Abstract
Small subunit 16S rRNA sequences, growth temperatures, and phylogenetic relationships have been established for 129 bacterial isolates recovered under aerobic growth conditions from different regions of a 22-m ice core from the Muztag Ata Mountain glacier on the Pamirs Plateau (China). Only 11% were psychrophiles (grew at 2°C or −2°C up to ∼20°C), although the majority (82%) were psychrotolerant (grew at 2°C or −2°C up to 37°C). The majority of the isolates had 16S rRNA sequences similar to previously determined sequences, ranging from 85% to 100% identical to database sequences. Based on their 16S rRNA sequences, 42.6% of the isolates were high-G+C (HGC) gram-positive bacteria, 23.3% were γ-Proteobacteria, 14.7% were α-Proteobacteria, 14.7% were Flavobacteria, and 4.7% were low-G+C (LGC) gram-positive bacteria. There were clear differences in the depth distribution, with Proteobacteria, HGC/Cytophaga-Flavobacterium-Bacteroides (CFB), Proteobacteria, LGC/CFB/HGC, Cryobacterium psychrophilum, HGC/CFB, Proteobacteria/HGC/CFB, and HGC/CFB being the predominant isolates from ice that originated from 2.7 to 3.8, 6.2, 7.5, 8.3, 9.0, 9.7, 12.5, and 15.3 m below the surface, respectively. This layered distribution of bacterial isolates presumably reflects both differences in bacteria inhabiting the glacier's surface, differences in bacteria deposited serendipitously on the glacier's surface by wind and snowfall, and nutrient availability within the ice.
Previous studies have recovered bacteria from ice cores retrieved from polar and low-middle-altitude mountain glaciers. For example, Proteobacteria, low-G+C (LGC) and high-G+C (HGC) gram-positive bacteria, Marinobacter, Flectobacillus, and Flavobacterium were recovered from the Malan and Guliya ice core from the Tibetan Plateau (7, 9, 42, 51) and members of the Proteobacteria, HGC, LGC, and Flavobacterium were also predominantly isolated from polar glacial ice (7, 8, 9, 11, 20, 25). Anaerobic Proteobacteria together with members of the Thermus, Bacteroides, Eubacterium, and Clostridium genera were isolated from the lowest region of the GISP 2 ice core drilled from the Greenland glacier (30). Based on their 16S rRNA sequences, most of the ice core isolates have close relatives in other terrestrial and marine environments, particularly in sea ice, permafrost soil, and cold deep marine sediments (7, 11, 43, 51). This observation suggests that there may be bacterial species that are ubiquitous in cold environments and predominant in glacial ice, but this has not been established. Few studies have addressed the variation in bacterial species that might be isolated from one glacial location at different depths within an ice core. To survive, the bacteria isolated from a glacial ice core have to overcome extreme cold, desiccation, and minimal nutrient availability. With increasing depth, the difference in the phylum of bacteria recovered may help understand the bacterial activities and their roles within the overall glacial environment.
The Muztag Ata glacier, located on the far western margin of China and east of the Pamirs Plateau, has a summit elevation of 7,546 m above sea level (a.s.l.). The mean annual air temperature is approximately −20°C at 6,300 m a.s.l.; however, in some regions, perennial glacial ice extends downwards to 4,300 m a.s.l. The Muztag Ata glacier is one of the world's most stable freshwater-ice environments with well-documented paleo-environmental records. Here we report the diversity of bacteria that were isolated from different depths within the Muztag Ata glacier. Previous studies related the microbial populations isolated from Arctic and Antarctic glaciers to past climate changes, and we also observed a correlation between high bacterial input, via atmospheric transport, and cold climate conditions revealed by δ-18O measurements in the Malan ice core drilled from the Tibetan plateau (47). But we did not determine if there were changes in the recoverable population of bacteria at different depths from within the ice core. We undertook this study to know what are the main bacteria isolated from the ice samples, identify our isolates, and present their characteristics, such as their growth temperatures and phylogenetic relatedness to each other and to known microbes. The results obtained revealed that the membership of the bacterial population that is recovered changes at different depths within the Muztag Ata glacier.
MATERIALS AND METHODS
Extraction of the ice core.
Snow (3 m) was removed from the surface of the Muztag Ata glacier, and the ice core (10-cm diameter, 22 m long) was then drilled at 6,350 m a.s.l. on the Pamirs Plateau (75°04′E, 38°17′N). The air temperature (August 2002) ranged from −15°C to 0°C, but the temperature of the ice in the borehole was −20.85°C. Visual inspection of the recovered ice core revealed many thin ice stratifications but no meltwater features consistent with particulates, including bacteria, being held permanently in position after immurement. The 22.4-m-long ice core should therefore provide a valuable chronological resource for climatological and microbiological studies.
Ice core sampling.
The ice core was split lengthwise into four sections, one of which was consumed for this study. The ice core contained both firn (granular, compacted snow) and ice and was therefore processed by a modification of the procedure described by Priscu et al. (25). Sterile gloves, clean laboratory clothing, and hair coverings were worn at all times during the ice core handling procedures, which were always undertaken at temperatures below 20°C within a sterile, positive-pressure laminar flow hood. An annulus (10 mm) was cut successively three times from the surface of each core sample using three clean, sterilized saw-tooth knifes. The remaining inner core was washed, and samples were allowed to melt at 4°C in covered, autoclaved containers. A control core was generated using frozen autoclaved water that was then exposed to all the storage and handling procedures. Plating water samples melted from this control core demonstrated that the level of contamination introduced during handling was below that detectable by the cultivation procedures employed.
Bacterial isolation.
The particulates in samples of meltwater (∼100 ml) were collected by filtration using 0.2-μm-pore-size filters over a period of 1 to 2 h and then resuspended in 4 ml of phosphate-buffered physiological saline. Aliquots (200 μl) of the suspensions generated were spread on the surface of agar-solidified low-nutrient peptone, yeast extract, glucose, and vitamin medium (38), as used previously to isolate bacteria from cold Antarctic soils and rocks (31, 32). The plates were incubated for 2 or 15 weeks at 4°C. To obtain pure cultures, isolates that formed colonies with visually different morphologies were restreaked on peptone, yeast extract, glucose, and vitamin medium and LB (0.2× concentration) agar plates. A total of 129 isolates were obtained in pure culture from samples of the Muztag Ata glacial ice. Cells generated in L broth cultures of each isolate were concentrated by centrifugation, frozen, and stored at −70°C in L broth containing 7% (vol/vol) dimethyl sulfoxide for use in inoculations in later studies.
