Abstract
Campylobacter jejuni and Campylobacter-specific bacteriophage were enumerated from broiler chicken ceca selected from 90 United Kingdom flocks (n = 205). C. jejuni counts in the presence of bacteriophage (mean log10 5.1 CFU/g) were associated with a significant (P < 0.001) reduction compared to samples with Campylobacter alone (mean log10 6.9 CFU/g).
Campylobacter jejuni is a major cause of acute bacterial enteritis worldwide. Domestic poultry are a reservoir for campylobacters and undercooked poultry meat, in particular chicken, is by implication an etiological source of human infections (11). One potential mechanism to control Campylobacter is the use of host-specific bacteriophage (3, 8). Previous studies have shown that there is a high prevalence of Campylobacter in broiler chicken flocks (5, 9, 13), which are able to survive abattoir processing and contaminate retail products (12). Similarly, we and others have shown that Campylobacter bacteriophages are present in the chicken intestinal tract (4, 7, 15, 16) and in all probability are the sources of bacteriophages demonstrated to survive processing (2). Although the chicken intestinal tract has been established as a source of Campylobacter and their phage, there has not been any attempt to enumerate naturally occurring populations of either in commercial broiler chickens. The aim of the present study was to investigate relationships between populations of Campylobacter and their bacteriophage found in the ceca of conventional barn-reared broiler chickens in the United Kingdom.
Commercial Ross broiler chickens (n = 205) from 90 flocks were obtained between August and September 2002 from 22 farms belonging to three national poultry producers based in the United Kingdom. The chickens were slaughtered between 22 and 46 days of age (mean 34 days), transported to the laboratory and stored at 4°C prior to dissection. All dissections were performed within 48 h of slaughter. The ceca of each chicken were removed individually, with thorough decontamination between dissections, and stored in sterile petri dishes at 4°C for up to 2 h prior to enumeration of bacteria and phage.
For the enumeration of bacterial populations, cecal contents (1 g) were diluted 1:10 in maximum recovery diluent (CODE CM733; Oxoid, Basingstoke, United Kingdom) and resuspended by pulse vortex mixing for ca. 2 min. This suspension was then serially diluted in maximum recovery diluent prior to spread plating 100-μl volumes of each dilution in duplicate on to the surface of modified charcoal-cefoperazone-deoxycholate agar (CCDA; Lab 112, Lab M, Bury, United Kingdom) with selective supplements (PL450; ProLab Diagnostics, Chester, United Kingdom). Plate count agar (0479-01-1; Difco, Oxford, United Kingdom) was used to enumerate the total aerobic, anaerobic, and microaerobic populations. CCDA plates were incubated under microaerobic conditions (5% H2, 5% O2, 10% CO2, 80% N2) at 42°C. Plate count agar plates were incubated at 37°C either aerobically, anaerobically, or microaerobically for the respective plates. Enumeration was performed after 24 to 48 h of incubation for all populations. Between four to eight presumptive Campylobacter colonies from each of 205 cecal samples were Gram stained and examined under a light microscope to confirm typical morphology prior to biochemical tests for oxidase, catalase, and hippurate hydrolysis. Macrorestriction profiles (SmaI and KpnI) of 124 Campylobacter chromosomal DNA were resolved by pulsed-field gel electrophoresis as described by Ribot et al. (14).
For the isolation of Campylobacter bacteriophage, cecal contents (1 g) were diluted 1:10 in SM buffer (50 mM Tris-HCl [pH 7.5], 0.1 M NaCl, 8 mM MgSO4 · 7H2O, 0.01% gelatin; Sigma, Gillingham, Dorset, United Kingdom) and resuspended by gentle inversion and pulse vortex mixing for up to 5 min. This suspension was then incubated aerobically at 4°C for 24 h on a gyratory platform shaker (60 rpm) to allow phage elution into the buffer. An aliquot (1 ml) of eluate was transferred to a sterile 1.5-ml microcentrifuge tube and subjected to centrifugation at 10, 000 × g for 3 min to remove bulk debris then passed through a 0.2-μm-pore-size membrane filter (Minisart; Sartorius, Gottingen, Germany) to remove any remaining bacterial cells. This filtrate was then serially diluted in SM buffer prior to enumeration on lawns of bacterial cells by using the surface droplet technique (16). Each bacteriophage isolate was subjected to a minimum of three successive rounds of serial plaque purification and propagation prior to lytic spectrum profiling (6). These purified isolates were then screened for their ability to infect Campylobacter strains, possessing different phage types, obtained from the National Collection of Type Cultures. Phage isolates with different lytic spectra were screened against a further 80 Campylobacter strains isolated from human sources and are distinguishable by their sensitivity to the phages used to form the basis of the United Kingdom phage typing scheme (6).
