Abstract
The psl gene cluster, comprising 15 cotranscribed genes from Pseudomonas aeruginosa, was recently identified as being involved in exopolysaccharide biosynthesis and biofilm formation. In this study, we investigated the regulation of the psl gene cluster and the function of the first gene in this cluster, the pslA gene. PslA shows strong similarities to UDP-glucose lipid carriers. An isogenic marker-free pslA deletion mutant of P. aeruginosa PAO1 deficient in attachment and biofilm formation was used for complementation studies. The expression of only the pslA gene, comprising a coding region of 1,437 bp, restored the biofilm-forming phenotype of the wild type, indicating that PslA is required for biofilm formation by nonmucoid P. aeruginosa. The promoter region of the psl gene cluster, which encodes PslA-PslO, was identified by rapid amplification of cDNA 5′ ends. Promoter assays using transcriptional fusions to lacZ and gfp indicated a constitutive expression of the psl cluster in planktonic cells and a highly regulated and localized expression in biofilms, respectively. Expression of the psl cluster in biofilms was almost exclusively found in the centers of microcolonies, as revealed by confocal laser scanning microscopy. These data suggest that constitutive expression of the psl operon enables efficient attachment to surfaces and that regulated localized psl operon expression is required for biofilm differentiation.
The gram-negative bacterium Pseudomonas aeruginosa is an important opportunistic human pathogen which causes several life-threatening nosocomial infections in immunocompromised patients and victims with burns (6, 29). Moreover, P. aeruginosa is the major and dominant pathogen in cystic fibrosis (4-8, 13, 15, 38, 39).
The ability to form biofilms is the crucial factor in fatal infections by P. aeruginosa and has made this bacterium a model organism with respect to biofilm formation. During chronic colonization, P. aeruginosa undergoes conversion from a nonmucoid to a mucoid phenotype (16). The most characteristic feature of the mucoid phenotype is the secretion of large amounts of highly viscous exopolysaccharides. The copolymer alginate, which is composed of mannuronic acid and guluronic acid, appears to be the major component of the secreted polysaccharide (9, 31), and besides nucleic acids and proteins, is the key factor in the development of mucoid biofilms which has been intensively studied (5, 16, 26, 30). Biofilms generated by P. aeruginosa have been shown to enhance the resistant colonization of cystic fibrosis-diseased lungs and to confer several advantages to the respective microorganism, such as a high-level resistance to antibiotics and to the killing effect of leukocytes (1, 18).
Recently, it was noted that alginate-negative mutants of P. aeruginosa as well as the nonmucoid wild type are also able to form biofilms. These nonmucoid biofilms showed a different architecture from that of biofilms formed by alginate-overproducing mucoid P. aeruginosa (25, 40). Moreover, evidence was found only recently of a novel exopolysaccharide involved in the formation of nonmucoid biofilms by P. aeruginosa strains (40). An analysis and comparison of the nonmucoid biofilm matrixes of P. aeruginosa wild-type strains and alginate-negative mutants clearly indicated that alginate is not required for biofilm formation and is not even a significant component of biofilms. Nonmucoid biofilms produced by P. aeruginosa wild-type strains or alginate-negative mutants showed no differences with respect to their architecture or antibiotic resistance (40). A detailed analysis of the P. aeruginosa PAO1 genome revealed three putative gene clusters, PA1381 to -1392, PA2231 to -2245, and PA3552 to -3558, harboring genes homologous to exopolysaccharide biosynthesis genes (37). Previously, first investigations showed that the gene cluster PA2231 to -2245 (designated psl) is involved in biofilm formation, since corresponding P. aeruginosa mutants with disrupted psl clusters were severely compromised in biofilm initiation and exhibited thin, unstructured, abnormal biofilms (11, 19, 22).
