Abstract
The NodD1 regulon of Sinorhizobium meliloti was determined through the analysis of the S. meliloti transcriptome in response to the plant flavone luteolin and the overexpression of nodD1. Nine new genes regulated by both NodD1 and luteolin were identified, demonstrating that NodD1 controls few functions behind nodulation in S. meliloti.
NodD regulators play an important role in the establishment of the rhizobium-legume symbiosis and the control of its specificity. NodD proteins belong to the LysR family of transcriptional regulators. Like most members of this family, they are activated by small molecules, specific flavonoids released in the rhizosphere by host plants. Flavonoids do not affect the binding affinity of NodDs for target promoters and instead enhance promoter activity by modifying DNA bending on conserved nod-boxes (8). Known targets of NodDs are the nodulation genes encoding proteins responsible for the synthesis and export of lipo-chito-oligosaccharides, the Nod factors, which trigger nodule organogenesis, root infection, and determine the host range.
In S. meliloti, the nodulation genes, nod, nol, and noe, are under the positive control of three NodD paralogues, NodD1 and NodD2, which are constitutively expressed and activated by specific flavonoids, and NodD3 whose expression is subject to a complex regulation involving the regulatory protein SyrM, the flavonoid-activated regulator NodD1, and the nitrogen status of the cell (7, 17). Although inactivation of the three nodD genes is required to abolish nodulation, the symbiotic activities of the three NodD regulators are host dependent. On Medicago sativa, the contribution of NodD1 to nodulation is more important than that of NodD2 and NodD3 (10).
In addition to Nod factor biosynthesis and transport, NodD proteins have been shown to control other functions in rhizobia. In Rhizobium sp. strain NGR234, Sinorhizobium fredii, and Bradyrhizobium japonicum, NodD1 activates a regulator of a type III secretion system (TTSS) that contributes to host range determination (14, 15, 25). NodD1 also controls exopolysaccharide biosynthesis genes in S. fredii (16), lipopolysaccharide modification through rhamnose biosynthesis genes, and indole-3-acetic acid synthesis genes in NGR234 (13, 22). In the latter broad-host-range rhizobium, 18 functional nod-boxes have been identified. Genes downstream of these nod-boxes are activated in a NodD1- and flavonoid-dependent manner and show a temporal hierarchy in the pattern of induction (13). The nodulation genes are rapidly induced by the flavonoid daidzein (1 h), whereas induction of TTSS and rhamnose biosynthesis genes is 6 to 24 h delayed.
In order to determine the extent of the NodD1 regulon in Sinorhizobium meliloti, we analyzed the transcriptome of bacteria in response (i) to the flavone luteolin, an activator of NodD1, and (ii) to the overexpression of NodD1 (Tables 1 and 2). Transcriptome experiments were complemented with quantitative reverse transcription-PCR (qRT-PCR) experiments and a bioinformatic search of nod-boxes in the genome. The combination of these approaches proved to be highly complementary for deciphering the NodD1 regulon, since none of them alone could identify the whole regulon. Very recently, the luteolin response and the NodD1 and NodD3 regulons were also determined in S. meliloti by using Affimetrix GeneChip (2), and the results are compared below.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| 1021 | Strr derivative of SU47 | 18 |
| A2012 | 1021 nodD1 nodD2 nodD3 | 11 |
| Plasmids | ||
| pRmM57a | 20-kb pSymA nod region, nodC-lacZ fusion | 19 |
| pMH901 | 2.7-kb insert from pSymA carrying nodD1 | 11 |
The 20-kb pSymA region includes SMa0861, nodABCIJ, nodD1, orf110 nifN, nodM nolFG nodN, SMa0882, SMa0883, SMa0886, and part of SMa0887.
TABLE 2.
General design of microarray experimentsa
| Exptb | Condition 1
|
Condition 2
|
Time (h)d | Expression profile | ||||
|---|---|---|---|---|---|---|---|---|
| Strain 1 | nodD1 statusc | Luteolin addition | Strain 2 | nodD1 status | Luteolin addition | |||
| A | 1021 | + | + | 1021 | + | − | 4 | Response to luteolin |
| B | 1021(pRmM57) | ++ | + | 1021(pRmM57) | ++ | − | 4 | Response to luteolin |
| C | A2012(pMH901) | ++ | + | A2012 | − | + | 4 | Response to NodD1 |
| D | 1021(pRmM57) | ++ | + | 1021(pRmM57) | ++ | − | 24 | Response to luteolin |
| E | A2012(pMH901) | ++ | + | A2012 | − | + | 24 | Response to NodD1 |
Strains and plasmids are described in Table 1. Comparisons of transcriptomes in conditions 1 and 2 are presented.
