Abstract
The control of protein conformation during translocation through the endoplasmic reticulum is often a bottleneck for heterologous protein production. The core pathway of the oxidative folding machinery includes two conserved proteins: Pdi1p and Ero1p. We increased the dosage of the genes encoding these proteins in the yeast Kluyveromyces lactis and evaluated the secretion of heterologous proteins. KlERO1, an orthologue of Saccharomyces cerevisiae ERO1, was cloned by functional complementation of the ts phenotype of an Scero1 mutant. The expression of KlERO1 was induced by treatment of the cells with dithiothreitol and by overexpression of human serum albumin (HSA), a disulfide bond-rich protein. Duplication of either PDI1 or ERO1 led to a similar increase in HSA yield. Duplication of both genes accelerated the secretion of HSA and improved cell growth rate and yield. Increasing the dosage of KlERO1 did not affect the production of human interleukin 1β, a protein that has no disulfide bridges. The results confirm that the ERO1 genes of S. cerevisiae and K. lactis are functionally similar even though portions of their coding sequence are quite different and the phenotypes of mutants overexpressing the genes differ. The marked effects of KlERO1 copy number on the expression of heterologous proteins with a high number of disulfide bridges suggests that control of KlERO1 and KlPDI1 is important for the production of high levels of heterologous proteins of this type.
In eukaryotes, the specific folding of proteins targeted to the secretory pathway or to extracellular space occurs in the endoplasmic reticulum (ER). For many secretory proteins, the proper folding requires the formation of intra- and intermolecular disulfide bonds (for reviews, see references 15, 21, and 39). The pathway of oxidative protein folding has been extensively studied in Saccharomyces cerevisiae. Genes have been identified that are involved in redox homeostasis within the ER. The protein folding process requires numerous chaperones and enzymes. The core pathway contains two conserved proteins: Pdi1p and Ero1p. Protein disulfide isomerase (PDI) catalyzes formation, isomerization, and reduction of disulfide bonds of substrate proteins (18, 22, 23, 33). The ER membrane-associated protein Ero1p (ER oxidoreduction) introduces oxidizing equivalents through a flavin-dependent mechanism, engaging thiol-disulfide exchange with Pdi1p (17, 38). Mutations in ERO1 and PDI1 result in cells that are sensitive to the reducing agent dithiothreitol (DTT) and that accumulate proteins that normally contain disulfide bonds in reduced form in the ER. The accumulation of reduced proteins induces the unfolded protein response (16, 19, 29, 30). Overexpression of ERO1 results in cells resistant to DTT (16). A few other proteins functionally related to Pdi1p or to Ero1p have also been identified in S. cerevisiae. Overproduction of Mpd1, Mpd2, Eug1, and Eps1 can partially complement the loss of Pdi1p, and overproduction of Erv2 partially complements the loss of Ero1. Unlike ERO1 and PDI1, however, the five related proteins are not essential genes (for a review, see reference 39).
Increasing Pdi1p activity in bacterial, yeast, insect, and mammalian expression systems can result in increased secretion of heterologous proteins containing disulfide bonds (9, 12, 20, 28, 31, 35, 41). In particular, in Kluyveromyces lactis, a yeast used as a host for secreted production of mammalian proteins (11, 13, 14, 40), duplication of KlPDI1 increases the amount of human serum albumin (HSA), a protein rich in disulfide bonds (4), that is secreted.
Our objectives in this study were (i) to isolate the KlERO1 gene of K. lactis and (ii) to evaluate the secretion of heterologous proteins with or without disulfide bridges by manipulating the key components of the oxidative folding machinery Ero1 and Pdi1. Our working hypothesis was that increasing Pdi-Ero activity would further increase the secretion of highly S-S bonded proteins. Our results provide additional support for the hypothesis that the expression of some classes of heterologous proteins may require significant manipulation of genes involved in protein secretion and processing in addition to increasing the copy number of the gene encoding the expressed protein.
MATERIALS AND METHODS
Strains and growth conditions.
