Abstract
Fourier transform infrared spectroscopy (FT-IR) has been used together with pattern recognition methodology to study isolates belonging to the species Campylobacter coli and Campylobacter jejuni and to compare FT-IR typing schemes with established genomic profiles based on enterobacterial repetitive intergenic consensus PCR (ERIC-PCR). Seventeen isolates were cultivated under standardized conditions for 2, 3, and 4 days to study variability and improve reproducibility. ERIC-PCR profiles and FT-IR spectra were obtained from strains belonging to the species Campylobacter coli and C. jejuni, normalized, and explored by hierarchical clustering and stepwise discriminant analysis. Strains could be differentiated by using mainly the first-derivative FT-IR spectral range, 1,200 to 900 cm−1 (described as the carbohydrate region). The reproducibility index varied depending on the ages of the cultures and on the spectral ranges investigated. Classification obtained by FT-IR spectroscopy provided valuable taxonomic information and was mostly in agreement with data from the genotypic method, ERIC-PCR. The classification functions obtained from the discriminant analysis allowed the identification of 98.72% of isolates from the validation set. FT-IR can serve as a valuable tool in the classification, identification, and typing of thermophilic Campylobacter isolates, and a number of types can be differentiated by means of FT-IR spectroscopy.
Campylobacter jejuni and Campylobacter coli constitute leading causes of food-borne infections, with a large number of cases worldwide and economic implications (2). Although different food products (poultry, pork, beef, and milk) and environmental samples (water and pets) are known to be sources of infection, the origin of contamination cannot be identified in a significant number of cases. The two species are difficult to distinguish because of their absence of reactivity in many conventional biochemical and physiological tests (9). For epidemiological purposes, methods capable of distinguishing between different subspecies of Campylobacter are very useful (4, 48, 50). Typing of isolates is of essential importance to assist in epidemiological surveillance and to study routes of transmission and risk factors. A number of methodologies have been developed in the last few decades. Most of them rely on the use of serotyping or DNA probes. Serotyping by means of the thermostable antigen (Penner scheme) or the heat-labile antigen (Lior scheme) is used as a means of characterization at the subspecies level, but the techniques are not routinely available, since they require specialized material and personnel. Other drawbacks are reproducibility problems with passive hemagglutination, agglutination to more than one antiserum, and inability to type a substantial number of strains (5, 48, 53).
Infrared (IR) spectroscopy has been successfully applied to the identification and classification of microorganisms at the species and subspecies levels. As early as the 1950s, several authors (8, 39, 43) reported the application of mid-IR spectroscopic data (4,000 to 400 cm−1) to the identification of microorganisms. Advances in the 1990s were favored by the development of interferometric IR spectroscopy and Fourier transform (FT) techniques. The reference work of Helm and colleagues (14) prompted a large amount of research, reviewed by Mariey and colleagues (26). Since 2001, more groups and genera have been studied: Lactobacillus (3, 36, 37), Carnobacterium (21), Bacillus (1), Brucella (27), Acinetobacter (52), yeasts (20, 41), enterococci (7, 10, 19), coryneforms (24, 34), and actinomycetes (54). In all of them, the technique has shown qualities, such as high discriminatory power and reproducibility, allowing the differentiation of taxonomic entities at the species or subspecies level on the basis of changes in the IR absorption patterns of the microbial cell. FT-IR spectroscopy is a physicochemical method that measures vibration properties of the chemical bonds when excited by the absorption of IR radiation. Thus, when applied to intact microbial cells, infrared spectra contain essentially qualitative information about the global biochemical composition of the cells. The interpretation of FT-IR spectra (made up of multiple, complex, overlapped absorbance bands of many different vibrational modes of all the cell components) requires the use of pattern recognition methods, such as hierarchical cluster analysis (HCA) or stepwise discriminant analysis (SDA), to disclose relationships and cluster elements on the basis of their perceived closeness (10, 13, 21, 23, 24, 36, 44, 45). SDA can eventually be used to create classification functions able to identify unknowns.
Enterobacterial repetitive intergenic consensus (ERIC)-PCR is a DNA-based typing technique that generates strain-specific fingerprinting using primers directed to specific nucleotide sequences, designated ERIC sequences. ERIC elements (small repetitive units of 126 bp containing a conserved central inverted repeat of 40 bp) have been detected in noncoding, intergenic regions of Escherichia coli and Salmonella enterica serovar Typhimurium (16). Discrimination among strains is achieved by analyzing individual electrophoretic profiles obtained from PCR products. The reproducibility and discriminatory power of the ERIC-PCR technique in the typing of thermophilic Campylobacter spp. favors its application in epidemiological studies (6, 42, 48, 49).
The objective of this study was to check the capability of FT-IR spectroscopy as an identification and typing method for Campylobacter strains. The isolates studied belonged to the two thermophilic species most commonly involved in food-borne infections, and they showed a degree of genomic diversity. Special emphasis was placed on comparison with an already established methodology, such as ERIC-PCR typing. Since chemotaxonomic methods depend strongly on incubation factors, the influence of culture age on reproducibility was also studied.
MATERIALS AND METHODS
Strains.