Since these previous investigations suggested an essential role of the psl gene cluster in the initial biofilm formation of P. aeruginosa, we focused on the functional assignment of the pslA gene (PA2231), which represents the first gene of this cluster, and on the temporally and spatially resolved regulation of the psl operon. An isogenic, marker-free pslA knockout mutant was generated and characterized. Complementation was achieved by heterologous expression of only the pslA gene. Rapid amplification of cDNA 5′ ends (5′-RACE) was used to identify the 5′ end of the mRNA and the promoter region of the psl operon. Reporter gene fusions containing lacZ and stable/unstable gfp as reporters were created in order to investigate the expression of psl in planktonic P. aeruginosa PAO1 and in biofilms, respectively.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth of bacteria.
The P. aeruginosa PAO1 and Escherichia coli strains and the plasmids used for this study are listed in Table 1. E. coli cells were grown at 37°C in Luria-Bertani (LB) broth. P. aeruginosa PAO1 cells were grown at 37°C in either LB broth or Pseudomonas isolation agar. If required, antibiotics were added at the following concentrations: for P. aeruginosa, 150 μg/ml carbenicillin and 150 μg/ml gentamicin; and for E. coli, 10 μg/ml gentamicin, 100 μg/ml ampicillin, and 50 μg/ml kanamycin.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides used for this study
| Strain, plasmid, or oligonucleotide | Relevant characteristics or sequence (5′-3′) | Source or reference |
|---|---|---|
| Strains | ||
| P. aeruginosa PAO1 | Prototroph, nonmucoid | ATCC 15692 |
| P. aeruginosa PAO1ΔpslA | PAO1 ΔpslA mutant, biofilm negative | This study |
| E. coli S17-1 | recA; harbors the tra genes of plasmid RP4 in the chromosome; proA thi-1 | 35 |
| E. coli XL1-Blue | recA1 endA1 gyrA96 thi1 hsdR17 (rK− mK+) supE44 relA1 λ−lac [F′ proAB lacIqZΔM15 Tn10(Tc)] | 3 |
| Plasmids | ||
| pBluescript SK(−) | AmprlacPOZ, T7 and T3 promoter | Stratagene |
| pEX100T | AmproriT sacB lacPOZ′ | 34 |
| pGEM-Teasy | AmprlacPOZ′ | Promega |
| pPS856 | Ampr GmrFRT | 17 |
| pEXpslA | pEX100T containing GM cassette flanked by 5′ and 3′ ends of the pslA gene | |
| pTZ110 | Broad-host-range lacZ fusion vector | 33 |
| pBBR1-MCS5 | GmrlacPOZ′ mob, broad-host-range vector | 20 |
| pBBR1-MCS5::pslA | pBBR1-MCS5 containing EcoRI/BamHI PCR fragment of pslA | This study |
| pSKPpsl | pBluescript SK(−) containing EcoRI/BamHI PCR fragment of psl promoter | This study |
| pTZ110::Ppsl | pTZ110 containing EcoRI/BamHI PCR fragment of psl promoter | This study |
| pProbeAT′ | Broad-host-range gfp (stable form) fusion vector | 24 |
| pProbeAT′::Ppsl | pProbeAT′ containing EcoRI/BamHI PCR fragment of psl promoter | This study |
| pProbe′-gfp[AAV] | Broad-host-range gfp (unstable form) fusion vector | |
| pProbe′-gfp[AAV]::Ppsl | pProbe′-gfp[AAV] containing EcoRI/BamHI PCR fragment of psl promoter | 24 |
| pProbe′-gfp[AAV]::ΩGm-Ppsl | pProbe′-gfp[AAV]::Ppsl containing a gentamicin restistance gene cassette in the opposite direction to that of the gfp gene | This study |
| pGEM-Teasy::RACE | pGEM-Teasy containing 5′-RACE PCR product | This study |
| Oligonucleotides | ||
| PA2231-KO-UA | GTAGATAGCCTGTCGCTGACACGGG | This study |
| PA2231-KO-DA | AAAAAGGATCCGATCACCGTCCGTTGCAGGTACATG | This study |
| PA2231-KO-UB | AAAAAGGATCCCGGCTGATCTGGGTCTTCAAGTTCC | This study |
| PA2231-KO-DB | TCAGTAGACTTCCTTGGTCAGGAG | This study |
| PA2231-UE | AAAAAGAATTCTGGACTGCCCGTGATCGGCAGAGC | This study |
| PA2231-DB | AAAAAGGATCCGCAGGGGATCAGCGGGGCGACGGC | This study |
| PpslUE | AAAAAGAATTCCCGAAATGGCACGAGGCGGCGCTG | This study |
| PpslDB | AAAAAGGATCCGCCGATCACGGGCAGTCCATTGA | This study |
| RACE-primer1 | CAGGCCCAGGCCCTGGTACATGAACAACAGCAGGC | This study |
| RACE-primer2 | CGTCGGAGTAGACGTCCAGTGCCTGGAAC | This study |
| RACE-primer3 | GCGCAGCTCCACGGGAACCAGGCCGGGG | This study |
For the cultivation of biofilms, M9 medium containing 48 mM Na2HPO4, 22 mM KH2PO4, 9 mM NaCl, 19 mM NH4Cl, 2 mM MgSO4, 100 μM CaCl2, and 5 mM glucose was used.