S. cerevisiae strains were CKY8, MATα leu2-3,112 ura3-52 (ERO1), and CKY559, MATα leu2-3,112 ura3-52 ero1-1 (16). The K. lactis strain was JA6, MATα ade1-600 adeT-600 trp1-11 uraA1-1 (7). YPD medium contained 5 g Difco (Detroit, Mich.) yeast extract, 10 g Difco Bacto peptone, and 20 g glucose per liter. Minimal medium (YNBD) contained 6.7 g liter−1 yeast nitrogen base without amino acids (Difco) supplemented with amino acids and bases as required to a final concentration of 40 μg ml−1 each and 20 g liter−1 glucose. For human serum albumin production, the antibiotic G418 was added to a final concentration of 200 μg ml−1 to provide stable maintenance of the replicative plasmid. The medium with low phosphate, for PHO5 promoter induction, was described previously by Morlino et al. (27). Media were solidified with 2% Bacto agar (Difco). The Escherichia coli strain JM83 [araD (lac-proAB) rpsL (= strA) ϕ80 lacZ DM15] was used for plasmid propagation and maintenance. This strain was grown in LB medium (32), and ampicillin was added to a final concentration of 100 μg ml−1 for plasmid maintenance.
Plasmids and DNA manipulation.
The plasmids used in this study are listed in Table 1. The KlERO1 gene was isolated as plasmid pE2, a clone of the K. lactis genomic library carried by the centromeric plasmid KCp491. The KlPDI1 gene was subcloned from the pCRS/P1 plasmid. The expression/secretion cassettes were carried in the multicopy plasmids pYG108 and pYGK44. In pYG108, human serum albumin expression/secretion was driven by the human pre-pro signal sequence under the control of the ScPGK promoter and terminator (14). In pYGK44, interleukin-1β expression/secretion was driven by the K. lactis killer toxin signal sequence under the control of the PHO5 promoter (13). Isolation and purification of plasmids from E. coli and agarose gel electrophoresis were performed according to the method of Sambrook and Russell (32). Yeast transformation was carried out by electroporation, according to the method of Wésolowsky-Louvel et al. (40), and E. coli transformation was done according to the method of Mandel and Higa (24).
TABLE 1.
Plasmids
| Plasmid | Hosta | Vector | Insert(s) | Remarks or reference |
|---|---|---|---|---|
| pE2 | K. lactis | KCp491 | DNA segment carrying the entire KIERO1 | Clone from K. lactis genomic library |
| pH48 | S. cerevisiae | pFL38 | KIERO1, HindIII-HindIII, 4.8 kb | Subcloned from pE2 insert |
| pCX48 | K. lactis | pCXJ11 | KIERO1, HindIII-HindIII, 4.8 kb | Subcloned from pE2 insert |
| pLCE | S. cerevisiae | pFL38 | KIERO1, SacI-HindIII, 3.8 kb | Subcloned from pE2 insert |
| pLCI | S. cerevisiae | pFL38 | KIPDI1, SacI-KpnI, 2.8 kb | Subcloned from pCRS/P1 insert |
| pLCEI | S. cerevisiae | pFL38 | KIEROI, SacI-HindIII, 3.8 kb, KIPDI1, SacI-KpnI, 2.8 kb | Subcloned from pE2 and pCRS/P1 inserts |
| pCRS/P1 | K. lactis | DNA segment carrying the entire KIPDI1 gene | 4 | |
| KCp491 | K. lactis | Centromere vector | 40 | |
| pFL38 | S. cerevisiae | Centromere vector | 6 | |
| pCXJ11 | K. lactis | Multicopy vector | 8 | |
| pYG108 | K. lactis | pKD1 with Kanr marker | Prepro-HSA gene under ScPGK promoter | 14 |
| pYGK44 | K. lactis | pKD1 with Kanr marker | Interleukin-1β gene under ScPHO5 promoter | 13 |
| pYG106 | K. lactis | pYG108 without prepro-HSA | 1.9-kb region deletion by HindIII digestion |
Note that plasmids whose normal host is K. lactis can replicate in S. cerevisiae with reduced stability. For example, the genomic library in KCp491 was used to transform an S. cerevisiae ero1 mutant (Fig. 1). The single-copy plasmid pFL38 whose normal host is S. cerevisiae can stably replicate in the JA6 K. lactis strain.
