Abstract
Insertional inactivation of the plasmid-encoded determinants for Mg2+ and Co2+ transport, orf18/corA, provides a tool for screening recombinant clones in Lactococcus, based on the observation that overexpression of orf18/corA results in cell growth inhibition on certain concentrations of CoCl2. The lacticin 3147 immunity gene, ltnI, was used to insertionally inactivate orf18/corA. The resulting clones were capable of growth on concentrations of CoCl2 that were inhibitory to the parent strain. Since only 3 of 17 lactococcal starters naturally harbor corA, the system has potential as a screen for selecting recombinant lactococcal clones.
Lactococci are among the most widely used bacteria in food fermentations, where they are often the principal starters in various cheeses and fermented drinks (2). These organisms have been the focus of intensive genetic investigation over the last three decades. To date, one strain, Lactococcus lactis IL-1403, has been completely sequenced (1), L. lactis MG1363 is close to completion (9), while a draft version of the L. lactis SK11 genome is now available (23).
Given that the ultimate aim of research on the biotechnology of food cultures has been to genetically improve strains for food use, much of the focus in recent years has been on the development of self-cloning systems, which rely on genetic elements naturally occurring in the genus. Examples include resistance to the heavy metal cadmium (10) and resistance to the bacteriocin nisin (20), as well as several complementation markers that include the amber suppressor supD (18), the lacF gene, involved in lactose metabolism (11), or the thymidylate synthase gene, thyA (16, 19). However, the presence of inherent background resistances associated with many of these food-grade markers has placed limitations on their use.
CorA is the principal Mg2+ transport system among the eubacteria. It is also involved in the uptake of Co2+, and hence its presence can be lethal where environmental Co2+ concentrations are above a certain threshold. Genetic determinants for CorA activity, comprising the genes orf18 and corA, are found on the lactococcal plasmid, pAH90. Insertionally inactivating the pAH90 orf18/corA determinants in the presence of Co2+ allows cells to grow in concentrations that are otherwise toxic to the cell. In the present study, we report on the potential of using the lactococcal Mg2+ and Co2+ transport determinants as a tool for genetic manipulation of lactococci to eliminate background resistances when they are used in conjunction with another marker. This system has the potential to provide an added advantage to current food-grade selectable markers often associated with background resistance by eliminating these resistances and increasing the rate of identification of recombinant clones.
Sequence analysis, cloning, and overexpression of corA.
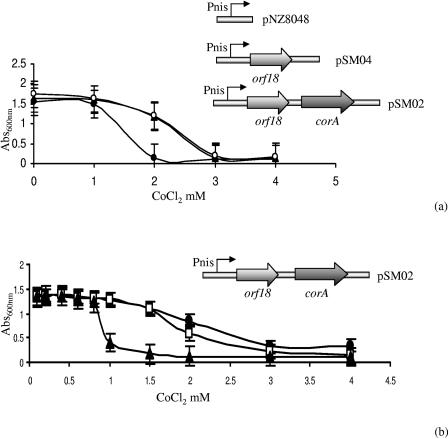
Analysis of the plasmid-encoded CorA protein using the DAS-transmembrane (8) and the HMMTOP prediction servers (21) showed that CorA is a membrane-associated protein (data not shown). Homologues of corA can also be found on other plasmids, for example, pNZ4000 (22) and pCIS3 (17). The corA gene in the present study was originally identified on the 26-kb phage resistance plasmid pAH90 (14), which is a cointegrate plasmid formed from the 20-kb plasmid pAH82 (accession number AF243383) and the 6-kb plasmid pAH33 (accession number AF207855) (7), the latter of which originally harbored the corA gene. In the present study, the nisin controlled expression (NICE) system (3, 4) was used to clone and overexpress corA; this system allows maximal protein expression at sublethal concentrations of nisin. The corA gene was initially amplified by using a proofreading polymerase with the primer pair CorF (5′-CGGGTACCCCCGACAAGGTTTCCGTTAT-3′) and CorR (5′-CCAAGCTTGCCCTATTCGGTTACAATCC-3′) and cloned into the KpnI/HindIII sites of the nisin expression vector pNZ8048, generating the plasmid pSM01. The resulting 1,848-bp product was found to be 100% identical to the nucleotide sequence information that was previously determined in this laboratory, (available in the GenBank sequence database under accession number AF207855). pSM01 was introduced into L. lactis NZ9800, an MG1614 derivative containing the nisRK signal transduction genes integrated on the chromosome. L. lactis NZ9800 harboring pSM01 was grown overnight in 0, 5, and 50 ng of nisin/ml in the presence of increasing concentrations of CoCl2, and CorA overexpression was analyzed by the growth inhibition assay. Exponentially grown cultures were inoculated into GM17 broth containing CoCl2 (0 to 6.0 mM) and were incubated for 16 h at 30°C, after which, growth was assessed by measuring the absorbance at 600 nm for each concentration of CoCl2. All experiments were carried out in triplicate. Surprisingly, L. lactis NZ9800 harboring pSM01 showed no activity under various concentrations of nisin after overnight growth in 0 to 4 mM CoCl2.
