Abstract
The objectives of this study were to isolate beneficial strains of microorganisms from the gastrointestinal tracts of healthy chickens and to screen them against Clostridium perfringens, a causative agent of necrotic enteritis in poultry. One of the bacteria isolated, a strain of Bacillus subtilis, was found to possess an anticlostridial factor that could inhibit the C. perfringens ATCC 13124 used in this study. The anticlostridial factor produced by B. subtilis PB6 was found to be fully or partially inactivated in the presence of pronase, trypsin, and pepsin. In contrast, the antimicrobial activity of the anticlostridial factor was not affected by treatment at 100 or 121°C or by treatment with any of the organic solvents used in the study. The optimum growth temperature and optimum pH for production of the anticlostridial factor were 37°C and 6.20, respectively. Using the mass spectroscopy-mass spectroscopy technique, the apparent molecular mass of the anticlostridial factor was estimated to be in the range from 960 to 983 Da. In terms of the antimicrobial spectrum, the anticlostridial factor was inhibitory toward various strains of C. perfringens implicated in necrotic enteritis in poultry, Clostridium difficile, Streptococcus pneumoniae, Campylobacter jejuni, and Campylobacter coli.
Necrotic enteritis, an enterotoxemic disease caused by Clostridium perfringens, leads to the development of necrotic lesions in the gut wall, resulting in mortality of poultry (30). This disease is also multifactorial with complex and partially unknown epidemiology and pathogenesis (18). The bacterium C. perfringens is commonly found in the gastrointestinal tract of poultry; the occurrence of necrotic enteritis is, however, sporadic (39). Nevertheless, feed contaminated with C. perfringens has been implicated in outbreaks of necrotic enteritis in chickens (18). Studies have also shown that a healthy chicken has a relatively low number of C. perfringens cells in its gastrointestinal tract, while an increase in the concentration of the bacterium has been correlated with necrotic enteritis (5). Bacitracin, linocomycin, and other growth-promoting antibiotics are commonly used to treat poultry suffering from necrotic enteritis (5). However, due to the isolation of antibiotic-resistant strains of C. perfringens from chickens and turkeys, other nonantibiotic alternatives are being sought (7).
The bacterial microflora can have both positive and negative effects on the intestinal health of a host and its susceptibility to disease (11). Microorganisms such as Lactobacillus spp., Streptococcus spp., and Escherichia coli have been isolated from the duodenum, jejunum, and ileum of the small intestine (23). The maintenance of some bacteria in the intestine, such as Lactobacillus spp. and Bifidobacterium spp., has been well recognized to have beneficial effects on the host animal (4). Substantial progress has been made in the development of probiotics, probiotics, and synbiotics, which are reported to be effective in increasing and maintaining lactic acid bacteria (LAB) in the intestine (20). Previous studies have suggested that Lactobacillus spp., Bifidobacterium spp., and Bacillus subtilis can be used to increase and maintain beneficial bacteria in the intestine (13). One of these organisms, B. subtilis, is used in the feed industry (33). In addition to stimulation of positive changes in the intestinal microflora (14), recovery from diarrhea (22), enhanced body weight gain, and feed efficiency (17) in the hosts were observed when animals were fed B. subtilis.
Antimicrobial peptides are produced by a wide variety of organisms, notably bacteria, and are believed to play an important protective role. When peptides from different organisms were compared (12), no consensus in the amino acid sequences was observed that could be used to design potent peptides targeted against specific organisms (12). In general, most antimicrobial peptides have at least 50% hydrophobic amino acid residues and between two and nine positively charged amino acids, such as arginine or lysine (12). Ribosomally synthesized peptides, including both cationic and neutral peptides, are secreted by gram-positive and gram-negative bacteria. Antimicrobial compounds, such as bacteriocins produced by gram-positive bacteria, are often membrane-permeabilizing cationic peptides with fewer than 60 amino acid residues (15).
For the last few decades, many studies have been focused on antagonistic metabolites produced by lactic acid bacteria (15). Besides bacteriocins, other common metabolites, such as organic acids (6), acetaldehyde, hydrogen peroxide, diacetyl, carbon dioxide, antibiotics, and amines (17, 31), are produced by lactic acid bacteria. In contrast, bacteriocins and other antagonistic metabolites of Bacillus spp. have attracted little attention even though some Bacillus spp., such as B. subtilis and Bacillus licheniformis, are “generally recognized as safe” bacteria (33).
In an attempt to develop probiotic strains to inhibit C. perfringens, we isolated B. subtilis PB6 from the gastrointestinal tracts of healthy chickens. The PB6 strain is able to produce a bacteriocin that is effective against both gram-negative and gram-positive bacteria that are potentially pathogenic for both humans and animals.
