Abstract
Phenylacetaldehyde reductase (PAR) is suitable for the conversion of various aryl ketones and 2-alkanones to corresponding chiral alcohols. 2-Propanol acts as a substrate solvent and hydrogen donor of coupled cofactor regeneration during the conversion of substrates catalyzed by PAR. To improve the conversion efficiency in high concentrations of substrate and 2-propanol, selection of a PAR mutant library and the subsequent rearrangement of mutations were attempted. With only a single selection round and following the manual combination of advantageous mutations, PAR was successfully adapted for the conversion of high concentrations of substrate with concentrated 2-propanol. This method will be widely applicable for the engineering of enzymes potentially valuable for industry.
The demand for enzymes in the chemical industry has expanded rapidly. Most enzymes can catalyze reactions at room temperature in a mild aqueous solution and have great substrate, stereo-, regio-, and chemoselectivity (12, 15, 16). Such properties are ideal for fine chemical production, particularly as chiral drug intermediates and agrochemicals.
Alcohol dehydrogenase (ADH) (EC 1.1.1.1) can catalyze the asymmetric reduction of aldehyde or ketone to chiral alcohols. Phenylacetaldehyde reductase (PAR) from styrene-assimilating bacteria Rhodococcus sp. (formerly identified as Corynebacterium sp.) ST-10 belongs to the family of zinc-containing medium-chain ADHs (8). PAR has broad substrate specificity and catalyzes asymmetric reduction at high enantioselectivity in an NADH-dependent manner (8-10).
Various examples of ADH applications for asymmetric reduction have been reported that use a cofactor regeneration system with additional enzymes such as formate dehydrogenase or glucose dehydrogenase (15). Recent reports have used the advantage of ADHs for NADH self regeneration with secondary alcohols as hydrogen donors (13, 21). We previously reported the ability of PAR to reduce various carbonyl compounds coupled with NADH self regeneration in the presence of 2-propanol as a proton donor (8). 2-Propanol can also profitably act as a solvent for the PAR substrates that cannot dissolve in aqueous media. However, at high concentrations of 2-propanol, the efficiency of substrate conversion by PAR clearly fell. Consequently, the conversion yields with high substrate concentrations were relatively low and insufficient for industrial application.
Here, we attempted to engineer PAR that can operate in relatively high concentrations of 2-propanol (>20% [vol/vol]) to achieve efficient conversion of concentrated substrates without altering the substrate specificity. Using our method with the combination of advantageous mutations, PAR was successfully improved with six amino acid replacements with significant enhancement of the substrate conversion. Although relative yield of conversion at high concentration of 2-propanol was enhanced, little change of substrate specificity was observed.
MATERIALS AND METHODS
Materials.
All chemicals were of reagent grade. Taq DNA polymerase and deoxynucleoside triphosphate (dNTP) were purchased from Roche Diagnostics (Mannheim, Germany). KOD Plus DNA polymerase was from Toyobo (Osaka, Japan). Ex Taq DNA polymerase was from TaKaRa (Shiga, Japan). Oligonucleotide primers were from ESPEC Oligo Service (Ibaraki, Japan) and SIGMA Genosis (Hokkaido, Japan). Restriction enzymes and DNA-modifying enzymes were from Toyobo, TaKaRa, and New England Biolabs (Beverly, MA). m-Chlorophenacyl chloride (m-CPC) was kindly provided by Sumitomo Chemical (Osaka, Japan).
Reidentification of Corynebacterium sp. ST-10.
PAR was derived from the microorganism formerly identified as Corynebacterium sp. ST-10 (11). Reidentification using a more detailed test for this organism was performed as follows. The nucleotide sequence was analyzed for 16S rRNA genes by NCIMB Japan (Shizuoka, Japan), and complete identity was found with the corresponding sequence of Rhodococcus erythropolis. The acid-fast stain was negative, and a rod-coccus cycle was observed. The fatty acid content was also analyzed by NCIMB Japan, and a monounsaturated normal chain composition was detected. Although these are typical properties of Rhodococcus erythropolis, a deficiency of glucose utilization was also observed (11). Thus, we concluded that this microorganism should be identified as Rhodococcus sp. ST-10.
Construction of PAR expression plasmid.
Unless otherwise stated, standard molecular biology techniques were used (17). The PAR gene on pUAR (20) was mutagenized by PCR to incorporate SfiI sites on both the 3′ end at the start codon and the 5′ end at the stop codon of the PAR gene without alteration of the deduced amino acid sequence. The PCR mixture (50 μl) was prepared with 5 μl of KOD Plus buffer, 0.2 mM concentration of deoxynucleoside triphosphates (dNTPs), 1 mM MgSO4, 300 nM each sense primer PAR207F (5′-AAGAATTCAAGGAGATAAGGCCATGAAGGCCATCCAGTAC-3′) and antisense primer PAR207R (5′-TTTCTGCAGGCCTCACAGGCCAGGGACCACAACCGC-3′), 5 ng of the pUAR plasmid as a template, and 1 U of KOD Plus DNA polymerase. The thermal cycling parameters were 94°C for 2 min and 30 cycles, each consisting of 94°C for 15 s, 55°C for 30 s, and 68°C for 2 min. The PCR product was treated with a phenol-chloroform solution, precipitated with ethanol, and resuspended in 10 μl of Tris-EDTA buffer. The resultant PCR product was cut with restriction enzymes of EcoRI and PstI and purified by agarose gel extraction (QIA Quick Gel Extraction kit; QIAGEN, Tokyo, Japan). The pUC118 vector plasmid was cut with the same restriction enzymes, treated with calf intestine alkaline phosphatase, and purified by agarose gel extraction. These two fragments were mixed and ligated with the TaKaRa DNA Ligation kit, version 2. After ethanol precipitation, the mixture was electroporated into Escherichia coli JM109 {recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB)/F′ [traD36 proAB+ lacIq lacZΔM15]}. The transformants were screened, and the DNA sequence was confirmed with an Applied Biosystems 310 automated fluorescent DNA sequencer (Applied Biosystems, Tokyo, Japan). The primers for sequencing were as follows: PARSQ-R1 (5′-CATTAGGCACCCCAGGCTTTACAC-3′), PARSQ-R2 (5′-AACTGTTGGCACTGCTCACAAGG-3′), PARSQ- R3 (5′-GCCGAGAACGTCCGCAAGATC-3′), PARSQ-F1 (5′-CAGCTGGCGAAAGGGGGATG-3′), PARSQ-F2 (5′-CGATGGTGGGCTGGTAGCCG-3′), and PARSQ-F3 (5′-GCACCGAGACCGGGAGGATTG-3′). Unless otherwise stated, the PAR nucleotide sequence was confirmed with all of these primers. This plasmid, named pEAR1, was further manipulated to incorporate the polyhistidine tag at the C terminus of the PAR polypeptide. The PCR mixture (100 μl) consisted of 10 μl of Ex Taq buffer, 0.2 mM dNTPs, 500 nM each PAR207F and PAR207RH primer (3′-TTTCTGCAGTCAGTGGTGGTGGTGGTGGTGGCCGGACAGGCCAGGGACCACAACCGC-5′), 1 ng of pEAR1 as a template, and 5 U of Ex Taq DNA polymerase. Thermal cycling parameters were 94°C for 5 min (1 cycle); 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min (30 cycles); and 72°C for 10 min (1 cycle). The PCR product was extracted, restricted, and purified as above, except for the restriction enzymes of KpnI and PstI. Plasmid pEAR1 was also treated with the same restriction enzymes, and the vector fragment was purified as described above. These fragments were ligated, electroporated, and screened, and the DNA sequence was confirmed as expected. This plasmid, named pEAR2, was further restricted with EcoT14I at two different sites in the PAR coding sequence. The sticky ends were subsequently blunted with T4 DNA polymerase (New England Biolabs) and were self ligated. Deletion of the central part of the PAR gene as designed was confirmed by DNA sequencing, and the resultant plasmid was named pEAR2s.
