Abstract
A biofiltration system inoculated with the mold Paecilomyces variotii CBS115145 showed a toluene elimination capacity (EC) of around 250 g/m3 of biofilter/h, which was higher than the values usually reported for bacteria. P. variotii assimilated m- and p-cresols but not the o isomer. Initial toluene hydroxylation occurred both on the methyl group and through the p-cresol pathway. These results were corroborated by detecting benzyl alcohol, benzaldehyde, and p-cresol as volatile intermediates. In liquid cultures with toluene as a substrate, the activity of toluene oxygenase (TO) was 5.6 nmol of O2/min/mg of biomass, and that of benzyl alcohol dehydrogenase was 16.2 nmol of NADH/min/mg of protein. Toluene biodegradation determined from the TO activity in the biofilter depended on the biomass distribution and the substrate concentration. The specific enzymatic activity decreased from 6.3 to 1.9 nmol of O2/min/mg of biomass along the reactor. Good agreement was found between the EC calculated from the TO activity and the EC measured on the biofilter. The results were confirmed by short-time biofiltration experiments. Average EC measured in different biofiltration experiments and EC calculated from the TO activity showed a linear relation, suggesting that in the biofilters, EC was limited by biological reaction. As the enzymatic activities of P. variotii were similar to those reported for bacteria, the high performance of the fungal biofilters can possibly be explained by the increased transfer of the hydrophobic compounds, including oxygen, from the gas phase to the mycelia, overcoming the transfer problems associated with the flat bacterial biofilms.
Studies of biofiltration show that bacterial biofilms can degrade low concentrations of volatile organic compounds from off-gas streams (7, 19). Recent work has demonstrated that biofilters containing fungi effectively eliminate volatile organic compounds, even under adverse environmental conditions such as low moisture content, low pH, and transient loadings (5, 18, 24, 26, 28). Previous results (9) have shown that a biofilter inoculated with a fungal strain (reported as Scedosporium apiospermum TB1 but recently reclassified as Paecilomyces variotii CBS115145) reached and maintained high elimination capacities (EC) of toluene (higher than 200 g/m3 of biofilter/h, with a removal efficiency of 98%). The maximum EC of toluene, around 245 g/m3 of biofilter/h, was obtained with the same strain growing on porous ceramic rings (1). The biofilter exhibited bacterial contamination, but fungal activity was responsible for about 70% of total removal. Woertz et al. (28) reported the performance of a biofilter containing Exophiala lecanii-cornii with an average toluene EC of around 80 g/m3 of biofilter/h and removal efficiencies greater than 95%. ECs up to 270 g/m3 of biofilter/h were attained for short periods. Exophiala oligosperma and Paecilomyces variotii have been recently reported to remove toluene with ECs of 55 and 80 g/m3 of biofilm/h (8).
Five metabolic pathways for toluene are known in bacteria (27). Toluene is initially hydroxylated on either the methyl group or the aromatic ring by an oxygenase. In fungi, initial hydroxylation on both molecular sites has also been reported. Some zygomycetes and deuteromycetes were shown to hydroxylate toluene at the aromatic ring, and the intermediates o-cresol and p-cresol were identified (21). Weber et al. (27) reported Cladosporium sphaerospermum growth on toluene with initial hydroxylation on the methyl group. Recently, Prenafeta-Boldú et al. (17) used isomeric fluorotoluenes as model substrates for toluene catabolism by five fungi that grow on toluene and two others that cometabolize toluene with glucose (Cunningamella echinulata and Aspergillus niger). With the toluene-utilizing fungi and A. niger, initial hydroxylation occurred only at the methyl group and resulted in fluorinated benzoates. In C. echinulata, hydroxylation was also initiated at the aromatic ring, and o-cresol and benzoate were detected. For toluene, it has been proposed that fungi preferentially hydroxylate the methyl group (13, 17, 27). Parallel pathways for the degradation of aromatic compounds have been shown elsewhere in fungi. Jones et al. (11) reported that in the p-cresol oxidation by Aspergillus fumigatus, both the methyl group and the ring hydroxylation were involved. Also with A. fumigatus, Jones et al. (12) showed that phenol degradation was initiated by both o and p hydroxylation. Similar results were found with Paecilomyces lilacinus on biphenyl degradation (10).
Enzymatic activities have seldom been used to understand the macroscopic behavior of biofilters. However, several authors (6, 15, 22) have correlated performance and metabolic measurements in bacterial biofilters.
The main objective of this work was to evaluate the relationship between the enzymatic activity and the performance of a biofilter for toluene degradation inoculated with Paecilomyces variotii CBS115145. The toluene metabolic pathway was first elucidated and the main enzyme activities and degradation products were evaluated in mycelia obtained from liquid cultures and biofilters. In a long-term biofiltration experiment, enzymatic activities were measured on samples collected along the biofilter and compared with the experimental macroscopic toluene elimination capacity.