Orf18 is required for CorA activity.
Amplification of corA with the preceding open reading frame (orf18) (also referred to as orf04 of pAH33) by using the primer pair CorF1 (5′-GGACTAGTCGTTATCTACTAGGTAGA-3′) and CorR1 (5′-CCAAGCTTGCCCTATTCGGTTACAATCC-3′) (italics indicate restriction enzyme sites), and cloning into the SpeI/HindIII sites of pNZ8048 resulted in the generation of pSM02. Unlike pSM01, this construct did result in functional activity as assessed by growth inhibition assays, even under conditions in which nisin was absent (Fig. 1a). Indeed, in the absence of nisin, NZ9800(pSM02) was sensitive to 2 to 2.5 mM CoCl2 in comparison to the control NZ9800(pNZ8048), which is normally capable of growth in up to 2.5 mM CoCl2 (Fig. 1a). This is presumably due to a basal level of expression associated with the NICE system in the absence of induction. In the presence of 50 ng of nisin/ml, the sensitivity of NZ9800(pSM02) to CoCl2 dramatically increased. In this case, CoCl2 concentrations in the range of 1 to 1.5 mM allowed negligible growth of the orf18/corA expressing clone (Fig. 1b). In comparison, growth of the vector control was not inhibited by the same concentrations of CoCl2 (results not shown). However, the phenotypic response of NZ9800(pSM02) to nisin was dramatically lower when concentrations of <10 ng/ml were used (Fig. 1b). To examine whether orf18 was responsible for the cobalt sensitivity phenotype observed with pSM02, orf18 was amplified with the primer pair Orf18F (5′-GGACTAGTCGTTATCTACTAGGTAG-3′) and Orf18R (5′-CCCAAGCTTCTGTTAGTTATTAACCTCTATTTTAGA-3′) and cloned into the SpeI/HindIII sites of pNZ8048 generating the plasmid pSM04. The gene did not confer sensitivity to cobalt (Fig. 1a). Therefore, the lactococcal corA gene can only function in the presence of orf18.
FIG. 1.
(a) Growth inhibition assays after 16 h of growth in 0 to 4.5 mM CoCl2 in the absence of nisin. Symbols: ▴, NZ9800(pNZ8048); ○, NZ9800(pSM04); •, NZ9800(pSM02). (b) Overexpression of corA. A growth inhibition assay of NZ9800(pSM02) after 16 h of growth in 0 to 4.5 mM CoCl2 in the presence of 0 ng of nisin/ml (•), 5 ng of nisin/ml (□), and 50 ng of nisin/ml (▴). For both graphs, construction schemes for the vectors are shown. The vector pNZ8048 is represented as a thick gray bar, the nisin inducible promoter is shown as a small thin black arrow, orf18 is represented as a light gray boxed arrow, and corA is represented as a dark gray boxed arrow.
Sequence analysis of pAH90-encoded orf18.
The gene product of orf18 does not exhibit homology to any protein of known function. Interestingly, the lactococcal exopolysaccharide-producing plasmid pNZ4000 (22), which harbors corA, also encodes a gene with homology to orf18 and is located directly upstream of corA. A similar arrangement is also seen on the lactococcal plasmid pCIS3 (17). This arrangement was not observed for the chromosomally encoded corA gene of L. lactis IL-1403, which instead is preceded by a gene involved in fatty acid metabolism, suggesting that the arrangement is specific to corA genes associated with plasmid DNA. Since Orf18 does not contain any transmembrane domains, its primary location is most likely in the cytoplasm, although it may be associated with the transmembrane transporter, by binding Mg2+, which has been transported across the membrane, delivering it into the cell. The absence of secretion signals associated with Orf18 supports this hypothesis.