PAR library construction.
The PAR library was generated by mutagenic PCR (5, 6). The 10× PCR buffer contained 70 mM MgCl2, 500 mM KCl, 100 mM Tris-HCl buffer (pH 8.3), and 0.1% (wt/vol) gelatin. The 10× dNTP mixture contained 2 mM dATP, 10 mM dTTP, 2 mM dGTP, and 10 mM dCTP. Each 100 μl of reaction mixture contained 10 μl each of 10× PCR buffer and dNTP mixture, 30 pmol each of RV-M and M13-47 primes (TaKaRa), 10 ng pEAR2 as template DNA, 5 U of Taq DNA polymerase, and 0.5 mM MnCl2. The thermal cycling parameters were 94°C for 1 min, 45°C for 1 min, and 72°C for 1 min (30 cycles). The PCR product was extracted, restricted with SfiI, and purified as above. The fragment was ligated with the pEAR2s vector and restricted with SfiI. The mixture was extracted and electroporated into JM109 as above. After a short incubation in SOC medium (17) at 37°C, 45% (vol/vol) glycerol in Luria-Bertani (LB) medium was added, and the mixture was stored as 15% (vol/vol) glycerol stock at −80°C until use.
Colorimetric screening.
The induction medium contained 100 μg/ml sodium ampicillin, 0.4 mM isopropyl-β-d-thiogalactopyranoside, 0.01% (wt/vol) ZnCl2, 10 g of Bacto tryptone (Difco, Detroit, Mich.), 5 g of Bacto yeast extract (Difco), and 10g of NaCl per liter (pH 7.0). A solid medium plate was also prepared with induction medium containing 15g/liter agarose. On this plate, library stock suspension was spread and incubated at 30°C for 24 h. The colorimetric assay with a modified procedure described previously (7, 14) was performed as follows. The colonies were transferred onto a nylon membrane (BiodyneA; Nihon Pall, Tokyo, Japan) by being placed on each plate. This membrane was then soaked for 30 min at room temperature in a reaction solution containing 50 mM 3-(N-morpholino) propanesulfonic acid (MOPS) buffer (pH 7.0), 1 mM NAD+, 200 μM nitroblue tetrazolium, 10 μM 1-methoxy-5-methylphenazinium methylsulfate (Dojindo Labratories, Kumamoto, Japan), and 20% (vol/vol) 2-propanol. The reaction was terminated by washing the membranes in distilled water, and eight colonies with significant purple color development per plate were visually selected. Eighty selected colonies, 8 colonies of JM109 harboring the plasmid pEAR2, and 8 colonies of JM109 harboring the plasmid pEAR2s were selected and transferred into each 96-well plate containing 100 μl of LB medium with 100 μg/ml of sodium ampicillin, and the lid was sealed to prevent vaporization. Two 96-well plates were then incubated at 37°C at 800 rpm for 18 h in a shaker (M-36; Taitec, Tokyo, Japan). After incubation, 50 μl of 45% (vol/vol) glycerol in LB medium was added to each well, mixed, and stored at −80°C as master plates.
Conversion assay.
From the master plates, 40 clones selected each time, with 8 clones harboring pEAR2 or pEAR2s, were transferred into 1 ml of induction medium in a 2-ml tube. Each lid was tightly closed, and the tubes were incubated at 37°C at 1,200 rpm for 22 h in a shaker. Cells were precipitated by centrifugation for 5 min at 20,000 × g at 4°C, and the supernatant was removed. Then, 400 μl of MOPS buffer (pH 7.0) containing NAD+ was added to each tube, followed by 100 μl of solution of 5% (wt/vol) m-CPC in 2-propanol. The final concentration of MOPS buffer, NAD+, and 2-propanol was 50 mM, 1 mM, and 19.5% (vol/vol), respectively. Each lid was tightly closed, and the reaction mixture was incubated at 30°C at 1,200 rpm for 22 h in a shaker. The mixture was extracted with 1 ml of ethyl acetate, and the organic phase was dried with anhydrous Na2SO4. The reaction components were then analyzed by the Shimadzu LC-10AT high-performance liquid chromatography (HPLC) system (Shimadzu, Kyoto, Japan) on a Chiralcel OB-H column (Daicel Chemical Industries, Osaka, Japan) with n-hexane/2-propanol (9:1 [vol/vol]) as the mobile phase at a flow rate of 0.8 ml/min. UV detection was carried out at 268 nm, and the retention times of m-CPC and (S)- and (R)-2-chloro-1-(3-chlorophenyl)ethanol (CCE) were 13.7, 9.6, and 8.4 min, respectively. For the conversion of phenacyl chloride (PC), the above conditions for m-CPC except for PC as the substrate were employed, and detected at 258 nm. The retention times of PC and (S)- and (R)-2-chloro-1-phenylethanol (CPE) were 17.4, 11.4, and 9.1 min, respectively. For the conversion of ethyl 4-chloro-3-oxobutanoate (ECOB), the above conditions for m-CPC except for ECOB as the substrate were employed. HPLC analysis of the reaction components was performed at a flow rate of 1.0 ml/min and detected at 220 nm. The retention times of ECOB and (S)- and (R)-ethyl 4-chloro-3-hydroxybutanoate (ECHB) were 11.6, 8.3, and 7.7 min, respectively. The reaction compounds of m-CPC were also analyzed by gas chromatography (GC) (HP6890; Yokogawa Analytical Systems, Tokyo, Japan) equipped with a CP-cyclodextrine-β-236 M-19 column (0.25 mm by 25 m) under the following conditions: a column temperature of 160°C, injection and detection temperatures of 240 and 250°C, and a flow rate of 0.4 ml/min of He. The retention times of m-CPC, (S)-CCE, and (R)-CCE were 8.5, 12.7, and 13.1 min, respectively.