Seventeen strains classified as Campylobacter coli and C. jejuni were included in the study (Table 1). Sixteen strains were isolated from animal feces (pigs and chickens) and one from human feces (a clinical case). The strains were isolated, identified, and DNA fingerprinted by means of the ERIC-PCR technique (30, 49). All strains were kept frozen (−80°C) in brain heart infusion (BHI) broth containing 20% glycerol during the experiments.
TABLE 1.
Coding, preliminary identification, ERIC-PCR profiles, and origins of strains included in the investigation
| Strain no. | Species | ERIC-PCR profilea | Source |
|---|---|---|---|
| Calibration setb | |||
| 222 | C. jejuni | B | Human feces |
| 503 | C. jejuni | B | Chicken feces |
| 299 | C. jejuni | D | Pig feces |
| 510 | C. coli | A | Pig feces |
| 360 | C. coli | C | Chicken feces |
| 425 | C. coli | C | Chicken feces |
| 444 | C. coli | C | Chicken feces |
| 457 | C. coli | C | Chicken feces |
| 459 | C. coli | C | Chicken feces |
| Validation setc | |||
| 371 | C. coli | C | Pig feces |
| 427 | C. coli | C | Chicken feces |
| 458 | C. coli | C | Chicken feces |
| 511 | C. coli | A | Pig feces |
| 512 | C. coli | A | Pig feces |
| 527 | C. jejuni | B | Chicken feces |
| 361 | C. jejuni | B | Chicken feces |
| 557 | C. jejuni | B | Chicken feces |
Campylobacter strains were subdivided into four types (A, B, C, and D) following banding patterns obtained by ERIC-PCR typing.
Strains used in the reproducibility analysis.
Additional strains used to establish and validate classification scheme.
ERIC-PCR conditions and primers.
ERIC-PCR was performed with the sequences ERIC1R and ERIC2 (47), using a Perkin-Elmer thermal cycler model 480. After the PCR was carried out, the PCR products were separated on 1.5% agarose gels and stained with ethidium bromide (6). Digitized images of the gels (tagged image file format) were obtained (One-scanner; Apple), and banding patterns were detected by using the RestrictoScan and RestrictoTyper programs from the Taxotron package (Taxolab, Institut Pasteur, Paris, France).
Preparation of samples and spectrum recording.
Cultures were cultivated in BHI broth (Oxoid) at 37°C for 24 h under microaerophilic conditions (5% O2, 10% CO2, 85% N2). An inoculum was then transferred with a sterile platinum loop from the broth to BHI agar and incubated for 48, 72, and 96 h to obtain a sufficient amount of cells. For each strain and cultivation time, at least three replicates (from each culture age) were grown and processed in independent assays. Cells (∼10 to 60 μg [dry weight]) were carefully removed with a platinum loop from regions of confluent colony growth in the third quadrant of the culture plate and suspended in 100 μl distilled sterile water, placed (20 μl) in a ZnSe window, and stove dried (5 min; 60°C). Infrared spectra were measured with a Fourier transform infrared spectroscope (Perkin-Elmer System 2000 FTIR). Measurements were recorded in the range of 4,000 to 500 cm−1, with an interval of 1 cm−1. The final spectrum of the sample was achieved by averaging 20 scans. Digitized infrared spectra were saved for further processing. Replicates were considered as such when their IR measurements stemmed from a microbial suspension prepared in a completely different assay (including strain resuscitation, culturing, sample preparation, and measurement).
Computer programs.
Two computer programs were written for database handling and spectral analysis. The “Transform.oy” program developed for the InfraRed Data Manager environment (v. 3.50; Perkin-Elmer Ltd.) was used for transformation, including normalization (zero settings for absorption at 1,800 cm−1; one setting at maximal absorption, around 1,650 cm−1), smoothing, and first derivative (Savitzky-Golay algorithm). After transformation, spectra were recorded in ASCII format and processed. The second program (“WinSpectra.exe” [25]) was written in Visual Basic (v. 3.0; Microsoft Corp.) for library management and calculation of reproducibility.
Mathematical analysis of reproducibility and discriminatory power.
To study the influence of culture conditions on the reproducibility of FT-IR spectra, a reduced group of strains (the calibration set [Table 1]) was processed nine times (three replicates per incubation time). The replicates were processed in independent assays. Reproducibility (within-strain variability) was calculated by averaging the three Pearson's product moment correlation coefficients (between replicates 1 and 2, 2 and 3, and 1 and 3), expressed as the differentiation index (D) (31),
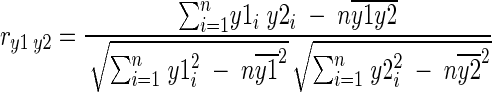 |
Where y1i and y2i are the individual absorbance values of the two spectra to be compared, n is the number of data points in the given range, and  and
and  are the arithmetic mean values of y1 and y2.
are the arithmetic mean values of y1 and y2.
From the correlation coefficient ry1y2, the differentiation index Dy1y2 may be defined according to the following equation: Dy1y2 = (1 − ry1y2) × 1,000. D may have values between 0 and 2,000, being 0 when spectral ranges are identical, 1,000 for completely noncorrelated spectra, and 2,000 for completely negatively noncorrelated spectra.