MATERIALS AND METHODS
Microorganisms and media.
The fungal strain was isolated from a peat biofilter treating toluene-contaminated air (3, 9). It was recently reclassified as Paecilomyces variotii CBS115145. Its preservation, cultivation conditions, and spore production have been reported by García-Peña et al. (9).
Chemicals.
Toluene (high-performance liquid chromatography [HPLC] grade; Baker), benzyl alcohol, catechol, m- and p-cresols, protocatehuate, NAD+ and NADP+ (99% pure; Sigma, St. Louis, MO), benzaldehyde, and o-cresol (99% pure; Aldrich Chemical Company, Milwaukee, WI) were used.
Liquid cultivation.
Growth experiments with the reported intermediates of toluene metabolism were performed in duplicate in 125-ml serum bottles with 30 ml of culture medium in a rotary shaker at 150 rpm and 30°C. The bottles were inoculated with a spore suspension to an initial concentration of 2 × 107 spores/ml (9) and sealed with Mininert Teflon valves (VICI; Precision Sampling Inc., Baton Rouge, LA). Initial headspace concentrations of around 35 mg/liter (depending on the density) of the various intermediates were used. Carbon dioxide (CO2) concentrations were periodically determined in duplicate from the headspace. Fungal biomass was evaluated by dry weight.
Biofilters.
Experiments were performed in a cylindrical biofilter (1 m high × 0.08-m inner diameter) with two intermediate gas sampling ports. The volume of the filter bed was 2.9 liters. Vermiculite, used as a support, was inoculated with a spore suspension to an initial concentration of 2 × 107 spores/g of initial dry mass (DM). Initial conditions were as follows: density of 138 g DM/liter of biofilter, pH 4.5, and moisture content of 70%. Air saturated with toluene was mixed with water-saturated air to obtain a toluene inlet concentration of 6 g/m3. The gas stream, 2.5 liters/min, was introduced at the top of the reactor. The empty-bed residence time was 1.15 min. Mineral medium was sprayed intermittently at the top.
Short-time biofiltration experiments (STBE).
Parallel biofilter experiments (packed volume, 0.25 liters; inlet flow rate, 0.08 liters/min; empty-bed residence time, 3.12 min) were performed for up to 15 days. The support was prepared as described above, and 55 g was packed in the biofilters (2). Glucose (20 g/liter) was included with the initial medium to favor spore germination and startup. In some columns, the volatile intermediates were condensed from the outlet air stream in a condenser at −5°C for 24 h.
Oxygen uptake rate with the various intermediates.
Oxygen consumption was measured with a dissolved oxygen probe (model 52; YSI, Yellow Springs, Ohio). The biomass, previously grown on toluene, was obtained from liquid culture, STBE, and the biofilter, and it was filtered and washed with distilled water before the assay. Samples were introduced in the respirometric cell, and the total volume was filled to 60 ml with phosphate buffer (0.05 M, pH 7.0). The biomass suspension was aerated for about 5 min until oxygen saturation was reached. The intermediates were then injected, and soluble oxygen uptake was measured during 20 min. The specific consumption rate was determined from the slope of dissolved oxygen concentration with time.
Determination of the metabolic intermediates of toluene oxidation.
Volatile intermediates produced in vivo from toluene oxidation were determined and quantified in the condensates from the STBE columns by gas and high-pressure chromatography as described below. The total mass of each volatile intermediate was obtained by multiplying its concentration by the condensate liquid volume. The specific production was obtained by dividing the total mass by the volume of the biofilter (0.25 liters) and the elapsed condensation time (24 h).
Enzymatic activity determination.
Enzyme assays were performed with complete cells and cell extract from the liquid cultures and the biofilters.
(i) Complete cells.
Fungal biomass previously grown in toluene for 5 days (between 25 and 30 mg for analysis) was harvested from liquid culture and STBE, filtered, washed, and resuspended in a phosphate buffer solution (0.05 M and pH 7.0). The toluene oxygenase activity from the cultures was assayed by soluble oxygen consumption.
(ii) Disrupted cells.
Mycelia, and in some cases samples from the biofilters, were physically disrupted via homogenization with an Ultra-turrax (GmbH & Co., Staufen, Germany) for 5 min at 20,000 rpm in a phosphate buffer solution (0.05 M and pH 7.0).
(iii) Cell extracts.
One gram of P. variotii mycelia was harvested and washed with distilled water. Cells were lysed by freezing in liquid nitrogen and resuspended in 2 ml of 4 mM dithiothreitol (Sigma, St. Louis, MO) in 0.1 M Tris-HCl buffer at pH 7.5. The lysates were centrifuged at 10,000 rpm at 4°C for 20 min to obtain cell extract.
Oxygenase activity.