Construction of plasmid pSarA.
Plasmid pSarA was constructed by the following procedure. Clones C38, C12, H23, and E9 were cultured in 4 ml of LB medium with 100 μg/ml of sodium ampicillin, and plasmids were purified with a QIAprep Spin Miniprep Kit (QIAGEN). The plasmid of the H23 clone was cut with restriction enzymes of AccI and HindIII, and the 3,384-bp fragment was purified by preparative agarose gel electrophoresis. The plasmid of the C38 clone was restricted with AccI and KpnI, and the 280-bp fragment was purified. In the same manner, the plasmid of the E9 clone was restricted with KpnI and HindIII, and the 550-bp fragment was purified. These three fragments were ligated, and the hybrid plasmid, named pSarP, was obtained. DNA sequencing analysis confirmed the six nucleotide substitutions, including A35G, A59G, A200G, A488G, A824G, and C980T. The plasmid pSarA was then constructed using the megaprimer method (3, 18). The primers for PCR were the following: PARA125T-comp (5′-CTCATGATGAAGACGTCCGAGTGGC-3′), PAR-T373A-sens (5′-GCACCCGGCGCGATGGCCGAGTTCA-3′), and PAR-T517C-comp (5′-CAACCGCGTACGGGCCTCCGCGAAG-3′). PCRs were performed with PARSQ-R1 and PAR-A125T-comp and PAR-T373A-sens and PAR-T517C-comp, with pSarP as a template and using KOD Plus DNA polymerase as described above. Each amplified fragment indicating 262 bp and 169 bp was purified by agarose gel electrophoresis, and a second PCR was performed with these fragments as megaprimers, pSarP as a template, and KOD Plus DNA polymerase. The reaction mixture was extracted and restricted with EcoRI and BsiWI, and the 537-bp fragment was purified. The 3,677-bp fragment of pSarP, restricted with the same enzymes, was ligated with a PCR fragment of 537 bp and plasmid pSarA was constructed. DNA sequence analysis confirmed that pSarA had nine amino acid substitutions with all nonsynonymous nucleotide substitutions found in the PAR sequences of the above four parent clones (listed in Table 1).
TABLE 1.
Substitutions of the nucleotide and deduced amino acid on the selected four top mutant clonesa
| Position of amino acid substitution | Nucleotide substitution | Amino acid substitution | Clone |
|---|---|---|---|
| 1 | A35G | E12G | H23 |
| 2 | A59G | K20R | H23 |
| 3 | A125T | D42V | C38 |
| 4 | A200G | K67R | H23 |
| A297T | — | C12 | |
| A303T | — | H23 | |
| 5 | T373A | L125M | C12 |
| T465G | — | C12 | |
| C466T | — | H23 | |
| 6 | A488G | K163R | C38 |
| 7 | T517C | S173P | C12 |
| C549T | — | H23 | |
| 8 | A824T | Q275L | E9 |
| 9 | C980T | A327V | E9 |
The nucleotide or amino acid is expressed as a single letter, and the replaced nucleotide or amino acid following the original and the number counted from the position of +1 is listed in the order of the number of nucleotide substitution. A dash indicates synonymous substitution without amino acid change. The order among all amino acid substitutions counted from the N terminus is also shown.
Construction of back mutants of pSarA.
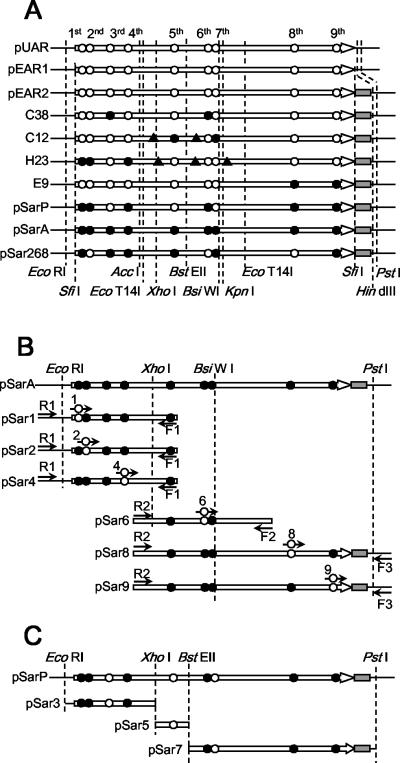
The rule for naming back mutants of pSarA was as follows. In the nucleotide sequence of pSarA, nine nonsynonymous mutations of PAR were numbered from 1 to 9 in the order of the deduced amino acid sequence from the N terminal to the C terminal (Table 1). Plasmid pSar1 is a substitution of the first mutation in pSarA with the wild-type sequence, and the other eight mutants were named based on this rule. The combinatorial back mutations were also indicated by the combination of the number of mutations (e.g., pSar268 for a mutant with second, sixth, and eighth back mutations). pSar1, -2, -4, -6, -8, and -9 were constructed as follows. The primers for PCR were as follows: PriSar1 (5′-GAATCGGCGCGGAACCCGAACTCAC-3′), PriSar2 (5′-CGGAGATTCCCAAACCCGAGCCCGG-3′), PriSar4 (5′-AAGGCGCAGGCAAGGTCGCCGCCGT-3′), PriSar6 (5′-ATCACGCGATCAAGCGTTCTCTGCC-3′), PriSar8 (5′-TCGGGGACGGCCAGGCCCACGCCAA-3′), and PriSar9 (5′-TCGACAACGGTGCCGAAGCGTATCG-3′). The first PCR was performed with pSarA as a template, primers, and KOD Plus DNA polymerase. The amplified product was purified, and a second megaprimer PCR using the purified PCR product and an additional primer was performed with pSarA as a template and KOD Plus DNA polymerase as above. The amplified product was restricted and ligated into pSarA, which was restricted with the same restriction enzymes. The construction procedure was schematically described in Fig. 1B, and the primers and restriction enzymes used for construction were also listed. pSar3, -5, and -7 were constructed by substitution of the partial DNA sequence of pSarA for the corresponding sequence of pSarP. The restriction enzymes used are listed and schematically represented in Fig. 1C. pSar26 was constructed by substitution of the XhoI-KpnI insert fragment of pSar2 for the corresponding fragment of pSar6. pSar68 was constructed by substitution of the XhoI-KpnI insert fragment of pSar8 for the corresponding fragment of pSar6. pSar268 was constructed by ligation of the three fragments: the XhoI-HindIII vector fragment of pSar2, the XhoI-KpnI insert fragment of pSar6, and the KpnI-HindIII insert fragment of pSar8.