Each type of experiment was repeated at least four times.
The nodD1 expression level increased ∼3-fold between strains 1021 and 1021(pRmM57) in experiments A and B, ∼5-fold between strains 1021 and 1021(pRmM57) in experiments A and D, >32-fold between strains A2012 and A2012(pMH901) in experiment C, and >22-fold between strains A2012 and A2012(pMH901) in experiment E.
That is, the time after luteolin addition.
In the present study, bacteria were cultivated in the presence or absence of luteolin in Vincent minimal medium (3) containing 6 mM glutamate as a nitrogen source and 7.4 mM succinate as the main carbon source. Luteolin (10 μM) was added to cultures grown to an optical density at 600 nm of 0.1 and incubated at 28°C for 4 or 24 h postinduction (hpi). Gene expression changes were measured by using whole-genome 70-mer oligonucleotide microarrays (21). Fluorescently labeled cDNA were prepared from 20 μg of total RNA as described previously (6). At least four independent hybridizations consisting of two biological and two technical replicates were performed for each comparison. Genes were considered as differentially expressed when the M value (log2 signal ratio) was greater than or equal to 1 or less than or equal to −1 and the P value (Student t test) was ≤0.05. Complete microarray results are available at http://bioinfo.genopole-toulouse.prd.fr/annotation/iANT/bacteria/rhime//DOC/Capela2004/index.html. A number of genes identified in the present study were tested for both luteolin inducibility and NodD1 dependency by qRT-PCR. Only genes that were induced by both luteolin and NodD1 were considered bona fide putative members of the NodD1 regulon.
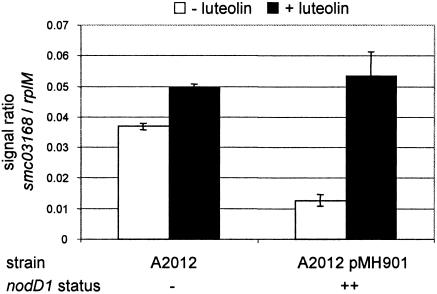
In the 1021 wild-type strain (Table 3, experiment A), low to medium levels of induction by luteolin were observed for many known nodulation genes. The most highly induced genes by luteolin in this experiment (16-fold) were SMc03167 and SMc03168, two adjacent genes on the chromosome encoding a multidrug efflux system. These two genes were also found to be induced by luteolin by Barnett et al. (2). Expression analyses of SMc03168 by qRT-PCR in a triple nodD mutant strain (strain A2012) overexpressing or not nodD1 on a plasmid (pMH901) indicated that this gene was actually repressed by NodD1 in the absence of luteolin and derepressed upon luteolin addition (Fig. 1). This result is fully consistent with the microarray data of experiment C (Table 3). A similar mechanism of repression that was not known for NodD1 was reported for another LysR-type regulator, CcpC of Bacillus subtilis, that derepresses citB expression in the presence of citrate (12). Whether the divergently transcribed transcription regulator, SMc03169, which was induced threefold by luteolin, takes part in this regulation remains to be determined.
TABLE 3.