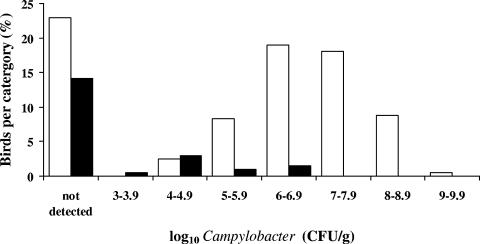
C. jejuni was isolated from 63% (129 of 205) of the ceca of birds tested and lytic bacteriophage of C. jejuni in 20% (41 of 205). No C. coli isolates were recovered in the present study. The enumeration of campylobacters from chicken ceca in the presence of bacteriophage (mean log10 5.1 CFU/g) was associated with a significant (P < 0.001) reduction in numbers compared to samples with Campylobacter alone (mean log10 6.9 CFU/g). The detection limit was determined as log10 2.0 Campylobacter CFU/g of cecal contents and similarly for bacteriophage log10 2.0 PFU/g of cecal contents. The calculation of mean colonization values excluded samples where Campylobacter was not detected. The Campylobacter-positive ceca were ranked according to their colonization titer recorded as the CFU/gram of cecal contents (Fig. 1). In the absence of phage, the colonization levels could be fitted to a normal distribution with a median of log10 6.9 CFU/g of cecal contents. Whereas when bacteriophages were present in conjunction with campylobacters, a reduction in titer of ca. 2 logs was observed. It was notable that viable Campylobacter cells could not be recovered from 29 of 41 (71%) of the phage-positive chicken ceca by using conventional selective plating techniques. This may be compared to 47 of 164 (29%) of the phage-negative ceca. The difference between these incidences may also be due to the influence of the phage populations acting to reduce the cecal host colonization levels below the limit of detection and possibly during experimental recovery. To investigate the possibility of other bacterial populations affecting the colonization of Campylobacter or the presence of bacteriophage, the total populations of aerobic, anaerobic, and microaerobic bacteria in the broiler chicken cecal contents were determined. No discernible differences evident in the total aerobic counts which remained constant (mean count of log10 7.4 ± 0.6 CFU/g of cecal contents). The microaerobic and anaerobic counts determined were more variable with mean counts of log10 7.5 ± 2.4 and log10 8.9 ± 2.1 CFU/g of cecal contents, respectively, but no significant differences were distinguishable between any of the subgroups.
FIG. 1.
Distribution of C. jejuni enumerated from chicken ceca with (▪) and without (□) phage expressed as a percentage of birds (n = 205) per category (intervals of 1 log10 CFU/g).
Macrorestriction profiling of six individual Campylobacter colonies from each of 12 phage-positive and 12 negative ceca was carried out. This analysis produced 19 unique profiles from 144 single-colony isolates. None of the profiles showed any significant association with either the phage-positive or -negative groups. However, it was notable that the ceca of the 5 of 12 phage-positive birds contained two or more Campylobacter types compared to 2 of 12 in which the phage were absent. The presence of phage in chicken ceca is likely to impose a considerable selective pressure on their hosts. The demise of Campylobacter strains sensitive to infection creates a niche for either phage-resistant subpopulations or new strains from the environment. This may ultimately result in a greater diversity of campylobacters in chickens which harbor phage. Recent studies have demonstrated the succession of phage-insensitive Campylobacter types within broiler flocks that naturally harbor phage (4).
A wide range of bacteriophage titers (log10 1.5 to log10 6.9 PFU/g of cecal contents) were recovered from chicken ceca. The range in titers probably reflects the availability of hosts due to the dynamic nature of Campylobacter populations in the chicken gut. Previous studies with Salmonella enterica serovar Typhi and Escherichia coli have shown that phage seldom eliminate their prey (1). Often in these cases, the numbers of predator and prey increase and decline with characteristic out-of-phase population oscillations (10, 18). Providing such interactions occur between phage and Campylobacter in the chicken gut, the numbers of phage recovered could vary according to the stage of the oscillatory cycle at which the sample was collected. The lytic spectra of the broiler phage isolates could be organized into nine lytic classes by using the phage typing scheme adopted in the United Kingdom (6). The assignment of phage to each lytic class was based on whether they are able to infect each of 80 Campylobacter type strains recovered from patients suffering from campylobacteriosis (6). The number of Campylobacter type strains infected by each phage lytic class varied considerably from 26 of 80 to 52 of 80.
Of the 41 birds from which bacteriophage were isolated, only 12 had recoverable campylobacters, and of these only four birds yielded sensitive Campylobacter hosts capable of propagating phage from the same source. The apparent absence of the host could have arisen due to the isolation procedures adopted, for example, if they were biased toward the recovery of certain phage and/or campylobacters, or be due to changes in host susceptibility that arose upon subculture. However, the oscillations in the predator and prey could also explain the observation if not all of the campylobacters present were sensitive to the phage. Depending on the amplitude of the predator and prey oscillation, the phage-sensitive hosts may become a minor component of the total Campylobacter population and present a lower stochastic chance of recovery from birds that are cocolonized. Given this advantage, an insensitive Campylobacter could displace the original host, resulting in the succession of a new population or of a subpopulation of the original in which phage resistance had developed. All other fitness characteristics being equal, the phage-resistant population could colonize the chicken gut with relative impunity. However, previous studies have shown phage-resistant mutants of E. coli to be less-virulent and less-efficient colonizers than the wild type (17). This may in part explain why fewer campylobacters were isolated from ceca also containing phage. For those birds harboring Campylobacter-specific phage but from which Campylobacter could not be recovered, explanations based on simple predator-prey relationships cannot easily be applied. This behavior could be a consequence of alternative bacterial flora competing for the same niche without the encumbrance of phage infection. If this was the case, it was not evident in the gross populations since the total aerobic, anaerobic, or microaerobic counts determined from Campylobacter-negative, phage-positive birds showed no significant differences compared to those determined without phage. However, it is possible the relative proportions of the bacterial species could change without altering the total counts.