MATERIALS AND METHODS
Isolation and identification of beneficial microorganisms.
Intestinal tracts of healthy chicken were obtained from a local market that was certified by the Agri-food & Veterinary Authority of Singapore. Freshly obtained intestinal tracts were dissected into four sections, the duodenum, the jejunum, the ileum, and the ceca.
Contents of each segment were collected in test tubes containing deMan Rogosa Sharpe (MRS) broth and then incubated at 37°C under 5% CO2 for 48 h. After 48 h of incubation, overnight cultures obtained from various intestinal segments were streaked onto MRS agar to obtain isolated colonies of lactic acid bacteria. Similarly, contents of various segments of the intestinal tracts were inoculated into tryptic soy broth supplemented with yeast extract (6 g/liter) (TSBYE broth) and then heated at 80°C for 20 min. After the heat treatment, portions from each test tube were streaked onto TSAYE agar and incubated at 37°C for isolation of Bacillus spp.
Biochemical tests using API 50CH and API 50CHB/L (bioMerieux) were performed to identify all bacteria, including Bacillus spp. and Lactobacillus spp., that were isolated from the intestinal tracts. Pure cultures of selected lactic acid bacteria and Bacillus spp. were further characterized by ribotyping their 16S RNA using RiboPrinter (Dupont, Wilmington, Del.).
Bacterial strains and culture conditions.
Isolated colonies of B. subtilis PB6 from TSAYE medium were transferred into 100 ml of TSBYE broth (Becton Dickenson and Company, Cockeysville, MD) supplemented with 0.6% yeast extract (Oxoid Limited, Basingstoke, England) and incubated at 37°C in a shaker incubator at 100 rpm. An overnight culture of B. subtilis PB6 was maintained and transferred weekly to fresh TSBYE broth and then kept at 4°C as a working culture.
Perfingens Agar Base (Oxoid Limited, Basingstoke, England) was prepared and sterilized at 121°C for 15 min. Two milliliters of Perfringens Selective Supplement A or B (Oxoid Limited, Basingstoke, England) was prepared and dissolved in sterile distilled water and then added to 500 ml of Perfringens Agar Base. Isolated colonies of C. perfringens ATCC 13124 from Perfringens Agar were inoculated into 10 ml of fluid thioglycolate broth (FTB) (Becton Dickenson and Company, Cockeysville, MD) and incubated at 37°C under anaerobic conditions using Anaerogen Pak (Oxoid Limited, Basingstoke, England). An overnight culture of C. perfringens ATCC 13124 was maintained and transferred weekly into fresh FTB. All freshly grown cultures of B. subtilis PB6 and C. perfringens ATCC 13124 were resuspended in 40% glycerol and kept at −80°C for long-term storage.
Antagonistic assays.
Putative strains of LAB were grown in MRS broth and incubated at 37°C under 5% CO2. Similarly, putative strains of Bacillus spp. were grown in TSBYE broth and incubated at 37°C under 5% CO2. C. perfringens ATCC 13124 was used as the indicator organism to screen microorganisms isolated from the intestinal tracts of chickens. Isolated colonies of C. perfringens ATCC 13124 were inoculated into FTB at 37°C under anaerobic conditions using Anaerogen Pak (Oxoid Limited, Basingstoke, England). An overnight culture of C. perfringens ATCC 13124 was streaked (perpendicularly) onto the surface of TSAYE agar using a sterile cotton swap.
Various overnight cultures containing strains of LAB and Bacillus spp. were then streaked across the same agar plates bisecting the streak line of C. perfringens ATCC 13124. All inoculated plates were incubated at 37°C under 5% CO2. After 24 h of incubation, antagonistic effects of the test organisms on the indicator bacteria were determined by the appearance of clear zones surrounding the junctions of the streak lines, which indicated the inhibitory effect of one organism on the other.
Well diffusion assay.
Molten TSAYE medium containing 0.7% agar at 45°C was inoculated with an 18-h culture of C. perfringens ATCC 13124 to obtain a final concentration of approximately 104 to 105 organisms per ml. Ten milliliters of the seeded agar was then dispensed aseptically into sterile polystyrene petri dishes (90 mm by 16 mm) containing 20 ml of solidified TSAYE medium (1% agar). When larger petri dishes (140 mm by 21 mm) were used, 70 ml of solidified 1% agar medium was overlaid with 30 ml of semisolid TSAYE medium (0.7% agar) containing the indicator organism. Upon solidification of both agar layers, the surface of the agar was perforated using a sterilized cork borer to create wells that were 8 mm in diameter. Aliquots (100 or 200 μl) containing the test samples were dispensed into the 8-mm wells and incubated at 37°C for 18 h under anaerobic conditions. The diameters of the zones of inhibition were measured using a vernier caliper. Each well diffusion assay used for testing an anticlostridial factor with various parameters was conducted three times in duplicate. Throughout this study, chloramphenicol (100 ppm) and sterile TSBYE broth (pH 6.3) were used as the positive and negative controls, respectively.