Oxygenase activity was measured with complete or disrupted cells by dissolved oxygen consumption. The oxygen uptake rate was measured with increasing toluene concentrations (29, 59, and 87 mg/liter) to determine the kinetic constants Vmax and Km. The rates were corrected by the endogenous respiration and normalized to the biomass content measured by dry weight. An enzymatic unit is defined as nanomoles of oxygen consumed per minute per milligram of mycelium. In some cases, the activity is reported as nanomoles of O2/min/mg of protein using a protein content of 0.18 mg of protein/mg of biomass experimentally determined with the Bradford protein assay (Bio-Rad, Hercules, CA).
Dehydrogenase activity.
The 3-ml reaction volume contained 0.3 ml of cell extract, 2.7 ml of Tris-HCl buffer (0.1 M, pH 7.5) with MgCl2 (5 mM), and NAD+ or NADP+ (0.4 mM). Reactions were performed at 30°C and were initiated with 2 μl of benzyl alcohol. The change in absorbance at 340 nm indicated the production of the reduced cofactor. The specific activity is the amount of enzyme required to reduce 1 mmol of NAD+ or NADP+ per minute per milligram of protein. Control experiments without substrate and with heat-denatured cell extract were also performed. Protein content in the enzymatic extracts was determined by the Bradford protein assay (Bio-Rad, Hercules, CA).
Analytical methods. (i) Toluene concentration.
Air samples (0.1 ml) were analyzed with a flame ionization detection gas chromatograph as reported previously by Morales et al. (16).
(ii) CO2 concentration.
CO2 concentration was measured in air with an infrared analyzer (3400 CO2 Gas Analyzer; California Analytical Instruments Inc., Orange, CA). Reported values correspond to the difference between the outlet and the inlet (ambient) CO2 concentrations.
(iii) Toluene metabolism intermediates.
Toluene metabolism intermediates were measured in the condensates by injecting 1 μl in a gas chromatograph (HP-5890, series II; Agilent Technologies, Palo Alto, CA) equipped with a flame ionization detector. Separation of toluene, benzaldehyde, and benzyl alcohol from the condensates was performed with an HP-624 column (30m × 0.32 mm × 1.5 μm). The oven temperature was 100°C for 2 min and then increased to 240°C at a rate of 10°C/min. Temperatures were 250°C for the injector and detector.
(iv) HPLC separation of metabolic products.
For detection and quantification of m- and p-cresol in the condensates, reversed-phase chromatography analysis was performed with an HPLC (Thermo Separation Products Inc., Riviera Beach, FL) equipped with a UV detector (SpectroMonitor 4100; Thermo Separation Products Inc., Riviera Beach, FL). The mobile phase was acetonitrile and water (30:70).
Calculations. (i) Elimination capacity.
Biofilter performance was evaluated with the toluene EC and removal efficiency (%Eff). EC (g/m3 of biofilter/h) is defined as (Sin − Sout) × air flow/Vbiofilter, and %Eff is equal to 100 × (Sin −Sout)/Sin, where Sin and Sout are the toluene concentrations in the inlet and the outlet air, respectively, and Vbiofilter is the biofilter volume.
(ii) Elimination capacity calculated from enzymatic activity values.
An estimated elimination capacity in biofilters (ECc) was calculated from the enzymatic activity using the following relation with the biomass from the biofilters: calculated ECc (mg/liter of biofilter/h or g/m3of biofilter/h) = biomass (mg of biomass/g of DM) × specific enzymatic activity (mg of toluene/mg of biomass/h) × biofilter packing density (138 g of DM/liter of biofilter).
RESULTS
Growth of P. variotii with intermediates of toluene metabolism.
P. variotii growth was evaluated with some of the reported intermediates of the toluene catabolic pathways and with glucose as a control (Table 1). In general, growth was detected from hour 12 and the substrates were utilized in the first 48 h. Biomass yield with toluene was the highest, and growth was detected with the intermediates of the methyl group oxidation, although it was lower for benzoate. Growth was also detected with m- and p- cresols and protocatehuate. The biomass and CO2 from o-cresol were similar to those obtained in the control without substrate and probably were due to substrate carried over with the inoculum.
TABLE 1.
Carbon balance for the various intermediates of the toluene metabolic pathways used as growth substrates for Paecilomyces variotii
| Carbon source | Substrate (mg) | Biomass (mg) | Biomass yield (mg/mg) | CO2 yield (mg/mg) |
|---|---|---|---|---|
| Glucose | 30.0 | 14.4 | 0.48 | 0.58 |
| Toluene | 6.26 | 5.2 | 0.83 | 1.58 |
| Benzyl alcohol | 6.26 | 3.1 | 0.50 | 1.87 |
| Benzaldehyde | 6.26 | 3 | 0.48 | 2.10 |
| Benzoate | 6.26 | 2.4 | 0.38 | 0.66 |
| Catechol | 6.49 | 3.8 | 0.59 | 2.25 |
| m-Cresol | 6.05 | 3 | 0.50 | 1.94 |
| p-Cresol | 6.05 | 3.6 | 0.60 | 1.84 |
| o-Cresol | 6.05 | 0.2 | 0.03 | 0.55 |
| Protocatehuate | 8.63 | 3.9 | 0.45 | 1.49 |
Respirometric oxygen consumption with toluene intermediates.