This statistic was measured for the whole working spectrum (range, 3,000 to 700 cm−1) and independently for the five spectral windows that offer the maximum information and discriminatory power (33): (i) the window between 3,000 and 2,800 cm−1, dominated by the influence of functional groups of fatty acids (w1); (ii) the window between 1,800 and 1,500 cm−1, with the influence of the amide groups belonging to proteins and peptides (w2); (iii) the window between 1,500 and 1,200 cm−1, a mixed region influenced by proteins, fatty acids, and other phosphate-carrying compounds (w3); (iv) the window between 1,200 and 900 cm−1, with predominant carbohydrate presence (w4); and (v) the window between 900 and 700 cm−1, which is named the true fingerprint because of very specific spectral patterns, characteristic at the species level (w5).
To compare the discriminatory powers of the ERIC-PCR and FT-IR typing methods, Simpson's index of diversity, which calculates the probability that any two isolates will be placed in two different typing groups, was computed (17).
Strain resemblance and grouping.
HCA is an unsupervised classification method that depicts similarity relationships between IR spectra without a priori knowledge about the microorganisms studied. For FT-IR data, a cluster analysis was done on 51 spectra (three replicates from 17 48-h-old cultures processed in independent assays). Pearson's product moment correlation coefficient was chosen as a measure of similarity between spectra. Similarity between ERIC-PCR profiles was measured with the Sorensen-Dice coefficient (fragment length error tolerance, 5%). In all cases, final clustering was achieved using Ward's algorithm, and for FT-IR data, using different spectral windows until an optimal discriminatory solution was found.
SDA (18) was applied to select the wavelengths with the highest discrimination power. Canonical analysis was carried out to create a classification function aimed at differentiating isolates at the subspecies level. In supervised methods, such as SDA, a classification of elements is made before analysis. In our study, the 17 strains were arranged into six groups, or classes, following the results of the HCA. This classification procedure maximizes the variance between categories and minimizes the variance within groups previously established. In the development of the discriminant function, variables are added or deleted based on their effect on Wilks' lambda (the ratio between the generalized within-category dispersion and the total dispersion) and on their significance as measured by a suitable F test. The linear functions (canonical variables) of the variables that provide the best discrimination of cases in two or more predefined groups are estimated, and cases are attributed to the group for which the classification function provides the highest value or, equivalently, to the group whose centroid is nearest. Canonical scores and Mahalanobis distances, M, were also calculated to present a graphical two-dimensional element. The Mahalanobis distance is defined as follows: M1,2 = (x1 − x2)S−1(x1 − x2), where S is the pooled estimate of the within-group covariance matrix and x1 and x2 are mean vectors for the two groups. Thus, M12 is the distance between groups in units of within-group standard deviations.
The analyses (including calculation of coefficients, joining of variables, canonical analysis, and graphical display) were carried out with Statistica for Windows v. 4.5 (Statsoft Inc., Tulsa, Okla.).
Examination of validation set.
To validate the classification functions yielded by the SDA, 54 spectra (72- and 96-h-old cultures from strains included in the calibration set [Table 1]) and another 24 spectra (from strains in the validation set) obtained in separate assays were submitted for identification. Strains were identified at the species and subspecies levels by using the linear functions obtained from SDA (canonical variables) of the variables previously reported, where unknowns are attributed to the group for which the classification function provides the highest value.
RESULTS
Study of reproducibility.
Reproducibility was measured for the whole working range (3,000 to 700 cm−1) and separately for the five window ranges described above by using the Pearson coefficient (expressed as the D value). This was performed for every incubation time. Instrumental variability was not calculated in a separate and meticulous way. Partial, inconclusive results suggest that variability had mainly a biological origin and that only a small part was due to instrumental sources. The lowest mean results of D for the nine strains belonging to the calibration set corresponded to windows w1 and w4 from 2-day cultures (w1, 2.20; w4, 3.57), with greater D values for 4-day cultures (w1, 3.22; w4, 8.28). Intermediate D values were obtained for windows w2 (range, 9.72 to 18.67) and w3 (range, 5.75 to 7.09). Window w5 presented the highest D values (range, 35.97 to 46.73). Individual D values for the nine strains (w4 spectral window) oscillated between 1.06 (strain 510; 2-day culture) and 16.33 (strain 459; 4-day culture). The results were taken into account in subsequent analyses. For HCA and SDA, only 48-h-incubation replicates were picked, but to validate the outcome of SDA, the 72- and 96-h-incubation replicates were used. Also, based on the results obtained, spectral range w4 (or part of it) was used for the analyses mentioned.
Cluster analysis and stepwise discriminant analysis.
Figure 1 shows the dendrogram obtained from the HCA carried out including three replicates each from 17 strains (48-h-incubation cultures processed in independent assays) to evaluate the ability of the method to differentiate at the species and subspecies levels. The most discriminating dendrogram from the cluster analysis was obtained with a subrange of window w4. Hierarchical classification of the infrared data yielded six distinct groups, or classes. An additional, internal differentiation among certain DNA groups was observed. C. coli strains belonging to DNA type C grouped into two clusters, one composed of three strains (numbers 360, 444, and 459) and another with five strains (371, 425, 427, 457, and 458). C. jejuni type B strains were located in two separate clusters, one including strains 222 and 503 and another with strains 361, 557, and 527. C. coli type A strains clustered together (isolates 510, 511, and 512). Strain 299 (C. jejuni type D), although close to C. jejuni type B (with 222 and 503), was considered a separate entity.