Effect of growth temperature and pH on the production of anticlostridial factor by B. subtilis PB6.
B. subtilis PB6 was grown aerobically in TSBYE medium at 37, 45, and 50°C for 18 h in a shaker incubator set at 100 rpm, and the overnight cultures were filtered through 0.20-μm sterile filters (Sartorius AG, Germany) to obtain cell-free filtrates.
Cells of B. subtilis PB6 were grown in TSBYE broth media adjusted to pH 5.0, 6.0, or 7.0 and incubated at 37°C for 18 h in a shaker incubator at 100 rpm. After the incubation period, all cells of B. subtilis PB6 were removed by filtration through 0.20-μm sterile filters. The negative controls for this experiment were sterile TSBYE broth adjusted to pH 5.0, 6.0, or 7.0. Aliquots (100 μl) of each filtrate fraction were examined for anticlostridial activity using the well diffusion assay described above. All inoculated agar plates were incubated at 37°C for 18 h under anaerobic conditions.
Sensitivity of anticlostridial factor to enzymes, temperature, and organic solvents.
B. subtilis PB6 was grown aerobically in TSBYE medium at 37°C for 18 h in a shaker incubator set at 100 rpm. The filtrate was collected and assayed for bacteriocins and hydrogen peroxide. For bacteriocins, the filtrate was treated with pronase and pepsin (Sigma Chemicals, St. Louis, MO) for 1 h at 37°C and with trypsin (Sigma Chemicals, St. Louis, MO) for 12 h at 37°C. The final concentration of pronase, pepsin, and trypsin was 1 mg per ml. To test for the presence of hydrogen peroxide, a filtrate was treated with catalase (final concentration, 0.5 mg per ml) for 1 h at 37°C. Treated and untreated filtrates, as well as controls containing pronase, pepsin, trypsin, and catalase at the appropriate concentrations, were then dispensed into the wells of the TSAYE media seeded with C. perfringens ATCC 13124 as the indicator organism. Filtrate from an 18-h culture of B. subtilis PB6 was collected and heated at 70, 80, 90, 100, and 121°C for 15 min. To ensure complete and uniform heating, the water level in the water bath was maintained above the level of the heating menstruum. After heating, the tubes were immediately cooled in an ice-water bath to terminate the reaction of the filtrate. Each treated filtrate was dispensed into the wells containing TSAYE medium and screened for antimicrobial activity against C. perfringens ATCC 13124. Chloramphenicol (100 ppm) and sterile TSBYE broth (pH 6.3) were used as positive and negative controls, respectively. The presence of inhibitory zones around the wells was determined after anaerobic incubation at 37°C for 18 h.
Anticlostridial active filtrates were mixed in ethyl alcohol, methanol, acetone, or acetonitrile (50%, vol/vol) and incubated at 25°C for 1.5 h. The solvents were then removed from the treated samples using a vacuum rotary evaporator set at 70°C for 15 min before the filtrates were tested for antimicrobial activity against C. perfringens ATCC 13124.
Estimation of the molecular weight of the anticlostridial factor using mass spectroscopy-mass spectroscopy (MS/MS).
B. subtilis PB6 was grown aerobically in TSBYE medium at 37°C for 18 h in a shaker incubator and filtered through a 0.20-μm filter. The filtrate was then extracted using chloroform-isopropyl alcohol (2:1) or ether. The mixture was allowed to stand at 25°C for 5 min before centrifugation at 1,000 × g for 5 min. The aqueous fraction was removed and tested for antimicrobial activity against C. perfringens ATCC 13124 using the well diffusion assay, but it showed no activity. The solvent fraction was evaporated using a Büchi Rotavapor (model R-124; Büchi Labortechnik AG, Switzerland) at 55°C under a vacuum. A portion of the residue was resuspended in 0.1 M phosphate buffer, pH 6.0. The remaining portion of the evaporated residue was dissolved in 10% formic acid before it was resuspended in a 0.1 to 0.2% formic acid-acetonitrile (6:4) solution. The mixture was centrifuged at 10,000 × g for 1 min before it was injected into an API 365 liquid chromatography-MS/MS system (Applied Biosystems/MDS Sciex, Canada) for mass spectroscopy-mass spectroscopy analysis with an electrospray ionization interface.
RESULTS
Identification of isolated bacteria.