DNA sequencing and sequence analysis.
The sequence of KlERO1 was obtained by primer extension with pH48 plasmid as the template. Sequence analysis was performed with the BLASTP program (1), and sequence alignment was performed with Clustal W (37).
Northern analysis.
Total RNA was prepared by extraction with hot acidic phenol (3). Northern analysis was carried out as described by Sherman et al. (34). The KLERO1 probe was produced by PCR amplification with primers EROA (5′-CCAGAGTATTGGCAGCCTG-3′) and EROC (5′-GTCTTGCCACATCGTCATCG-3′) and genomic DNA from strain JA6 as the template. The KlACT1 probe corresponded to the HindIII 1.4-kb region derived from the KlACT1-containing pUC19 plasmid (10). The probes were labeled with [α-32P]dCTP using the ReadyPrime DNA labeling system (Amersham Biosciences, Little Chalfont, England) according to the manufacturer's instructions.
Analysis of secreted HSA and IL-1β.
Ten milliliters of culture supernatant, corresponding to 2 × 109 cells, was mixed with an equal volume of 10% trichloroacetic acid. The precipitated proteins were collected by centrifugation (10 min, 4°C, 14,000 × g). The pellets were washed with 10% acetone, air dried for 20 min, resuspended in 10 μl H2O, and mixed with 2 μl of sample buffer (0.2 M Tris-HCl [pH 6.8], 30% glycerol, 1.2% sodium dodecyl sulfate [SDS], 30% β-mercaptoethanol, and 0.05% bromophenol blue). After 3 min at 95°C, the solution was resolved by 12% SDS-polyacrylamide gel electrophoresis (PAGE). For evaluation of interleukin-1β (IL-1β) secretion, a 1:10 dilution was used. After gel electrophoresis, the amounts of secreted HSA and IL-1β were evaluated by densitometry after silver staining or Coomassie blue R-250 staining, respectively. For Western analysis, 2.5 μl of culture media was loaded directly onto 12% SDS-PAGE gels and, after electrophoresis, electroblotted onto a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA) in Towbin buffer at 200 V for 40 min. Primary polyclonal antibodies were used at a dilution of 1:50,000 (Sigma-Aldrich Corporation, St. Louis, MO). The secondary antibody was anti-rabbit immunoglobulin G conjugated with peroxidase (Bio-Rad). An ECL detection kit (Amersham) was used according to the manufacturer's instructions. The densitometric analysis was performed with an image analyzer (Phoretix 1D; Non Linear Dynamics Ltd., New Castle upon Tyne, United Kingdom) and normalized against HSA (Sigma) standard.
Statistical analysis.
Cell growth rates and yield means of strains overexpressing HSA in the presence or absence of different Ero-Pdi activities were compared by using univariate analysis (GLM, SPSS, version 12.0; SPSS Inc., Chicago, IL).
RESULTS
Isolation and sequence analysis of the KlERO1 gene of K. lactis.
We searched for the K. lactis ortholog of the S. cerevisiae ERO1 gene by functional complementation of the ts phenotype of a Scero1 mutant (16). After transformation of the mutant with a K. lactis genomic library constructed in a single-copy vector, a positive clone that could grow at 37°C was selected. The complementing plasmid, pE2, contained an insert of 6.5 kb. The minimal DNA region able to complement the ts phenotype of the mutant, a 4.8-kb HindIII-HindIII fragment, was subcloned in the single-copy vector pFL38 to yield pH48. Both the pE2 and pH48 plasmids also could relieve the hypersensitivity of the reducing agent DTT of the Scero1 mutant (Fig. 1). The insert in the pH48 plasmid was sequenced. It contained a gene (KlERO1, EMBL accession number AJ489319) that codes for a protein of 562 amino acids that is 67% similar and 59% identical to ScEro1p. The protein is cysteine rich and contains the conserved CXXCXXC motif, which resembles the CXXC thioredoxin-like motif of eukaryotic and prokaryotic disulfide exchange proteins (2, 25). No homology was detected between ScERO1 and KlERO1 in the N-terminal region, probably corresponding to the leader sequence for import into the ER.