Growth at different temperatures.
The ability of each isolate to grow and form colonies on the plates containing 0.2× L medium incubated at −2°C, 4°C, 15°C, 25°C, 37°C, and 45°C was determined. The growth of representative isolates at each temperature in liquid culture was also determined. Cultures were grown, with shaking (100 rpm), in side arm flasks incubated in a temperature-regulated water bath, with the optical density at 600 nm (OD600) measured at regular intervals.
Amplification of small subunit 16S rRNA genes.
Genomic DNA was isolated using the chloroform-isoamyl alcohol extraction procedure (12) from the cells grown in 1.5 ml L broth cultures of each isolate. 16S rRNA genes were PCR amplified from 20-ng aliquots of this genomic DNA using the protocols described by Voytek and Ward (39) in 25-μl reaction mixtures with the universal bacterial primers 8f (5′-AGAGTTTGATCATGGCTCAG) and 1492 (5′-CGGTTACCTTGTTACGACTT) (16, 40), which correspond to regions 8 through 27, and 1492 through 1511, of the Escherichia coli 16S rRNA molecule, respectively (5).
ARDRA.
Amplified rRNA restriction analysis (ARDRA) was used to evaluate the overall diversity of the isolates. A 1.5-kbp region of 16S rRNA gene was amplified and subjected to HaeIII (TakaRa, Japan) digestion (15 U HaeIII/200 to 400 ng for 3 h at 37°C). The restriction fragments generated were separated by electrophoresis through 1% agarose gels, stained with ethidium bromide, and visualized by UV irradiation.
Sequencing of PCR products and phylogenetic analysis.
PCR products were passed through PCR purification columns (TakaRa) and ligated into pMD-T (TakaRa), and the resulting plasmids were transformed into competent E. coli JM109 cells and partially sequenced directly using primer 8f with an ABI PRISM 377-96 sequencer. To evaluate diversity, 4 to 10 isolates from each ARDRA pattern were sequenced. All sequences were validated using the CHECK-CHIMERA software of the Ribosomal Database Project (18). For further phylogenetic analyses, the 16S rRNA sequences (650 to 800 bases) of the isolates were matched with those in the National Center for Biotechnology Information nucleotide database by using BLAST searching (1) and were assigned to major groups (α-Proteobacteria, γ-Proteobacteria, and everything else) (2). The most similar reference sequences were downloaded and aligned with the isolate sequences using the Clustal X (36). The multiple alignments were used in maximum-parsimony and distance analyses utilizing the Mega (Molecular Evolutionary Genetics Analysis, 1.01) (14) package. Phylogenetic relationships of the sequences were constructed by using the maximum-parsimony method (heuristic search) and the distance method (neighbor-joining algorithm [28] and p-distance model in the Mega package), with bootstrap analysis (100 replicates) performed by using the p-distance model (13) in the Mega package (14) for the last method. The sequence of the Halobacterium salinarum 16S rRNA gene AB074299 was used as an out-group. The previous reports showed that the phylogenetic assignments obtained from the partial and full-length sequences were very similar (3, 15, 19, 29). Since we were interested only in determining the dominant groups of glacial bacteria, a partial sequence analysis was justified.
Nucleotide sequence accession number.
The sequences obtained have been deposited in the GenBank nucleotide sequence database under accession numbers AY526633 to AY526716.
RESULTS
Effects of temperature on growth.
During the initial isolation, colonies were observed on some plates after 2 weeks at 4°C, but in most cases, colonies were first visible ∼1 month after plating. There were, however, some plates on which colonies were never observed (or where these samples had microbial biomasses too low for detection by using this method) even after extended periods of incubation at 4°C. A total of 129 isolates were chosen for further study that originated from ice from 2.89 to 15.59, 17.99, 21.38, and 21.64 m below the surface (mbs). Most of these (82%) grew well at all temperatures from 2°C (or −2°C) to 37°C, consistent with psychrotolerant species, 14 (11%) grew at temperatures up to ∼20°C, consistent with psychrophilic species, and 9 isolates (7%) grew at temperatures ranging up to 45°C, representing mesophiles (Table 1).
TABLE 1.
Isolates from the Muztag Ata ice core and growth at different temperatures
| Depth (mbs) | Isolatea | Affiliationc | Growth temp range (°C) | Depth (mbs) | Isolatea | Affiliationc | Growth temp range (°C) | |
|---|---|---|---|---|---|---|---|---|
| 2.67 | B21 | γ | −2-45 | |||||
| B22 | α | 2-37 | ||||||
| 2.89 | B31 | γ | 2-37 | |||||
| B32 | HGC | 2-37 | ||||||
| B33 | α | 2-37 | ||||||
| B34* | HGC | 2-37 | ||||||
| B35 | HGC | 2-37 | ||||||
| 3.15 | B4 | α | 2-45 | |||||
| 3.30 | NDb | |||||||
| 3.55 | ND | |||||||
| 3.84 | B7 | HGC | −2-37 | |||||
| 3.99 | B81* | CFB | ||||||