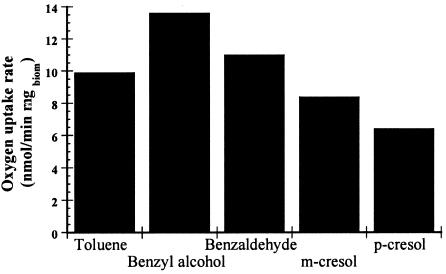
Respirometry was evaluated with several intermediates using biomass previously grown in toluene in the STBE. Compared with toluene (9.9 nmol/min/mg of biomass), higher rates were obtained with benzyl alcohol (+36%) and benzaldehyde (+12%) and lower rates were obtained with m- and p-cresols (−8% and −35%, respectively) (Fig. 1). No activity was detected with o-cresol.
FIG. 1.
Oxygen uptake rates of homogenized Paecilomyces variotii biomass from STBE with various intermediates.
Enzymatic activities related to toluene degradation in P. variotii.
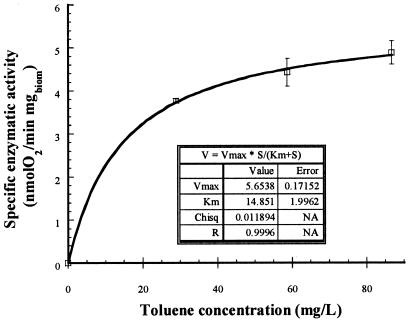
Oxygenase activity was measured in washed P. variotii mycelia grown with toluene in liquid culture and from the STBE. The results obtained with biomass from liquid culture in Fig. 2 show Vmax for the toluene oxygenase of 5.6 nmol of O2/min/mg of biomass (or 31.1 nmol of O2/min/mg of protein) and a Km of 14.8 mg/liter (9.6 μM). The activity from the biomass grown in the biofilters (STBE) was higher, as shown in Table 2. Partial homogenization of the mycelia improved the activity in the biofilter samples.
FIG. 2.
Toluene oxygenase activity with different toluene concentrations. Vmax and Km are the constants from the Michaelis-Menten model. Chisq, chi-square test; NA, not applicable.
TABLE 2.
Comparison of the enzymatic activity of Paecilomyces variotii biomass produced in liquid culture and in STBE
| Experimental system | Oxygenase sp act (nmol O2/min/mg biomass)a | Dehydrogenase sp act (nmol NADH/min/mg protein)b |
|---|---|---|
| Biomass from liquid culture | 4.7 | ND |
| Cell extract | ND | 16.2 (NAD+) |
| 4.6 (NADP+) | ||
| Biomass from STBE | 6.3 | ND |
| Homogenized | 11.9 | ND |
| Cell extract | ND | 36.6 (NAD+) |
| 0.2 (NADP+) |
Measured with a toluene concentration of 87 mg/liter.
Benzyl alcohol dehydrogenase activity was measured in cell extracts obtained from the liquid culture and the STBE. ND, not determined.
Benzyl alcohol dehydrogenase activity was measured in cell extracts from biomass grown on toluene in liquid culture and from biofilters (STBE). Both NAD+ and NADP+ served as electron acceptors, but higher rates were measured with NAD+ (Table 2). As with the oxygenase test, the specific activity was higher in the extracts produced in biofilters.
Intermediates involved in toluene catabolism by P. variotii.
The intermediates benzyl alcohol, benzaldehyde, and p-cresol were consistently identified in the condensates (Table 3). Toluene consumption started on day 3 of the process, and during startup, no intermediates were detected. At the seventh day, when the toluene removal efficiency (%Eff) was around 50%, the intermediates were detected in the condensate (around 6.2 mg/liter of biofilter in 24 h), indicating that they were transiently accumulating. The total amount of volatilized intermediates decreased to 1.2 mg/liter of biofilter in 24 h as the %Eff increased to 95% on day 13. When a %Eff of >98% was reached on days 14 and 15, no intermediates were detected in the condensates.
TABLE 3.
Volatile intermediates produced from toluene consumption in biofilters by Paecilomyces variotiib
| Time (days) | Toluene EC (g/m3 biofilm/h) and REa (%) | Recovered intermediate (mg/liter biofilter/h)
|
Carbon recovered as intermediate (%)
|
Yield (%C)c | ||||
|---|---|---|---|---|---|---|---|---|
| BOH | BA | p-C | BOH | BA | p-C | |||
| 7 | 62 (53) | 0.15 | 0.054 | 0.054 | 0.206 | 0.076 | 0.076 | 0.357 |
| 11 | 126 (94) | 0.018 | 0.022 | 0.05 | 0.012 | 0.015 | 0.034 | 0.003 |
| 13 | 124 (95) | 0.012 | 0.018 | 0.02 | 0.008 | 0.013 | 0.014 | 0.002 |
RE, removal efficiency.