Insertional inactivation of orf18/corA with ltnI.
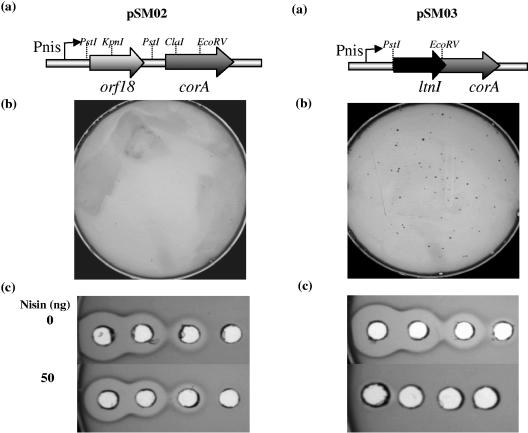
Based on the exhibited sensitivity to CoCl2, we investigated whether the orf18/corA determinants could be used as a tool for screening recombinant clones in Lactococcus, in conjunction with another selectable marker, chloramphenicol. To this end, the gene encoding immunity to the lantibiotic lacticin 3147, ltnI, was amplified from the 60-kb lactococcal plasmid pMRC01 (5) by using the primer pair LtnIF (5′-AACTGCAGATGAAGAATGAAAATATC-3′) and LtnIR (5′-GGATATCTTATTTATTATCTTTAATA-3′). This product was cloned into the PstI/EcoRV restriction sites in the orf18/corA determinants to form the plasmid pSM03, whereby orf18 was replaced with ltnI and corA was truncated in the strategy (Fig. 2a). After electroporation of this plasmid into L. lactis NZ9800, transformants were plated onto agar containing 2.5 mM CoCl2 and 5 μg of chloramphenicol/ml and were obtained at a frequency of 3 × 102 CFU/μg of DNA (Fig. 2b). Eight colonies were analyzed for the presence of the ltnI gene by the PCR; all eight colonies were shown to harbor ltnI (results not shown). In contrast, no growth was observed for the control transformation, wherein pSM02 was directly transformed into L. lactis NZ9800 (Fig. 2b). This insertional inactivation of the orf18/corA determinants is associated with improved growth at higher CoCl2 concentrations. Indeed, the scheme was effective without the addition of nisin. Moreover, background resistance of the orf18/corA-expressing strain to 2.5 mM CoCl2 was <10−9 CFU, allowing the selection to be used directly after transformation on solid media. Furthermore, the ltnI gene, which was inserted into the orf18/corA determinants, was found to be functionally active and overexpressed. Indeed, in the presence of 50 ng of nisin/ml, the strain was fourfold less sensitive to lacticin. This compares favorably to the previous results of McAuliffe et al. (12), who cloned and overexpressed the ltnI gene and demonstrated that increasing the level of inducer resulted in a concomitant increase in the level of immunity of NZ9800 to lacticin 3147.
FIG. 2.
(a) Restriction map of the vectors pSM02 and pSM03. Boxed arrows represent the genes; the light gray arrow represents orf18, the dark gray arrow represents corA, and the black arrow represents ltnI. The thick gray line represents the vector pNZ8048. The nisin-inducible promoter is shown as a black arrow. (b) Selection plates after transformation of competent L. lactis NZ9800 with pSM02 and pSM03 plated onto 5 μg of chloramphenicol/ml and 2.5 mM CoCl2 after incubation overnight at 30°C. (c) Well diffusion assays demonstrating immunity of NZ9800(pSM02) and NZ9800(pSM03) exhibited against lacticin 3147. Immunity in the absence of nisin is shown in the top panel and in the presence of nisin is shown in the bottom panel.
Distribution of plasmid-encoded corA.