| B82* | HGC | |||||||
| 4.17 | B9 | CFB | 2-37 | |||||
| 4.37 | ND | |||||||
| 4.55 | C11 | HGC | 2-37 | |||||
| C12 | HGC | −2-25 | ||||||
| C13 | HGC | 2-45 | ||||||
| 4.72 | C21 | Cry. | ||||||
| C22* | γ | |||||||
| 4.99 | C4 | HGC | 2-37 | |||||
| 5.19 | C5* | HGC | 2-37 | |||||
| 5.37 | C6 | α | 2-37 | |||||
| 5.53 | C71 | CFB | ||||||
| C72 | LGC | |||||||
| C73 | HGC | |||||||
| 5.69 | C8 | HGC | ||||||
| 5.99 | C91* | HGC | ||||||
| C92* | α | |||||||
| 6.26 | D01 | HGC | 2-37 | |||||
| D02* | γ | |||||||
| D03* | CFB | |||||||
| 6.42 | D1* | γ | 2-37 | |||||
| 6.63 | D2* | α | ||||||
| 9.99 | D3* | γ | ||||||
| 7.30 | D41 | CFB | 2-37 | |||||
| D42 | α | 2-37 | ||||||
| D43* | γ | 2-37 | ||||||
| D44* | CFB | 2-37 | ||||||
| 7.56 | D5 | LGC | −2-37 | |||||
| 7.82 | D61* | CFB | 2-37 | |||||
| D62 | HGC | 2-37 | ||||||
| D63* | α | 2-37 | ||||||
| D64* | γ | 2-37 | ||||||
| 7.99 | D7 | HGC | −2-37 | |||||
| 8.30 | D81 | Cry. | −2-25 | |||||
| D82 | HGC | −2-37 | ||||||
| D83 | HGC | −2-25 | ||||||
| D84 | LGC | 2-45 | ||||||
| D85 | α | 2-37 | ||||||
| 8.62 | D91 | Cry. | −2-25 | |||||
| D92 | α | 2-37 | ||||||
| D93 | HGC | −2-25 | ||||||
| D94* | HGC | 2-37 | ||||||
| D95 | γ | 2-45 | ||||||
| 8.82 | C31 | Cry. | −2-25 | |||||
| C32 | HGC | −2-25 | ||||||
| C33 | Cry. | −2-25 | ||||||
| 9.09 | E01 | CFB | −2-25 | |||||
| E02 | γ | 2-37 | ||||||
| E03 | HGC | 2-37 | ||||||
| E04 | HGC | 2-37 | ||||||
| E05 | LGC | 2-37 | ||||||
| E06* | HGC | −2-25 | ||||||
| 9.21 | E11 | γ | 2-45 | |||||
| E12* | HGC | 2-37 | ||||||
| 9.21 | E13 | HGC | 2-37 | |||||
| E14 | α | 2-37 | ||||||
| 9.47 | E21 | α | −2-25 | |||||
| E22 | Cry. | −2-25 | ||||||
| E23 | HGC | −2-37 | ||||||
| 9.70 | E3* | γ | 2-37 | |||||
| 10.09 | E41* | HGC | 2-37 | |||||
| E42* | HGC | 2-37 | ||||||
| 10.30 | E51* | γ | 2-37 | |||||
| E52* | CFB | 2-37 | ||||||
| 10.50 | E61 | γ | 2-45 | |||||
| E62 | CFB | −2-25 | ||||||
| 10.75 | E71* | CFB | 2-37 | |||||
| E72* | HGC | 2-37 | ||||||
| E73* | α | 2-37 | ||||||
| 11.03 | E8* | HGC | 2-37 | |||||
| 11.33 | E91 | γ | 2-45 | |||||
| E92* | HGC | 2-37 | ||||||
| 11.65 | F01 | HGC | 2-37 | |||||
| F02 | γ | 2-37 | ||||||
| 11.81 | F11 | γ | −2-37 | |||||
| F12 | HGC | 2-37 | ||||||
| F13 | CFB | 2-37 | ||||||
| F14* | HGC | 2-37 | ||||||
| 12.03 | F21 | γ | −2-25 | |||||
| F22 | CFB | 2-37 | ||||||
| F23 | γ | −2-37 | ||||||
| 12.50 | F31* | HGC | 2-37 | |||||
| F32 | HGC | 2-37 | ||||||
| 12.64 | F41* | HGC | 2-37 | |||||
| F42* | HGC | 2-37 | ||||||
| 12.84 | F5* | α | 2-37 | |||||
| 13.03 | F6 | HGC | 2-37 | |||||
| 13.20 | F71* | γ | 2-37 | |||||
| F72 | HGC | 2-37 | ||||||
| 13.51 | F81 | CFB | 2-37 | |||||
| F82* | γ | 2-37 | ||||||
| 13.74 | F91 | α | 2-37 | |||||
| F92 | CFB | 2-37 | ||||||
| F93 | γ | 2-37 | ||||||
| F94* | γ | 2-37 | ||||||
| F95 | HGC | 2-37 | ||||||
| 14.03 | G01 | γ | 2-37 | |||||
| 14.33 | G11* | HGC | 2-37 | |||||
| G12* | LGC | −2-37 | ||||||
| 14.66 | G21* | HGC | 2-37 | |||||
| G22* | HGC | 2-37 | ||||||
| 14.95 | G31 | γ | 2-37 | |||||
| G32 | HGC | −2-37 | ||||||
| 15.17 | G4 | HGC | 2-37 | |||||
| 15.32 | G51 | γ | 2-37 | |||||
| G52 | CFB | 2-37 | ||||||
| 15.59-15.79 | G61* | LGC | 2-37 | |||||
| G62* | γ | 2-37 | ||||||
| 17.99-18.24 | H6 | γ | 2-37 | |||||
| 18.75-18.87 | 101 | γ | 2-37 | |||||
| I02 | α | −2-37 | ||||||
| I03 | CFB | 2-37 | ||||||
| I04* | HGC | 2-37 | ||||||
| 20.35-20.65 | I71 | HGC | 2-37 | |||||
| I72 | CFB | 2-37 | ||||||
| I73 | α | 2-37 | ||||||
| 21.38-21.64 | J11 | α | 2-37 | |||||
| J12 | HGC | 2-37 | ||||||
| 21.64-21.99 | J21 | γ | 2-37 | |||||
| J22 | α | 2-37 |
*, similar isolate with the same ARDRA pattern but 16S rRNA sequence not determined.
ND, no detection of the isolate sequences from ice depth at 3.30 to 3.55 and 4.37 to 4.55 mbs.
α, α-Proteobacteria; γ, γ-Proteobacteria; Cry., C. psychrophilum.
Clustering isolates by ARDRA.
Fifty-five isolates were related to the HGC gram-positive bacteria, although this cluster exhibited 29 different ARDRA patterns. The second (30 isolates) and third (19 and 19 isolates) largest clusters of isolates were related to the γ- and α-Proteobacteria and Cytophaga-Flavobacterium-Bacteroides (CFB) group, respectively. A small number of LGC gram-positive bacteria were also isolated.
16S rRNA-based phylogenetic relationships.
Many isolates with the same HaeIII-generated ARDRA pattern had sufficiently different 16S rRNA sequences that phylogenetic analyses placed them in different evolutionary lineages (Fig. 1 to 3). For example, the 16S rRNA sequences obtained from the cluster of isolates represented by strain Acinetobacter sp. strain ANT9054 (GenBankaccession no. AY16723) placed many on separate branches within the γ-Proteobacteria lineage. To explore and document this diversity, 16S rRNA sequences were obtained from 4 to 10 different isolates that had the same HaeIII-generated ARDRA pattern.
