Abstract
Concentrations of (E,E)-farnesol needed to inhibit germ tube formation were determined for Candida albicans strains A72 and SC5314 by using six different conditions known to trigger germination. For defined media, 1 to 2 μM farnesol was sufficient. However, with serum at 2 to 20%, up to 250 μM farnesol was required. Farnesol blocked germ tube formation but did not block elongation of existing germ tubes.
Fungal yeast-mycelium dimorphism is of interest because of the economic and medical importance of dimorphic fungi and because these organisms may serve as model systems for studying differentiation. Farnesol is a fungal quorum-sensing molecule with intriguing regulatory properties in Candida albicans (7). This compound is excreted continuously by C. albicans (7), and when it accumulates beyond a threshold level, it blocks the yeast-to-mycelium conversion (7). Farnesol blocks mycelial development initiated by any of three chemically distinct triggers for germ tube formation: l-proline, N-acetylglucosamine, and serum (7). Of these, serum (10 to 20% [vol/vol] at 37°C) has been used most commonly (9, 15). Serum has even been described as the “magic potion” for the induction of germ tubes by C. albicans (5). Thus, both farnesol's mode of action and the therapeutic potential of farnesol analogs are of interest.
By stationary phase, 2 to 4 μM farnesol, which is at a concentration well above the 1.2 μM concentration needed to block germ tube formation in an N-acetylglucosamine-stimulated differentiation assay (17), has accumulated in cultures of C. albicans (7, 8). However, some recent studies (10, 11, 16, 18, 19) with farnesol and C. albicans have used farnesol concentrations (250 to 500 μM) that we believe are much higher than those that are physiologically relevant. Farnesol is a lipophilic molecule that can accumulate in membranes, and the farnesol effects observed at these concentrations may be nonspecific. In some cases, these higher concentrations may result in physiological artifacts. Indeed, Kim et al. (10) found that 450 μM farnesol inhibited growth of C. albicans by 35%.
Our objectives in this study were threefold: (i) to identify the minimum levels of farnesol needed to block germ tube formation in six media commonly used to trigger germination; (ii) to establish the physiological relevance of the production levels we have observed, 2 to 4 μM farnesol (7, 8); and (iii) to test our hypothesis that much higher levels of farnesol are needed to block germ tube formation in serum. This hypothesis was developed because of the presence of albumins in serum; albumins are noted for their nonspecific lipid binding capacity (20).
Procedures for the growth and storage of C. albicans were as described previously (7, 8, 17). Strain A72 is also ATCC MYA-2430. Strain SC5314 was used for the genomic sequence for C. albicans (www.candidagenome.org). (E,E)-Farnesol (Sigma Chemical, St. Louis, MO) was freshly prepared as a stock solution (10 mM) in methanol and stored under nitrogen to prevent oxidation (17).
All resting cell preparations were washed three times in 50 mM potassium phosphate buffer (pH 6.5) prior to use. Resting cells were inoculated into 5 ml of prewarmed (37°C) medium at a cell density of 2 × 107 per ml and incubated in 25-ml flasks at 37°C with aeration by shaking at 200 rpm on a G2 shaker (New Brunswick Scientific Co., Edison, NJ). Samples were removed periodically for microscopic examination.
The liquid media used were (i) 2.5 mM N-acetylglucosamine with imidazole and magnesium (7), (ii) glucose phosphate proline (GPP) (7, 12), (iii) Lee's medium (13), (iv) yeast nitrogen base without amino acids but supplemented with 2.5 mM N-acetylglucosamine (1), (v) RPMI 1640 (1) (Invitrogen), and (vi) serum (2 to 20%) in distilled, deionized water. Pig, horse, sheep, and bovine sera were obtained from the Meat Animal Research Center, Clay Center, Nebr. The solid medium used was GPP with 1.5% agar. After being autoclaved, the molten medium was cooled to 45°C and then added to 100- by 15-mm plastic petri dishes (20 ml medium/dish) containing appropriate aliquots of farnesol. The dishes were swirled to mix the farnesol into the medium before the agar solidified. Because the 20 ml per petri dish was estimated by eye, the resulting farnesol concentrations also differ somewhat. We believe the medium volumes are accurate to ±1 ml, and thus, the farnesol concentrations would be accurate to ±5%. Germ tube formation was quantified on plates by using a Labophot-2 microscope (Nikon Inc. Instrument Group, Melville, NY) modified as a tetrad dissection microscope (Micro Video Instruments, Avon, MA) using a Plan 10/0.30 objective lens.
Farnesol concentrations with and without serum.