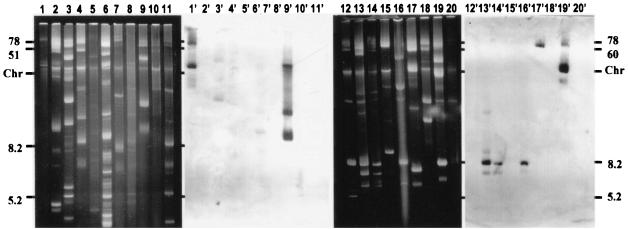
CorA systems with high identity (>97%) to the pAH33 system have already been identified on the 42-kb plasmid pNZ4000 from the extracellular polysaccharide-producing strain L. lactis NIZO B40 (22), the 6-kb plasmid pCIS3 from the dairy starter L. lactis UC509.9 (17), and the 9.2-kb Lactobacillus plantarum plasmid pLP9000 (15). In order to establish the general applicability of this system to various starter strains, the distribution of corA was examined in the plasmid complements of 17 different strains of lactococci. Plasmid isolation and electrophoresis was followed by Southern blot analysis with the pAH33-corA PCR product (generated with primers CorP1 (5′-ATGATCAAACCCGAAAAGAC-3′) and CorP2 (5′-TCACATCTTCCGCCAGAACTTC-3′)) as a probe. This showed that the corA determinant was only linked to plasmid DNA in 3 of the 17 selected strains. These were L. lactis SK11G, DPC1014, and DPC3886, which harbored the corA determinant on plasmids of approximately 26, 6, and 78 kb, respectively (Fig. 3). We have previously observed that lactococcal plasmids pAH33 and pAH90 contain a corA gene proximal to the phage resistance determinants (14). Therefore, as expected, there was also hybridization to a 6-kb plasmid in strain 425A corresponding to pAH33 and a 26-kb plasmid in strain DPC721 corresponding to pAH90. Strain DPC3290 derived from L. lactis MG1614 containing pAH90 was used as a positive control. The physiological function of these plasmid-encoded genes still remains cryptic.
FIG. 3.
Plasmid profiles of L. lactis strains and corresponding Southern blots with the pAH33 corA probe. Lanes 1 to 11 and 1′ to 11′: DPC3290, DPC4935, Z8, 17, E8, HP, 320, 19B(d), SK11G, DPC4987, DRC3(d). Lanes 12 to 20 and 12′ to 20′: DRC3(d), DPC1014(d), DPC1014(d), AM1, 425A(d), DPC3886, DPC4270, DPC721(d), DRC3(d). Molecular masses are indicated in kilobases and are based on the mobilities of plasmids from strain DRC3. The superscript “(d)” indicates biovar diacetylactis.
To summarize, we overexpressed the plasmid-borne Mg2+/Co2+ transporter and investigated its potential as a marker gene for direct insertional inactivation in lactococci and can make the following overall conclusions. (i) CorA requires the preceding Orf18 for Co2+ transport activity, and they function as a gene pair, which occurs in at least three other lactococcal strains. (ii) Strains overexpressing the orf18/corA determinants cannot grow on CoCl2 concentrations of >2.0 mM, whereas strains in which orf18 has been replaced with ltnI and corA has been truncated can grow in concentrations greater than this value. (iii) Since the spontaneous resistance of lactococci expressing orf18/corA on solid media after transformation (in the absence of nisin) is less than 1 in 109 CFU, the system could be used to identify clones in which the gene has been disrupted. (iv) The system should be applicable to the vast majority of dairy lactococci given the low incidence of plasmid-borne corA.
Acknowledgments
This research has been funded in part by grant aid under the Food Sub-Programme of the Operational Programme for Industrial Development, which is administered by the Department of Agriculture, Food, and Forestry and supported by national and European Union funds. S.M. was supported by a Teagasc Walsh Fellowship.
REFERENCES
- 1.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis subsp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cogan, T., and J. Accolas. 1996. Dairy starter cultures. VCH Publishers, Inc., New York, N.Y.
