field and to reuse them. When dispersed, the coated cells experienced minimal mass transfer problems. Thus, this technique has advantages over conventional immobilization by adsorption to carrier materials such as Celite. Furthermore, it can overcome drawbacks such as limitations in biomass loading and in the loss of cells from the carrier associated with conventional immobilization by adsorption. To be effective, Pseudomonas delafieldii R-8 coated by magnetic Fe3O4 nanoparticles should have the same desulfurization activity as free R-8 cells.
MATERIALS AND METHODS
Chemicals.
Dibenzothiophene (DBT) was purchased from Acros Organics (United States). 2-Hydroxy-biphenyl (2-HBP) and n-dodecane were purchased from Tokyo Kasei Kogyo Co., Ltd. (TCI; Tokyo, Japan). The methanol used was liquid chromatography grade. Celite (about 100 to 200 μm) was purchased from Beijing Chemical Reagents Company (China). All other materials were analytical grade and were available commercially.
Strain and medium.
P. delafieldii R-8 (9) was isolated from the sewage pool in the Shengli oil field in China and has the ability to convert dibenzothiophene (DBT), a model sulfur-containing compound, to 2-HBP and sulfate. This organism was grown on a culture medium composed of 2.44 g KH2PO4, 12.03 g Na2HPO4 · 12H2O, 2.0 g NH4Cl, 0.4 g MgCl2 · 6H2O, 0.75 mg CaCl2, 1 mg FeCl3 · 6H2O, 4 mg MnCl2 · 4H2O, 10 g glycerol, and 0.1 mmol DBT in 1 liter of deionized water. One milliliter of a 100 mM solution of DBT in ethanol (anhydrous) was added to the medium.
Synthesis and modification of magnetic nanoparticles.
We synthesized and modified magnetic nanoparticles by the following method. We dissolved 23.5 g FeCl3 · 6H2O and 8.6 g FeCl2 · 4H2O in 600 ml deionized water under N2 with mechanical stirring at 800 rpm and 85°C and then quickly added 30 ml of 7.1 M NH4OH. To the resulting suspension we added dropwise 16 ml of oleic acid over a period of 30 min. After several minutes, we separated the magnetic precipitate by magnetic decantation and washed it with deionized water several times. We modified the magnetic precipitate with about 4 ml of 7.1 M NH4OH to form the hydrophilic magnetic nanoparticles, which in an aqueous solution were monodisperse. The magnetic nanoparticle concentration was expressed in terms of dry weight per unit volume of suspension medium.
Microbial cells coated by magnetic nanoparticles.
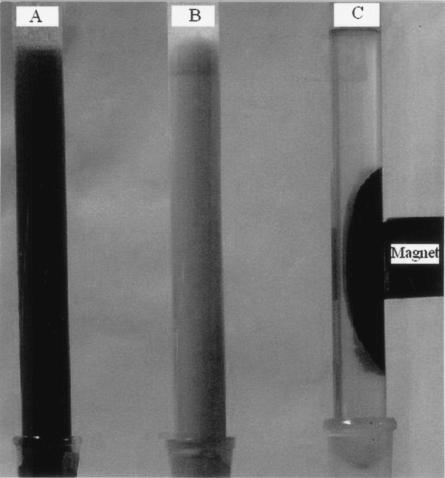
Fifteen milliliters of a magnetic suspension (15 g Fe3O4 nanoparticles per liter of saline water) was mixed with 100 ml of a cell suspension (25 g [dry weight] of cells per liter of saline water). The microbial cells were coated by adsorbing the magnetic nanoparticles. The coated cells could be concentrated on the side of the vessel containing the suspension and separated from the suspension medium by decantation (see Fig. 4).
FIG. 4.
Photographs of P. delafieldii cell suspensions in columns under three different conditions. (A) Cells coated with magnetite nanoparticles; (B) free cells; (C) coated cells concentrated and collected by an external magnet.
Biodesulfurization of model oil.
We prepared a model oil consisting of 2.0 mmol · liter−1 DBT in n-dodecane. The reaction mixtures contained 0.255 g (dry weight) of cells not treated with magnetic nanoparticles for control setups or 0.255 g (dry weight) of cells treated with a defined quantity of magnetic nanoparticles before introduction into the reaction mixtures of experimental setups or 0.255 g (dry weight) of cells immobilized by Celite before introduction into the reaction mixtures of experimental setups. Each cell preparation was suspended in 15 ml phosphate buffer (0.1 M, pH 7.0), and the suspension was mixed with 8 ml of model oil. The reactions were carried out in 100-ml flasks at 30°C on a rotary shaker at 180 rpm.