BOH; benzyl alcohol; BA, benzaldehyde, p-C, p-cresol.
Percentage of carbon from the consumed toluene recovered as carbon in the intermediates.
Toluene removal and CO2 production in the biofilters.
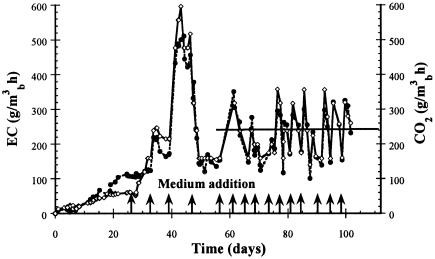
Figure 3 shows the toluene elimination capacity and the CO2 production in a biofilter inoculated with P. variotii. Toluene consumption started 12 days after inoculation, attaining an EC of 100 g/m3 of biofilter/h. After three medium additions, starting on day 26, the EC attained an EC of around 500 g/m3 of biofilter/h, which was maintained during 7 days. The EC then decreased to an average of 250 g/m3 of biofilter/h. Similarly to previous experiments, CO2 production was associated to toluene elimination capacity (9). At day 104, when the average EC was around 250 g/m3 of biofilter/h, the experiment was stopped, and biomass from various heights of the biofilter was used to measure oxygenase activity.
FIG. 3.
Evolution of the toluene EC (•) and CO2 (⋄) production in a 2.5-liter biofilter inoculated with Paecilomyces variotii. Arrows indicate the time of mineral medium additions, and the diagonal line shows the average EC and the average CO2 production.
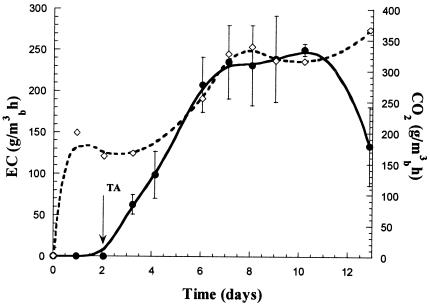
Toluene removal and CO2 production obtained in one of the STBE is presented in Fig. 4. Initial glucose addition shortened the adaptation period compared with the experiment described in Fig. 3. Toluene consumption started a few hours after it was fed to the system, reaching an EC of 200 g/m3 of biofilter/h on day 6. A maximum EC of approximately 250 g/m3 of biofilter/h was obtained, with a diminution from day 12, yielding an average EC of 146 g/m3 of biofilter/h for the duration of the experiment. A high CO2 production was initially detected due to glucose consumption and thereafter to toluene oxidation. The total biomass produced was 44.5 mg/g of DM. Some drying of the system was observed with an average moisture content decreasing from 70% to 65%.
FIG. 4.
Evolution of the toluene EC (•) and CO2 (⋄) production in the STBE inoculated with Paecilomyces variotii (TA, start of toluene addition).
Correlation between enzymatic activity and fungal biofilter performance.
Table 4 shows the axial distribution of different variables after 104 days of biofilter operation. The difference in humidity reflects the fact that the experiment was stopped 3 days after the last medium addition. Biomass content was lower at the inlet and attained a value of 143.4 mg/g of DM at the outlet. The oxygenase enzymatic activity profile, measured from standardized samples (2 g of wet mass), was similar in the first four levels and slightly higher at the bottom of the reactor, where biomass overgrowth was observed. The enzymatic activities were therefore normalized to the total amount of dry biomass in each level, and it was found that the specific activity was higher at the inlet (6.3 nmol of O2/min/mg of biomass) and decreased threefold down to 1.9 nmol of O2/min/mg of biomass at the outlet. Using the enzymatic activity and the amount of biomass in each section, an expected EC was calculated and compared with the experimental value obtained with the partial EC from the intermediate gas samples. Both the calculated ECc and the experimental EC were lower at the inlet of the biofilter and higher at the outlet, with the experimental ECs being consistently higher. In the STBE, good agreement was found with an ECc of 143 g/m3 of biofilter/h (obtained from enzymatic activity of 6.3 nmol of O2/min/mg of biomass [Table 2]) and the average experimental EC of 146 g/m3 of biofilter/h.
TABLE 4.
Correlation between the experimental EC measured in the biofilter and the calculated EC obtained through the enzymatic activity values
| Biofilter level | Humidity (%) | Biomass concn in each level (mg/g DM) | Volumetric enzymatic activity (nmol O2/min/g WM)a | Specific enzymatic activity (nmol O2/min/mg biomass) | Calculated ECc (g/m3 biofilm/h) | Experimental EC (g/m3 biofilm/h)b |
|---|---|---|---|---|---|---|
| Inlet | 31 | 15.1 | 65.6 | 6.3 | 83.1 | 117 |
| Level 2 | 42 | 21.6 | 56.2 | 4.5 | 85.3 | ND |
| Level 3 | 51 | 36.6 | 56.2 | 3.1 | 102.5 | 262 |
| Level 4 | 59 | 39.3 | 46.9 | 2.9 | 102.4 | ND |
| Outlet | 73 | 143.4 | 76.2 | 1.9 | 283.7 | 317 |
| Global | 131.4 | 232 |
WM, wet mass.