Isolation, analysis, manipulation, and transfer of DNA.
Plasmid DNA and DNA restriction fragments were isolated and analyzed by standard methods (32). Competent E. coli cells were prepared and transformed by using the CaCl2 procedure (14). Conjugations of E. coli S17-1 (donor) harboring hybrid plasmids and P. aeruginosa PAO1 (recipient) were performed on solidified NB medium as described by Friedrich et al. (12). DNA sequences of new plasmid constructs were confirmed by DNA sequencing according to the chain termination method using an ABI370 automatic sequencer (Applied Biosystems).
Generation of isogenic pslA knockout mutant of P. aeruginosa PAO1.
All oligonucleotides used for this study are summarized in Table 1, and PCRs were performed with high-fidelity Pfx polymerase. The isogenic pslA knockout mutant was obtained according to the method of Pham et al. (28). The following primers were used for PCR: the pslA 5′-end region was amplified using PA2231-KO-UA and PA2231-KO-DA, and the pslA 3′-end region was amplified using PA2231-KO-UB and PA2231-KO-DB. The isogenic mutant was verified by PCR using the primers PA2231UE and PA2231DB, which hybridize to 31 bp upstream and 32 bp downstream of the pslA start and stop codons, respectively. The PCR product was analyzed by restriction site mapping and DNA sequencing. The inserted gentamicin cassette was removed by using plasmid pFLP2 as described elsewhere (17), resulting in P. aeruginosa PAO1ΔpslA.
Plasmid constructions.
For complementation of the isogenic knockout mutant P. aeruginosa PAO1ΔpslA, the pslA gene was amplified by a tailed PCR using the primers PA2231UE and PA2231DB together with genomic DNA from P. aeruginosa PAO1 as template DNA. The PCR product was isolated, hydrolyzed with EcoRI (for which a site was present in the upstream primer) and BamHI (for which a site was present in the downstream primer), and cloned into the broad-host-range vector pBBR1-MCS5. In the resulting plasmid, pBBR1-MCS5::pslA, the pslA gene was arranged colinear to and downstream of the lacZ promoter. The mobilizing strain E. coli S17-1 was transformed with pBBR1-MCS5::pslA and was then used as a donor strain in a conjugation experiment, by which the plasmid was transferred to P. aeruginosa PAO1ΔpslA.
For construction of the broad-host-range promoter-probe vectors pTZ110::Ppsl, pProbe-AT′::Ppsl, and pProbe′-gfp[AAV]::ΩGm-Ppsl, the following procedures were employed. A 1,007-bp upstream region of pslA comprising the putative promoter region of the psl gene cluster was amplified by a tailed PCR using the primers PpslUE and PpslDB (Table 1) and genomic DNA from P. aeruginosa PAO1 as template DNA. The PCR product was digested with EcoRI (for which a site was present in the upstream primer) and BamHI (for which a site was present in the downstream primer) and cloned into correspondingly hydrolyzed pBluescript SK(−), resulting in the plasmid pSKPpsl. The PCR product was analyzed by restriction site mapping and DNA sequencing.