FIG. 1.
Schematic representation of mutations of each clone. The structural gene of PAR is indicated by an open arrow. Amino acid substitutions for each clone are indicated on PAR by either an open circle (wild type) or a closed circle (mutation). The order of amino acid substitutions counted from N-terminal of amino acid sequence is demonstratively shown in panel A. The restriction site is shown by a dashed line. (A) Mutations of representative clones. Restriction sites described in Materials and Methods are also shown. A closed triangle indicates the synonymous substitution of nucleotide without amino acid substitution. The polyhistidine tag is indicated by a gray box. (B) The amplified fragment by megaprimer PCR for construction of pSar1, 2, 4, 6, 8, and 9. The primers used are displayed as arrows and shown close to each fragment. R1, R2, F1, F2, and F3 indicate the primers PARSQ-R1, -R2, -F1, -F2, and -F3, respectively. Numbers 1, 2, 4, 6, 8, and 9 indicate the primers PriSar1, -2, -4, -6, -8, and -9, respectively; an open circle on each primer represents the back mutations introduced. The R1 or R2 primer was used for the second (megaprimer) PCR with the product of the first PCR with the other two primers. After megaprimer PCR, each fragment was restricted by enzymes, indicated by two dashed lines on each fragment, and each back mutant was constructed by replacing the corresponding fragment on pSarA with it. (C) The fragment of pSarP for construction of pSar3, -5, and -7. Each restriction fragment of pSarP, harboring back mutations at the third, fifth, or seventh position, was prepared with enzymes indicated by two dashed lines on each fragment. Each back mutant was constructed by replacing the corresponding fragment on pSarA with it.
Conversion of concentrated m-CPC.
The fresh colony of E. coli JM109, harboring each plasmid of pUAR, pEAR2, pEAR2s, pSarA, and pSar268, was cultured in 4 ml LB medium at 37°C overnight. The 1-ml culture was transferred into 100 ml of induction medium in a 500-ml Sakaguchi flask and incubated at 37°C at 121 rpm of reciprocal shaking for 22 h. The cells were harvested by centrifugation (5,000 × g for 15 min at 4°C), resuspended in ice-cold buffer containing 50 mM MOPS buffer (pH 7.0)-50 mM NaCl, and adjusted to a wet cell concentration of 120 mg/ml. The 60-mg cell suspension (500 μl) was transferred to a microtube and precipitated by centrifugation for 5 min at 20,000 × g at 4°C, and the supernatant was carefully removed. Otherwise, a smaller volume of suspension was transferred in proportion to the lower final amount of cells in each tube, and the pellet was similarly prepared. The tubes were stored at −80°C until use. For conversion of m-CPC, 100 mg of m-CPC powder and 1 ml of buffer containing 50 mM MOPS buffer (pH 7.0), 1 mM NAD+, and 5 to 30% (vol/vol) of 2-propanol was added to a tube containing the defrosted cells. After incubation at 30°C at 1,200 rpm for 22 h in a shaker, the mixture was extracted three times with 500 μl of ethyl acetate, and the organic phase was dried over anhydrous Na2SO4. The reaction products were analyzed as described above.
Analysis of enzyme expression.
In the tube containing 60 mg of cells prepared as described above, 500 mg of 0.1-mm-diameter glass beads and 500 μl of 50 mM MOPS buffer (pH 7.0) were added. The tube was then applied three times to a multibead shocker (Yasui Kikai, Osaka, Japan) with shaking for 30 s at 30-s intervals at 4°C, and the cells were disrupted. After centrifugation for 5 min at 20,000 × g at 4°C, the supernatant was collected and protein concentration was determined with a BCA Protein Assay kit (Pierce, Illinois). Thirty micrograms of each protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 12% acrylamide by the standard method (17). Low-range standards were purchased from Bio-Rad Laboratories (California).
Nucleotide sequence accession number.
The nucleotide sequence of the 1,217-bp BamHI-PstI fragment in pUAR encoding PAR has been deposited in the DDBJ database under accession no. AB190261.
RESULTS
Construction of PAR mutant library.
Although PAR was successfully cloned and expressed in E. coli with plasmid pUAR (20), the expression and activity of resting cells were not sufficient for industrial application. To improve PAR, we used random mutagenesis and the subsequent selection of an advantageous mutation(s). We found that the previously reported nucleotide sequence of PAR (20) was inconsistent with the sequence of PAR on pUAR, and we therefore sequenced PAR on pUAR repeatedly here. The deduced amino acid sequence for the resultant nucleotide sequence of PAR contained no elongated C-terminal sequence, compared with other homologous ADHs. The single nucleotide insertion at position +949 from the start codon that causes the frameshift and C-terminal elongation was found in the old sequence. Furthermore, the analysis by matrix-assisted laser desorption ionization-time of flight mass spectrometry (AXIMA-CFR; Shimadzu Biotech, Kyoto, Japan) (data not shown) confirmed that the molecular weight of the recombinant PAR protein expressed in the E. coli harboring pUAR was consistent with the deduced amino acid sequence based on the newly elucidated nucleotide sequence (accession number AB190261). We therefore employed the new sequence, and all experiments described in this paper were performed based on the revised sequence. The plasmid vector pEAR2, which has SfiI restriction sites on both ends of the reading frame of PAR and polyhistidine tag at the C terminus, was constructed as described in Materials and Methods. No significant differences in the efficiency of conversion of m-CPC were observed between the E. coli clones harboring plasmid pUAR and pEAR2 (see Fig. 5A). To prevent the appearance of active PAR after the mutant gene was cloned, the pEAR2s vector was constructed from pEAR2 by removing the central part of the PAR gene, the fragment between two EcoT14I sites (Fig. 1A), and the resultant plasmid also had a flameshift at the self-ligated position. E. coli harboring pEAR2s completely lost PAR activity. The random mutations were introduced with the standard mutagenic PCR method with pEAR2 as a template, and the amplified fragment was cloned into the pEAR2s plasmid vector.