Analytical methods.
The size and morphology of magnetic nanoparticles were determined by transmission electronic microscopy (TEM) (Hitachi 8100, 200 kV). Each sample was prepared by evaporating a drop of a very dilute nanoparticle suspension on a carbon copper grid.
The cells coated with magnetic nanoparticles were fixed with 3% glutaraldehyde in 0.1 M phosphate buffer, pH 7.0, for 2 h, dehydrated in an alcohol series for 2 h, embedded in an acrylic resin, and allowed to polymerize for 2 days at 60°C. Ultrathin cell sections were viewed and photographed with a TEM at 200 kV. The morphology of cells immobilized on Celite was determined using a scanning electron microscope (SEM) (QUANTA 200; FEI).
Magnetization curves for the coated cells were obtained with a vibrating sample magnetometer (model 155; Digital Measurement System, Inc.).
High-performance liquid chromatography was performed with an Agilent 1100 (Agilent, United States) liquid chromatograph equipped with an autosampler, a reversed-phase Zorbax SB C18 column (4.6 mm by 150 mm; 3.5 μm), and a diode array detector for quantitative assays of DBT (retention time, 5.49 min) and 2-HBP (retention time, 3.29 min) in the dodecane phase. The mobile phase was 90% (vol/vol) methanol in water at a flow rate of 1.0 ml · min−1, using the external standard method at 280 nm.
The specific desulfurization rate was expressed as the amount of sulfur (millimoles) consumed by 1 kg (dry weight) of cells per hour.
RESULTS
Characteristics of magnetic nanoparticles.
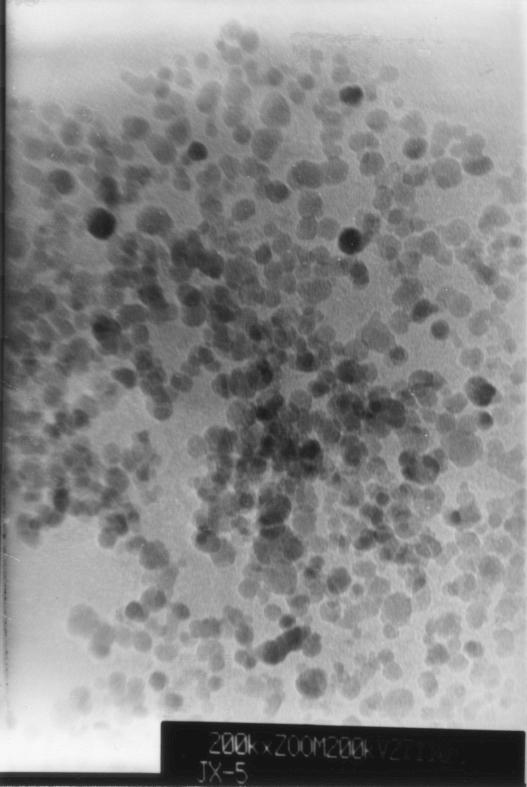
A TEM photomicrograph is shown in Fig. 1. The particle sizes ranged from 10 to 15 nm. Figure 1 shows that the magnetic nanoparticles had a narrow size distribution in aqueous solution. The magnetic nanoparticles in suspension did not settle in over 18 months of storage.
FIG. 1.
TEM images of magnetite nanoparticles.
Characteristics of coated cells and immobilized cells.