FIG. 1.
Dendrogram, obtained from FT-IR spectral data of thermophilic Campylobacter strains, with cluster analysis performed with the Pearson product moment correlation coefficient (r) and by the Ward algorithm method. Three replicates each (48-h-old culture), measured independently, belonging to 17 strains were included in the analysis (30 wavelengths from the w4 spectral window).
These results were corroborated by the stepwise discriminant analysis and the canonical analysis (Table 2). Mahalanobis distances proved that the two clusters with C. jejuni type B strains were differentiated from the rest (high scores) and that the two clusters of C. coli type C were close to each other. In general, all the distances are large enough for there to be negligible overlap of any of the clusters of spectra in the canonical-variate space.
TABLE 2.
Mahalanobis distances between groups in discriminant analysis
| Species and type (strain no.) | Distance
|
|||||
|---|---|---|---|---|---|---|
| C. jejuni D (299) | C. coli C (360, 444, 459) | C. jejuni B (361, 557, 527) | C. coli A (510, 511, 512) | C. jejuni B (222, 503) | C. coli C (371, 425, 427, 457, 458) | |
| C. jejuni D (299) | 0.00 | |||||
| C. coli C (360, 444, 459) | 29.02 | 0.00 | ||||
| C. jejuni B (361, 557, 527) | 24.15 | 148.20 | 0.00 | |||
| C. coli A (510, 511, 512) | 33.38 | 48.77 | 104.46 | 0.00 | ||
| C. jejuni B (222, 503) | 113.75 | 133.32 | 60.09 | 39.46 | 0.00 | |
| C. coli C (371, 425, 427, 457, 458) | 16.18 | 6.69 | 146.53 | 33.03 | 22.72 | 0.00 |
Figure 2 shows the dendrogram obtained from the ERIC-PCR banding patterns. C. coli strains were positioned in two separated clusters (types A and C), while C. jejuni grouped in three clusters, two of them very close (types B and D).
FIG. 2.
Dendrogram, obtained from ERIC-PCR profiles of 17 Campylobacter strains, with cluster analysis performed with the Sorensen-Dice coefficient and by the Ward algorithm method.
Examination of validation set.
Differentiation between FT-IR types is achieved on the basis of a few wavelengths, which are elucidated by means of discriminant analysis. Twelve variables (wavelengths) in the range of 1,200 to 900 cm−1 (window w4) were selected. As a result of the identification process, 77 out of 78 (98.72%) spectra belonging to the validation set were assigned to the correct library group by means of the classification functions elucidated by the SDA (function coefficients not shown). One replicate from strain 299 was not correctly allocated. Figure 3 gives the projection of the first and second canonical variates of several spectra from the five-dimensional canonical-variate space. The misidentified strain (a replicate of C. jejuni type B 299) was positioned far from its respective centroid.
FIG. 3.
Projection onto a plane of the scores of the first and second canonical dimensions from the canonical discriminate analysis carried out on spectra (first derivative in the 1200 to 900 cm−1 range) of Campylobacter cultures.
Discriminatory power.
The discriminatory power of a typing method is the ability to distinguish between unrelated strains, and it depends on the number of types defined and on the relative frequencies of these types. Simpson's index of diversity, which includes these two discrimination concepts, was found to be 0.853 and 0.698 for FT-IR and ERIC-PCR, respectively.
DISCUSSION
Study of reproducibility.
Factors influencing spectral reproducibility are medium preparation and batch, growth temperature, incubation time, sample preparation, and spectrum measurement. Variability due to the last factor can be minimized by an appropriate spectral normalization. According to Naumann (31), mean D values between 7 and 10 are considered normal when analyzing the first derivative of samples prepared from cultures grown in independent assays. Kummerle and colleagues (20) have attained values as low as 0.1 in yeasts, but this is probably because of the type of microorganism studied. D values can be as high as 300 when microorganisms from different genera are compared. Despite poorer reproducibility values obtained for some strains, FT-IR types could still be differentiated and the validation set correctly identified. Our results indicate that reproducibility should be studied separately for each window range and for each strain because differences between them may be significant. Strains reporting low D values for a given range presented compact clusters (i.e., strain 510). Although no spectrum selection based on reproducibility parameters was done in our study, our findings indicate that implementing it as a step in the spectrum acquisition and analysis would improve the final results.