Using biochemical and molecular techniques, the predominant bacterial strains that were isolated from the jejunum, duodenum, and ileum segments of the chicken intestinal tract were identified as Pediococcus pentosaceous, Pediococcus acidilactici, Lactobacillus acidophilus, Lactobacillus fermentum, B. licheniformis, and B. subtilis (data not shown).
The Bacillus strains isolated in the present study are gram-positive rods and possess catalase activity. Malachite green staining confirmed that these microorganisms possess endospores and are spore formers. These microorganisms were also “swarmers” because upon extended incubation they tended to spread over the entire agar surface. In terms of relatedness to known Bacillus spp., the API biochemical profiles showed that all Bacillus strains isolated in this study exhibited between 92.0 and 99.9% identity (data not shown). Using the RiboPrinter ID system, B. subtilis PB6 has a DNA profile that is a 90% match with the DNA profiles of other strains of B. subtilis in the database.
Antagonistic screening with C. perfringens.
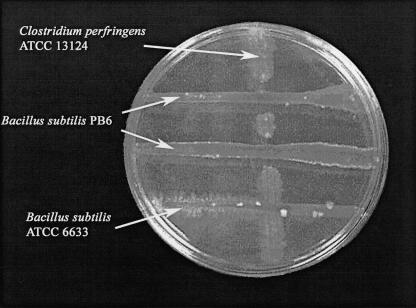
In contrast to B. subtilis ATCC 6633, B. subtilis PB3 and B. subtilis PB6 were antagonistic toward C. perfringens ATCC 13124. After 24 h of incubation, the antagonistic effect of B. subtilis PB3 or B. subtilis PB6 on the growth of C. perfringens ATCC 13124 could be observed by the appearance of truncated clear zones surrounding the intersections of the streak lines of the test and indicator organisms (Fig. 1). In contrast, none of the LAB strains isolated was inhibitory toward C. perfringens ATCC 13124. Studies in our laboratory have indicated that fermented extracts of B. subtilis PB6 were also inhibitory toward growth of C. perfringens ATCC 13124 (data not shown).
FIG. 1.
Antagonism assay with B. subtilis PB6, B. subtilis ATCC 6633, and C. perfringens ATCC 13124.
Effect of growth temperature and pH on the production of anticlostridial factor.
When B. subtilis PB6 was tested at various growth temperatures, no zone of inhibition was observed with filtrates extracted from cultures grown at 45 and 50°C (Table 1). There was no zone of inhibition with filtrate extracted from a bacterial culture grown at 25°C.
TABLE 1.
Factors affecting the anticlostridial activity of B. subtilis PB6
| Treatment | Activity (%) |
|---|---|
| Enzymes (1 mg per ml) | |
| Untreated filtrate | 100 |
| Catalase | 100 |
| Pepsin | 91 |
| Trypsin | 80 |
| Pronase | 0 |
| Heat (15 min) | |
| 70°C | 100 |
| 80°C | 100 |
| 90°C | 98 |
| 100°C | 98 |
| 121°C | 95 |
| Organic solvents (50%, vol/vol) | |
| Ethyl alcohol | 100 |
| Methanol | 100 |
| Acetone | 100 |
| Acetonitrile | 100 |
| Growth temp | |
| 25°C | 0 |
| 30°C | 55 |
| 37°C | 100 |
| 45°C | 0 |
| 50°C | 0 |
| pH of growth medium | |
| 5.0 | 0 |
| 6.0 | 68 |
| 6.2 | 100 |
| 7.0 | 91 |
| Antimicrobial spectrum | |
| Clostridium perfringens ATCC 13124 | 100 |
| Clostridium difficilea | 100 |
| Streptococcus pneumoniaea | 100 |
| Campylobacter jejuni ATCC 35918 | 100 |
| Campylobacter coli ATCC 51798 | 100 |
Strains were isolates from patients in Singapore.
Results of the well diffusion assay indicated that there was no zone of inhibition with filtrate from B. subtilis PB6 grown in TSBYE broth at pH 5.0 (Table 1). However, when B. subtilis PB6 was grown in TSBYE broth at pH 6.0, 6.2, or 7.0, various degrees of antimicrobial activity against C. perfringens ATCC 13124 were observed (Table 1). The maximum anticlostridial activity was observed at pH 6.2 (Table 1). No zone of inhibition was detected with negative controls using sterile TSBYE broth at pH 5.0, 6.0, and 7.0.
Effects of enzymes, temperature, and solvents on anticlostridial activity.
When the filtrates of B. subtilis PB6 were treated with pepsin, trypsin, and pronase, the sizes of the zones of inhibition decreased significantly (8, 20, and 100%, respectively) (Table 1). However, compared to the anticlostridial effect of hydrogen peroxide, little or no change in the diameter of the zone of inhibition was observed when the same filtrates were treated with catalase (Table 1). No zones of inhibition were observed with controls containing pepsin, trypsin, pronase, and catalase (data not shown).