Understanding the dynamics of the interaction between bacteriophage and their hosts in vivo is necessary if bacteriophages are to be used to reduce the incidence of pathogens in animal reservoirs. The results of the present study suggest that phage may affect the numbers of C. jejuni in commercial broiler chickens to various degrees, in that we demonstrate a correlation between the presence of natural environmental phage and a reduction in the numbers of Campylobacter colonizing broiler chicken ceca. These observational data are encouraging in that they provide evidence that phage-mediated control of Campylobacter in poultry could be utilized under commercial operating conditions. However, further studies are required to understand the interaction of Campylobacter-phage and their hosts in chicken poultry production if practical intervention measures are to be established.
Acknowledgments
This study was supported in part by the United Kingdom Department for the Environment, Food, and Rural Affairs. R.J.A. acknowledges the financial support of the University of Nottingham.
REFERENCES
- 1.Alexander, M. 1981. Why microbial predators and parasites do not eliminate their prey and hosts. Annu. Rev. Microbiol. 35:113-133. [DOI] [PubMed] [Google Scholar]
- 2.Atterbury, R. J., P. L. Connerton, C. E. R. Dodd, C. E. D. Rees, and I. F. Connerton. 2003. Isolation and characterization of Campylobacter bacteriophages from retail poultry. Appl. Environ. Microbiol. 69:4511-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atterbury, R. J., P. L. Connerton, C. E. R. Dodd, C. E. D. Rees, and I. F. Connerton. 2003. Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 69:6302-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connerton, P. L., C. M. Loc Carrillo, C. Swift, E. Dillon, A. Scott, C. E. D. Rees, C. E. R. Dodd, J. Frost, and I. F. Connerton. 2004. A longitudinal study of Campylobacter jejuni bacteriophage and their hosts from broiler chickens. Appl. Environ. Microbiol. 70:3877-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans, S. J., and A. R. Sayers. 2000. A longitudinal study of Campylobacter infection of broiler flocks in Great Britain. Prev. Vet. Med. 46:209-223. [DOI] [PubMed] [Google Scholar]
- 6.Frost, J. A., J. M. Kramer, and S. A. Gillanders. 1999. Phage typing of Campylobacter jejuni and Campylobacter coli and its use as an adjunct to serotyping. Epidemiol. Infect. 123:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grajewski, B. A., J. W. Kusek, and H. M. Gelfand. 1985. Development of a bacteriophage typing system for Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 22:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goode, D., V. M. Allen, and P. A. Barrow. 2003. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69:5032-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey, T. J., A. Henley, and D. G. Lanning. 1993. The colonization of broiler chickens with Campylobacter jejuni: some epidemiological investigations. Epidemiol. Infect. 110:601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizoguchi, K., M. Morita, C. R. Fischer, M. Yoichi, Y. Tanji, and H. Unno. 2003. Coevolution of bacteriophage PP01 and Escherichia coli O157:H7 in continuous culture. Appl. Environ. Microbiol. 69:170-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newell, D. G., and C. Fearnley. 2003. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 69:4343-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newell, D. G., J. E. Shreeve, M. Toszeghy, G. Domingue, S. Bull, T. Humphrey, and G. Mead. 2001. Changes in the carriage of Campylobacter strains by poultry carcasses during processing in abattoirs. Appl. Environ. Microbiol. 67:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newell, D. G., and J. A. Wagenaar. 2000. Poultry infections and their control at the farm level, p. 497-509. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter. ASM Press, Washington, D.C.
- 14.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sails, A. D., D. R. Wareing, F. J. Bolton, A. J. Fox, and A. Curry. 1998. Characterisation of 16 Campylobacter jejuni and C. coli typing bacteriophages. J. Med. Microbiol. 47:123-128. [DOI] [PubMed] [Google Scholar]
- 16.Salama, S., F. J. Bolton, and D. N. Hutchinson. 1989. Improved method for the isolation of Campylobacter jejuni and Campylobacter coli bacteriophages. Lett. Appl. Microbiol. 8:5-7. [Google Scholar]
- 17.Smith, H. W., and M. B. Huggins. 1983. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets, and lambs. J. Gen. Microbiol. 129:2659-2675. [DOI] [PubMed] [Google Scholar]
- 18.Van den Ende, P. 1973. Predator-prey interactions in continuous culture. Science 181:562-564. [DOI] [PubMed] [Google Scholar]