Control cultures with no farnesol always had 98 to 100% germ tubes. For each of the defined media, the level of farnesol needed to reduce germ tube formation to 50% was ∼1 μM for both strains of C. albicans (Table 1). Also, the levels of farnesol needed were the same for liquid and solid GPP media (Table 1); they were not influenced by the presence of agar. However, the farnesol levels needed were influenced dramatically by the presence of serum. Serum increased the amount of farnesol needed in a dose-dependent manner (Table 1), which reached 150 to 250 μM farnesol at 10 to 20% serum. Virtually identical results were obtained with pig serum, horse serum, sheep serum, and bovine serum (Table 1).
TABLE 1.
Concentration of farnesol needed to reduce germ tube formation to 50%
| Germ tube trigger | Concn of farnesol (μM)a for indicated strain
|
|
|---|---|---|
| A72 | SC5314 | |
| N-Acetylglucosamine | 1 | 0.9 |
| GPP medium | 1 | 1 |
| Solid GPP medium | 0.75 | 1 |
| Lee's medium | 1 | 3 |
| YNB plus N-acetylglucosamine | 1 | 1 |
| RPMI 1640 | 0.9 | 1 |
| Serum (%) | ||
| 2 | 9 (P) | 17.5 (P), 10 (H) |
| 5 | ND | 25 (P), 50 (B) |
| 10 | 150 (P) | 175 (P), 150 (B) |
| 20 | ND | 250 (P), 250 (S) |
Concentration of farnesol (in μM) needed to reduce germ tube formation to 50%. Replicate experiments (two to four) were conducted at 37°C. Germ tube formation was measured at 4 hours for all media except solid GPP, which was measured at 5 hours. All controls without farnesol had ≥98% germ tube formation. Values indicated are the means of two to four separate experiments. All values are within ±10% of the mean. Sera were pig (P), horse (H), sheep (S), or bovine (B) sera. YNB, yeast nitrogen base without amino acids; ND, not determined.
Commitment.
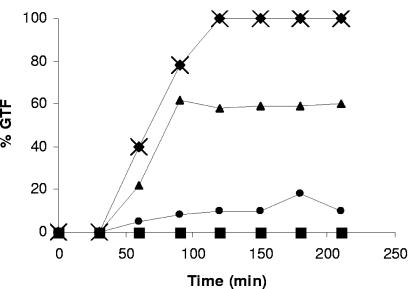
We also sought to clarify the effect of farnesol on preexisting hyphae (Fig. 1). Germ tube formation was blocked completely when farnesol was added at any time up to 30 min after inoculation. However, it was not blocked at all when the farnesol was added 90 min after inoculation, and it was stopped at an intermediate value (ca. 60% germ tubes) when the farnesol was added 60 min after inoculation (Fig. 1). These experiments were done with both strain A72 and strain SC5314 in both the N-acetylglucosamine differentiation medium and the GPP growth medium. The data shown in Fig. 1 are for SC5314 in GPP. In all cases, farnesol blocked germ tube formation by cells that did not already have visible germ tubes. These cells formed buds instead. However, farnesol neither reversed nor altered germ tube formation by cells that already had visible germ tubes. Those cells were committed to mycelial development (2, 14). Thus, farnesol does not halt the growth or elongation of existing mycelia, even at concentrations as high as 50 μM. When farnesol was added 2 hours after the inoculation of C. albicans A72 in N-acetylglucosamine, the germ tubes continued to elongate for at least the next 3.5 hours (6). Ramage et al. (16) reported a similar phenomenon during biofilm formation by C. albicans. Once hyphal formation had been initiated, it was not inhibited by the addition of farnesol (16). The present work extends their finding to include other, nonbiofilm germ tube-forming conditions.
FIG. 1.
Kinetics of germ tube formation (GTF) with farnesol added at various time points. C. albicans SC5314 was grown in GPP medium at 37°C, and farnesol (30 μM) was added at time zero (▪), 30 min (•), 60 min (▴), and 90 min (⧫). X, no farnesol addition. Values shown are the averages of duplicate measurements conducted on separate days which agreed within ±5%. The values for the samples with farnesol added at 90 min were the same as those with no farnesol addition.
The disparity in farnesol concentrations needed with and without serum (Table 1) is consistent with our hypothesis on the farnesol binding capacity of serum albumins. Albumins play an important role in the transport of sparingly soluble metabolic products from one tissue to another (20). Substances such as dyes, fatty acids, bilirubin, sulfonamides, and naphthoquinone derivatives, all of which are sparingly soluble in water, are readily dissolved in albumin solutions (20), and in each instance, it has been demonstrated that the dissolved substance is actually bound to the protein (20). Farnesol is also sparingly soluble in water (7). These differences with and without serum further illustrate the advantages of working with chemically defined media. Moreover, the fatty acid binding abilities of serum albumins can be exploited; adding fatty acid-deficient bovine serum albumin (0.4%) is a common mechanism for avoiding the toxic effects of excess fatty acids. The added albumins create a slow-release fatty acid buffer (3). We expect that the influences of farnesol on cell morphology will differ greatly during pathogenesis depending on location within the body. Two relevant factors would be the presence of albumins and the degree of anaerobicity, since C. albicans does not excrete farnesol under strictly anaerobic conditions (4).