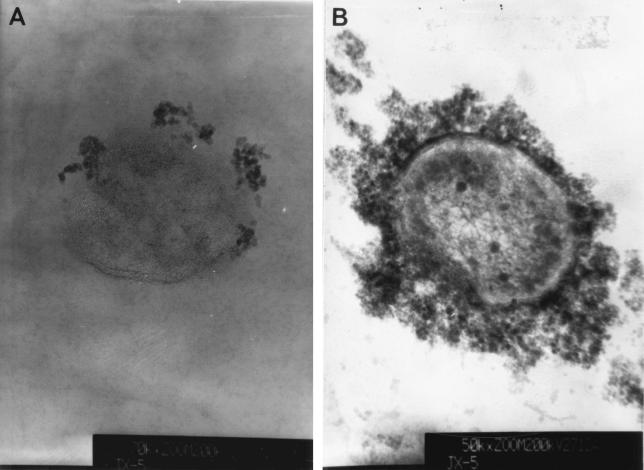
Figure 2 shows TEM images of ultrathin cross sections of cells coated with Fe3O4 nanoparticles. Cells with adsorbed magnetic Fe3O4 nanoparticles, such as those shown in Fig. 2A, were produced by mixing 2.0 g (dry weight) of cells with 100 ml of a preparation containing 0.10 g of Fe3O4 nanoparticles per liter of magnetic suspension, whereas the cells shown in Fig. 2B were produced by mixing 2.0 g (dry weight) of cells with 100 ml of a magnetic suspension containing 25 g of Fe3O4 nanoparticles per liter.
FIG. 2.
TEM images of ultrathin cross sections of cells coated with magnetite nanoparticles.
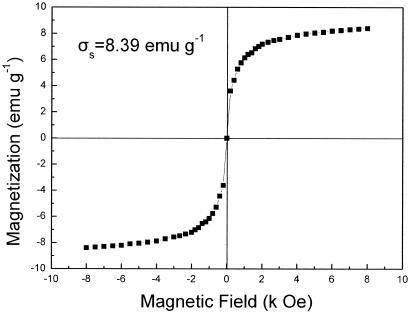
The cells coated with Fe3O4 nanoparticles were superparamagnetic with zero remanence and coercivity, as shown in Fig. 3. The magnetization (δs) was 8.39 emu · g−1. Therefore, the cell-nanoparticle aggregates in aqueous suspension could be easily concentrated with an externally supplied magnetic field and redispersed by gentle shaking after the magnetic field was removed. Figure 4 is a photograph of P. delafieldii cell suspensions in columns under three different conditions taken with a digital camera. When a magnet was touched to the side of a column containing a suspension of cells coated with Fe3O4 nanoparticles, the cells aggregated in the region where the magnet touched the column (Fig. 4C). In this way, the magnetized cells were easily collected. When the magnet was withdrawn from the side of the column and the column was shaken, the cells were readily resuspended, and the suspension had the appearance shown in Fig. 4A. The results of our experiments show that 0.086 g of nanoparticles · g (dry weight) of cells−1 was the optimal ratio. When the ratio was smaller, the cells could not be effectively concentrated in a magnetic field, and when it was larger, an excess of Fe3O4 particles affected the separation of the coated cells by application of a magnetic field.
FIG. 3.
Magnetization curves for the coated cells as determined with a vibrating sample magnetometer. σs, saturation magnetization; emu, electromagnetic unit; Oe, Oersted.
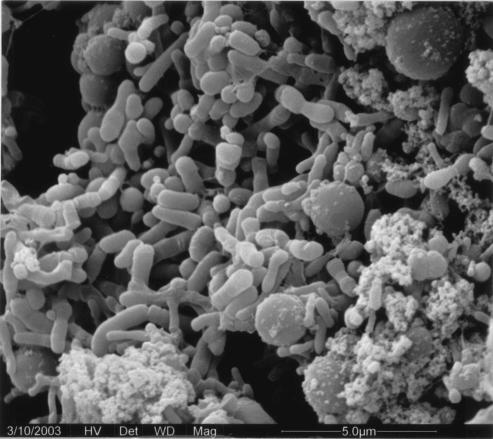
Figure 5 shows an SEM image of P. delafieldii R-8 cells immobilized on Celite. This figure shows that the cells were adsorbed on the surface of the Celite and that some cells partially penetrated pores in the Celite.
FIG. 5.
SEM image of P. delafieldii R-8 immobilized by Celite.
Desulfurization of DBT.