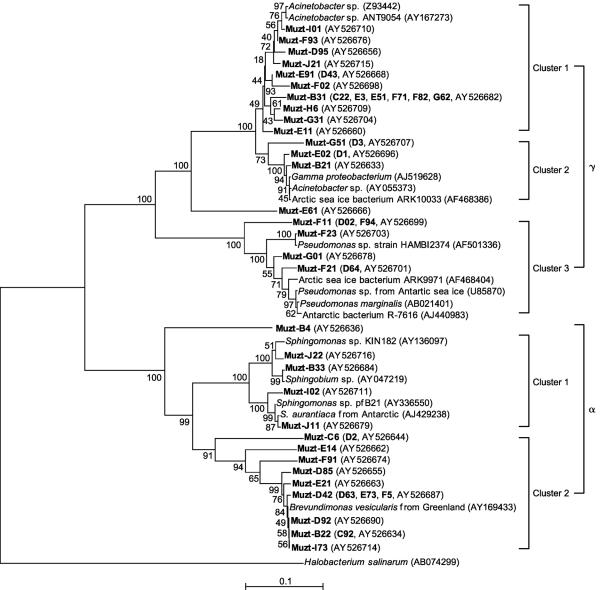
FIG. 1.
Neighbor-joining tree indicating the phylogenetic relationships of the α- and γ-Proteobacteria isolated from the Muztag Ata ice core and their nearest relatives based on GenBank 16S rRNA sequences. Bootstrap values of >50% (of 100 iterations) were obtained by maximum-parsimony analysis for bootstrap sampling of 100. Scale bars indicate p distances. Additional similar isolates (shown in boldface in parentheses) had the same ARDRA patterns as those sequences of the representative strains, but the 16S rRNA sequences were not determined.
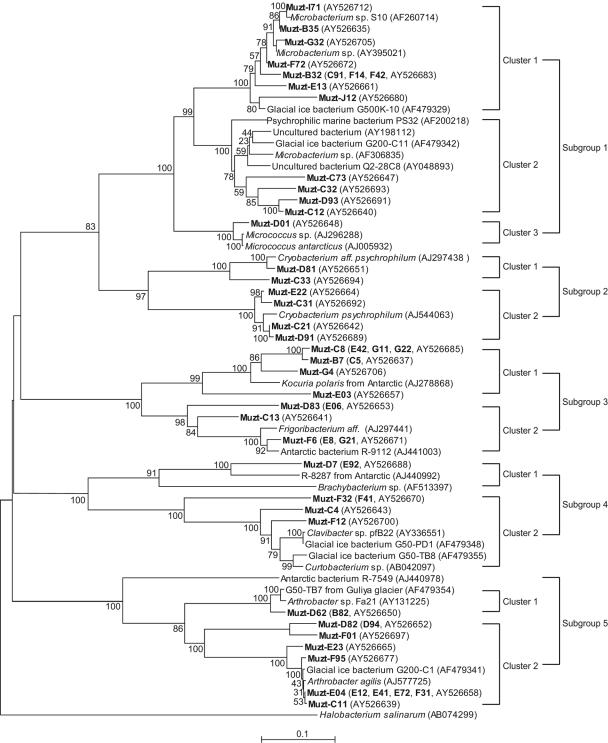
FIG. 3.
Neighbor-joining tree indicating phylogenetic relationships of the HGC isolates from the Muztag Ata ice core and their nearest relatives based on GenBank 16S rRNA sequences. Bootstrap values of >50% (of 100 iterations) were obtained by maximum-parsimony analysis for bootstrap sampling of 100. Scale bars indicate p distances. Additional similar isolates (shown in boldface in parentheses) had the same ARDRA patterns as those sequences of the representative strains, but the 16S rRNA sequences were not determined.
The most frequently recovered cluster of isolates (30 in total; 23.3% of all isolates) was the member of the γ-Proteobacteria (Fig. 1; Table 1). They were distributed throughout the ice core section (Table 1) and were subdivided into 22 isolates related to Acinetobacter species, 7 isolates related to Pseudomonas species, and 1 isolate (E61) not closely related to previously established species. Of the 22 Acinetobacter-related isolates, 17 were most closely related to strain ANT9054 from polar sea ice (AY167273), and 5 were related to an anoxic Acinetobacter species and to strain ARK10033 from Arctic sea ice. The 7 Pseudomonas isolates were most closely related to Pseudomonas oryzihabitans strain HAMB12374 and a Pseudomonas species from Antarctic sea ice.
The second most frequently recovered cluster of isolates (19 in total; 14.7% of all isolates) were members of the α-Proteobacteria (Fig. 1; Table 1). These isolates were also distributed throughout the ice core, 4 of which had 16S rRNA sequences almost identical to those reported previously for Sphingomonas species. Isolates Muzt-I02 and J11 had 16S rRNA sequences identical to that reported for Sphingomonas strain pfB21 (GenBank accession no. AY336550), and isolates Muzt-B33 and J22 had 16S rRNA sequences 99% identical to that of Sphingobium yanoikuyae (GenBank accession no. AY047219). Furthermore, all four 16S rRNA sequences were very similar to the 16S rRNA sequence determined from the Antarctic isolate Sphingomonas aurantiaca strain MA405 (EMBL accession no. AJ429238). Fourteen of the α-Proteobacteria isolates, with Muzt-D63, E73, and F5 represented by Muzt-D42, Muzt-C92 represented by Muzt-B22, and Muzt-D2 represented by Muzt-C6, grouped with Brevundimonas vesicularis (GenBank accession no. AY169433), which was isolated from a Greenland glacier. Isolate Muzt-B4 grouped with Sphingomonas and Brevundimonas in the α-Proteobacteria (Fig. 1).