FIG. 1.
Complementation of Scero1 hypersensitivity to the reducing agent DTT by the KlERO1 gene. The S. cerevisiae ero1 mutant (CKY559) transformed with pE2, pH48, or the insert-less plasmid KCp491 and its isogenic ERO1 CKY8 strain transformed with KCp491 were spotted onto YNBD medium plates in presence of different DTT concentrations. The plates were incubated at 28°C for 5 days. −, without.
Effect of KlERO1 on the growth of K. lactis.
High gene dosage of KlPDI1 is toxic to K. lactis (5), but the dose effect of KlERO1 is not known. The KlERO1 gene was cloned in the multicopy vector pCXJ11 (to produce pCX48), which was introduced into K. lactis strain JA6. The transformed strains with either the single-copy or multicopy plasmid had doubling times (5.3 h) and final cell yields (∼2.2 × 108 cells ml−1) similar to those of the wild-type strain in YNBD medium. This similarity indicates that the presence of additional copies of the gene was not toxic to K. lactis cells. Unlike in S. cerevisiae (16), overexpression of KlERO1 does not confer resistance to dithiothreitol on K. lactis cells (data not shown). KlERO1 expression was strongly induced by DTT and induced to a lesser extent when the HSA gene was expressed (Fig. 2).
FIG. 2.
Transcriptional regulation of KlERO1 gene. (A) Effect of DTT on KlERO1 mRNA levels. Total RNA was prepared from JA6 cells growing in YPD medium before (lane 1) and 1 h after the shift to 2 mM (lane 2) or 4 mM (lane 3) DTT. RNA was hybridized with labeled probes for the KlERO1 and KlACT1 genes. Each lane contained 20 μg of total RNA. (B) Effect of HSA on KlERO1 mRNA levels. Total RNA was prepared from JA6 transformed with the plasmid carrying the cassette for secreted expression of rHSA (pYG108) (lane 1) or the insert-less plasmid (pYG106) (lane 2). Cells were grown in YPD medium supplemented with 200 μg ml−1 of G418. RNA was hybridized with labeled probes for KlERO1 and KlACT1 genes. Each lane contained 20 μg of total RNA.
Effect of KlERO1 duplication on secretion of HSA.
The stability of single-copy (pE2) and multicopy (pCx48) plasmids carrying KlERO1 was tested in YPD plus 200 μg/ml G418. pE2 was highly stable; after 72 h of shake culture at 28°C (in stationary phase), 95 to 97% of cells maintained the plasmid. pCX48 was not as stable, with only 20 to 40% of the transformed cells retaining the plasmid under similar conditions.
Cells carrying pE2 have two copies of KlERO1 (one on the plasmid and one in the genome) and have a different protein expression pattern (Fig. 3), including a strong stimulation of HSA secretion. After 48 h, an intense band corresponding to HSA is visible in the culture medium of the strain carrying pE2 that is not evident in the culture medium of the nontransformed strain. After 72 h, differences in band intensity were also evident. A second copy of KlERO1 in the presence and absence of the HSA expression/secretion cassette also increased the secretion of endogenous proteins (Fig. 3, compare lanes 2 and 4 with lanes 3 and 5).
FIG. 3.
Secretion of human serum albumin from K. lactis strain JA6 transformed with pYG108 carrying the HSA cassette. After 48 and 72 h of culture, the proteins of the supernatant were separated by SDS-PAGE and visualized by silver staining. Lane 1: human serum albumin reference (250 ng). Lane 2: transformant with an additional copy of KlERO1 (pE2 plasmid). Lane 3: transformant with the insert-less plasmid KCp491 (lacks KlERO1). Lanes 4 and 5: controls, i.e., transformants with the HSA cassette-less version of pYG108 (pYG106) in the presence and absence of pE2, respectively.