The putative promoter region Ppsl was isolated from EcoRI/BamHI-hydrolyzed pSKPpslA and cloned into the corresponding sites of pTZ110, pProbe-AT′, and pProbe′-gfp[AAV]. In the resulting plasmids, pTZ110::Ppsl, pProbe-AT′::Ppsl, and pProbe′-gfp[AAV]::Ppsl, respectively, the putative promoter region was arranged colinear to and upstream of the lacZ or gfp reporter gene. To enable stable propagation, a gentamicin resistance cassette was inserted into the EcoRI site of pProbe′-gfp[AAV]::Ppsl. In the resulting hybrid plasmid, pProbe′-gfp[AAV]::ΩGm-Ppsl, the gentamicin resistance gene was cloned upstream of and in the opposite direction to the gfp gene. Cells of E. coli S17-1 were transformed with pTZ110::Ppsl, pProbe-AT′::Ppsl, and pProbe′-gfp[AAV]::ΩGm-Ppsl, and the resulting transformants were then used as donor strains in a conjugation experiment, by which the plasmids were transferred to P. aeruginosa PAO1.
Plasmid stability was confirmed by plasmid isolation over many subcultures.
β-Galactosidase assay.
β-Galactosidase activity was measured as described by Miller (23) and is expressed in Miller units (MU). The data presented below are the results obtained from three independent experiments. The variance is indicated by error bars in the figures.
5′-RACE.
5′-RACE was used to determine the 5′ end of the pslA mRNA transcript. It was carried out using a commercial 5′/3′-RACE kit (Roche) according to the manufacturer's instructions. Total RNA was isolated from a P. aeruginosa PAO1 culture grown in LB medium with an optical density at 600 nm of 1.0 by using an SV total RNA isolation kit (Promega). The primer used for the synthesis of cDNA (RACE-primer1) and the first and second nested primers (RACE-primer2 and RACE-primer3) are listed in Table 1. The cDNA was purified using a High Pure PCR product purification kit (Roche). The final PCR product of the 5′-RACE assay was cloned into the pGEM-Teasy vector (Promega) and analyzed by DNA sequencing.
Biofilm experiments.
Abiotic solid surface assay (SSA) biofilm formation was analyzed in polyethylene 96-well microtiter plates after 20 h of incubation at 37°C as described previously (10, 27). After crystal violet staining, the absorbance was measured at 595 nm using a microtiter plate reader (Anthos Labtec, Austria).
For the temporal and spatial resolution of psl operon expression in biofilms, biofilms were grown in continuous-culture flow cells as described previously (28). P. aeruginosa strains were grown in continuous-culture flow cells (channel dimensions, 1 × 4 × 40 mm) at room temperature as described previously (28). Channels were inoculated with 0.5 ml of early-stationary-phase cultures containing approximately 1 × 109 cells ml−1 and incubated without flow for 1 h at room temperature. Flow was then started with a mean flow velocity in the flow cells of 0.2 mm s−1, corresponding to laminar flow with a Reynolds number of 0.02. Cells expressing the psl-gfp transcriptional fusion in biofilms were visualized using a confocal laser scanning microscope (FluoView500; Olympus) with fluorescein isothiocyanate optical filters. The magnification used was ×1,000 for all images.
The attachment assay was carried out in wells of tissue culture plates. Overnight cultures (16 h at 37°C) were adjusted to an optical density at 600 nm of 2.0 and inoculated into wells. Cells were allowed to adhere for 1 h before being washed three times using a multichannel pipette to remove unattached cells. Attached cells were stained using SYTO9 (Molecular Probes Inc., Eugene, Oreg.). The number of attached cells was analyzed by obtaining the relative fluorescence using a plate fluorimeter (Wallac Victor 2; Perkin-Elmer). Mean adhesion values for each strain were determined for 16 wells, and similar results were observed in three replicate attachment experiments.
RESULTS
Molecular characterization of the pslA gene.