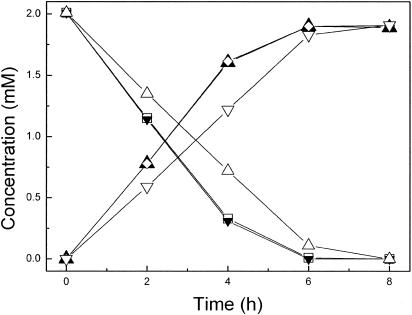
The effect of the magnetic nanoparticles on the desulfurizing activity of P. delafieldii R-8 was studied. Cells were grown in the culture medium described in Materials and Methods. Complete consumption of the sulfur source in 450 ml of medium containing 1.0 × 10−4 mM DBT yielded a cell suspension containing the equivalent of 0.765 g (dry weight) of cells. This suspension was divided into three equal portions, and each portion contained the equivalent of 0.255 g (dry weight) of cells. The cells from one portion of the cell suspension that had been coated with magnetic nanoparticles were concentrated and collected by application of a magnetic field. A second portion of the cell suspension was centrifuged, and the cell pellet was collected. The cells from the third portion of the cell suspension were immobilized by Celite. Each of the three cell preparations was washed three times with saline water (8.5% [wt/vol] NaCl), dried, and then tested separately for biodesulfurization activity in 8 ml of model oil. Figure 6 shows the time courses of consumption of DBT and production of 2-HBP by each of the three cell preparations. The results showed that the coated cells and the free cells exhibited similar time courses and the same desulfurizing activities during the first 4 h, 16.4 mmol · kg (dry weight) of cells−1 · h−1. DBT at a concentration of 2.0 mM was completely desulfurized in 6 h. By contrast, the cells immobilized by Celite desulfurized only 12.6 mmol · kg−1 · h−1 during the first 4 h. The findings for the cells immobilized by Celite were the result of a small loss of cells from the Celite during washing with the saline solution. By comparison, no cells coated with magnetic nanoparticles appeared to have been lost during washing. They thus had greater operational stability than cells immobilized on Celite.
FIG. 6.
Time course of desulfurization of dibenzothiophene by different cell preparations. □, residual DBT concentration after consumption by free cells; ▴, 2-HBP concentration produced by free cells; ▾, residual DBT concentration after consumption by magnetic nanoparticle-coated cells; ⋄, 2-HBP concentration produced by magnetic nanoparticle-coated cells; ▵, residual DBT concentration after consumption by Celite-immobilized cells; ▿, 2-HBP concentration produced by Celite-immobilized cells.
Repeated biodesulfurization by coated cells.
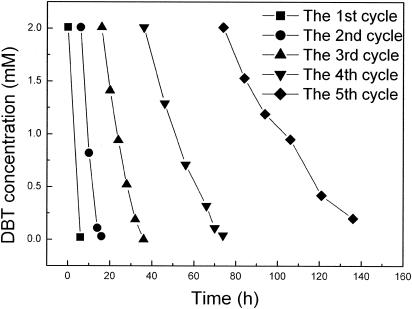
The same cells coated with magnetic nanoparticles were tested repeatedly in a reaction mixture containing 8 ml of model oil and 15 ml of phosphate buffer. Each test was performed until the DBT was consumed completely. At the end of each test, the coated cells were collected by application of a magnetic field and then reused in another test. As shown in Fig. 7, the desulfurization activity of coated cells decreased gradually after a few test cycles. For example, in the first cycle, DBT in 8 ml of model oil was completely consumed in 6 h; in the second cycle, the same amount of DBT was consumed in 10 h; and in the fifth cycle, the DBT was not completely consumed in 60 h. In contrast to cells coated with magnetic nanoparticles, uncoated, free cells could be used only once. They exhibited no desulfurizing activity when they were reused.
FIG. 7.
Repeated biodesulfurization by coated cells.
DISCUSSION
Magnetic nanoparticles.
Magnetic Fe3O4 nanoparticles were prepared by a coprecipitation method. The displacement of air by N2 gas during preparation prevented oxidation of ferrous ion in the aqueous solution and also controlled the particle size. As Fig. 1 shows, the average particle diameter was found to be about 10 to 15 nm, and the particles had superparamagnetic properties (Fig. 3) because the particle diameter was less than 30 nm (8).
It is well known that magnetic Fe3O4 prepared by the coprecipitation method has a large number of hydroxyl groups on its surface in contact with the aqueous phase. The OH groups on the surface of Fe3O4 particles react readily with carboxylic acid head groups of oleic acid molecules. Excess oleic acid is then adsorbed to the prebound oleic acid layer to form a hydrophobic shell. When the magnetic nanoparticles are put into an NH4OH solution, the outer layer of oleic acid on the Fe3O4 surface is transformed into an ammonium salt of oleic acid, which modifies the magnetic nanoparticles so that they exist in a dispersed state in an aqueous phase. Indeed, the magnetic nanoparticles become monodisperse because their surfaces are now hydrophilic.
Cell immobilization and cells coated by magnetic nanoparticles.