Effect of simultaneous duplication of KlERO1 and KlPDI1.
The centromeric vectors pLCE, pLCI, and pLCEI (Table 1), which contain KlERO1, KlPDI1, and KlERO1 plus KlPDI1, respectively, are retained by 93 to 95% of the cells in cultures grown on YPD plus G418 for 72 h (Fig. 4). The simultaneous duplication of both genes led to a significantly (P < 0.001) higher growth rate and yield than those observed if only a single gene was duplicated (Fig. 4). The level of the secreted HSA was similar after 48 and 72 h of growth in strains with two copies of either ERO1 or PDI1 (Fig. 5). In both cases, secretion was strongly stimulated (about 15-fold) relative to the parental strain. After 48 h of culture, the signal corresponding to HSA was strongly enhanced in the supernatant of the strain with two copies of both genes, indicating that the highest amount of total protein was produced in this condition. If normalized to cell density, however, the secretion level was similar to that of strains carrying a duplication of either ERO1 or PDI1 (data not shown). After 72 h, the amount of HSA secreted was similar if ERO1 alone, PDI1 alone, or ERO1 and PDI1 were duplicated. The final amount of secreted HSA was estimated to be ∼50 mg/liter.
FIG. 4.
Cellular growth in the presence of KlERO1 and/or KlPDI1 duplication. Growth curves of the K. lactis strain JA6 transformed with pYG108 (carrying the HSA cassette) in the presence of an additional copy of KlERO1 (pLCE) (□), an additional copy of KlPDI (pLCI) (▵), additional copies of both KlERO1 and KlPDI (pLCEI) (•), or the insert-less plasmid (pFL38) (⋄) on YPD containing 200 μg ml−1 of G418. The results are means of the results from four independent experiments with standard errors for all points of <10%. The comparison of average values was done by means of statistical univariate analysis (GLM).
FIG. 5.
Secretion of HSA. After 48 and 72 h of culture, 2.5 μl of the supernatant was examined by Western blotting. Lane 1: human serum albumin reference (50 ng). Lanes 2 and 6: transformant with the insert-less pFL38 plasmid. Lanes 3 and 7: transformant with the pLCE plasmid. Lanes 4 and 8: transformant with the pLCI plasmid. Lanes 5 and 9: transformant with the pLCEI plasmid.
Secretion of IL-1β.
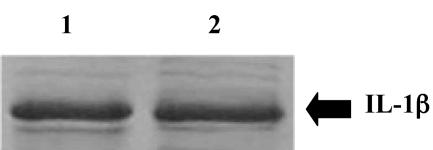
Strain JA6 (with and without KlERO1 duplication) was transformed with plasmid pYGK44 containing an expression cassette corresponding to IL-1β cDNA fused to the secretion signal of the K. lactis killer toxin (13) and placed under the control of the phosphate-repressible PHO5 promoter. After 3 days of culture growth on phosphate-less YPD to allow induction of IL-1β transcription, IL-1β in the supernatant was analyzed by SDS-PAGE. Densitometric measurements of the IL-β bands (Fig. 6) indicated that the amount of secreted IL-1β, a protein that has no disulfide bridges (26), was not significantly different in the presence or in the absence of the KlERO1 duplication.
FIG. 6.
Secretion of IL-1β in the presence of KlERO1 duplication. Strain JA6 transformed with the plasmid pYGK44 (carrying the IL-1β cassette), in the presence or absence of an additional copy of KlERO1, was grown for 72 h on YPD depleted of phosphate. An aliquot of supernatant, corresponding to 2 × 108 cells, was examined by SDS-PAGE. Protein bands were stained with Coomassie blue. Lane 1: transformant with the insert-less KCp491 plasmid. Lane 2: transformant with a second copy of KlERO1 (pE2 plasmid).