Recently, it has been shown that pslA (PA2231) is part of the psl operon comprising 15 cotranscribed genes which are involved in the synthesis of an exopolysaccharide that is essential for P. aeruginosa PAO1 biofilm formation (11, 19, 22). To evaluate the essential role of pslA in biofilm formation, we generated a nonpolar isogenic pslA knockout mutant of P. aeruginosa PAO1 as described in Materials and Methods. The corresponding isogenic, marker-free pslA knockout mutant, P. aeruginosa PAO1ΔpslA, contained a deletion of 0.52 kbp in the chromosomal pslA gene and was impaired in attachment and biofilm formation. The mutant showed about 30% less attachment to tissue culture plates than the respective wild type (data not shown). The expected biofilm-negative phenotype of this mutant strain was confirmed by an abiotic SSA biofilm formation assay (10). P. aeruginosa PAO1ΔpslA was not able to initiate biofilm formation when grown overnight in the wells of a microtiter dish.
For the complementation of P. aeruginosa PAO1ΔpslA, the coding region of the pslA gene was cloned into the broad-host-range vector pBBR1-MCS5, resulting in plasmid pBBR1-MCS5::pslA. This plasmid was introduced into P. aeruginosa PAO1ΔpslA. The ability to produce biofilms was successfully restored in this mutant strain harboring pBBR1-MCS5::pslA (data not shown).
An analysis of the PslA sequence using TMHMM (21, 36) revealed five putative transmembrane helices, at amino acid positions 26 to 45, 54 to 72, 88 to 104, 117 to 137, and 289 to 310. A putative cleavage site between amino acid positions 34 and 35 is detectable using SignalP 3.0 (2). A Pfam database search revealed conserved protein domains, Pfam 02397 and Pfam 02719, which represent conserved regions of different bacterial sugar transferases and diverse bacterial polysaccharide biosynthesis proteins and putative epimerases, respectively.
Identification of the promoter region of the psl operon upstream of the pslA gene.
To determine the transcription start site of the psl gene cluster, 5′-RACE was performed as described in Materials and Methods, using RNA isolated from P. aeruginosa cells in the late logarithmic growth stage. The final 5′-RACE PCR product was cloned into pGEM-Teasy (Promega), resulting in pGEM-Teasy::RACE, and sequenced. The result of the 5′-RACE experiment is shown in Fig. 1.
FIG. 1.
Promoter region of psl operon. The transcription start site A (+1) is located 41 bp upstream of the translation start site of pslA. A putative σ70-dependent promoter is indicated.
Evidence was provided that the transcription start site is located 41 nucleotides (nt) upstream of the pslA translational start site. A putative σ70 promoter region was found 26 nt upstream of the transcription start site (Fig. 1).
Expression of psl operon in planktonic cells.
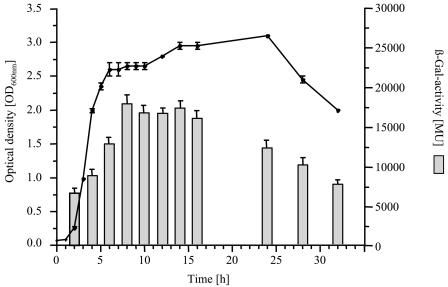
In order to investigate the expression of the psl operon in P. aeruginosa PAO1, we constructed plasmid pTZ110::Ppsl, which contains the identified promoter region of the psl operon upstream of lacZ as a transcriptional reporter fusion. P. aeruginosa PAO1(pTZ110::Ppsl) was grown under planktonic growth conditions. Growth and the β-galactosidase activity were measured spectrophotometrically over a period of 32 h and are shown in Fig. 2. During the complete experiment, high levels of β-galactosidase activity were measured, ranging from 7,000 MU at the beginning of the exponential growth phase to 18,000 MU at the beginning of the stationary growth phase, suggesting a constitutive expression under planktonic growth conditions. Cells of P. aeruginosa PAO1 harboring only the vector pTZ110 showed a similar growth behavior and revealed a β-galactosidase activity of <200 MU (data not shown).
FIG. 2.
Analysis of psl operon regulation using a transcriptional fusion of the identified promoter region to lacZ. The growth and β-galactosidase activity of P. aeruginosa PAO1(pTZ110::Ppsl) under planktonic culture conditions are shown.
Expression of psl operon in biofilms.