Luteolin-inducible and NodD1-dependent genes 4 h after addition of luteolin
| Genea | Microarrayb
|
qRT-PCRc
|
Description | ||||
|---|---|---|---|---|---|---|---|
| MA | MB | MC | MA | MB | MC | ||
| SMa0772NB | 1.57 | 2.34 | 6.41 | NodL Nod factor acetyltransferase | |||
| SMa0773 | 1.06 | 1.97 | 5.58 | NoeA host-specific nodulation protein | |||
| SMa0774 | 0.50 | 2.37 | 4.78 | NoeB host-specific nodulation protein | |||
| SMa0848 | 0.05 | 1.20 | 2.61 | −0.85 | 2.24 | 2.63 | Hypothetical protein |
| SMa0849NB | 0.53 | 0.71 | 2.25 | 2.74 | 2.64 | SyrM transcriptional regulator | |
| SMa0850 | 1.98 | 2.39 | 6.58 | 1.44 | 5.57 | 6.83 | Hypothetical protein |
| SMa0851NB | 2.22 | 3.55 | 6.97 | NodH sulfotransferase | |||
| SMa0852NB | 2.27 | 2.63 | 7.00 | NodF acyl carrier protein | |||
| SMa0853 | 1.70 | 4.21 | 7.23 | NodE beta ketoacyl ACP synthase | |||
| SMa0854 | 1.46 | 3.66 | 7.02 | NodG 3 oxoacyl reductase | |||
| SMa0855 | 0.10 | 2.16 | 4.24 | NodP1 ATP sulfurylase small subunit | |||
| SMa0857 | 0.33 | 1.48 | 4.40 | NodQ1 ATP sulfurylase large subunit | |||
| SMa0866 | 2.15 | 0.84 | 6.64 | 2.65 | 6.17 | NodC N-acetyl-glucosaminyl-transferase | |
| SMa0868 | 1.83 | 5.14 | 6.85 | NodB chitooligosaccharide deacetylase | |||
| SMa0869NB | 2.37 | 4.44 | 7.12 | 2.62 | 6.11 | 7.01 | NodA N-acyltransferase |
| SMa0875 | 0.89 | 2.15 | 4.60 | NolG efflux transporter | |||
| SMa0876 | 0.05 | 2.82 | 4.18 | NolF secretion protein | |||
| SMa0878NB | 0.31 | 1.92 | 1.27 | NodM Glutamine aminotransferase | |||
| SMa21223* | 0.16 | 1.40 | 4.46 | NodP2 ATP sulfurylase small subunit | |||
| SMa21224* | 0.12 | 1.55 | 4.35 | NodQ2 ATP sulfurylase large subunit | |||
| SMa21566 | −0.59 | 0.93 | 1.84 | −0.08 | 2.07 | 3.52 | GroEL5 chaperonin |
| SMbR2 | −0.32 | 1.32 | 1.44 | −0.04 | 1.82 | 2.50 | Putative GroES chaperonin |
| SMc03151 | 0.02 | 0.64 | 1.27 | −0.01 | 1.23 | 1.48 | Hypothetical protein |
| SMc03167 | 4.16 | 0.52 | −0.06 | Putative multidrug efflux system | |||
| SMc03168 | 4.08 | 1.01 | −0.16 | 3.22 | 2.00 | 0.11 | Putative multidrug efflux system |
| SMc03169 | 1.71 | 0.61 | 0.00 | Putative transcription regulator | |||
Superscript NB indicates genes containing a nod-box in their promoter region. Detection of genes indicated with an asterisk may be due to a cross hybridization with nodP1Q1.
Changes in gene expression are expressed as log2 signal ratios (M values) obtained in experiments A, B, and C as described in Table 2. All differentially expressed genes (M > 1) had P values of <0.05 except for the MB value for SMbR2. New genes regulated by NodD1 and luteolin are in boldface. Empty cells indicate nondetermined values.
Changes in gene expression measured by qRT-PCR are expressed as log2 signal ratios normalized to the constitutively expressed gene rplm.
FIG. 1.
SMc03168 derepression by NodD1 in the presence of luteolin. Expression levels of SMc03168 were measured in strains expressing nodD1 [A2012(pMH901)] or not (A2012) in the presence or absence of luteolin by qRT-PCR and were normalized to the constitutively expressed gene rplM.
Upon nodD1 overexpression (Table 3, experiments B and C), all nodulation genes except nodI, nodJ, and nodN were highly induced within 4 h after luteolin addition. Besides nodulation genes, six additional NodD1- and luteolin-regulated genes were identified at 4 hpi. Among them, two hypothetical genes, SMa0848 and SMa0850, are located in the pSymA nod region, upstream from syrM and downstream from nodH, respectively. These two genes might be cotranscribed with syrM and nodH and controlled by the nod-boxes present in the syrM and nodH promoters (1). Unexpectedly, we found that the regulatory gene syrM (SMa0849), which is known to be activated by NodD3, could be slightly induced by NodD1 (about twofold induction). This activation was luteolin dependent, as shown by qRT-PCR data (Table 3). Two other NodD1- and luteolin-dependent genes were two adjacent genes on pSymB, groEL5 and SMbR2, homologous to genes encoding the GroEL and GroES chaperonins, respectively. GroEL chaperonins were shown previously to be induced by luteolin (4) and to modulate in vitro activity of NodD in S. meliloti (26). GroEL proteins may be involved in the folding, assembly, or activity of NodD proteins. The last NodD1-regulated gene was a chromosomal gene of unknown function SMc03151. This gene was induced by luteolin, as shown by qRT-PCR data (Table 3). Among these new luteolin- and NodD1-responsive genes, only SMa0850 and groEL5 were found to be regulated by NodD1 by Barnett et al.; no other new NodD1 target was identified in that study. Interestingly, SMc03151 was found to be regulated by NodD3 (2).