Immobilization of a biocatalyst in an appropriate reactor involves its localization on a support medium (5), which allows economic reuse of the biocatalyst under stable conditions. Immobilization helps in making continuous processes more economic by allowing automation and optimization of the investment/capacity ratio.
Adsorption is perhaps the simplest of all the immobilization techniques without mass transfer problems. However, conventional immobilization by adsorption has some drawbacks, such as a loss of cells, a decrease in biomass loading over time, and consequent operational instability. In the process described in the present paper, Fe3O4 nanoparticles were strongly adsorbed on the surfaces of microbial cells and thus coated the cells (Fig. 2) because of the large specific surface area and the high surface energy of the nanoparticles. Furthermore, the presence of a variety of functional groups at the microbial cell surface that interact with ammonium ions of the modified Fe3O4 nanoparticles makes the adsorption even stronger. In our experiment, the Fe3O4 nanoparticles on the cell surface were not washed out by deionized water or other reagents, such as ethanol, saline water, or phosphate buffer. Thus, no cell loss or decrease in biomass loading occurred when cells coated with magnetic nanoparticles were used, as occurs with cells immobilized by adsorption to a carrier (Fig. 6). As Fig. 6 shows, both magnetic nanoparticle-coated cells and free cells had the same desulfurizing activity, which was greater than that of cells immobilized on Celite; i.e., the coated cells did not experience a mass transfer problem. Thus, the new technique not only has an advantage over conventional immobilization by adsorption but also makes the cells magnetic and can overcome some limitations associated with the conventional immobilization by adsorption. The new technique involves cells coated with magnetic nanoparticles (Fig. 2) that can be immobilized by application of a magnetic field. This is in contrast to permanently immobilized cells that are adsorbed to the surface of a support material (Fig. 5).
We found in our experiments that the cells coated with surface-modified magnetic nanoparticles did not settle spontaneously in deionized water, saline water, culture medium, and phosphate buffer. The adsorbed magnetic nanoparticles did not change the density of the cells significantly because the dry weight ratio of the particles to cells was only 0.086 g · g−1 and the volume of the coated cells was only slightly greater than that of uncoated cells.
Biodesulfurization.
Biodesulfurization is a promising technology for the removal of organic sulfur from fossil fuels. It is increasingly attractive because of its high selectivity for organic sulfur compounds in fossil fuels, especially polycyclic and polyheterocyclic sulfur compounds (2, 11). Moreover, it has the potential benefits of low capital and operating costs because of mild conditions (7).
However, the treatment of oils using a biocatalyst suspension has some limitations, such as low volumetric ratios of oil to aqueous suspension and difficulties in biocatalyst recycling. The use of immobilized biocatalysts has been considered to be a potential alternative (13). Desulfurization of oils by immobilized cells has some advantages, including relatively high oil/water volumetric ratios, good storage stability, and low risk of contamination.
So far, only a few reports on the use of biocatalyst immobilization, such as entrapment or adsorption on Celite in biodesulfurization, have been published (4, 10, 15). However, the methods have some limitations, such as a mass transfer problem, loss of cells, and separation of biocatalysts from the treated oils. In the present study, the new technique in which magnetic nanoparticles were used to coat the cells not only had an advantage over conventional cell immobilization by adsorption, but it could also successfully overcome difficulties of conventional cell immobilization, such as mass transfer problems, cell loss, and separation of carrier with adsorbed cells from the reaction mixture at the end of a desulfurization treatment. Cells coated by magnetic nanoparticles appear not to experience a mass transfer problem because we found that the desulfurizing activities of coated and uncoated cells exhibited the same time course (Fig. 6). This is because DBT transfer from the oil to the water and then from the water to the cells limits the rate of DBT metabolism (14). Our findings are consistent with a coating layer of nanoparticles that does not change the hydrophilicity of the cell surface. They are also consistent with an effect of the coating layer on mass transfer that is negligible because the structure of the layer is looser than that of the cell wall. Thus, the coating layer does not interfere with mass transfer of DBT. The coated cells have good stability and can be reused (Fig. 7). The new technique has the advantage of magnetic separation (3).
Therefore, the new technique is rather convenient and easy to perform, and it may be promising for large-scale industrial applications.
Acknowledgments
We acknowledge Mooson Kwauk for revising the manuscript.