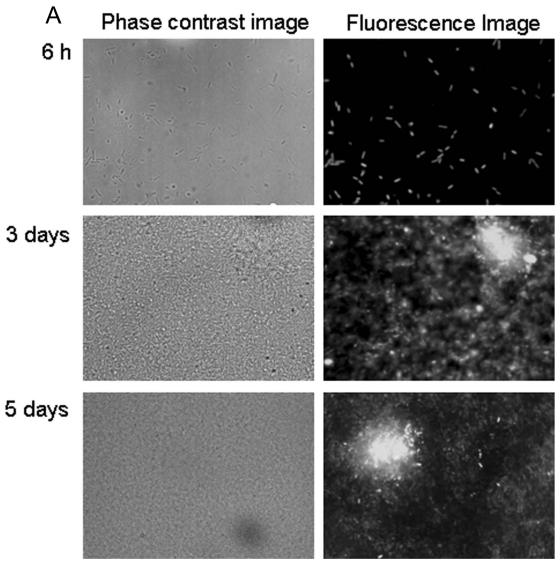
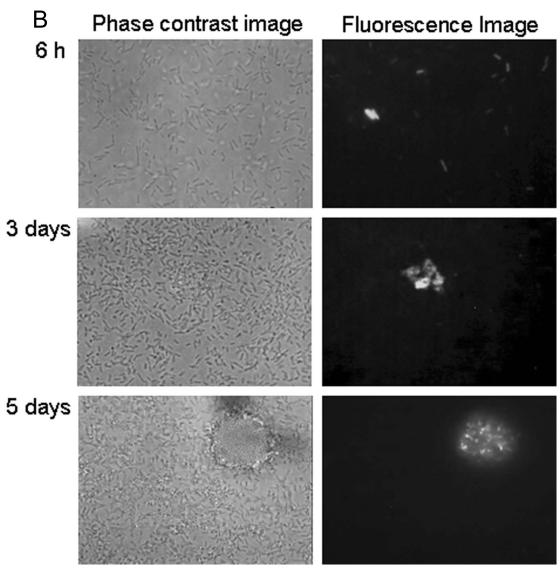
To monitor the expression of the psl operon in biofilms, the identified promoter region upstream of the pslA gene was transcriptionally fused to genes encoding stable and unstable green fluorescent protein (GFP). With stable GFP, nearly all cells were fluorescent 6 h after inoculation (Fig. 3A). After 3 and 5 days, GFP fluorescence was considerably and increasingly brighter in the microcolonies. Enhanced GFP expression in microcolonies is visible at the single-cell level, i.e., individual cells in microcolonies have clearly upregulated GFP production compared to those in the bulk biofilm (Fig. 3A). With unstable GFP, these cells exhibited weak fluorescence. The exposure times necessary to capture the images were 2 to 4 seconds, in contrast to 0.2 to 0.4 seconds for stable GFP. There was some variation in GFP fluorescence after 6 h; most cells showed weak fluorescence, but some cells were very bright, and others showed no fluorescence at all (Fig. 3B). After 3 and 5 days of biofilm development, GFP production was detectable only inside the microcolonies (Fig. 3B). Many regions of the biofilm without microcolonies showed no fluorescence at all. The production of unstable GFP occurred almost exclusively in the microcolonies.
FIG. 3.
Expression of stable gfp (A) and unstable gfp (B) under control of the psl promoter in P. aeruginosa PAO1 biofilms.