DISCUSSION
Secreted production of proteins from heterologous hosts, often a yeast or Aspergillus strain, is still largely dependent on empirically developed protocols. Although the construction of heterologous gene expression cassettes may follow rational schemes, the control of protein secretion pathways remains uncertain. When a large amount of foreign protein enters a secretory pathway, the cell's chaperone activities may be insufficient, triggering stress responses with unpredictable consequences. By manipulating a few key components of the secretory system, the stress response may not be triggered. The present study focused on KlERO1, whose product directly interacts with the Pdi1 protein.
The expression of KlERO1 was strongly induced by DTT, which is consistent with a role for this gene in oxidative protein folding. KlERO1 overexpression, i.e., from multiple copies of the gene, did not increase the DTT resistance of the K. lactis wild-type strain. This lack of response may be due to the very high level of induction of KlERO1 transcription by DTT, which would make it more difficult to observe the gene dosage effect. Overexpression of HSA also increased ERO1 transcription 1.5-fold.
Duplication of either KlERO1 or KlPDI1 resulted in a striking increase (∼15-fold) in the amount of HSA secreted by K. lactis. Since oxidizing equivalents flow directly from Ero1p to secretory proteins via Pdi1p, the observed increase in protein production suggests that neither Ero1p nor Pdi1p is a limiting factor when either PDI1 or ERO1 is duplicated. The simultaneous duplication of both genes accelerated the secretion of HSA and increased both growth rate and cell yield. The stimulatory effects of the duplication of PDI1 (or its orthologs) confirm several earlier reports (9, 12, 20, 28, 31, 35, 41), but the similarly marked effects of the KlERO1 copy number is a new finding.
One hypothesis is that only the secretion of highly S-S bonded proteins is improved by increasing Pdi-Ero activities. However, there are examples in which secretion of non-disulfide-bonded proteins also can be stimulated by PDI1 duplication (for an example, see reference 36). Duplication of KlERO1 did not influence the production of Il-1β, which suggests that Ero1p does not have a chaperone-like function such as that suggested for Pdi1p in S. cerevisiae. Information on the effects of PDI1/ERO1 duplication and the expression of other secreted proteins is needed to predict the impact of these proteins on the secretion of heterologous proteins in this system.
Acknowledgments
We thank A. Cabibbo (DIBIT-HSR, Milan, Italy) for the S. cerevisiae mutant ero1-1 CKY559 and its isogenic wild type, CKY8, M. Wésolowsky-Louvel for the K. lactis genomic library constructed in the single-copy vector KCp491, H. Fukuhara (Institut Curie, Orsay, France) for plasmids pRCS, pYG108, and pYGK44, and Roberto Silva for skillful technical assistance.
This work was supported by Cofin 2002 grant 2002052349_003 from the Ministero Università e Ricerca Scientifica e Tecnologica.
REFERENCES
- 1.Altshul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Aslund, F., and J. Beckwith. 1999. The thioredoxin superfamily: redundancy, specificity, and gray-area genomics. J. Bacteriol. 181:1375-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Current protocols in molecular biology. Wiley, New York, N.Y.