We acknowledge the financial support of the State Major Basic Research Development Program (China) (grant G2000048004), the National High Technology Research and Development Program (China) (grant 2002AA213041), and the National Natural Science Foundation of China (grant 30370046).
REFERENCES
- 1.Amanda, K. Y., and K. D. Wisecarver. 1992. Cell immobilization using PVA crosslinked with boric acid. Biotechnol. Bioeng. 39:447-449. [DOI] [PubMed] [Google Scholar]
- 2.Beverly, L. M. F. 1999. Biodesulfurization. Curr. Opin. Microbiol. 2:257-264. [DOI] [PubMed] [Google Scholar]
- 3.Býlkova, Z., M. Slovakova, D. Horak, J. Lenfeld, and J. Churácek. 2002. Enzymes immobilized on magnetic supports: efficient and selective system for protein modification. J. Chromatogr. B 770:177-181. [DOI] [PubMed] [Google Scholar]
- 4.Chang, J. H., Y. K. Chang, H. W. Ryu, and H. N. Chang. 2000. Desulfurization of light gas oil in immobilized-cell systems of Gordona sp. CYKS1 and Nocardia sp. CYKS2. FEMS Microbiol. Lett. 182:309-312. [DOI] [PubMed] [Google Scholar]
- 5.Chotani, G. K., and A. Constaninides. 1984. Immobilized cell cross-flow reactor. Biotechnol. Bioeng. 26:217-220. [DOI] [PubMed] [Google Scholar]
- 6.Gill, I. S., and A. Ballesteros. 2000. Bioencapsulation within synthetic polymers (part 1): sol-gel encapsulation of biologicals. Trends Biotechnol. 18:282-296. [DOI] [PubMed] [Google Scholar]
- 7.Hartdegen, F. J., J. M. Coburn, and R. L. Roberts. 1984. Microbial desulfurization of petroleum. Chem. Eng. Prog. 5:63-67. [Google Scholar]
- 8.Lee, J., T. Isobe, and M. Senna. 1996. Preparation of ultrafine Fe3O4 particles by precipitation in the presence of PVA at high pH. J. Colloid Interf. Sci. 177:90-494. [Google Scholar]
- 9.Luo, M. F., J. M. Xing, Z. X. Gou, S. Li, H. Z. Liu, and J. Y. Chen. 2003. Desulfurization of dibenzothiophene by lyophilized cells of Pseudomonas delafieldii R-8 in the presence of dodecane. Biochem. Eng. J. 13:1-6. [Google Scholar]
- 10.Naito, M., T. Kawamoto, K. Fujino, M. Kobayashi, K. Maruhashi, and A. Tanaka. 2001. Long-term repeated biodesulfurization by immobilized Rhodococcus erythropolis KA2-5-1 cells. Appl. Microbiol. Biotechnol. 55:374-378. [DOI] [PubMed] [Google Scholar]
- 11.Ohshiro, T., and Y. Izumi. 1999. Microbial desulfurization of organic sulfur compounds in petroleum. Biosci. Biotechnol. Biochem. 63:1-9. [DOI] [PubMed] [Google Scholar]
- 12.Pakula, R., and A. Freeman. 1996. A new continuous biofilm bioreactor for immobilized, oil degrading filamentous fungi. Biotechnol. Bioeng. 49:20-25. [DOI] [PubMed] [Google Scholar]
- 13.Setti, L., G. Lanzarini, and P. G. Pifferi. 1996. Immobilized cells for applications in non-conventional systems pp. 777-784. In R. H. Wijffels, R. M. Buitelaar, C. Bucke, and J. Tramper (ed.), Progress in biotechnology. Immobilized cells: basics and applications, vol. 11. Elsevier, Amsterdam, The Netherlands.
- 14.Setti, L., P. Farinelli, S. Di Martino, S. Frassinetti, G. Lanzarini, and P. G. Pifferi. 1999. Developments in destructive and non-structive pathways for selective desulfurization in oil biorefining process. Appl. Microbiol. Biotechnol. 52:111-117. [DOI] [PubMed] [Google Scholar]
- 15.Shan, G. B., J. M. Xing, M. F. Luo, H. Z. Liu, and J. Y. Chen. 2003. Immobilization of Pseudomonas delafieldii R-8 with magnetic polyvinyl alcohol beads and its application in biodesulfurization. Biotechnol. Lett. 25:1977-1981. [DOI] [PubMed] [Google Scholar]