FIG. 5.
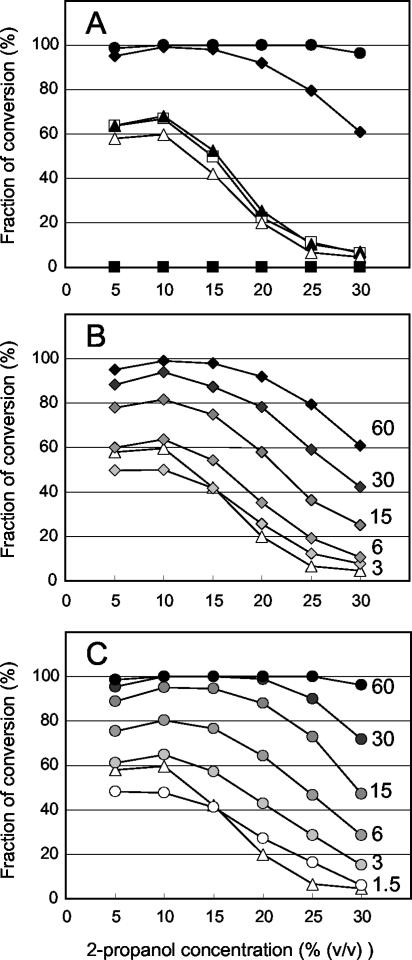
The conversion of concentrated m-CPC under different concentrations of 2-propanol. One hundred milligrams of m-CPC was converted with 1 ml of reaction medium by a clone harboring pUAR (open squares), pEAR1 (closed triangles) pEAR2 (open triangles), pEAR2s (closed squares), pSarA (closed diamonds), and pSar268 (closed circles) in the presence of 5 to 30% (vol/vol) of 2-propanol. The GC area of m-CPC and (R)-CCE was measured, and the fraction of conversion was calculated as the area of (R)-CCE divided by the total area, m-CPC plus (R)-CCE. The enantiopurities of (R)-CCE for all reactions were ≫99% e.e. (A) Sixty milligrams of cells was subjected to each reaction. (B) Different amounts of cells with pSarA were tested for each trace. The amount of cells used (in milligrams) is shown on the right side of each trace, and the contrast (indicated by diamonds) varied in proportion to the amount of cells. The same traces with 60 mg of cells expressing pEAR2 and pSarA as in panel A were also displayed as references. (C) Conversions with different amounts of cells with pSar268 are indicated as in panel B. Traces of pSar268 are indicated by circles.
Library screening.
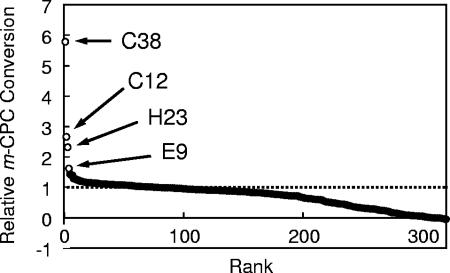
The substrate reducing/oxidizing activity of PAR is coupled with NADH oxidation/reduction activity. To omit the inactive mutants or vectors without inserts, the PAR library was prescreened by measuring NAD+ reducing activity in the presence of 20% (vol/vol) 2-propanol on a nylon membrane. On average, about 1/20 of colonies were detected as positive by color development, and the 8 most significant colonies among 250 to 300 colonies per plate were picked up. At a lower concentration of 2-propanol, color development was observed on almost all colonies (data not shown), due to intrinsic enzymes that could regenerate NADH. In a previous paper, we reported that the conversion efficiency of m-CPC into (R)-CCE was lowered as the concentration of 2-propanol increased from 10% (vol/vol) to 20% (vol/vol) (8). Since m-CPC is barely soluble in aqueous medium but partially soluble in aqueous organic medium, it is suitable as a test substrate to improve the enzymes for conversion in an aqueous-organic solvent. (R)-CCE is also expected to be utilized as a pharmaceutical intermediate. We therefore employed m-CPC as a substrate and attempted to screen PAR mutants from the above library that can efficiently convert it under high concentrations of 2-propanol. The preliminarily selected 320 clones were cultured in 1 ml of medium, harvested, and tested for their ability to convert 1% (wt/vol) m-CPC under 19.5% (vol/vol) of 2-propanol. Significant activity of better than average was observed for several clones (Fig. 2), and the nucleotide sequences of four top clones (C38, C12, H23, and E9) were analyzed. In the deduced amino acid sequence of these clones, nine amino acid substitutions were found. The mutations and corresponding amino acid substitutions are listed in Table 1 and schematically represented in Fig. 1A.
FIG. 2.
The conversion of 1% (wt/vol) m-CPC with 320 clones selected by color development assay. The area of HPLC charting for m-CPC and (R)-CCE was measured, and relative conversion was calculated as the area of (R)-CCE divided by the total area, m-CPC plus (R)-CCE. The values were normalized with the averaged values of positive (pEAR2) and negative (pEAR2s) controls and arranged in descending order. The four top clones, C38, C12, H23, and E9, are specifically represented by open circles, and the rest are represented by closed circles. The level of positive control is shown as a broken line.
Combination of mutations.