Very small or no significant decreases in the anticlostridial activities of the filtrates from B. subtilis PB6 were observed when they were heated at 70, 80, 90, 100, and 121°C for 15 min, compared to the unheated filtrate (Table 1). When filtrates from B. subtilis PB6 were treated with ethyl alcohol, methanol, acetone, and acetonitrile, no loss of anticlostridial activity was observed (Table 1).
Estimation of the molecular weight of anticlostridial factor using mass spectroscopy-mass spectroscopy.
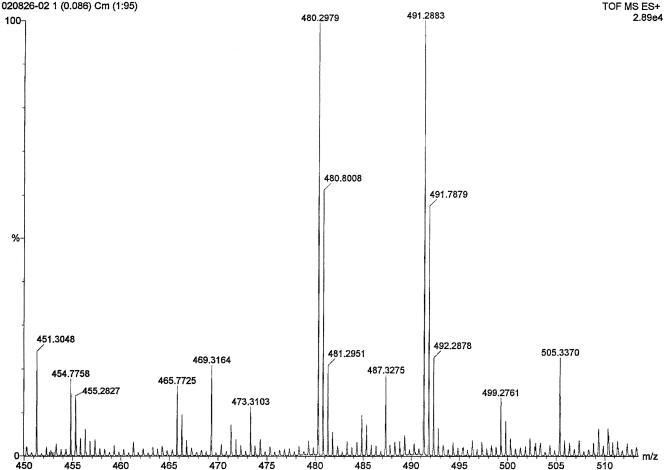
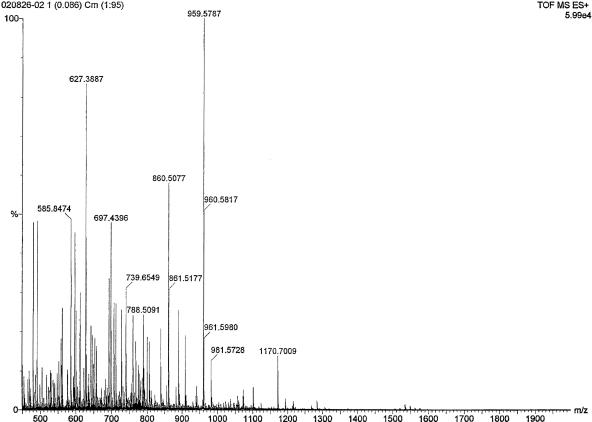
A preliminary scan study of the active CHCl3-isopropyl alcohol-extracted fraction revealed two double-charged (M + 2H)2+ species at m/z 480.3 and 491.29 as major fragments along with a few other minor fragments (Fig. 2). Another MS/MS scan study of the ether-extracted fraction also revealed that a fragment at m/z 959.58 was the major fragment (Fig. 3).
FIG. 2.
Electrospray ionization liquid chromatography-MS/MS spectrum of the bacteriocin produced by B. subtilis PB6 with two double-charged (M+2H)2+ species at m/z 480.2979 and 491.2883.
FIG. 3.
MS/MS spectrum of B. subtilis PB6 bacteriocin 1 ion at m/z 959.5787.
DISCUSSION
The purposes of this study were to isolate beneficial bacteria from various segments of the chicken intestinal tract and to screen them as potential probiotics for use against C. perfringens. This study was based on previous studies which demonstrated that the ability of probiotic microorganisms to adhere to and colonize the epithelial cells of the gastrointestinal tract is largely dependent on the specific site of isolation from a specific animal source (8). There are many beneficial effects associated with the use of microbial probiotics in animal feeds (10). These beneficial effects include competitive exclusion of pathogenic strains of E. coli (38), Campylobacter jejuni (24), and Salmonella enterica serovar Enteritidis (28); enhancing the growth and viability of beneficial gut microflora (14); and improved digestion and absorption of nutrients (36) in chickens. Other criteria used for isolating and defining probiotic bacteria include bile and acid stability (13), production of antimicrobial factors (32), and meeting safety or generally recognized as safe status (Scientific Committee on Animal Nutrition). A number of lactic acid bacteria have been shown to exhibit various degrees of antimicrobial activity against Clostridium spp. Previous studies have shown that Lactobacillus rhamnosus (1), Lactobacillus plantarum (40), Lactococcus lactis subsp. lactis (35), and Pediococcus pentosaceus (9) are bactericidal for Clostridium spp.