ND, not determined.
DISCUSSION
Growth and oxygen consumption by P. variotii with reported intermediates of the toluene metabolic pathways suggest that the hydroxylation of the methyl group might be the preferred route, but an alternate pathway through the meta or para ring hydroxylation cannot be discarded. In contrast, Cladosporium sphaerospermum showed an increase in oxygen consumption only after the addition of benzyl alcohol, benzaldehyde, and catechol, and no metabolic activity was observed with the cresol isomers (27).
Cryogenic condensation of the volatiles from the STBE allowed the identification of benzyl alcohol, benzaldehyde, and p-cresol. The presence of p-cresol can be attributed to the ring hydroxylation. Prenafeta-Boldú et al. (17) reported that the hydroxylation of fluorotoluene by Cunninghamela echinulata resulted in fluorinated o-cresol and fluorobenzoate. Those authors suggested, based on results with several strains, that fungi may have less metabolic versatility than bacteria for toluene assimilation. Nevertheless, the results with P. variotii indicate that this microorganism has more than one toluene metabolic pathway. The production of the intermediates was lower when the efficiency of the system increased, suggesting that the fungus was capable of overcoming the metabolic bottlenecks.
The average concentrations of condensed intermediates in the gas phase on day 7 were 0.008 mg/liter of air for benzyl alcohol, 0.0028 mg/liter of air for benzaldehyde, and 0.0028 mg/liter of air for p-cresol. Using the partition coefficients for these compounds (2.45 × 10−5, 1.7 × 10−3, and 2.86 × 10−5, respectively) (4), the calculated concentrations in the liquid phase were 326, 1.7, and 97 mg/liter of water, respectively. These correspond to 12.4, 0.06, and 3.7 mg in the STBE calculated for a moisture content of 69%. These concentrations were transitory and diminished as the efficiency increased. The fraction of toluene converted into its volatile intermediates was very low (<0.4%) and diminished as the efficiency increased (Table 3).
The higher activity with benzyl alcohol and benzaldehyde than that with toluene suggests that the limiting step for toluene oxidation was its initial hydroxylation. The experiments did not allow us to establish if this reduced rate was due to cell transport or kinetics.
The maximum specific toluene monooxygenase activity for P. variotii (5.6 nmol of O2/min/mg of biomass or 31.1 nmol of O2/min/mg of protein) was similar to that obtained for Cladosporium sphaerospermum (20.5 nmol/min/mg of protein) (13) and higher than the activity reported for benzopyrene hydroxylase from Pleurotus pulmonarius (13.6 nmol/min/mg of protein) and Pseudomonas putida F1 (7 nmol/min/mg of protein) (14, 20). On the other hand, they were lower than the catechol dioxygenases measured for Cladosporium sphaerospermum and for Aspergillus fumigatus (12, 27). The literature shows strong discrepancies in the oxygenase activity, depending on the strain and the assay conditions. Higher activity was measured with the biomass produced in the biofilters than that in liquid culture. This difference was probably due to an increased enzymatic induction favored by the higher bioavailability through the aerial mycelia. The affinity constant (Km = 9.6 μM) was higher than the value of 0.9 μM reported for the toluene monooxygenase from Cladosporium sphaerospermum (13) obtained from cell extracts. The lower specificity can be attributed partially to the membrane and cell wall transport which was improved almost twofold when the mycelium was partially homogenized.
The results from the biofilter demonstrated that operation can be maintained for long periods with sustained performance. The specific oxygenase enzymatic activity profile, determined with samples from the biofilter after 104 days of operation, showed the highest activity at the inlet of the bioreactor (6.3 nmol of O2/min/mg of biomass). This value was slightly higher than the maximum enzymatic activity (Vmax, 5.6 nmol of O2/min/mg of biomass [Fig. 2]) obtained with biomass from the STBE (Table 2). Higher biomass content and lower toluene concentration in the gas phase towards the outlet may induce less availability of toluene and possibly oxygen to the biomass, thus reducing specific activity. Song and Kinney (22) reported that excess biomass accumulation can lead to a loss of activity in vapor phase bioreactors.
The reduction in microbial activity as a function of inlet substrate concentration in fixed-bed bioreactors was reported previously by Deront et al. (6). Other authors have estimated specific microbial activity from enzymatic assays and from toluene uptake rates (15). Song and Kinney (22) demonstrated that biodegradation determined by dehydrogenase activity depended on biomass accumulation and reflected pollutant removal profiles along the biofilter.