- 4.Bao, W.-G., and H. Fukuhara. 2001. Secretion of human proteins from yeast: stimulation by duplication of polyubiquitin and protein disulfide isomerase genes in Kluyveromyces lactis. Gene 272:103-110. [DOI] [PubMed] [Google Scholar]
- 5.Bao, W.-G., K. K. Huo, Y. Y. Li, and H. Fukuhara. 2000. Protein disulfide isomerase genes of Kluyveromyces lactis. Yeast 16:329-341. [DOI] [PubMed] [Google Scholar]
- 6.Bonneaud, N., O. Ozier-Kalogeropoulos, G. Li, M. Labouesse, L. Minvielle-Sebastia, and F. Lacroute. 1991. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast 7:609-615. [DOI] [PubMed] [Google Scholar]
- 7.Breunig, K. D. 1989. Glucose repression of LAC gene expression in yeast is mediated by the transcriptional activator LAC9. Mol. Gen. Genet. 216:422-427. [DOI] [PubMed] [Google Scholar]
- 8.Chen, X. J. 1996. Low- and high-copy-number shuttle vectors for replication in the budding yeast Kluyveromyces lactis. Gene 172:131-136. [DOI] [PubMed] [Google Scholar]
- 9.Davis, R., K. Schooley, B. Rasmussen, J. Thomas, and P. Reddy. 2000. Effect of PDI overexpression on heterologous protein secretion in CHO cells. Biotechnol. Prog. 16:736-743. [DOI] [PubMed] [Google Scholar]
- 10.Deshler, J. O., G. P. Larson, and J. J. Rossi. 1989. Kluyveromyces lactis maintains Saccharomyces cerevisiae intron-encoded splicing signals. Mol. Cell. Biol. 9:2208-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnini, C., F. Farina, B. Neglia, M. C. Compagno, D. Uccelletti, P. Goffrini, and C. Palleschi. 2004. Improved production of heterologous proteins by a glucose repression defective mutant of Kluyveromyces lactis. Appl. Environ. Microbiol. 70:2632-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn, A., J. M. Luz, D. Natalia, J. A. Gamble, R. B. Freedman, and M. F. Tuite. 1995. Protein disulphide isomerase (PDI) is required for the secretion of a native disulfide-bonded protein from Saccharomyces cerevisiae. Biochem. Soc. Trans. 23:78S. [DOI] [PubMed] [Google Scholar]
- 13.Fleer, R., X. J. Chen, N. Amellal, P. Yeh, A. Fournier, F. Guinet, N. Gault, D. Faucher, F. Folliard, H. Fukuhara, and J. F. Mayaux. 1991. High-level secretion of correctly processed recombinant human interleukin-1β in Kluyveromyces lactis. Gene 107:285-295. [DOI] [PubMed] [Google Scholar]
- 14.Fleer, R., P. Yeh, N. Amellal, I. Maury, A. Fournier, F. Bacchetta, P. Baduel, G. Jung, H. L'Hote, J. Becquart, H. Fukuhara, and J. F. Mayauax. 1991. Stable multicopy vectors for high-level secretion of recombinant human serum albumin by Kluyveromyces lactis. Biotechnology 9:968-975. [DOI] [PubMed] [Google Scholar]
- 15.Frand, A. R., J. W. Cuozzo, and C. A. Kaiser. 2000. Pathways for protein disulphide bond formation. Trends Cell Biol. 10:203-210. [DOI] [PubMed] [Google Scholar]
- 16.Frand, A. R., and C. A. Kaiser. 1998. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell 1:161-170. [DOI] [PubMed] [Google Scholar]
- 17.Frand, A. R., and C. A. Kaiser. 1999. Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol. Cell 4:469-477. [DOI] [PubMed] [Google Scholar]
- 18.Freedman, R. B., T. R. Hirst, and M. F. Tuite. 1994. Protein disulphide isomerase: building bridges in protein folding. Trends Biochem. Sci. 19:331-336. [DOI] [PubMed] [Google Scholar]
- 19.Holst, B., C. Tachibana, and J. R. Winther. 1997. Active site mutations in yeast protein disulfide isomerase cause dithiothreitol sensitivity and a reduced rate of protein folding in the endoplasmic reticulum. J. Cell Biol. 138:1229-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu, T. A., S. Watson, J. J. Eiden, and M. J. Betenbaugh. 1996. Rescue of immunoglobulins from insolubility is facilitated by PDI in the baculovirus expression system. Protein Expr. Purif. 7:281-288. [DOI] [PubMed] [Google Scholar]
- 21.Huppa, J. B., and H. L. Ploegh. 1998. The eS-Sence of -SH in the ER. Cell 92:145-148. [DOI] [PubMed] [Google Scholar]