For thermophilic campylobacters, the shape of the cell (coccoid-spiral forms), as well as its chemical composition, changes as the culture becomes old (12, 15). Moran and Upton (28) described coccoid forms as a degenerative state caused by stress factors such as decreased nutrient availability. For the window range employed in the classification (w4, which reflects carbohydrate composition), reproducibility decreased as incubation time progressed, except for strain 222. Carbohydrate composition, which is influenced by the structure of the cell membrane and the metabolic state of the cells at the moment of measuring, is apparently more stable in young cultures, where only spiral forms are detected. Conversely, for w2, reproducibility improved, perhaps because some structural components, such as proteins (detected by w2), reach a balanced composition level in older cultures. No uniform trend was observed in windows w1, w3 (reproducibility remained almost unchanged), and w5 (considered “the true fingerprinting region”), but the last window consistently yielded the highest D values, and possibly for this reason, dendrograms based on this window were not congruent.
Several authors have reported that discrimination is not greatly affected by changes in growth conditions (22, 32, 48). Wenning and colleagues (51) found that the age of yeast colonies had to be considered to achieve a high degree of reproducibility. For Campylobacter isolates, reproducibility is strongly influenced by culture age and depends on the window range studied.
Cluster analysis and stepwise discriminant analysis.
Despite using different mathematical analyses (including two different similarity coefficients for HCA) and data types, cluster arrangements (Fig. 1, 2, and 3) showed large similarities. Arrangements were somehow different depending on the type of data employed in the analysis. Not surprisingly, the species C. coli and C. jejuni appeared combined in the FT-IR dendrogram and in the scatter plot (Fig. 1 and 3). This is not infrequently observed in thermophilic Campylobacter, where the borders between the two species are not apparent, and it has been noted by several authors (29, 38, 50), whose results presented the two species combined in dendrograms stemming from ERIC-PCR and restriction fragment length polymorphism DNA profiles. Groupings obtained from ERIC-PCR resemble in some ways the clusters formed from FT-IR data, although different arrangements are constructed (C. jejuni types B and D join first) and only one type of C. coli type C is detectable, while FT-IR data distinguishes two variants. To explain these results, the fact that the natures of the data are completely different (a set of wavelengths versus banding patterns from DNA fragments) should be taken into account, and as new strains are included in the classification process, a better delineation between the species and subspecies is achieved.
It has been shown that classifications obtained with FT-IR spectroscopy at the genus level cannot be expected to be taxonomically relevant in all cases (20, 31, 35). By the same token, taxonomic relationships between microbial genera or evolutionary relationships are not always in accordance with schemes based on DNA profiles.
Examination of validation set.
The identification process correctly assigned 98.72% of spectra from the external validation set to already established FT-IR classes. This is an excellent indication that classes included in the reference library are well separated (Table 2) and that replicates from the validation set closely resemble those from the calibration set, even though reproducibility is not optimal. Development of the reference database is a problem inherent in any classification and identification method where correct assignment of unknowns to the right class is as important as detection of unrelated elements.
Discriminatory power.
Results from Simpson's index of diversity confirm that FT-IR has good discriminatory power, at least comparable to that of a genetic typing method like ERIC-PCR. In our study, the lower score attained by ERIC-PCR was due to the existence of one large group (C. coli type C, comprising eight isolates). The fact that the isolates studied are not representative of the existing diversity in C. jejuni and C. coli should be taken into account.
FT-IR spectroscopy is a physicochemical methodology that fingerprints the whole cell and is able to detect subtle compositional changes that cannot be revealed by the ERIC-PCR method. A comparison of ERIC-PCR and FT-IR procedures shows that the first methodology depends less on preparation conditions, although its large number of protocol steps influences reproducibility and rapidity. Furthermore, intensities of band patterns are sometimes variable, and the interpretation and analysis of results become difficult. On the other hand, FT-IR is easier to carry out and allows the analysis of a large number of strains, although it requires a high degree of standardization and complex data processing. Differences between ERIC-PCR types are due to differences in generated DNA fragments, and sometimes this difference is due to methodological variability or strain modification. Since Campylobacter is a bacterium that can show significant genetic instability over a period of time or after passage through the intestine (11, 48), results based on genotype profiling could be influenced by this fact. ERIC-PCR, which generates a few fragments from a large DNA section, could undergo changes in profiles due to rearrangements of genomic segments. The properties of FT-IR recommend its use when high numbers of strains are studied (i.e., characterization of environmental samples) (46) or when longitudinal studies are carried out, since Campylobacter could show variability in its genomic profile in the long run (11, 40, 48). Typing methods for Campylobacter with extremely high discriminatory power, such as pulsed-field gel electrophoresis (42), may also be unsatisfactory for such studies due to the large numbers of types detected, while FT-IR yields reasonably good figures for both discriminatory power and reproducibility.
These results demonstrate the potential usefulness of FT-IR spectroscopy, either alone or in combination, in the typing and identification of strains belonging to the species C. coli and C. jejuni. They also show that high reproducibility obtained by means of a careful standardization of procedure (standardization of cell culture conditions, spectroscopic measurements, and mathematical analysis) is necessary to obtain sound classifications and correct identifications. The IR technique is rapid and easy to perform and has proved to be reproducible, possessing a discriminatory power similar to that of genetic methods, such as ERIC-PCR. It is also important to consider its limitations: it does not reflect changes in key molecules, such as 16S rRNA, and there is not an understandable connection between the differences in the spectra and the changes in biochemical composition.