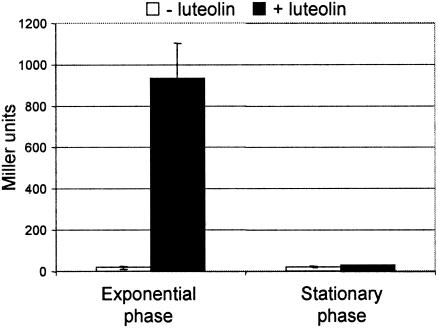
To our surprise, at 24 hpi the NodD1 regulon of S. meliloti was restricted to a few nod genes in contrast to what happens in NGR234. Significant activation of only five nod genes—nodAB, nodH, nodF, and nodL—was detectable (Table 4). In searching for the reason for this, we found that a nodC-lacZ fusion was not induced by the addition of 10 μM luteolin to bacteria in late stationary phase, i.e., bacteria cultivated 24 h after inoculation at an optical density at 600 nm of 0.05 (Fig. 2), indicating that nodulation genes in S. meliloti are not inducible or are weakly inducible during stationary phase in our experimental conditions.
TABLE 4.
Luteolin-inducible and NodD1-dependent genes 24 h after addition of luteolin
| Gene | Microarraya
|
Description | |
|---|---|---|---|
| MD | ME | ||
| SMa0851 | 2.45 | 5.12 | NodH sulfotransferase |
| SMa0772 | 1.30 | 3.84 | NodL Nod factor acetyltransferase |
| SMa0868 | 1.19 | 3.76 | NodB chitooligosaccharide deacetylase |
| SMa0869 | 1.15 | 4.11 | NodA N-acyltransferase |
| SMa0852 | 1.14 | 3.46 | NodF acyl carrier protein |
Changes in gene expression are expressed as log2 signal ratios (M values) obtained in experiments D and E as described in Table 2. All differentially expressed genes had P values of <0.05.
FIG. 2.
Inducibility of a nodC-lacZ fusion by luteolin in strain 1021(pRmM57) in the exponential phase and stationary phase of growth. β-galactosidase activity was measured 4 h after luteolin addition (10 μM).
Nod-boxes were searched within regions from −600 to +50 bp around the start codons of the S. meliloti genes by using the motifSampler/MotifScanner programs (23, 24) trained with known nod-boxes from Rhizobium sp. strain NGR234, Rhizobium etli, and Mesorhizobium loti. The detailed procedure and the sequences used for this research are available on our website (http://bioinfo.genopole-toulouse.prd.fr/annotation/iANT/bacteria/rhime//DOC/Capela2004/index.html). The five canonical nod-boxes upstream from nodA, nodL, nodF, nodH, and nodM and the less-conserved nod-box upstream from syrM were indeed predicted, but no new nod-box was identified in the promoter regions of the new NodD1 targets. The only nodulation genes lacking a nod-box in their promoter are the pSymB nodP2Q2 genes. Actually, we suspect these genes may not be NodD1 regulated since microarray signals may be due to cross hybridization with nodP1Q1 (99% identical at the nucleotide level) genes that are under the control of the nodF nod-box (5). The absence of induction of SMb21225, which is in the operon with nodP2Q2, supports this hypothesis. The lack of conspicuous nod-box in the promoter regions of SMc03151, SMc03167, SMc03168, SMc03169, and groEL5 suggests an indirect mechanism of activation of these genes by NodD1. The transcriptional regulators activated by NodD1 in these experiments, the SMc0169 and SyrM proteins, might be responsible for the induction of these genes, although the SMc03169 protein belongs to a family of repressors (TetR) and no syrM-box was found upstream of these genes.
In Rhizobium etli, genes homologous to SMc03167 and SMc03168, the rmrAB genes, were previously reported to be inducible by root exudates (9). Mutants of these genes formed 40% fewer nodules and had enhanced sensitivity to flavonoids, phytoalexins, and salicylic acid than the wild-type strain. To investigate the potential symbiotic role of the SMc03167-SMc03168 multidrug efflux system in S. meliloti, a null mutant of the SMc03167 gene was constructed by integration of the pVO155 plasmid in the genome (20), and its symbiotic phenotype was assessed on M. sativa cv. Europe. The mutant was Nod+ Fix+ and was as competitive as the wild-type strain for nodulation (data not shown). In addition, unlike the R. etli rmrAB mutants, the SMc03167 mutant did not show an altered growth at high concentrations of luteolin (10 and 100 μM) compared to the wild-type strain. We ruled out that induction of this transporter could be triggered by Nod factors per se by showing that SMc03168 was still induced in a nodB mutant that does not produce Nod factors (data not shown).