- 22.Laboissiere, M. C., S. L. Sturley, and R. T. Raines. 1995. The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J. Biol. Chem. 270:28006-28009. [DOI] [PubMed] [Google Scholar]
- 23.LaMantia, M. L., and W. J. Lennarz. 1993. The essential function of yeast protein disulfide isomerase does not reside in its isomerase activity. Cell 74:899-908. [DOI] [PubMed] [Google Scholar]
- 24.Mandel, J. K., and S. Higa. 1970. Calcium dependent bacteriophage DNA infection. J. Mol. Biol. 53:159-162. [DOI] [PubMed] [Google Scholar]
- 25.Martin, J. L. 1995. Thioredoxin—a fold for all reasons. Structure 3:245-250. [DOI] [PubMed] [Google Scholar]
- 26.Meyers, C. A., K. O. Johanson, L. M. Miles, P. J. McDevitt, P. L. Simon, R. L. Webb, M. J. Chen, B. P. Holskin, J. S. Lillquist, and P. R. Young. 1987. Purification and characterization of human recombinant interleukin-1 beta. J. Biol. Chem. 262:11176-11181. [PubMed] [Google Scholar]
- 27.Morlino, G. B., L. Tizzani, M. M. Bianchi, and L. Frontali. 1999. Inducible amplification of gene copy number and heterologous protein production in the yeast Kluyveromyces lactis. Appl. Environ. Microbiol. 65:4808-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostermeier, M., K. De Sutter, and G. Georgiou. 1996. Eukaryotic protein disulfide isomerase complements Escherichia coli dsbA mutants and increases the yield of a heterologous secreted protein with disulfide bonds. J. Biol. Chem. 271:10616-10622. [DOI] [PubMed] [Google Scholar]
- 29.Pagani, M., M. Fabbri, C. Benedetti, A. Fassio, S. Pilati, N. J. Bulleid, A. Cabibbo, and R. Sitia. 2000. Endoplasmic reticulum oxidoreductin 1-lβ (ERO1-Lβ), a human gene induced in the course of the unfolded protein response. J. Biol. Chem. 275:23685-23692. [DOI] [PubMed] [Google Scholar]
- 30.Pollard, M. G., K. J. Travers, and J. S. Weissman. 1998. Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol. Cell 1:171-182. [DOI] [PubMed] [Google Scholar]
- 31.Robinson, A. S., V. Hines, and K. D. Wittrup. 1994. Protein disulfide isomerase overexpression increases secretion of foreign proteins in Saccharomyces cerevisiae. Biotechnology 12:381-384. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Scherens, B., E. Dubois, and F. Messenguy. 1991. Determination of the sequence of the yeast YCL313 gene localized on chromosome III. Homology with the protein disulfide isomerase (PDI gene product) of other organisms. Yeast 7:185-193. [DOI] [PubMed] [Google Scholar]
- 34.Sherman, F., G. R. Fink, and J. B. Hicks. 1986. Laboratory course manual for methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Shusta, E. V., R. T. Raines, A. Pluckthun, and K. D. Wittrup. 1998. Increasing the secretory capacity of Saccharomyces cerevisiae for production of single-chain antibody fragments. Nat. Biotechnol. 16:773-777. [DOI] [PubMed] [Google Scholar]
- 36.Smith, J. D., B. C. Tang, and A. S. Robinson. 2004. Protein disulfide isomerase, but not binding protein, overexpression enhances secretion of a non-disulfide-bonded protein in yeast. Biotechnol. Bioeng. 85:3403-3450. [DOI] [PubMed] [Google Scholar]
- 37.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tu, B. P., and J. S. Weissman. 2002. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol. Cell 10:983-994. [DOI] [PubMed] [Google Scholar]
- 39.Tu, B. P., and J. S. Weissman. 2004. Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 164:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wésolowsky-Louvel, M., K. D. Breunig, and H. Fukuhara. 1996. Kluyveromyces lactis, p. 139-201. In K. Wolf (ed.), Nonconventional yeasts in biotechnology. Springer-Verlag, Berlin, Germany.
- 41.Wunderlich, M., and R. Glockshuber. 1993. In vivo control of redox potential during protein folding catalyzed by bacterial protein disulfide-isomerase (DsbA). J. Biol. Chem. 268:24547-24550. [PubMed] [Google Scholar]