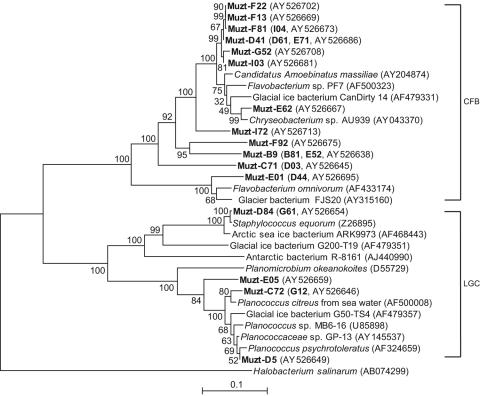
As illustrated in Fig. 2, members of the low-G+C group of gram-positive bacteria were not isolated from all sections of the ice core. Four isolates, Muzt-D5, C72 and E05, and Muzt-12, were recovered from ice from 5.53 to 5.69, 7.56 to 7.82, and 9.09 to 9.21 mbs, respectively. They have 16S rRNA sequences identical to that reported for Planomicrobium okeanokoites (DDBJ accession no. D55729). Two isolates, Muzt-D84 and G61, were isolated from 8.30 to 8.62 and 15.59 to 15.79 mbs, respectively, and have 16S rRNA sequences identical to those of Staphylococcus equorum (EMBL accession no. Z26895) and strain ARK9973 (GenBank accession no. AF468443) isolated from Antarctic and Arctic sea ice, respectively.
FIG. 2.
Neighbor-joining tree indicating phylogenetic relationships of the LGC and CFB isolates from the Muztag Ata ice core and their nearest relatives based on GenBank 16S rRNA sequences. Bootstrap values of >50% (of 100 iterations) were obtained by maximum-parsimony analysis for bootstrap sampling of 100. Scale bars indicate p distances. Additional similar isolates (shown in boldface in parentheses) had the same ARDRA patterns as those sequences of the representative strains, but the 16S rRNA sequences were not determined.
Nineteen isolates (14.7% of total) (Fig. 2) were members of the CFB group. Isolates Muzt-E01 and the similar isolate Muzt-D44 were most closely related to Flavobacterium omnivorum from the China no. 1 glacier (GenBank accession no. AF433174) and strain FJS20 (GenBank accession no. AY315160) from an Antarctic glacier. The 16 remaining isolates grouped most closely with, but positioned on separate phylogenetic branches from, Flavobacterium species strain PF7 (GenBank accession no. AF500323), a phenylacetic acid-degrading soil isolate, and strain CanDirty14 (GenBank accession no. AF479331) from a southern hemisphere glacier.
High-G+C gram-positive bacteria were isolated from all regions of the ice core and formed eight clusters. The largest cluster was related to Microbacterium represented by strain S10 (GenBank accession no. AF260714), including 10 isolates, Muzt-B32, C91, and F14, etc. Within this largest cluster, four isolates, Muzt-C12, C32, C73, and D93, grouped with uncultured clones Q2-28C8 (GenBank accession no. AY048893) and AY1981122 (Fig. 3). The second largest cluster was related to Arthrobacter and formed two branches in cluster 8. One of the two branches was the two isolates Muzt-B82 and D62 and their closest-related glacial bacterium G50-TB7 AF49354. The other was the branch represented by glacial bacterium G200-C1 AF479341, containing 12 isolates, Muzt-B34, C11, and D82, etc. The third largest cluster was closely similar to the Cryobacterium group, containing six isolates recovered from ice layers from 4.72 to 4.99, and 8.30 to 9.09 and 9.47 to 9.7 mbs. All 6 had 16S rRNA sequences 100% identical to that of Cryobacterium psychrophilum DSM 4854 (EMBL accession no. AJ544063) and that of Cryobacterium aff. psychrophilum (EMBL accession no. AJ297438) from Lake Fryxell in Antarctica (Fig. 3). The remaining five clusters belonged to the Micrococcus, Kocuria, Frigoribacterium, Brachybacterium, and Clavibacter/Curtobacterium genera. One isolate, Muzt-D01, contained sequences that grouped within cluster 2, with 100% similarity to Micrococcus antarcticus (EMBL accession no. AJ005932). Cluster 4 was 99% to 100% related to Kocuria polaris from an Antarctic pond (EMBL accession no. AJ278868), containing four isolate sequences, Muzt-B7, C8, E03, and G4, and four additional similar isolates, Muzt-C5, E42, G11, and G22. Cluster 5 was related to Frigoribacterium aff. (EMBL accession no. AJ297441) and the Antarctic bacterium R-9112 (EMBL accession no. AJ441003) and had three isolate sequences, Muzt-C13, D83, and E06, and three additional similar isolates, Muzt-E06, E8, and G21. Muzt-D7 and the similar Muzt-E92 belonged to Brachybacterium and were closely related to R-8287 from an Antarctic lake (EMBL accession no. AJ440992). Four isolates, Muzt-C4, F12, F32, and Muzt-F41, fell into the Clavibacter/Curtobacterium group in cluster 7.
Vertical distribution of the most frequent isolates.
Members of different phylogenetic groups were predominantly isolated in ice from different core depths. Figure 4 shows the most frequently isolated bacterial phylotype, expressed as a proportion of total number of isolates recovered at each depth. Proteobacteria predominated among the isolates from 2.7 to 3.80 mbs and from 6.2 to 7.5 mbs, HGC or CFB group bacteria predominated among isolates from 3.8 to 6.2 mbs and from 9.0 to 9.7 mbs, LGC, CFB group, or HGC bacteria predominated among the isolates from 7.5 to 8.3 mbs and from 9.4 to 9.7 mbs, Proteobacteria or HGC bacteria predominated among the isolates from 9.7 to 12.5 mbs, and HGC bacteria, Proteobacteria, or CFB group bacteria were most frequently isolated from 12.5 to 15.3 mbs. Cryobacterium psychrophilum was isolated only from ice from 8.3 to 9.0 mbs (Fig. 4).
FIG. 4.
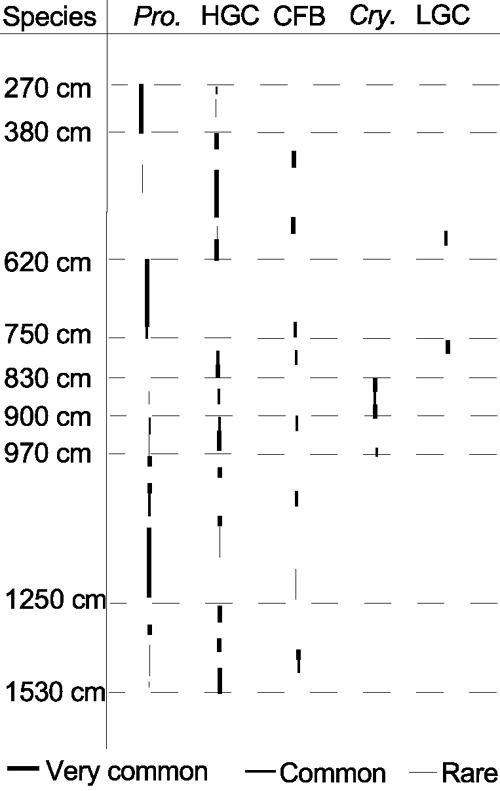
Predominant bacteria isolated and their relative abundances at different depths within the ice core. Pro., Proteobacteria; Cry., Cryobacterium psychrophilum.