We also constructed a null mutant of the SMc03151 gene and tested its symbiotic phenotype on M. sativa cv. Europe. However, the mutant was not altered in its nodulation and nitrogen fixation efficiencies compared to the wild-type strain (data not shown).
In conclusion, the present transcriptome study, as well as that of Barnett et al., demonstrates that NodD1 controls few functions behind nodulation in the narrow host range rhizobium, Sinorhizobium meliloti. This conclusion was further reinforced here by the computer-assisted evidence for a small number of canonical nod-boxes in the genome. The NodD1 regulon of S. meliloti is thus much less extended than that of the broad-host-range rhizobium NGR234, in which at least 75 genes are cascade regulated by NodD1. The NodD3 regulon of S. meliloti appeared to be much larger than the NodD1 regulon since hundreds of genes were induced or repressed upon NodD3 overexpression (2). This indicates that the NodD paralogs are not functionally equivalent in S. meliloti, with NodD1 essentially controlling nodulation function, whereas the physiological function of the genes under NodD3 control largely remains to be determined.
Acknowledgments
We thank Anke Becker at Bielefeld University for access to S. meliloti whole-genome oligo-slides and Claude Bruand for valuable discussions and critical reading of the manuscript.
This study was supported by a grant from the Toulouse Génopôle.
REFERENCES
- 1.Barnett, M. J., B. G. Rushing, R. F. Fisher, and S. R. Long. 1996. Transcription start sites for syrM and nodD3 flank an insertion sequence relic in Rhizobium meliloti. J. Bacteriol. 178:1782-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett, M. J., C. J. Toman, R. F. Fisher, and S. R. Long. 2004. A dual-genome symbiosis chip for coordinate study of signal exchange and development in a prokaryote-host interaction. Proc. Natl. Acad. Sci. USA 101:16636-16641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, A., H. Berges, E. Krol, C. Bruand, S. Ruberg, D. Capela, E. Lauber, E. Meilhoc, F. Ampe, F. J. de Bruijn, J. Fourment, A. Francez-Charlot, D. Kahn, H. Kuster, C. Liebe, A. Puhler, S. Weidner, and J. Batut. 2004. Global changes in gene expression in Sinorhizobium meliloti 1021 under microoxic and symbiotic conditions. Mol. Plant-Microbe Interact. 17:292-303. [DOI] [PubMed] [Google Scholar]
- 4.Chen, H., J. Higgins, I. J. Oresnik, M. F. Hynes, S. Natera, M. A. Djordjevic, J. J. Weinman, and B. G. Rolfe. 2000. Proteome analysis demonstrates complex replicon and luteolin interactions in pSyma-cured derivatives of Sinorhizobium meliloti strain 2011. Electrophoresis 21:3833-3842. [DOI] [PubMed] [Google Scholar]
- 5.Demont, N., F. Debelle, H. Aurelle, J. Denarie, and J. C. Prome. 1993. Role of the Rhizobium meliloti nodF and nodE genes in the biosynthesis of lipo-oligosaccharidic nodulation factors. J. Biol. Chem. 268:20134-20142. [PubMed] [Google Scholar]
- 6.DeRisi, J. L., V. R. Iyer, and P. O. Brown. 1997. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278:680-686. [DOI] [PubMed] [Google Scholar]
- 7.Dusha, I., and A. Kondorosi. 1993. Genes at different regulatory levels are required for the ammonia control of nodulation in Rhizobium meliloti. Mol. Gen. Genet. 240:435-444. [DOI] [PubMed] [Google Scholar]
- 8.Feng, J., Q. Li, H. L. Hu, X. C. Chen, and G. F. Hong. 2003. Inactivation of the nod box distal half-site allows tetrameric NodD to activate nodA transcription in an inducer-independent manner. Nucleic Acids Res. 31:3143-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Pasayo, R., and E. Martinez-Romero. 2000. Multiresistance genes of Rhizobium etli CFN42. Mol. Plant-Microbe Interact. 13:572-577. [DOI] [PubMed] [Google Scholar]
- 10.Györgypal, Z., N. Iyer, and A. Kondorosi. 1988. Three regulatory nodD alleles of diverged flavonoid-specificity are involved in host-dependent nodulation by Rhizobium meliloti. Mol. Gen. Genet. 212:85-92. [Google Scholar]