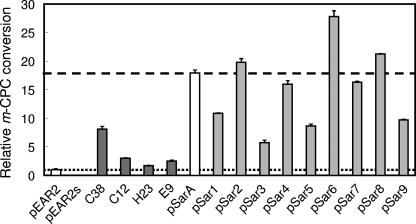
To generate a more efficient mutant, we subsequently applied a combination of advantageous mutations. All amino acid substitutions found in the top four clones selected were combined in the same plasmid. The E. coli-harboring resultant plasmid, pSarA (Fig. 1A), showed more efficient conversion than any of the four parent clones (Fig. 3). In parent clones, several mutations coexisted in a gene, and not all of these were assumed necessarily advantageous. Furthermore, the combination of mutations from different clones might cause cooperative damage. We therefore attempted to omit the disadvantageous mutations from nine amino acid-substituting mutations on pSarA. First, the nine back mutant plasmids, pSar1 to -9, were constructed (Fig. 1B and C). Each has a back mutation to a wild-type sequence at the first to ninth positions of mutations numbered from the N terminal of the amino acid sequence. A higher conversion of m-CPC than with pSarA was observed for pSar2, -6, and -8; thus, mutations at the second, sixth, and eighth positions were assumed to be disadvantageous. Combinatorial back mutations were then attempted. To exclude the possibility of a cooperative advantageous effect of these back mutations, the conversion of several combinations of the back mutants at the second, sixth, and eighth positions was examined. The constructed plasmids (pSar26, pSar68, and pSar268) showed more efficient conversion of m-CPC than any one of pSar1 to -9. Among these mutants, the most efficient conversion was observed with pSar268 (Fig. 4A).
FIG. 3.
The conversion of 1% (wt/vol) m-CPC by each mutant. The clones harboring pEAR2 and pEAR2s, as positive and negative controls, were selected from the library (C38, C12, H23, and E9), and the clones with pSarA and pSar1 to -9 were subjected to the reaction. The relative conversions were calculated as described in the legend to Fig. 2, and the average of three independent conversion experiments with standard deviation is shown. The levels for pEAR2 and pSarA are shown as a broken and a dashed line, respectively. The enantiopurities of (R)-CCE for all reactions were ≫99% e.e.
FIG. 4.
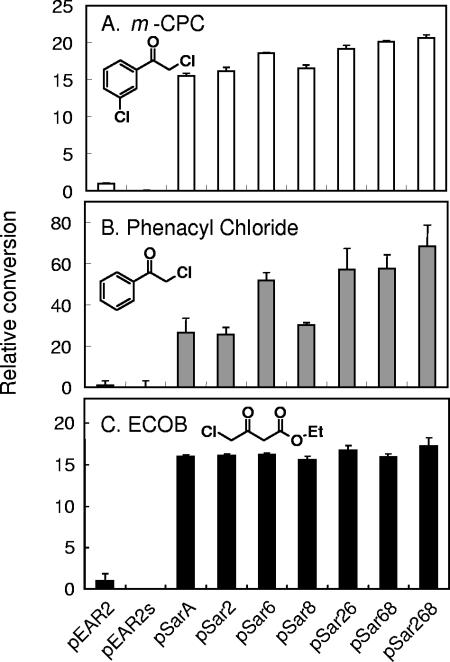
The conversion of some substrates with clones harboring pSar268 or its ancestors. pEAR2, pEAR2s, pSarA, and pSar2, -6, -8, -26, -68, and -268 were subjected to the reaction for each substrate (1% [wt/vol]). Normalized relative conversions were calculated as described in the legend to Fig. 2, and the average of three independent conversion experiments with standard deviations is shown. There was no loss of enantioselectivity observed with pEAR2 or any mutants for these substrates as previously reported: ≫99% e.e. for (R)-CCE (8), 99% e.e. for (R)-CPE (9), and 99% e.e. for (R)-ECHB (8). The structural formula of each substrate is also shown.
Substrate specificity of pSar268.
To test whether the substrate specificity of mutated PAR (pSar268) had been altered, the conversion of several substrates was attempted (Fig. 4). Phenacyl chloride and ECOB were also efficiently converted to (R)-CPE and (R)-ECHB with pSar268 in comparison with pEAR2 and other mutants. Although the conversions for ECOB were almost saturated with all mutants, the order of conversion efficiency for mutants was similar to that observed for m-CPC.
2-Propanol concentration dependency.
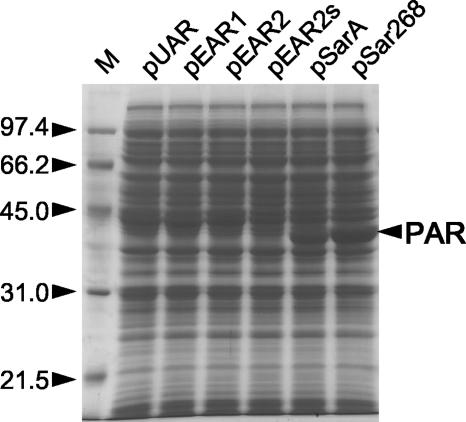
The conversion efficiency depending on the concentration of 2-propanol with concentrated m-CPC was measured (Fig. 5). As reported previously (8), the maximum conversion was observed at around 3 to 10% (vol/vol) 2-propanol concentration with E. coli cells harboring pUAR, and conversions dropped when the 2-propanol concentration increased by >10% (vol/vol). Almost the same profile (conversion efficiency versus 2-propanol concentration) was observed for pEAR1 and pEAR2, suggesting an additional polyhistidine-tag at the C-terminal or the synonymous modification of other nucleotide sequences virtually did not affect the efficiency. Significant enhancements were observed for E. coli harboring pSarA and pSar268 with the same number of cells (Fig. 5A). Under the test conditions, complete conversion was observed between 10 and 25% (vol/vol) of 2-propanol with pSar268. Good conversions were observed with pSarA or pSar268 even at concentrations of <30% (vol/vol) of 2-propanol, where the conversion was hardly detected with pUAR or pEAR2 and the yield with pSarA or pSar268 was much higher than that with pUAR or pEAR2 over the range of 2-propanol concentration. Since the 2-propanol concentration dependency with pSarA or pSar268 was not proportional because of the conversion saturation, it was difficult to distinguish whether the relative performance of the enzyme at a high concentration of 2-propanol was actually enhanced by pSarA or pSar268. Firstly, the enzyme expression levels were analyzed by SDS-PAGE (Fig. 6). Although the expression of PAR with pUAR, pEAR1, or pEAR2 was hardly detected, apparent expressions were found with pSarA and more with pSar268. Thus, the increased expression level was identified as one of the causes of conversion enhancement with the same amount of cells. Then, to assess the possibility of the variation of enzymatic properties in a high concentration of 2-propanol, we further tested the same conversion with fewer cells, canceling the effect of an increase in expression level. When cells harboring pSarA were gradually restricted to 3 mg, the lower yields of conversions, compared to those of pEAR2, were observed at 5 or 10% (vol/vol) 2-propanol (Fig. 5B). However, more conversions than pEAR2 were observed above 15% (vol/vol) 2-propanol with the same number of cells. With 6 mg of cells, apparent enhancement of conversions from pEAR2 was observed above 10% (vol/vol) 2-propanol, even though slight increases were found at 5 or 10% (vol/vol) 2-propanol. Furthermore, significant enhancements at levels above 15% (vol/vol) 2-propanol were observed even with 1.5 mg of the cells harboring pSar268 (Fig. 5C). Although almost the same conversions were observed with 6 mg of pSarA cells and 3 mg of pSar268 cells at 5 to 10% (vol/vol) 2-propanol, greater conversions were achieved above 10% (vol/vol) 2-propanol. Thus, the increase in relative performance at a high concentration of 2-propanol was also identified with pSarA and more with pSar268. Conversion with pSar268 was further tested with more concentrated m-CPC and analyzed by GC. Over 99% molar conversion to (R)-CCE with the enantiomeric purity of ≫99% enantiomeric excess (e.e.) was observed under the same reaction conditions with 60 mg cells, except with 20% (vol/vol) 2-propanol and 200 mg m-CPC.