DISCUSSION
Globally distributed ice core isolates and regional characteristics.
Overall, our results are very consistent with the results of previous bacterial isolation studies from glacial ice, although all of the most similar sequences in the database were downloaded and used for phylogenetic analysis. Members of the α- and γ-Proteobacteria and the LGC and HGC bacterial lineages were similarly isolated from a Guliya (Tibet) ice core (9), α- and β-Proteobacteria, LGC, HGC, and CFB group bacteria were also isolated from a Vostok (Antarctica) ice core (8), and 16S rRNA molecules that were amplified directly from melt water from the Dunde, Puruogangri, Malan (Tibet), Sajama (Bolivia), Taylor Dome, and Siple (Antarctic) glaciers had sequences revealing origins from α-, β-, γ-, and ɛ-Proteobacteria, HGC, LGC, and CFB group bacteria (7, 8, 9, 50). The majority of the Muztag Ata ice core isolates have 16S rRNA sequences ranging from 85% similarity to 100% identity to database sequences (Table 1; Fig. 1 to 3), and the closest relatives of many of the Muztag Ata ice core isolates originated from other glaciers or from sea ice. Most of the Muztag Ata ice isolates had temperature growth profiles consistent with adaptation to growth under cold growth conditions and most likely were transferred by wind or snow from local ecosystems onto the glacier's surface (Table 1). The evidence suggests that cold environments favor the growth and survival of similar organisms on a worldwide basis, given that very similar isolates are obtained from Arctic and Antarctic ice (4, 7).
BLAST searches revealed that, in addition to isolates from cold environments, many of the Muztag Ata ice core isolates also have relatives that were isolated from more temperate terrestrial and freshwater environments (Table 1; Fig. 1 to 3). Isolate Muzt-D62, for example, has a 16S rRNA sequence identical to that from both a glacier isolate, bacterium G50-TB7 (GenBank accession no. AF479354) and the epiphytic Arthrobacter sp. strain Fa21 (GenBank accession no. AY131225). The 10 isolates typified by Muzt-J12 have 16S rRNA sequences very similar to those of both the glacier isolate G500K-10 (GenBank accession no. AF479329) and Microbacterium strain VKM Ac-2050, a species also typically associated with plant surfaces. This suggests the complicated resources of glacial bacteria entrapped in ice or the global distribution of these bacteria.
Based on the criterion that the bacteria with 16S rRNA sequences that are 93 to 97% identical should be considered members of the same genus (21, 33), some of the Muztag Ata ice core isolates may belong to new genera. For example, Muzt-C13, C21, and D91 loosely grouped with the known bacterial species (Fig. 3), the sequences of which had 84% to 91% similarity to those for reported species. This was also the case in other studies with members of several new genera isolated from glacial and sea ice from Puruogangri, Tunde, and Malan ice cores (43, 50) and other ice samples (6, 11, 17, 20, 21, 26, 30). Moreover, most isolates obtained from different regions of the Muztag Ata ice core grouped together more closely than with the reported sequences from different environments (Fig. 1 to 3). This is consistent with the suggestion that a substantial percentage of the bacteria endemic to these remote cold environments are unique to these environments (10, 22, 41).
Relationship of differences in bacterial isolates at different ice core depths to climate and environment changes.
There were very clear differences in the bacteria isolated from different regions of the Muztag Ata ice core. Acinetobacter species (members of the γ-Proteobacteria) were isolated from 17 segments of ice core section but not from all sections (Fig. 1). Flavobacterium species were also found at many, but not all, locations throughout the length of the core (Fig. 2). Many members of the CFB group have the capacity to degrade complex organic substrates (2, 21, 24), and such compounds were detected in Himalayan Dasuopu glacial ice (44), consistent with particle-borne and/or volatilized organics being trapped and providing nutrients to CFB-related species immured in ice. Brevundimonas, Sphingomonas, Pseudomonas, Microbacterium, and Arthrobacter species dominated at some depths. However, all six isolates belonging to the phylum Cryobacterium originated from a continuous ice layer at 8.30 to 9.09 mbs (Fig. 3). This may have resulted from the specific source and environmental conditions during their deposition on the surface of the glacier. The Cryobacterium isolates from the ice core grew well at temperatures below 25°C, making them psychrophiles, which was favorable for their survival in ice. This specific source and physiological property of the Cryobacterium isolates found in ice may be the main reasons for their occurrence at specific ice depth ranges.
There were clear differences in the predominant aerobes isolated at different depths from the Muztag Ata ice core (Fig. 4), implying that there were differences in the bacteria deposited and/or that survived on the ice surface under different past climate conditions. Yoshimura et al. (48, 49) and Takeuchi et al. (34, 35) demonstrated that the membership of the snow algal community changed with environmental conditions on the Yala, AX010 (Himalaya), and Gulkana (Alaska) glaciers, and annual layers of algal growth were apparent in a shallow ice core from the Yala glacier. Our earlier studies similarly revealed a layered distribution of bacteria in a Malan (Tibet) ice core (43), and it seems likely that differences in the microorganisms recovered reflect the differences in climate, local and possibly remote environments (23, 37, 45, 46), and the nutrients trapped within the ice. Given this explanation, the layered distribution of bacteria recovered from the Muztag Ata ice core reflects the effect of the climatic and environmental changes on the microbial distribution in the glacier. Rutter and Nedwell (27) obtained results that indicate that bacterial survival reflects a competition between psychrophiles and psychrotolerant species, and this could help explain these features of the bacteria isolated from the Muztag Ata glacier. It is not, however, clear how well the surviving microbial community correlates with climate and environmental conditions rather than properties of specific bacteria, as the fate of microorganisms trapped intentionally in glacial ice sheets has yet to be investigated. Experiments to differentiate between these parameters are needed. We need to determine the survival and subsequent recovery of well-characterized bacteria, intentionally inoculated on the surface of a glacier, over time and at increasing depths within a glacier.