- 3.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ruyter, P. G., O. P. Kuipers, W. C. Meijer, and W. M. de Vos. 1997. Food-grade controlled lysis of Lactococcus lactis for accelerated cheese ripening. Nat. Biotechnol. 15:976-979. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty, B. A., C. Hill, J. F. Weidman, D. R. Richardson, J. C. Venter, and R. P. Ross. 1998. Sequence and analysis of the 60 kb conjugative, bacteriocin-producing plasmid pMRC01 from Lactococcus lactis DPC3147. Mol. Microbiol. 29:1029-1038. [DOI] [PubMed] [Google Scholar]
- 6.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington, A., and C. Hill. 1992. Plasmid involvement in the formation of a spontaneous bacteriophage insensitive mutant of Lactococcus lactis. FEMS Microbiol. Lett. 75:135-141. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda, M., M. Arai, D. M. Lao, and T. Shimizu. 2002. Transmembrane topology prediction methods: a re-assessment and improvement by a consensus method using a dataset of experimentally-characterized transmembrane topologies. In Silico Biol. 2:19-33. [PubMed] [Google Scholar]
- 9.Klaenhammer, T. R., E. Altermann, F. Arigoni, A. Bolotin, F. Breidt, J. Broadbent, R. Cano, S. Chaillou, J. Deutscher, M. Gasson, M. van de Guchte, J. Guzzo, A. Hartke, T. Hawkins, P. Hols, R. Hutkins, M. Kleerebezem, J. Kok, O. Kuipers, M. Lubbers, E. Maguin, L. McKay, D. Mills, A. Nauta, R. Overbeek, H. Pel, D. Pridmore, M. Saier, D. van Sinderen, A. Sorokin, J. Steele, D. O'Sullivan, W. de Vos, B. Weimer, M. Zagorec, and R. Siezen. 2002. Discovering lactic acid bacteria by genomics. Antonie Leeuwenhoek 82:29-58. [DOI] [PubMed] [Google Scholar]
- 10.Liu, C. Q., V. Leelawatcharamas, M. L. Harvey, and N. W. Dunn. 1996. Cloning vectors for lactococci based on a plasmid encoding resistance to cadmium. Curr. Microbiol. 33:35-39. [DOI] [PubMed] [Google Scholar]
- 11.MacCormick, C. A., H. G. Griffin, and M. J. Gasson. 1995. Construction of a food-grade host/vector system for Lactococcus lactis based on the lactose operon. FEMS Microbiol. Lett. 127:105-109. [DOI] [PubMed] [Google Scholar]
- 12.McAuliffe, O., C. Hill, and R. P. Ross. 2000. Identification and overexpression of ltnl, a novel gene which confers immunity to the two-component lantibiotic lacticin 3147. Microbiology 146(Pt. 1):129-138. [DOI] [PubMed] [Google Scholar]
- 13.McKay, L. L., and K. A. Baldwin. 1984. Conjugative 40-megadalton plasmid in Streptococcus lactis subsp. diacetylactis DRC3 is associated with resistance to nisin and bacteriophage. Appl. Environ. Microbiol. 47:68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Sullivan, D., R. P. Ross, D. P. Twomey, G. F. Fitzgerald, C. Hill, and A. Coffey. 2001. Naturally occurring lactococcal plasmid pAH90 links bacteriophage resistance and mobility functions to a food-grade selectable marker. Appl. Environ. Microbiol. 67:929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren, D., Y. Wang, Z. Wang, J. Cui, H. Lan, and J. Zhou. 2003. Complete DNA sequence and analysis of two cryptic plasmids isolated from Lactobacillus plantarum. Plasmid 50:70-73. [DOI] [PubMed] [Google Scholar]
- 16.Ross, P., F. O'Gara, and S. Condon. 1990. Thymidylate synthase gene from Lactococcus lactis as a genetic marker: an alternative to antibiotic resistance genes. Appl. Environ. Microbiol. 56:2164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seegers, J. F., D. van Sinderen, and G. F. Fitzgerald. 2000. Molecular characterization of the lactococcal plasmid pCIS3: natural stacking of specificity subunits of a type I restriction/modification system in a single lactococcal strain. Microbiology 146(Pt. 2):435-443. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen, K. I., R. Larsen, A. Kibenich, M. P. Junge, and E. Johansen. 2000. A food-grade cloning system for industrial strains of Lactococcus lactis. Appl. Environ. Microbiol. 66:1253-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steidler, L., S. Neirynck, N. Huyghebaert, V. Snoeck, A. Vermeire, B. Goddeeris, E. Cox, J. P. Remon, and E. Remaut. 2003. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat. Biotechnol. 21:785-789. [DOI] [PubMed] [Google Scholar]
- 20.Takala, T. M., and P. E. Saris. 2002. A food-grade cloning vector for lactic acid bacteria based on the nisin immunity gene nisI. Appl. Microbiol. Biotechnol. 59:467-471. [DOI] [PubMed] [Google Scholar]
- 21.Tusnady, G. E., and I. Simon. 2001. The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849-850. [DOI] [PubMed] [Google Scholar]
- 22.van Kranenburg, R., M. Kleerebezem, and W. M. de Vos. 2000. Nucleotide sequence analysis of the lactococcal EPS plasmid pNZ4000. Plasmid 43:130-136. [DOI] [PubMed] [Google Scholar]
- 23.Weimer, B., and L. McKay. 2002. Lactococcus lactis subsp. cremoris SK11. Joint Genome Institute, Walnut Creek, Calif.