Fungi degrade aromatic compounds in biofilters better than bacteria. As toluene oxygenase and benzyl alcohol dehydrogenase activities were in the range of those reported in bacteria, it is suggested that the characteristic mycelial growth of fungi enhances toluene availability and, consequently, its degradation. Aerial mycelia, in direct contact with the gas, can uptake hydrophobic compounds directly and faster than bacterial biofilms (25) by the increased surface for toluene sorption which reduces the limitations observed with the deep bacterial biofilms. The hydrophobic nature of the mycelial surface may further improve toluene sorption directly from the air stream.
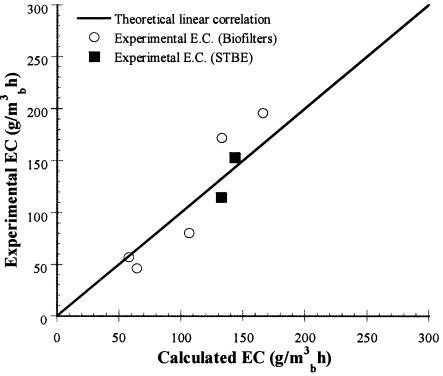
To extrapolate the results from the enzymatic activities, the calculated ECc and the average experimental EC of the biofilter, the STBE, and four biofiltration experiments previously reported (9) were compared (Fig. 5). The calculated EC was obtained for each biofilter by multiplying the measured final biomass by the average of the oxygenase activity (3.7 nmol/min/mg of biomass). The experimental average EC was obtained not considering the adaptation time, which was variable for each experiment. A good correlation was obtained between the calculated and the average ECs. The enzymatic activity reflects the metabolic activity in the system, as it was shown for a bacterial fixed-film bioreactor degrading trichloroethylene and for a toluene-degrading bioreactor reported previously by Sun and Wood (23) and by Song and Kinney (22). The linear correlation suggests that globally the biofiltration processes, under the established conditions, were limited mainly by the biological reaction. These results would support the hypothesis that in the fungal reactors, the mass transfer limitations, usually found in bacterial biofilms due to low substrate solubility and low transfer area, were surmounted by the mycelial nature of fungal growth.
FIG. 5.
Correlation between the average experimental EC measured in different biofiltration systems and the calculated elimination capacity, ECc, obtained through the enzymatic activity values. ○, five different biofiltration experiments (9); ▪, STBE. The line corresponds to EC = ECc.
Acknowledgments
We acknowledge CONACYT (Consejo Nacional de Ciencia y Tecnología, Mexico) for the grant given to the project and for the scholarship of I.G.-P.
We thank Carmen Fajardo and Pierre Roger for their technical assistance.
REFERENCES
- 1.Aizpuru, A., B. Dunat, P. Christen, R. Auria, I. García-Peña, and S. Revah. 2005. Fungal biofiltration of toluene on ceramic rings. J. Environ. Eng. 13:396-402. [Google Scholar]
- 2.Auria, R., M. Morales, E. Villegas, and S. Revah. 1993. Influence of mould growth on the pressure drop in aerated solid-state fermentors. Biotechnol. Bioeng. 41:1007-1013. [DOI] [PubMed] [Google Scholar]
- 3.Auria, R., G. Frere, M. Morales, M. E. Acuña, and S. Revah. 2000. Influence of mixing and water addition on the removal rate of toluene vapors in a biofilter. Biotechnol. Bioeng. 68:448-455. [DOI] [PubMed] [Google Scholar]
- 4.Card, T. R. 1998. Fundamentals: chemistry and characteristics of odors and VOCs, p. 2.1-2.36. In H. Rafson (ed.), Odor and VOC control handbook. McGraw Hill, New York, N.Y.
- 5.Cox, H. H. J., R. E. Moerman, S. Van Baalen, W. N. M. van Heiningen, H. J. Doddema, and W. Harder. 1997. Performance of styrene-degrading biofilter containing the yeast Exophiala jeanselmei. Biotechnol. Bioeng. 53:259-266. [DOI] [PubMed] [Google Scholar]
- 6.Deront, M., F. M. Samb, N. Adler, and P. Peringer. 1998. Biomass growth monitoring using pressure drop in a cocurrent biofilter. Biotechnol. Bioeng. 60:97-104. [DOI] [PubMed] [Google Scholar]
- 7.Devinny, J. S., M. A. Deshusses, and T. S. Webster. 1999. Biofiltration for air pollution control, p. 299. Lewis Publishers, New York, N.Y.
- 8.Estévez, E., M. C. Veiga, and C. Kennes. 2004. Fungal biodegradation of toluene in gas-phase biofilters, p. 337-340. In W. Verstraete (ed.), Proceedings of the European Symposium on Environmental Biotechnology, ESEB 2004. Taylor Francis Group, London, United Kingdom.