In the present study, five strains of lactic acid bacteria were tested for antagonism toward C. perfringens ATCC 13124 without production of a zone of inhibition (data not shown). However, two strains of B. subtilis, PB3 and PB6, exhibited antimicrobial activity against C. perfringens ATCC 13124. Findings obtained in our laboratory showed that filtrates of these Bacillus species contained a proteinaceous antimicrobial factor that was stable in the presence of high heat and in the presence of bile salt and solvents (Table 1) (34). Production of bacteriocins by Bacillus spp. has been reported previously, and the best-characterized bacteriocins are subtilin of B. subtilis (16), megacin of Bacillus megaterium (37), lichenin of B. licheniformis (29), tochicin of Bacillus thuringiensis (27), and the bacteriocins of Bacillus cereus (26). Despite extensive screening of these bacteriocins against a wide spectrum of pathogenic microorganisms, there has been no report on the direct effect of metabolites of B. subtilis on C. perfringens (34). Studies in our laboratory revealed for the first time the anticlostridial bacteriocin of B. subtilis. In addition, the bacteriocins of B. subtilis PB3 and B. subtilis PB6 were also inhibitory toward Clostridium difficile, C. jejuni, Campylobacter coli, and Streptococcus pneumoniae (Table 1) (34).
Bacteriocins produced by lactic acid bacteria can be classified as either class I or II bacteriocins based on their molecular weights (19). Although many class II antimicrobial peptides are produced by LAB, it is probable that these nonlantibiotic bacteriocins may also be produced by other gram-positive bacteria (25). Nevertheless, to date, no bacteriocins from the genus Bacillus have been characterized as members of class II. Subtilin, a linear lantibiotic produced by B. subtilis, is a class I bacteriocin (16). However, subtilosin, also produced by B. subtilis, is a posttranslationally modified cyclic polypeptide that is unlike the polypeptides of the class I or class II bacteriocins (41). In the present study, the antimicrobial activity of B. subtilis PB6 was susceptible to pronase and partially sensitive to trypsin, suggesting that it is proteinaceous, as described previously (15). The fact that the antimicrobial substance retains most of its activity after exposure to trypsin also provides indirect evidence that the molecule may contain lipid or carbohydrate moieties as well. It can also be inferred that the antimicrobial activity of the catalase-treated filtrate could not be due to the production of hydrogen peroxide by B. subtilis PB6 (Table 1). The inhibitory activity of the antimicrobial factor was maintained after heating at 100 or 121°C for 15 min (Table 1), indicating that it is likely to have a low molecular weight (2). Although the thermal properties of the antimicrobial factor were very similar to those described by Baquero and Moreno (2), the molecular weight of the putative bacteriocin described in this study is considerably higher (2). Our initial finding showed that the molecular mass of the putative bacteriocin produced by B. subtilis PB6 was estimated to be in the range from 960 to 983 Da (Fig. 3). As far as we know, no bacteriocin in this molecular mass range has been reported to withstand heat treatment at 121°C for 15 min and still maintain its activity.
In the present study, maximum antimicrobial activity was observed when cells of B. subtilis PB6 were grown in broth at pH 6.2 and incubated at 37°C (Table 1). Unlike LAB bacteriocins, which have a narrow antimicrobial spectrum (6), bacteriocins produced by Bacillus spp. have been reported to exhibit distinct diversity in their inhibitory activities (3). In the current study, the antimicrobial factor produced by B. subtilis PB6 is typical of gram-positive bacteriocins (15) in being broadly active against various strains of Clostridium spp., Streptococcus spp., and Campylobacter spp. (Table 1). Specifically, the spectrum of activity of this antimicrobial peptide includes strains of C. perfringens which were implicated in outbreaks of necrotic enteritis among poultry in Australia (21). The proteinaceous nature of the antimicrobial factor and the retention of the inhibitory activity after heat treatment are desirable characteristics that make B. subtilis PB6 an attractive probiotic candidate with potential application for prevention of necrotic enteritis in poultry. In view of this, an in vivo broiler trial is currently under way to test samples containing cells of B. subtilis PB6 and its anticlostridial substance in a necrotic enteritis disease model developed by researchers at the University of New England in Australia (21).