FIG. 6.
The extract of cells with pUAR, pEAR1, pEAR2, pEAR2s, pSarA, and pSar268 for 100 mg m-CPC conversion was prepared and analyzed by SDS-PAGE. The molecular masses (in kilodaltons) of the low-range standards (M) and PAR are indicated by arrowheads.
DISCUSSION
Enzymes that can accommodate the harsh industrial process are in great demand, because industrial chemical processes require many types of substrates or solvents. One of the most necessary properties of enzyme catalysts is chemical resistance. PAR has an advantage by regenerating NADH coupled with the oxidation of secondary alcohols, such as 2-propanol, essentially required for the reduction of ketone substrates. However, our PAR expression system was not sufficient to convert concentrated substrates or 2-propanol. We therefore attempted to improve the conversion efficiency of substrates by using a resting-cell system expressing PAR in the presence of a high concentration of substrates and 2-propanol; we successfully created a PAR mutant that can reduce several chemicals in a solvent containing a high concentration of 2-propanol (over ∼20% [vol/vol]) without altering the substrate specificity of PAR.
The original bacterial species from which we cloned the PAR gene was reidentified as Rhodococcus species, and the nucleotide sequence of PAR on pUAR was revised. An efficient (S)-specific alcohol dehydrogenase isolated from Rhodococcus erythropolis DSM 43297 was previously reported (1), and a 99.6% identity of the nucleotide was found with the revised sequence of PAR on pUAR.The only difference in the amino acid sequence was Ser in PAR on pUAR to Ala at position 317. Considering the difference of substrate specificity or thermostability between these enzymes (1), it is possible that this difference in amino acid changes the properties of the enzyme.
Directed evolution is a very powerful technique for modifying enzymes, because it does not require extra information about the enzyme (4). By this method, advantageous mutations can be accumulated by repetitive cycles of mutation and selection. The key to the successful creation of a modified enzyme with directed properties is a sufficient size of the mutant library and an efficient technique of screening. The color development of the colonies with appropriate substrates is one technique that fulfills the above requirement. However, the enzymes required for industry cannot always be selected by such easily accessible methods, and this makes it difficult to apply repeated and high-throughput screening. The procedure employed here was the combinatorial mutagenesis technique. Initially, about 5,000 colonies were screened, a relatively small number in contrast to other molecular evolution techniques. The color development technique employed to select these clones was not used to select highly active clones but to exclude inactive clones or self-ligated vectors. We then screened only 320 clones, selected by the first screening, to identify mutants that could efficiently convert m-CPC. The efficiency of asymmetric reduction is generally restricted for evaluatation by HPLC, GC, or other methods. Thus, the selection of such a library of mutants is conceivably restricted with a limited number of clones in practical application. The clones selected from THE 320 mutants showed relatively small enhancement of activity compared to their parents. However, significant enhancement was observed simply by combining the mutations found in multiple clones. Furthermore, successful advancement of activation was also achieved by manual exclusion of disadvantageous mutations, not by selecting a library constructed by random shuffling. The manual combination method of scanning limited mutants might appear rather insufficient. However, if a library consisting of 9 mutations is constructed, 512 mutant combinations are expected. Although this is a relatively small number, significant effort is required for complete scanning of the library by HPLC or GC analysis. On the other hand, we scanned the nine back mutants and some of their combination mutants. The mutational effect of protein is reported to be roughly additive, at least for free energy change by ligand binding or structural stability (2). Although our results might originate in activity or solvent resistance, the method of scanning mutants with a single back mutation for each and further compensation of the risk of combination of back mutations by surveying several combinations of mutations, based on the assumption of the additivity of mutational effect, was almost sufficient to optimize the mutation combinations. Consequently, efficient improvement was achieved with the six remaining advantageous mutations, in spite of the relatively small library size and single selection of the library. The procedure developed here is suitable for potentially applicable enzymes, particularly those that catalyze asymmetric conversion, to improve efficiently with relatively little effort.
Efficient asymmetric reduction of various ketones was reported with alcohol dehydrogenase A from Rhodococcus ruber DSM 44541, with coupled redox reactions using 2-propanol as a hydrogen donor (19). Its activity persists at concentrations of up to 20% (vol/vol) acetone and 50% (vol/vol) 2-propanol. Although the engineered PAR, pSar268, appears to be less efficient than ADH A, the efficiency was significantly enhanced from that of the original PAR, even with a heterologous recombinant expression system in E. coli as a host. If pSar268 could be reintroduced into the Rhodococcus species, known as bacteria with high organic solvent resistance, increased enhancement might be expected. Furthermore, the advent of enzyme engineering has advantages for upgrading enzyme properties, alteration of substrate specificity, and optimization of expression in the heterologous host. The method developed here, successfully demonstrated with PAR, points the way to the rapid development of enzymes equipped with such properties, even from natural enzymes.