- 11.Honma, M. A., M. Asomaning, and F. M. Ausubel. 1990. Rhizobium meliloti nodD genes mediate host-specific activation of nodABC. J. Bacteriol. 172:901-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, S. I., C. Jourlin-Castelli, S. R. Wellington, and A. L. Sonenshein. 2003. Mechanism of repression by Bacillus subtilis CcpC, a LysR family regulator. J. Mol. Biol. 334:609-624. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi, H., Y. Naciri-Graven, W. J. Broughton, and X. Perret. 2004. Flavonoids induce temporal shifts in gene-expression of nod-box controlled loci in Rhizobium sp. NGR234. Mol. Microbiol. 51:335-347. [DOI] [PubMed] [Google Scholar]
- 14.Krause, A., A. Doerfel, and M. Gottfert. 2002. Mutational and transcriptional analysis of the type III secretion system of Bradyrhizobium japonicum. Mol. Plant-Microbe Interact. 15:1228-1235. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan, H. B., J. Lorio, W. S. Kim, G. Jiang, K. Y. Kim, M. DeBoer, and S. G. Pueppke. 2003. Extracellular proteins involved in soybean cultivar-specific nodulation are associated with pilus-like surface appendages and exported by a type III protein secretion system in Sinorhizobium fredii USDA257. Mol. Plant-Microbe Interact. 16:617-625. [DOI] [PubMed] [Google Scholar]
- 16.Machado, D., and H. B. Krishnan. 2003. nodD alleles of Sinorhizobium fredii USDA191 differentially influence soybean nodulation, nodC expression, and production of exopolysaccharides. Curr. Microbiol. 47:134-137. [DOI] [PubMed] [Google Scholar]
- 17.Maillet, F., F. Debelle, and J. Denarie. 1990. Role of the nodD and syrM genes in the activation of the regulatory gene nodD3, and of the common and host-specific nod genes of Rhizobium meliloti. Mol. Microbiol. 4:1975-1984. [DOI] [PubMed] [Google Scholar]
- 18.Meade, H. M., S. R. Long, G. B. Ruvkun, S. E. Brown, and F. M. Ausubel. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulligan, J. T., and S. R. Long. 1989. A family of activator genes regulates expression of Rhizobium meliloti nodulation genes. Genetics 122:7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oke, V., and S. R. Long. 1999. Bacterial genes induced within the nodule during the Rhizobium-legume symbiosis. Mol. Microbiol. 32:837-849. [DOI] [PubMed] [Google Scholar]
- 21.Ruberg, S., Z. X. Tian, E. Krol, B. Linke, F. Meyer, Y. Wang, A. Puhler, S. Weidner, and A. Becker. 2003. Construction and validation of a Sinorhizobium meliloti whole genome DNA microarray: genome-wide profiling of osmoadaptive gene expression. J. Biotechnol. 106:255-268. [DOI] [PubMed] [Google Scholar]
- 22.Theunis, M., H. Kobayashi, W. J. Broughton, and E. Prinsen. 2004. Flavonoids, NodD1, NodD2, and nod-Box NB15 modulate expression of the y4wEFG locus that is required for indole-3-acetic acid synthesis in Rhizobium sp. strain NGR234. Mol. Plant-Microbe Interact. 17:1153-1161. [DOI] [PubMed] [Google Scholar]
- 23.Thijs, G., M. Lescot, K. Marchal, S. Rombauts, B. De Moor, P. Rouze, and Y. Moreau. 2001. A higher-order background model improves the detection of promoter regulatory elements by Gibbs sampling. Bioinformatics 17:1113-1122. [DOI] [PubMed] [Google Scholar]
- 24.Thijs, G., K. Marchal, M. Lescot, S. Rombauts, B. De Moor, P. Rouze, and Y. Moreau. 2002. A Gibbs sampling method to detect overrepresented motifs in the upstream regions of coexpressed genes. J. Comput. Biol. 9:447-464. [DOI] [PubMed] [Google Scholar]
- 25.Viprey, V., A. Del Greco, W. Golinowski, W. J. Broughton, and X. Perret. 1998. Symbiotic implications of type III protein secretion machinery in Rhizobium. Mol. Microbiol. 28:1381-1389. [DOI] [PubMed] [Google Scholar]
- 26.Yeh, K. C., M. C. Peck, and S. R. Long. 2002. Luteolin and GroESL modulate in vitro activity of NodD. J. Bacteriol. 184:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]