REFERENCES
- 1.Alander, M., R. Satokari, R. Korpela, M. Saxelin, T. Vilpponen-Salmela, T. Mattila-Sandholm, and A. von Wright. 1999. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl. Environ. Microbiol. 65:351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baquero, F., and F. Moreno. 1984. The microcins. FEMS Microbiol. Lett. 23:117-124. [Google Scholar]
- 3.Cordovilla, P., E. Valdivia, A. Gonzalez-Segura, A. Galvez, M. Martinez-Bueno, and M. Maqueda. 1993. Antagonistic action of the bacterium Bacillus licheniformis M-4 toward amoeba Naegleria fowleri. J. Eukaryot. Microbiol. 323-328. [DOI] [PubMed] [Google Scholar]
- 4.Cowen, B. S., L. D. Schwartz, R. A. Wilson, and S. I. Ambrus. 1987. Experimentally induced necrotic enteritis in chickens. Avian Dis. 31:904-906. [PubMed] [Google Scholar]
- 5.Craven, S. E., N. J. Stern, N. A. Cox, J. S. Bailey, and M. Berrang. 1999. Cecal carriage of Clostridium perfringens in broiler chicken given Mucosal Starter Culture. Avian Dis. 43:484-490. [PubMed] [Google Scholar]
- 6.Daeschel, M. A. 1989. Antimicrobial factors from lactic acid bacteria for use as food preservatives. Food Technol. 43:164-167. [Google Scholar]
- 7.Devriese, L., A. G. Daube, J. Hommez, and F. Haesebrouck. 1993. In vitro susceptibility of Clostridium perfringens isolated from farm animals to growth-enhancing antibiotics. J. Appl. Bacteriol. 75:55-57. [DOI] [PubMed] [Google Scholar]
- 8.Fuller, R. 1989. Probiotics in man and animals. J. Appl. Bacteriol. 66:365-378. [PubMed] [Google Scholar]
- 9.Graham, D. C., and L. L. McKay. 1985. Plasmid DNA in strains of Pediococcus cerevisiae and Pediococcus pentosaceus. Appl. Environ. Microbiol. 50:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gusils, C., S. N. Gonzalez, and G. Oliver. 1999. Some probiotic properties of chicken lactobacilli. Can. J. Microbiol. 45:981-987. [DOI] [PubMed] [Google Scholar]
- 11.Halzapfel, W. H., P. Haberer, J. Snel., U. Schillinger, and J. H. J. Huis in't Veld. 1998. Overview of gut flora and probiotics. Int. J. Microbiol. 41:85-101. [DOI] [PubMed] [Google Scholar]
- 12.Hancock, R. E. W., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoa, N. T., L. Baccigalupi, A. Huxham, A., Smertenko, P. H. Van, S. Ammendola, E. Ricca, and S. M. Cutting. 2000. Characterization of Bacillus species used for oral bacteriotherapy and bacterio-prophylaxis of gastrointestinal disorders. Appl. Environ. Microbiol. 66:5241-5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosoi, T., A. Ametani, K. Kiuchi, and S. Kaminogawa. 2000. Improved growth and viability of lactobacilli in the presence of Bacillus subtilis (natto) or subtilin. Can. J. Microbiol. 46:892-897. [DOI] [PubMed] [Google Scholar]
- 15.Jack, R. W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen, E. F., and D. J. Hirschmann. 1944. Subtilin, an antibacterial factor of Bacillus subtilis: culturing condition and properties. Arch. Biochem. 4:297-309. [Google Scholar]
- 17.Jiraphocakul, S., T. W. Sullivan, and K. M. Shahani. 1990. Influence of a dried Bacillus subtilis culture and antibiotics on performance and intestinal microflora in turkeys. Poult. Sci. 69:1966-1973. [DOI] [PubMed] [Google Scholar]
- 18.Kaldhusdal, M. I. 2000. Necrotic enteritis as affected by dietary ingredients. World Poult. 16:42-43. [Google Scholar]
- 19.Klaenhammer, T. R. 1993. Genetics of bacteriocins by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-86. [DOI] [PubMed] [Google Scholar]
- 20.Klein, G., A. Pack, C. Bonaparte, and G. Reuter. 1998. Taxonomy and physiology of probiotic lactic acid bacteria. Int. J. Food Microbiol. 41:103-125. [DOI] [PubMed] [Google Scholar]
- 21.Kocher, A., M. Choct, A. Teo, H. M. Tan, and R. R. Carter. 2004. Effectiveness of alternative feed supplements to broiler diets using a necrotic enteritis challenge model. Aust. Poult. Sci. Symp. 16:84. [Google Scholar]
- 22.Maruta, K., H. Miyazaki, Y. Tadano, S. Masuda, A. Suzuki, H. Takahashi, and M. Takahashi. 1996. Effects of Bacillus subtilis C-3102 intake on fecal flora of sows and on diarrhea and mortality rate of their piglets. Anim. Sci. Technol. 67:403-409. [Google Scholar]