For Campylobacter, no single typing method embraces all advantages sought (maximum discriminatory power and reproducibility, typeability, equipment requirements, and affordability), and a combination of methods is the best solution (4, 42). More research is needed to improve reproducibility, to link patterns with biochemical compounds or structures, and to establish how many FT-IR types of a concrete species exist.
Acknowledgments
We thank Jesús Cachón and Isabel García Natal from the Hospital de León for their skillful technical assistance.
Part of the work was funded by the Junta de Castilla y León (Project LE09-98) and the Spanish Ministerio de Ciencia y Tecnología (INIA; Project CAL-00-30).
REFERENCES
- 1.Beattie, S. H., C. Holt, D. Hirst, and A. G. Williams. 1998. Discrimination among Bacillus cereus, B. mycoides and B. thuringiensis and some other species of the genus Bacillus by Fourier transform infrared spectroscopy. FEMS Microbiol. Lett. 164:201-206. [DOI] [PubMed] [Google Scholar]
- 2.Buzby, J. C., T. Roberts, C. T. J. Lin, and J. M. MacDonald. 1997. Bacterial foodborne disease: medical costs and productivity losses. Agricultural Economic Report no. 741. U.S. Department of Agriculture, Washington, D.C.
- 3.Curk, M. C., F. Peladan, and J. C. Hubert. 1994. Fourier transform infrared (FTIR) spectroscopy for identifying Lactobacillus species. FEMS Microbiol. Lett. 123:241-248. [Google Scholar]
- 4.de Boer, P., B. Duim, A. Rigter, J. van der Plas, W. F. Jacobs-Reitsma, and J. A. Wagenaar 2000. Computer-assisted analysis and epidemiological value of genotyping methods for Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 38:1940-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frost, J. A., A. N. Oza, R. T. Thwaites, and B. Rowe. 1998. Serotyping scheme for Campylobacter jejuni and Campylobacter coli based on direct agglutination of heat-stable antigens. J. Clin. Microbiol. 36:335-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giesendorf, B. A., H. Goossens, H. G. Niesters, A. van Belkum, A. Koeken, H. P. Endtz, H. Stegeman, and W. G. Quint. 1994. Polymerase chain reaction-mediated DNA fingerprinting for epidemiological studies on Campylobacter spp. J. Med. Microbiol. 40:141-147. [DOI] [PubMed] [Google Scholar]
- 7.Goodacre, R., E. M. Timmins, P. J. Rooney, J. J. Rowland, and D. B. Kell. 1996. Rapid identification of Streptococcus and Enterococcus species using diffuse reflectance-absorbance Fourier transform infrared spectroscopy and artificial neural networks. FEMS Microbiol. Lett. 140:233-239. [DOI] [PubMed] [Google Scholar]
- 8.Goulden, J. D. S., and M. E. Sharpe. 1958. The infra-red absorption spectra of lactobacilli. J. Gen. Microbiol. 19:76-86. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths, P. L., G. S. Moreno, and R. A. Park. 1992. Differentiation between thermophilic Campylobacter species by species-specific antibodies. J. Appl. Bacteriol. 72:467-474. [DOI] [PubMed] [Google Scholar]
- 10.Guibet, F., C. Amiel, P. Cadot, C. Cordevant, M. H. Desmonts, M. Lange, A. Marecat, J. Travert, C. Denis, and L. Mariey. 2003. Discrimination and classification of enterococci by Fourier transform infrared (FT-IR) spectroscopy. Vib. Spectrosc. 33:133-142. [Google Scholar]
- 11.Hanninen, M. L., M. Hakkinen, and H. Rautelin. 1999. Stability of related human and chicken Campylobacter jejuni genotypes after passage through chick intestine studied by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 65:2272-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazeleger, W. C., J. D. Janse, P. J. Koenraad, R. R. Beumer, F. M. Rombouts, and T. Abee. 1995. Temperature-dependent membrane fatty acid and cell physiology changes in coccoid forms of Campylobacter jejuni. Appl. Environ. Microbiol. 61:2713-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helm, D., H. Labischinski, and D. Naumann. 1991. Elaboration of a procedure for identification of bacteria using Fourier-transform IR spectral libraries: a stepwise correlation approach. J. Microbiol. Methods 14:127-142. [Google Scholar]
- 14.Helm, D., H. Labischinski, G. Schallehn, and D. Naumann. 1991. Classification and identification of bacteria by Fourier-transform infrared spectroscopy. J. Gen. Microbiol. 137:69-79. [DOI] [PubMed] [Google Scholar]
- 15.Hoeller, C., D. Witthuhn, and B. B. Janzen. 1998. Effect of low temperatures on growth, structure, and metabolism of Campylobacter coli SP10. Appl. Environ. Microbiol. 64:581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulton, C. S. J., C. F. Higins, and P. M. Sharp. 1991. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol. Microbiol. 5:825-834. [DOI] [PubMed] [Google Scholar]
- 17.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenrich, R. I. 1960. Stepwise discriminant analysis, p. 76-95. In K. Enslein, A. Ralston, and H. S. Wilf (ed.), Statistical methods for digital computers. John Wiley & Sons, New York, N.Y.