DISCUSSION
The psl gene cluster, comprising 15 cotranscribed genes (pslA to pslO) encoding proteins involved in exopolysaccharide biosynthesis, was recently identified by three independent research groups as playing an important role in biofilm formation by nonmucoid P. aeruginosa (11, 19, 22). In this study, we focused on the role of pslA, encoding a putative UDP-glucose carrier protein, in biofilm formation and on the regulation of the entire psl operon in planktonic and biofilm cells. A marker-free pslA deletion mutant was constructed to enable complementation studies by expressing only the pslA gene. A comparative attachment analysis indicated that P. aeruginosa PAO1ΔpslA was impaired in attachment, suggesting a role of the psl operon-mediated exopolysaccharide in the attachment process. This mutant was also strongly impaired in biofilm formation, as observed for other mutants with mutations affecting psl genes (11, 19, 22). A plasmid mediating the expression of only the 1,437 bp comprising the coding region of the pslA gene under the control of the lac promoter showed a restoration of biofilm development in P. aeruginosa PAO1ΔpslA to the wild-type level. In a previous study, complementation of a pslA null mutant of P. aeruginosa could not be achieved with a plasmid containing the genes pslA to pslC (22). Jackson and coworkers (19) isolated a pslA-pslB insertion mutant which could be restored to wild-type biofilm development by using a cosmid with a 22-kb insert comprising the genes pslA to pslF. In this study, we identified the designated pslA gene as a functional gene essential for biofilm development in nonmucoid P. aeruginosa and capable of complementing an isogenic pslA deletion mutant to have wild-type biofilm formation.
Since the psl genes are cotranscribed and the pslA gene is the first gene in this operon, we identified the promoter region upstream of pslA. 5′-RACE was used to locate the transcriptional start point 41 nt upstream of the start codon of pslA. Similarities to σ70 promoter sequences indicated that the psl operon is constitutively expressed (Fig. 1).
In order to understand the regulation of the psl operon, which plays a crucial role in biofilm development, we constructed transcriptional fusions of reporter genes with the identified psl promoter region comprising 1,007 bp upstream of the translational start codon of pslA. With planktonic cells, an analysis of the lacZ fusion indicated a deregulated constitutive expression of the psl operon (Fig. 2). A similar observation was made when a stable gfp fusion was investigated to study the localized regulation of the psl operon in biofilms. Almost all cells showed a rather high level of fluorescence after early attachment to the surface (Fig. 3A). However, after 3 and 5 days of biofilm development, the induction of the psl operon appears to be increasingly localized to the centers of microcolonies (Fig. 3A). To achieve a higher temporal resolution of psl operon expression, we used unstable gfp fusions. These data indicated that after attachment to the surface, the psl operon is repressed (Fig. 3B). However, with increasing biofilm development and microcolony formation, the psl operon is induced and localized to the centers of microcolonies (Fig. 3B). Since we found an important role for pslA in attachment, the constitutive expression of the psl operon might be required for efficient attachment to surfaces mediated by the synthesized exopolysaccharide. After attachment, psl operon expression is repressed and increasingly localizes to the centers of microcolonies, which strongly suggests an important role in biofilm differentiation. Future experiments will comprise the identification of factors involved in the regulation of the psl operon.
Acknowledgments
This study was supported by the Massey University Research Fund and by research grant Re1097/6-1 to B.R. from the Deutsche Forschungsgemeinschaft.
We thank H. P. Schweizer, W. G. Miller, and S. E. Lindow for the provision of various very useful vectors.
REFERENCES
- 1.Bayer, A. S., D. P. Speert, S. Park, J. Tu, M. Witt, C. C. Nast, and D. C. Norman. 1991. Functional role of mucoid exopolysaccharide (alginate) in antibiotic-induced and polymorphonuclear leukocyte-mediated killing of Pseudomonas aeruginosa. Infect. Immun. 59:302-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 16:783-795. [DOI] [PubMed] [Google Scholar]
- 3.Bullock, W. O., J. M. Fernandez, and J. M. Stuart. 1987. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques 5:376-379. [Google Scholar]
- 4.Collins, F. S. 1992. Cystic fibrosis: molecular biology and therapeutic implications. Science 256:774-779. [DOI] [PubMed] [Google Scholar]