Conversion enhancement with pSarA or pSar268 at high concentrations of 2-propanol was achieved by both increased expression level and improved relative performance of enzyme. It was shown that the expression level of recombinant enzyme can be increased even when only the structural gene is mutated. Thus, the hardly detectable expression of other recombinant enzymes could also be improved through a similar procedure. The engineering procedure employed here also worked well in increasing the relative enzyme performance at a high concentration (over ∼10% [vol/vol]) of 2-propanol. Although both effects could be obtained without rational designing, it is unclear how mutants can work efficiently under high concentrations of substrate and 2-propanol. Elucidating the mechanism of resistance and the efficiency of enzymes in high concentrations of aqueous organic solvents, including concentrated substrates and products, is one of the key issues for rational engineering of industrial enzymes. The effects of the mutations described above, such as the expression, activity, resistance to organic solvents, and the mechanism of changes in properties by mutations, are now under investigation.
Acknowledgments
We thank Sumitomo Chemical for the supply of m-CPC.
REFERENCES
- 1.Abokitse, K., and W. Hummel. 2003. Cloning, sequence analysis, and heterologous expression of the gene encoding a (S)-specific alcohol dehydrogenase from Rhodococcus erythropolis DSM 43297. Appl. Microbiol. Biotechnol. 62:380-386. [DOI] [PubMed] [Google Scholar]
- 2.Aita, T., and Y. Husimi. 2000. Theory of evolutionary molecular engineering through simultaneous accumulation of advantageous mutations. J. Theor. Biol. 207:543-556. [DOI] [PubMed] [Google Scholar]
- 3.Angelaccio, S., and M. C. Bonaccorsi di Patti. 2002. Site-directed mutagenesis by the megaprimer PCR method: variations on a theme for simultaneous introduction of multiple mutations. Anal. Biochem. 306:346-349. [DOI] [PubMed] [Google Scholar]
- 4.Arnold, F. H., and A. A. Volkov. 1999. Directed evolution of biocatalysts. Curr. Opin. Chem. Biol. 3:54-59. [DOI] [PubMed] [Google Scholar]
- 5.Cadwell, R. C., and G. F. Joyce. 1992. Randomization of genes by PCR mutagenesis. PCR Methods Appl. 2:28-33. [DOI] [PubMed] [Google Scholar]
- 6.Cadwell, R. C., and G. F. Joyce. 1994. Mutagenic PCR. PCR Methods Appl. 3:S136-S140. [DOI] [PubMed] [Google Scholar]
- 7.El Hawrani, A. S., R. B. Sessions, K. M. Moreton, and J. J. Holbrook. 1996. Guided evolution of enzymes with new substrate specificities. J. Mol. Biol. 264:97-110. [DOI] [PubMed] [Google Scholar]
- 8.Itoh, N., M. Matsuda, M. Mabuchi, T. Dairi, and J.-C. Wang. 2002. Chiral alcohol production by NADH-dependent phenylacetaldehyde reductase coupled with in situ regeneration of NADH. Eur. J. Biochem. 269:2394-2402. [DOI] [PubMed] [Google Scholar]
- 9.Itoh, N., N. Mizuguchi, and M. Mabuchi. 1999. Production of chiral alcohols by enantioselective reduction with NADH-dependent phenylacetaldehyde reductase from Corynebacterium strain, ST-10. J. Mol. Catal. B Enzym. 6:41-50. [Google Scholar]
- 10.Itoh, N., R. Morihama, J.-C. Wang, K. Okada, and N. Mizuguchi. 1997. Purification and characterization of phenylacetaldehyde reductase from a styrene-assimilating Corynebacterium strain, ST-10. Appl. Environ. Microbiol. 63:3783-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itoh, N., K. Yoshida, and K. Okada. 1996. Isolation and identification of styrene-degrading Corynebacterium strains, and their styrene metabolism. Biosci. Biotechnol. Biochem. 60:1826-1830. [DOI] [PubMed] [Google Scholar]
- 12.Koeller, K. M., and C. H. Wong. 2001. Enzymes for chemical synthesis. Nature 409:232-240. [DOI] [PubMed] [Google Scholar]
- 13.Kosjek, B., W. Stampfer, M. Pogorevc, W. Goessler, K. Faber, and W. Kroutil. 2004. Purification and characterization of a chemotolerant alcohol dehydrogenase applicable to coupled redox reactions. Biotechnol. Bioeng. 86:55-62. [DOI] [PubMed] [Google Scholar]
- 14.Mayer, K. M., and F. H. Arnold. 2002. A colorimetric assay to quantify dehydrogenase activity in crude cell lysates. J. Biomol. Screen. 7:135-140. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura, K., R. Yamanaka, T. Matsuda, and T. Harada. 2003. Recent developments in asymmetric reduction of ketones with biocatalysis. Tetrahedron Asymmetry 14:2659-2681. [Google Scholar]
- 16.Patel, R. N. 2003. Microbial/enzymatic synthesis of chiral pharmaceutical intermediates. Curr. Opin. Drug Discov. Devel. 6:902-920. [PubMed] [Google Scholar]
- 17.Sambrook, J., E. F. Fritsch, and T. Maniatis 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Sarkar, G., and S. S. Sommer 1990. The “megaprimer” method of site-directed mutagenesis. BioTechniques 8:404-407. [PubMed] [Google Scholar]
- 19.Stampfer, W., B. Kosjek, C. Moitzi, W. Kroutil, and K. Faber. 2002. Biocatalytic asymmetric hydrogen transfer. Angew. Chem. Int. Ed. Engl. 41:1014-1017. [DOI] [PubMed] [Google Scholar]
- 20.Wang, J.-C., M. Sakakibara, J.-Q. Liu, T. Dairi, and N. Itoh. 1999. Cloning, sequence analysis, and expression in Escherichia coli of the gene encoding phenylacetaldehyde reductase from styrene-assimilating Corynebacterium sp. strain ST-10. Appl. Microbiol. Biotechnol. 52:386-392. [DOI] [PubMed] [Google Scholar]
- 21.Wolberg, M., W. Hummel, C. Wandrey, and M. Müller. 2000. Highly regio- and enantioselective reduction of 3,5-dioxocarboxylates. Angew. Chem. Int. Ed. Engl. 39:4306-4308. [DOI] [PubMed] [Google Scholar]