- 19.Kirschner, C., K. Maquelin, P. Pina, N. A. Ngo Thi, L. P. Choo-Smith, G. D. Sockalingum, C. Sandt, D. Ami, F. Orsini, S. M. Doglia, P. Allouch, M. Mainfait, G. J. Puppels, and D. Naumann. 2001. Classification and identification of enterococci: a comparative phenotypic, genotypic, and vibrational spectroscopic study. J. Clin. Microbiol. 39:1763-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kummerle, M., S. Scherer, and H. Seiler. 1998. Rapid and reliable identification of food-borne yeasts by Fourier-transform infrared spectroscopy. Appl. Environ. Microbiol. 64:2207-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai, S., R. Goodacre, and L. N. Manchester. 2004. Whole-organism fingerprinting of the genus Carnobacterium using Fourier transform infrared spectroscopy (FT-IR). Syst. Appl. Microbiol. 27:186-191. [DOI] [PubMed] [Google Scholar]
- 22.Lefier, D., D. Hirst, C. Holt, and A. G. Williams. 1997. Effect of sampling procedure and strain variation in Listeria monocytogenes on the discrimination of species in the genus Listeria by Fourier transform infrared spectroscopy and canonical variates analysis. FEMS Microbiol. Lett. 147:45-50. [DOI] [PubMed] [Google Scholar]
- 23.Maquelin, K., C. Kirschner, L.-P. Choo-Smith, N. van den Braak, H. P. Endtz, D. Naumann, and G. J. Puppels. 2002. Identification of medically relevant microorganisms by vibrational spectroscopy. J. Microbiol. Methods 51:255-271. [DOI] [PubMed] [Google Scholar]
- 24.Maquelin, K., C. Kirschner, L. P. Choo-Smith, N. A. Ngo-Thi, T. van Vreeswijk, M. Stammler, H. P. Endtz, H. A. Bruining, D. Naumann, and G. J. Puppels. 2003. Prospective study of the performance of vibrational spectroscopies for rapid identification of bacterial and fungal pathogens recovered from blood cultures. J. Clin. Microbiol. 41:324-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maradona, M. P. 1996. Software for microbial fingerprinting by means of the infrared spectra. Comput. Appl. Biosci. 12:353-356. [DOI] [PubMed] [Google Scholar]
- 26.Mariey, L., J. P. Signolle, C. Amiel, and J. Travert. 2001. Discrimination, classification, identification of microorganisms using FTIR spectroscopy and chemometrics. Vib. Spectrosc. 26:151-159. [Google Scholar]
- 27.Miguel Gomez, M. A., M. A. Bratos Perez, F. J. Martin Gil, A. Duenas Diez, J. F. Martin Rodriguez, P. Gutierrez Rodriguez, A. Orduna Domingo, and A. Rodriguez Torres. 2003. Identification of species of Brucella using Fourier transform infrared spectroscopy. J. Microbiol. Methods 55:121-131. [DOI] [PubMed] [Google Scholar]
- 28.Moran, A. P., and M. E. Upton. 1986. A comparative study of the rod and coccoid forms of Campylobacter jejuni ATCC 29428. J. Appl. Bacteriol. 60:103-110. [DOI] [PubMed] [Google Scholar]
- 29.Moreno, Y., M. A. Ferrus, A. Vanoostende, M. Hernandez, R. M. Montes, and J. Hernandez. 2002. Comparison of 23S polymerase chain reaction-restriction fragment length polymorphism and amplified fragment length polymorphism techniques as typing systems for thermophilic campylobacters. FEMS-Microbiol. Lett. 211:97-103. [DOI] [PubMed] [Google Scholar]
- 30.Mouwen, D. J. M., and M. Prieto. 1999. Fourier-transform infrared (FT-IR) spectroscopy for identifying and typing Campylobacter jejuni and C. coli strains. Abstr. Proc. 17th Int. Conf. Food Microbiol. Hyg., p. 928-930. Zeist, Veldhoven, The Netherlands.
- 31.Naumann, D. 2000. Infrared spectroscopy in microbiology, p. 1-29. In R. A. Meyers (ed.), Encyclopedia of analytical chemistry. John Wiley & Sons Ltd., Chichester, United Kingdom.