Acknowledgments
We thank John N. Reeve at the Department of Microbiology of the Ohio State University for work on the improvement of language. We thank Nozomu Takeuchi from Research Institute for Humanity and Nature (Japan) for helpful comments on this paper. We also thank Z. Li, G. J. Wu, B. Q. Xu, and other members of the Muztag Ata glacial expedition for help in the recovery of the ice core used in this study.
This study was supported by grant no. 2001CCB00300, 2001CB711001, 40471025, KZCX2-SW-118, 40121101, and 90102005.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Bano, N., and J. T. Hollibaugh. 2002. Phylogenetic composition of bacterioplankton assemblages from the Arctic Ocean. Appl. Environ. Microbiol. 68:505-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borneman, J., P. W. Skroch, K. M. O'Sullivan, J. A. Palus, N. G. Rumjanek, J. L. Jansen, and J. Nienhuis. 1996. Molecular microbial diversity of an agricultural soil in Wisconsin. Appl. Environ. Microbiol. 62:1935-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmeyer, R., K. Knittel, J. Jürgens, H. Weyland, R. Amann, and E. Helmke. 2003. Diversity and structure of bacterial communities in arctic versus Antarctic pack ice. Appl. Environ. Microbiol. 69:6610-6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosius, J., M. L. Palmer, P. J. Kennedy, and H. R. Noller. 1978. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc. Natl. Acad. Sci. USA 7:4801-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busse, H. J., E. B. Denner, S. Buczolits, M. Salkinoja-Salonen, A. Bennasar, and P. Kampfer. 2003. Sphingomonas aurantiaca sp. nov., Sphingomonas aerolata sp. nov., and Sphingomonas faeni sp. nov., air- and dustborne and Antarctic, orange-pigmented, psychrotolerant bacteria, and emended description of the genus Sphingomonas. Int. J. Syst. Evol. Microbiol. 53:1253-1260. [DOI] [PubMed] [Google Scholar]
- 7.Christner, B. C., E. Mosley-Thompson, L. G. Thompson, V. Zagorodnov, K. Sandman, and J. N. Reeve. 2000. Recovery and identification of viable bacteria immured in glacier ice. Icarus 144:479-485. [Google Scholar]
- 8.Christner, B. C., E. Mosley-Thompson, L. G. Thompson, and J. N. Reeve. 2001. Isolation of bacteria and 16S rDNAs from lake Vostok accretion ice. Environ. Microbiol. 3:570-577. [DOI] [PubMed] [Google Scholar]
- 9.Christner, B. C., E. Mosley-Thompson, L. G. Thompson, and J. N. Reeve. 2003. Bacterial recovery from ancient glacial ice. Environ. Microbiol. 5:433-436. [DOI] [PubMed] [Google Scholar]
- 10.Fenchel, T. 2003. Biogeography for bacteria. Science 301:925-926. [DOI] [PubMed] [Google Scholar]
- 11.Foght, J., J. Aislabie, S. Turner, C. E. Brown, J. Ryburn, D. J. Saul, W. Lawson. 2004. Culturable bacteria in subglacial sediments and ice from two southern hemisphere glaciers. Microb. Ecol. 47:329-340. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, J. L. 1981. Genetic characterization, p. 450-472. In R. G. E. Murray, R. N. Costilow, E. W. Nester, W. A. Wood, N. R. Krieg, and G. B. Phillips, (ed.), Manual of methods for general bacteriology. American Society for Microbiology, Washington, D.C.
- 13.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, New York, N.Y.
- 14.Kumar, S., and K. M. N. Tamura. 1993. MEGA molecular evolutionary genetics analysis, version 1.01. Pennsylvania State University, University Park.
- 15.Lane, D. J., B. Pace, G. J. Olsen, D. A. Stahl, M. L. Sogin, and N. R. Pace. 1985. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 82:6955-6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. Academic Press, Chichester, England.
- 17.Liu, H., Y. Xu, Y. Ma, and P. Zhou. 2000. Characterization of Micrococcus antarcticus sp. nov., a psychrophilic bacterium from Antarctica. Int. J. Syst. Evol. Microbiol. 50:715-719. [DOI] [PubMed] [Google Scholar]
- 18.Maidak, B. L., G. J. Olsen, N. Larsen, R. Overbeek, M. J. McCaughey, and C. R. Woese. 1996. The ribosomal database project (RDP). Nucleic Acids Res. 24:82-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massana, R., A. E. Murray, C. M. Preston, and E. F. Delong. 1997. Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara channel. Appl. Environ. Microbiol. 63:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miteva, V. I., P. P. Sheridan, and J. E Brenchley. 2004. Phylogenetic and physiological diversity of microorganisms isolated from a deep Greenland glacier ice core. Appl. Environ. Microbiol. 70:202-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullins, T. D., T. B. Britschgi, R. L. Krest, and S. J. Giovannoni. 1995. Genetic comparisons reveal the same unknown bacterial lineages in Atlantic and Pacific bacterioplankton communities. Limnol. Oceanogr. 40:148-158. [Google Scholar]
- 22.Papke, R. T., N. B. Ramsing, M. M. Bateson, and D. M. Ward. 2003. Geographical isolation in hot spring cyanobacteria. Environ. Microbiol. 5:650-659. [DOI] [PubMed] [Google Scholar]
- 23.Petit, J. R., J. Jouzel, D. Raynaud, N. I. Barkov, J. M. Barnola, I. Basile, M. Benders, J. Chappellaz, M. Davis, G. Delaygue, M. Delmotte, V. M. Kotlyakov, M. Legrand, V. Y. Lipenkov, C. Lorius, L. Pepin, C. Ritz, E. Saltzman, and M. Stievenard. 1999. Climate and atmospheric history of the past 420,000 years from the Vostock ice core, Antarctica. Nature 399:429-436. [Google Scholar]
- 24.Pinhassi, J., F. Azam, J. Hempälä, R. A. Long, J. Martinez, U. L. Zweifel, and Å. Hagström. 1999. Coupling between bacterioplankton species composition, population dynamics, and organic matter degradation. Aquat. Microb. Ecol. 17:13-26. [Google Scholar]