Acknowledgments
This work was supported by grants from the National Science Foundation (MCB-0110999) and the University of Nebraska Tobacco Settlement Biomedical Research Enhancement Fund. D. D. Mosel, an undergraduate student at Nebraska Wesleyan University, Lincoln, was supported by a grant from the Howard Hughes Medical Institute to Nebraska Wesleyan University.
We thank Susan Hassler and Joe Ford, Meat Animal Research Center, Clay Center, Nebr., for providing the serum samples.
REFERENCES
- 1.Atlas, R. M. 1993. Handbook of microbiological media. CRC Press, Boca Raton, Fla.
- 2.Chaffin, W. L., and D. E. Wheeler. 1981. Morphological commitment in Candida albicans. Can. J. Microbiol. 27:131-137. [DOI] [PubMed] [Google Scholar]
- 3.Das, D. V. M., and G. Weeks. 1979. Effects of polyunsaturated fatty acids on the growth and differentiation of the cellular slime mould, Dictyostelium discoideum. Exp. Cell Res. 118:237-243. [DOI] [PubMed] [Google Scholar]
- 4.Dumitru, R., J. M. Hornby, and K. W. Nickerson. 2004. Defined anaerobic growth medium for studying Candida albicans basic biology and resistance to eight antifungal drugs. Antimicrob. Agents Chemother. 48:2350-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ernst, J. F. 2000. Transcription factors in Candida albicans—environmental control of morphogenesis. Microbiology 146:1763-1774. [DOI] [PubMed] [Google Scholar]
- 6.Hornby, J. M. 2003. Quorum sensing and the regulation of morphology in the dimorphic fungus Candida albicans. Ph.D. thesis. University of Nebraska—Lincoln, Lincoln.
- 7.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hornby, J. M., and K. W. Nickerson. 2004. Enhanced production of farnesol by Candida albicans treated with four azoles. Antimicrob. Agents Chemother. 48:2305-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson, D. A., Q. L. Sciascia, R. J. Sanders, G. E. Norris, P. J. B. Edwards, P. A. Sullivan, and P. C. Farley. 2004. Identification of the dialyzable serum inducer of germ-tube formation in Candida albicans. Microbiology 150:3041-3049. [DOI] [PubMed] [Google Scholar]
- 10.Kim, S., E. Kim, D.-S. Shin, H. Kang, and K.-B. Oh. 2002. Evaluation of morphogenic regulatory activity of farnesoic acid and its derivatives against Candida albicans dimorphism. Bioorg. Med. Chem. Lett. 12:895-898. [DOI] [PubMed] [Google Scholar]
- 11.Kruppa, M., B. P. Krom, N. Chauchan, A. V. Bambach, R. L. Cihlar, and R. A. Calderone. 2004. The two-component signal transduction protein Chk1p regulates quorum sensing in Candida albicans. Eukaryot. Cell 3:1062-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkarni, R. K., and K. W. Nickerson. 1981. Nutritional control of dimorphism in Ceratocystis ulmi. Exp. Mycol. 5:148-154. [Google Scholar]
- 13.Lee, K. L., H. R. Buckley, and C. C. Campbell. 1975. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia 13:148-153. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell, L. H., and D. R. Soll. 1979. Commitment to germ tube or bud formation during release from stationary phase in Candida albicans. Exp. Cell Res. 120:167-179. [DOI] [PubMed] [Google Scholar]
- 15.Odds, F. C. 1988. Candida and candidosis, 2nd ed. Bailliere Tindall, London, United Kingdom.
- 16.Ramage, G., S. P. Saville. B. L. Wickes, and J. L. Lopez-Ribot. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68:5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schepin, R., J. M. Hornby, E. Burger, T. Niessen, P. Dussault, and K. W. Nickerson. 2003. Quorum sensing in Candida albicans: probing farnesol's mode of action with 40 natural and synthetic farnesol analogs. Chem. Biol. 10:743-750. [DOI] [PubMed] [Google Scholar]
- 18.Soto, T., T. Watanabe, T. Mikami, and T. Matsumoto. 2004. Farnesol, a morphogenetic autoregulatory substance in the dimorphic fungus Candida albicans, inhibits hyphae growth through suppression of a mitogen-activated protein kinase cascade. Biol. Pharm. Bull. 27:751-752. [DOI] [PubMed] [Google Scholar]
- 19.Uppuluri, P., and L. Chaffin. 2004. Effect of farnesol on expression of Candida albicans stationary phase genes SNO1 and SNZ1. Abstr. 7th Am. Soc. Microbiol. Candida Candidiasis Conf., Austin, Tex., abstr. 243.
- 20.White, A., P. Handler, and E. L. Smith. 1959. Principles of biochemistry, 3rd ed. McGraw-Hill, New York, N.Y.