- 5.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 280:1318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Cunha, B. A. 2001. Nosocomial pneumonia. Diagnostic and therapeutic considerations. Med. Clin. N. Am. 85:79-114. [DOI] [PubMed] [Google Scholar]
- 7.De Braekeleer, M., and J. Daigneault. 1992. Spatial disruption of the DF508 mutation in cystic fibrosis: a review. Hum. Biol. 64:167-174. [PubMed] [Google Scholar]
- 8.Eberl, L., and B. Tummler. 2004. Pseudomonas aeruginosa and Burkholderia cepacia in cystic fibrosis: genome evolution, interaction and adaptation. Int. J. Med. Microbiol. 294:123-131. [DOI] [PubMed] [Google Scholar]
- 9.Evans, L. R., and A. Linker. 1973. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J. Bacteriol. 116:295-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman, L., and R. Kolter. 2004. Genes involved in matrix formation of Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 51:675-690. [DOI] [PubMed] [Google Scholar]
- 11.Friedman, L., and R. Kolter. 2004. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol. 186:4457-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedrich, B., C. Hogrefe, and H. G. Schlegel. 1981. Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J. Bacteriol. 147:198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govan, J. R., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 15.Haussler, S. 2004. Biofilm formation by the small colony variant phenotype of Pseudomonas aeruginosa. Environ. Microbiol. 6:546-551. [DOI] [PubMed] [Google Scholar]
- 16.Hentzer, M., G. M. Teitzel, G. J. Balzer, A. Heydorn, S. Molin, M. Givskov, and M. R. Parsek. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 183:5395-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 18.Hoiby, N. 1993. Antibiotic therapy for chronic infection of Pseudomonas in the lung. Annu. Rev. Med. 44:1-10. [DOI] [PubMed] [Google Scholar]
- 19.Jackson, K. D., M. Starkey, S. Kremer, M. R. Parsek, and D. J. Wozniak. 2004. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 186:4466-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovach, M. E., P. H. Elzer, S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 21.Krogh, A., B. Larsson, G. von Heijne, and E. L. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 22.Matsukawa, M., and E. P. Greenberg. 2004. Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J. Bacteriol. 186:4449-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 24.Miller, W. G., J. H. J. Leveau, and S. E. Lindow. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant-Microbe Interact. 13:1243-1250. [DOI] [PubMed] [Google Scholar]
- 25.Nivens, D. E., D. E. Ohman, J. Williams, and M. J. Franklin. 2001. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J. Bacteriol. 183:1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 27.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 28.Pham, T. H., J. S. Webb, and B. H. A. Rehm. 2004. The role of polyhydroxyalkanoate biosynthesis by Pseudomonas aeruginosa in rhamnolipid and alginate production as well as stress tolerance and biofilm formation. Microbiology 150:3405-3413. [DOI] [PubMed] [Google Scholar]
- 29.Pruitt, B. A., Jr., A. T. McManus, S. H. Kim, and C. W. Goodwin. 1998. Burn wound infections: current status. World J. Surg. 22:135-145. [DOI] [PubMed] [Google Scholar]
- 30.Rehm, B. H. A. 2002. Alginates from bacteria. Biopolymers Polysaccharides 5:179-212. [Google Scholar]
- 31.Rehm, B. H. A., and S. Valla. 1997. Bacterial alginates: biosynthesis and applications. Appl. Microbiol. Biotechnol. 48:281-288. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 33.Schweizer, H. P., and R. Chuanchuen. 2001. Small broad-host-range lacZ operon fusion vector with low background activity. BioTechniques 31:1258-1262. [DOI] [PubMed] [Google Scholar]
- 34.Schweizer, H. P., and T. T. Hoang. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15-22. [DOI] [PubMed] [Google Scholar]
- 35.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-831. [Google Scholar]
- 36.Sonnhammer, E. L. L., G. von Heijne, and A. Krogh. 1998. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 6:175-182. [PubMed] [Google Scholar]
- 37.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. F. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen. J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 306:959-964. [DOI] [PubMed] [Google Scholar]
- 38.Tümmler, B., and C. Kiewitz. 1999. Cystic fibrosis: an inherited susceptibility to bacterial respiratory infections. Mol. Med. Today 5:351-358. [DOI] [PubMed] [Google Scholar]
- 39.Welsh, M. J., and A. E. Smith. 1995. Cystic fibrosis. Sci. Am. 273:52-59. [DOI] [PubMed] [Google Scholar]
- 40.Wozniak, D. J., T. J. O. Wyckhoff, M. Starkey, R. Keyser, P. Azadi, G. A. O'Toole, and M. R. Parsek. 2003. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 100:7907-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]