- 23.Mead, G. C. 1997. Bacteria in the intestinal tract of birds, p. 216-240. In R. I. Mackie, B. A. White, and R. E. Isaacsson (ed.), Gastrointestinal microbiology, vol. 2. Gastrointestinal microbes and host interactions. Chapman & Hall, New York, N.Y. [Google Scholar]
- 24.Morishita, T. Y., P. P. Aye, B. S. Harr, C. W. Cobb, and J. R. Clifford. 1997. Evaluation of an avian-specific probiotic to reduce the colonization and shedding of Campylobacter jejuni in broilers. Avian Dis. 41:850-855. [PubMed] [Google Scholar]
- 25.Nes, L. F., D. B. Diep, L. S. Håvarstein, M. B. Brurberg, V. Eijsink, and H. Holo. 1996. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek 70:113-128. [DOI] [PubMed] [Google Scholar]
- 26.Paik, H.-D., N.-K. Lee, H.-K. Lee, Y.-I. Hwang, and J.-C. Pan. 2000. Identification and partial characterization of cerein BS229, bacteriocin produced by Bacillus cereus BS229. J. Microbiol. Biotechnol. 10:195-200. [Google Scholar]
- 27.Paik, H.-D., S.-S. Bae, S.-H. Park, and J.-G. Pan. 1997. Identification and partial characterization of tochicin, a bacteriocin produced by Bacillus thuringiensis subsp. tochigiensis. J. Ind. Microbiol. Biotechnol. 19:294-298. [DOI] [PubMed] [Google Scholar]
- 28.Pascual, M., M. Hugas, J. I. Badiola, J. M. Monfort, and M. Garriga. 1999. Lactobacillus salivarius CTC2197 prevents Salmonella enteritidis colonization in chickens. Appl. Environ. Microbiol. 65:4981-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pattnaik, P., J. K. Kaushik, S. Grover, and V. K. Batish. 2001. Purification and characterization of a bacteriocin-like compound (lichenin) produced anaerobically by Bacillus licheniformis isolated from water buffalo. J. Appl. Microbiol. 91:636-645. [DOI] [PubMed] [Google Scholar]
- 30.Paulus, C., and J. P. Ruckebusch. 1996. Necrotic enteritis (NE). Zootec. Int. 19:40-42. [Google Scholar]
- 31.Piard, J. C., and M. Desmazeaud. 1992. Inhibiting factors produced by lactic acid bacteria. 2. Bacteriocins and other antimicrobial factors. Lait 72:113-142. [Google Scholar]
- 32.Salminen, S., E. Isolauri, and E. Salminen. 1996. Clinical uses of probiotics for stabilizing the gut mucosal barrier: successful strains and future challenges. Antonie Leeuwenhoek 70:347-358. [DOI] [PubMed] [Google Scholar]
- 33.Scientific Committee on Animal Nutrition. 2000. Opinion of the Scientific Committee on Animal Nutrition on the safety use of Bacillus species in animal nutrition. http://europa.eu.int/comm/food/fs/sc/scan/index_en.html.
- 34.Seah, A. H.-L., A. Y.-L. Teo, H.-M. Tan. November 2002. Antimicrobial compounds from Bacillus subtilis for use against animal and human pathogens. U.S. Patent and Trademark Office serial no. 10/306,365.
- 35.Spelhaug, S. R., and S. K. Harlander. 1989. Inhibition of foodborne bacterial pathogens by bacteriocins from Lactococcus lactis and Pediococcus pentosaceous. J. Food Prot. 52:856-862. [DOI] [PubMed] [Google Scholar]
- 36.Thomke, A., and K. Elwinger. 1998. Growth promotants in feeding pigs and poultry. III. Alternatives to antibiotic growth promotants. Ann. Zootech. (Paris) 47:245-271. [Google Scholar]
- 37.Von Tersch, M. A., and B. C. Carlton. 1983. Bacteriocin from Bacillus megaterium ATCC 19213: comparative studies with megacin A216. J. Bacteriol. 155:866-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins, B. A., B. F. Miller, and D. H. Neil. 1982. In vivo effects of Lactobacillus acidophilus against pathogenic Escherichia coli in gnotobiotic chicks. Poult. Sci. 61:1298-1308. [DOI] [PubMed] [Google Scholar]
- 39.Watkins, K. L., T. R. Shryock, R. N. Dearth, and Y. M. Saif. 1997. In-vitro antimicrobial susceptibility of Clostridium perfringens from commercial turkey and broiler chicken origin. Vet. Microbiol. 54:195-200. [DOI] [PubMed] [Google Scholar]
- 40.West, C. A., and P. J. Warner. 1988. Plantacin, B, a bacteriocin produced by Lactobacillus plantarum NCDO 1193. FEMS Microbiol. Lett. 49:163. [Google Scholar]
- 41.Zheng, G., L. Z. Yan, J. C. Vederas, and P. Zuber. 1999. Genes of the sbo-alb locus of Bacillus subtilis are required for production of the antilisterial bacteriocin subtilosin. J. Bacteriol. 181:7346-7355. [DOI] [PMC free article] [PubMed] [Google Scholar]