- 32.Naumann, D., V. Fijala, H. Labischinski, and P. Giesbrecht. 1988. The rapid differentiation and identification of pathogenic bacteria using Fourier transform infrared spectroscopic and multivariate statistical analysis. J. Mol. Struct. 174:165-170. [Google Scholar]
- 33.Naumann, D., D. Helm, and H. Labischinski. 1991. Microbiological characterizations by FT-IR spectroscopy. Nature 351:81-82. [DOI] [PubMed] [Google Scholar]
- 34.Oberreuter, H., H. Seiler, and S. Scherer. 2002. Identification of coryneform bacteria and related taxa by Fourier-transform infrared (FT-IR) spectroscopy. Int. J. Syst. Evol. Microbiol. 52:91-100. [DOI] [PubMed] [Google Scholar]
- 35.Oberreuter, H., J. Charzinski, and S. Scherer. 2002. Intraspecific diversity of Brevibacterium linens, Corynebacterium glutamicum and Rhodococcus erythropolis based on partial 16S rDNA sequence analysis and Fourier-transform infrared (FT-IR) spectroscopy. Microbiology 148:1523-1532. [DOI] [PubMed] [Google Scholar]
- 36.Oust, A., T. Moretro, C. Kirschner, J. A. Narvhus, and A. Kohler. 2004. Evaluation of the robustness of FT-IR spectra of lactobacilli towards changes in the bacterial growth conditions. FEMS Microbiol. Lett. 239:111-116. [DOI] [PubMed] [Google Scholar]
- 37.Oust, A., T. Moretro, C. Kirschner, J. A. Narvhus, and A. Kohler. 2004. FT-IR spectroscopy for identification of closely related lactobacilli. J. Microbiol. Methods 59:149-162. [DOI] [PubMed] [Google Scholar]
- 38.Owen, R. J., A. Fayos, J. Hernandez, and A. Lastovica. 1993. PCR-based restriction fragment length polymorphism analysis of DNA sequence diversity of flagellin genes of Campylobacter jejuni and allied species. Mol. Cell. Probes 7:471-480. [DOI] [PubMed] [Google Scholar]
- 39.Riddle, J. W., P. W. Kabler, B. A. Kenner, R. H. Bordner, S. M. Rockwood, and H. J. R. Stevenson. 1956. Bacterial identification by infrared spectrophotometry. J. Bacteriol. 72:593-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sails, A. D., B. Swaminathan, and P. I. Fields. 2003. Utility of multilocus sequence typing as an epidemiological tool for investigation of outbreaks of gastroenteritis caused by Campylobacter jejuni. J. Clin. Microbiol. 41:4733-4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandt, C., G. D. Sockalingum, D. Aubert, H. Lepan, C. Lepouse, M. Jaussaud, A. Leon, J. M. Pinon, M. Manfait, and D. Toubas. 2003. Use of Fourier-transform infrared spectroscopy for typing of Candida albicans strains isolated in intensive care units. J. Clin. Microbiol. 41:954-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinbrueckner, B., F. Ruberg, and M. Kist. 2001. Bacterial genetic fingerprint: a reliable factor in the study of the epidemiology of human Campylobacter enteritis? J. Clin. Microbiol. 39:4155-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas, L. C., and J. E. S. Greenstreet. 1954. The identification of micro-organisms by infrared spectrophotometry. Spectrochim. Acta 6:302-319. [Google Scholar]
- 44.Timmins, E. M., S. A. Howell, B. K. Alsberg, W. C. Noble, and R. Goodacre. 1998. Rapid differentiation of closely related Candida species and strains by pyrolysis-mass spectrometry and Fourier transform-infrared spectroscopy. J. Clin. Microbiol. 36:367-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmins, E. M., D. E. Quain, and R. Goodacre. 1998. Differentiation of brewing yeast strains by pyrolysis mass spectrometry and Fourier transform infrared spectroscopy. Yeast 14:885-893. [DOI] [PubMed] [Google Scholar]
- 46.Tindall, B. J., E. Brambilla, M. Steffen, R. Neumann, R. Pukall, R. M. Kroppenstedt, and E. Stackebrandt. 2000. Cultivatable microbial biodiversity: gnawing at the Gordian knot. Environ. Microbiol. 2:310-318. [DOI] [PubMed] [Google Scholar]
- 47.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wassenaar, T. M., and D. G. Newell. 2000. Genotyping of Campylobacter spp. Appl. Environ. Microbiol. 66:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weijtens, M. J., R. D. Reinders, H. A. Urlings, and J. van der Plas. 1999. Campylobacter infections in fattening pigs; excretion pattern and genetic diversity. J. Appl. Microbiol. 86:63-70. [DOI] [PubMed] [Google Scholar]
- 50.Weijtens, M. J., J. van der Plas, P. G. Bijker, H. A. Urlings, D. Koster, J. G. van Logtestijn, and J. H. Huis in't Veld. 1997. The transmission of Campylobacter in piggeries; an epidemiological study. J. Appl. Microbiol. 83:693-698. [DOI] [PubMed] [Google Scholar]
- 51.Wenning, M., H. Seiler, and S. Scherer. 2002. Fourier-transform infrared microspectroscopy, a novel and rapid tool for identification of yeasts. Appl. Environ. Microbiol. 68:4717-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winder, C. L., E. Carr, R. Goodacre, and R. Seviour. 2004. The rapid identification of Acinetobacter species using Fourier transform infrared spectroscopy. J. Appl. Microbiol. 96:328-339. [DOI] [PubMed] [Google Scholar]
- 53.Woodward, D. L., and F. G. Rodgers. 2002. Identification of Campylobacter heat-stable and heat-labile antigens by combining the Penner and Lior serotyping schemes. J. Clin. Microbiol. 40:741-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao, H., Y. Kassama, M. Young, D. B. Kell, and R. Goodacre. 2004. Differentiation of Micromonospora isolates from a coastal sediment in Wales on the basis of Fourier transform infrared spectroscopy, 16S rRNA sequence analysis, and the amplified fragment length polymorphism technique. Appl. Environ. Microbiol. 70:6619-6627. [DOI] [PMC free article] [PubMed] [Google Scholar]