- 25.Priscu, J. C., E. E. Adams, W. B. Lyons, M. A. Voytek, D. W. Mogk, R. L. Brown, C. P. McKay, C. D. Takacs, K. A. Welch, C. F. Wolf, J. D. Kirshtein, and R. Avci. 1999. Geomicrobiology of subglacial ice above lake Vostok, Antarctica. Science 286:2141-2144. [DOI] [PubMed] [Google Scholar]
- 26.Reddy, G. S., J. S. Prakash, V. Prabahar, G. I. Matsumoto, E. Stackebrandt, and S. Shivaji. 2003. Kocuria polaris sp. nov., an orange-pigmented psychrophilic bacterium isolated from an Antarctic cyanobacterial mat sample. Int. J. Syst. Evol. Microbiol. 53:183-187. [DOI] [PubMed] [Google Scholar]
- 27.Rutter, M., and D. B. Nedwell. 1994. Influence of changing temperature on growth rate and competition between two psychrotolerant Antarctic bacteria: competition and survival in non-steady-state temperature environments. Appl. Environ. Microbiol. 60:1993-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for constructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt, T. M., E. F. DeLong, and N. R. Pace. 1991. Analysis of a marine phytoplankton community by 16S rRNA gene cloning and sequencing. J. Bacteriol. 173:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheridan, P. P., V. I. Miteva, and J. E. Brenchley. 2003. Phylogenetic analysis of anaerobic psychrophilic enrichment cultures obtained from a Greenland glacier ice core. Appl. Environ. Microbiol. 69:2153-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi, T., R. H. Reeves, D. A. Gilichinsky, and E. I. Friedmann. 1997. Characterization of viable bacteria from Siberian permafrost by 16S rDNA sequencing. Microb. Ecol. 33:169-179. [DOI] [PubMed] [Google Scholar]
- 32.Siebert, J., and P. Hirsch. 1988. Characterization of 15 selected coccal bacteria isolated from Antarctic rock and soil samples from the McMurdo-Dry Valleys (southern Victoria Land). Polar Biol. 9:37-44. [DOI] [PubMed] [Google Scholar]
- 33.Stackebrandt, E., and B. M. Goebel. 1994. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 34.Takeuchi, N., S. Kohshima, and K. Fujita. 1998. Snow algae community on a Himalayan glacier, glacier AX010 East Nepal: relationship with glacier summer mass balance. Bull. Glacier. Res. 16:43-50. [Google Scholar]
- 35.Takeuchi, N. 2001. The altitudinal distribution of snow algae on an Alaska glacier (Gulkana Glacier in the Alaska Range). Hydrol. Process. 15:3447-3459. [Google Scholar]
- 36.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL-X Windows interface—flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson, L. G., T. Yao, E. Mosley-Thompson, M. E. Davis, K. A Henderson, and P. N. Lin. 2000. A high-resolution millennial record of the South Asian monsoon from Himalayan ice cores. Science 289:1916-1919. [DOI] [PubMed] [Google Scholar]
- 38.Van, E. M., and J. T. Staley. 1971. Gas-vacuolated strains of Microcyclus aquaticus. J. Bacteriol. 108:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voytek, M. A., and B. B. Ward. 1995. Detection of ammonium-oxidizing bacteria of the beta-subclass of the class Proteobacteria in aquatic samples with the PCR. Appl. Environ. Microbiol. 61:2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weisenburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitaker, R. J., D. W. Grogan, and J. W. Taylor. 2003. Geographic barriers isolate endemic populations of hyperthermophilic Archaea. Science 301:976-978. [DOI] [PubMed] [Google Scholar]
- 42.Xiang, S. R., T. D. Yao, L. Z. An, B. Q. Xu, Z. Li, G. J. Wu, Y. Q. Wang, S. Ma, and X. R. Chen. 2004. Bacterial diversity in Malan Ice Core from the Tibetan Plateau. Folia Microbiol. 49:269-276. [DOI] [PubMed] [Google Scholar]
- 43.Xiang, S. R., T. D. Yao, L. Z. An, Z. Li, G. J. Wu, Y. Q. Wang, B. Q. Xu, and J. X. Wang. 2004. Community change of bacteria in the Malan Ice Core and its relation to climate and environment. Chin. Sci. Bull. 49:1869-1875. [Google Scholar]
- 44.Xie, S., T. D. Yao, S. Kang, B. Xu, K. Duan, and L. G. Thompson. 2000. Geochemical analyses of a Himalayan snowpit profile: implications for atmospheric pollution and climate. Org. Geochem. 31:15-23. [Google Scholar]
- 45.Yao, T., and L. G. Thompson. 1992. Trends and features of climatic changes in the past 5000 years recorded by the Dunde ice core. Ann. Glaciol. 16:21-24. [Google Scholar]
- 46.Yao, T., V. Masson, J. Jouzel, M. Stievenard, S. Weizhen, and J. Keqin. 1999. Relationships between δ18O in precipitation and surface air temperature in the Urumqi River Basin, east Tianshan Mountains, China. Geophys. Res. Lett. 26:3473-3476. [Google Scholar]
- 47.Yao, T. D., S. R. Xiang, X. J. Zhang, and J. C. Pu. 2003. Microbiological characteristics recorded by Manlan and Puruogangri ice core. Quaternary Sci. 23:193-199. [Google Scholar]
- 48.Yoshimura, Y., S. Kohshima, and S. Ohtani. 1997. A community of snow algae on a Himalayan glacier: change of algal biomass and community structure with altitude. Arct. Antarct. Alp. Res. 29:126-137. [Google Scholar]
- 49.Yoshimura, Y., S. Kohshima, N. Takeuchi, K. Seko, and K. Fujita. 2000. Himalayan ice-core dating with snow algae. J. Glaciol. 46:335-340. [Google Scholar]
- 50.Zhang, X. J. 2002. Diversity of microorganisms and DNA entrapped in glacier ice of Qinghai-Tibet Plateau and its relation with environment. Ph.D. thesis. Cold and Arid Regions Environmental Engineering Research Institute, Chinese Academy of Science, Lanzhou, China.
- 51.Zhang, X. J., X. J. Ma, T. D. Yao, and G. S. Zhang. 2003. Diversity of 16S rDNA and environmental factor affecting microorganisms in Malan ice core. Chin. Sci. Bull. 48:947-957. [Google Scholar]