- 9.García-Peña, E. I., S. Hernández, E. Favela-Torres, R. Auria, and S. Revah. 2001. Toluene biofiltration by the fungus Scedosporium apiospermum TB1. Biotechnol. Bioeng. 76:61-69. [DOI] [PubMed] [Google Scholar]
- 10.Gesell, M., E. Hammer, M. Specht, W. Francke, and F. Schauer. 2001. Biotransformation of biphenyl by Paecilomyces lilacinus and characterization of ring cleavage products. Appl. Environ. Microbiol. 67:1551-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones, K. H., P. W. Trudgill, and D. J. Hopper. 1993. Metabolism of p-cresol by fungus Aspergillus fumigatus. Appl. Environ. Mirobiol. 59:1125-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, K. H., P. W. Trudgill, and D. J. Hopper. 1995. Evidence of two pathways for the metabolism of phenol by Aspergillus fumigatus. Arch. Microbiol. 163:176-181. [DOI] [PubMed] [Google Scholar]
- 13.Luykx, D. M. A. M., F. X. Prenafeta-Boldú, and J. A. M. de Bont. 2003. Toluene monooxygenase from the fungus Cladosporium sphaerospermum. Biochem. Biophys. Res. Commun. 312:373-379. [DOI] [PubMed] [Google Scholar]
- 14.Masaphy, S., D. C. Lamb, and S. L. Kelly. 1999. Purification and characterization of a penzo-pyrene hydroxylase from Pleurotus pulmonarius. Biochem. Biophys. Res. Commun. 266:326-329. [DOI] [PubMed] [Google Scholar]
- 15.Mirpuri, R., W. Jones, and J. D. Bryers. 1997. Toluene degrading kinetics for planktonic and biofilm-growth cells of Pseudomonas putida 54G. Biotechnol. Bioeng. 53:535-546. [DOI] [PubMed] [Google Scholar]
- 16.Morales, M., S. Revah, and R. Auria. 1998. Start-up and the effect of gaseous ammonia additions on a biofilter for the elimination of toluene vapors. Biotechnol. Bioeng. 60:483-491. [DOI] [PubMed] [Google Scholar]
- 17.Prenafeta-Boldú, F. X., D. M. A. M. Luykx, J. Vervoort, and J. A. M. de Bont. 2001. Fungal metabolism of toluene: monitoring of fluorinated analogs by 19F nuclear magnetic resonance spectroscopy. Appl. Environ. Microbiol. 67:1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi, B., W. M. Moe, and K. A. Kinney. 2002. Biodegradation of volatile organic compounds by five fungal species. Appl. Microbiol. Biotechnol. 58:684-689. [DOI] [PubMed] [Google Scholar]
- 19.Shareefdeen, Z., and A. Singh. 2005. Biotechnology for odor and air pollution control. Springer-Verlag, Berlin, Germany.
- 20.Shields, M. S., S. O. Montgomery, S. M. Cushey, P. J. Chapman, and P. H. Pritchard. 1989. Novel pathway of toluene catabolism in trichloroethylene-degrading bacterium G4. Appl. Environ. Microbiol. 55:1624-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, R. V., and J. P. Rosazza. 1974. Microbial models of mammalian metabolism. Aromatic hydroxylation. Arch. Biochem. Biophys. 161:551-558. [DOI] [PubMed] [Google Scholar]
- 22.Song, J., and K. Kinney. 2000. Effect of vapor-phase bioreactor operation on biomass accumulation, distribution, and activity: linking biofilm properties to bioreactor performance. Biotechnol. Bioeng. 68:508-516. [DOI] [PubMed] [Google Scholar]
- 23.Sun, A. K., and T. K. Wood. 1997. Trichloroethylene mineralization in a fixed-film bioreactor using a pure culture expressing constitutively toluene ortho-monooxygenase. Biotechnol. Bioeng. 55:674-685. [DOI] [PubMed] [Google Scholar]
- 24.van Groenestijn, J. W., and P. G. M. Hesselink. 1993. Biotechniques for air pollution control. Biodegradation 4:283-301. [Google Scholar]
- 25.van Groenestijn, J. W., and J. X. Liu. 2002. Removal of alpha-pinene from gases using biofilters containing fungi. Atmospheric Environ. 36:5501-5508. [Google Scholar]
- 26.Weber, F., and S. Hartmans. 1996. Prevention of clogging in a biological trickle-bed reactor removing toluene from contaminated air. Biotechnol. Bioeng. 50:91-97. [DOI] [PubMed] [Google Scholar]
- 27.Weber, F. J., K. C. Hage, and J. A. M. de Bont. 1995. Growth of the fungus Cladosporium shaerospermum with toluene as the sole carbon and energy source. Appl. Environ. Microbiol. 61:3562-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woertz, J. R., K. A. Kinney, N. D. P. McIntosh, and P. J. Szaniszlo. 2001. Removal of toluene in a vapor-phase bioreactor containing a strain of the dimorphic black yeast Exophiala lecanii-corni. Biotechnol. Bioeng. 75:550-558. [DOI] [PubMed] [Google Scholar]