Abstract
Extracellular vesicles, particularly exosomes, play a pivotal role in the cellular mechanisms underlying cancer. This review explores the various functions of exosomes in the progression, growth, and metastasis of cancers affecting the male and female reproductive systems. Exosomes are identified as key mediators in intercellular communication, capable of transferring bioactive molecules such as microRNAs, proteins, and other nucleic acids that influence cancer cell behavior and tumor microenvironment interactions. It has been shown that non-coding RNAs transported by exosomes play an important role in tumor growth processes. Significant molecules that may serve as biomarkers in the development and progression of male reproductive cancers include miR-125a-5p, miR-21, miR-375, the miR-371 ~ 373 cluster, and miR-145-5p. For female reproductive cancers, significant microRNAs include miR-26a-5p, miR-148b, miR-205, and miRNA-423-3p. This review highlights the potential of these noncoding RNAs as biomarkers and prognostics in tumor diagnostics. Understanding the diverse roles of exosomes may hold promise for developing new therapeutic strategies and improving treatment outcomes for cancer patients.
Keywords: extracellular vesicles, exosomes, cancers, reproductive cancers, microRNA, reproduction
Summary Sentence
Research into exosomal ncRNAs in reproductive cancers is crucial because it combines fundamental biological insights with immediate clinical applications. It holds the potential to revolutionize early detection, treatment personalization, and the understanding of metastatic and resistance mechanisms, ultimately improving outcomes for patients with these challenging malignancies.
Graphical Abstract
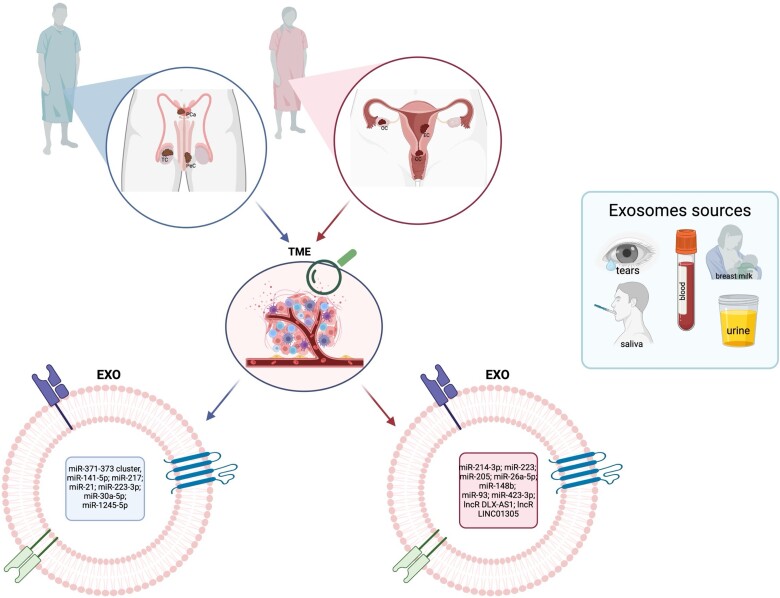
Graphical Abstract.
Introduction
Cancers represent a major global health challenge that consistently ranks as a leading cause of death and disease worldwide [1]. These pathophysiological conditions emerge from the abnormal differentiation and proliferation of cells, primarily driven by genetic mutations. Such cells exhibit a dysregulated cell cycle, resist programmed cell death (apoptosis), and are characterized by uncontrolled growth and the capacity to metastasize [2]. A tumor is a vivid example of gender as an indicator of the pathogenesis, prognosis, and even diagnosis of many diseases [3]. Factors that expose the body to cancer include hormonal changes, age, obesity, genetic defects, inflammation, ultraviolet exposure, viral and bacterial infections, well-being, and general sanitary conditions, as well as the availability of health care [4, 5]. Other significant risk factors include smoking, alcohol consumption, environmental pollution, and a lack of physical activity [6–8]. For non-reproductive cancers, the prevalence in men is twice as high as in women. Some cancers, such as bladder cancer, appear up to four times more often in men than in women [9].
As a major public health concern of the 21st century, cancer statistics from 2022 identify lung cancer as the most prevalent, representing 12.4% of all diagnosed cases, followed by breast cancer at 11.6%, colorectal cancer at 9.6%, prostate cancer (PCa) at 7.3%, and stomach cancer at 4.9% (Figure 1) [1]. Meanwhile, lung cancer is characterized by the highest mortality rate (18.7%), followed by colorectal (9.3%), liver (7.8%), breast (6.8%), stomach (6.8%), and pancreatic cancers (4.8%) [10].
Figure 1.
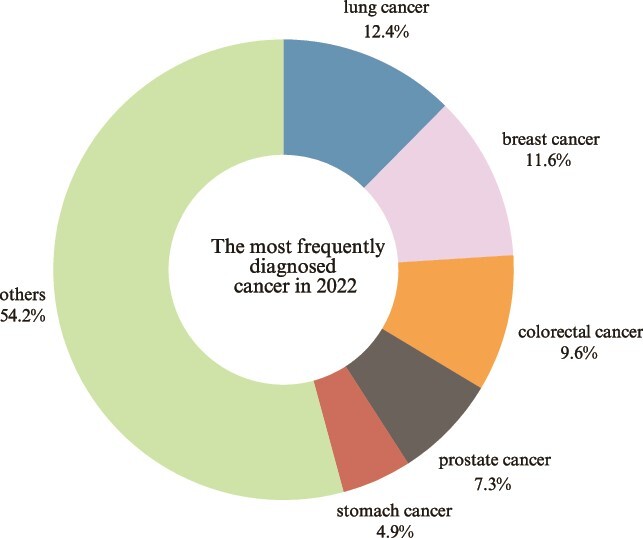
The frequency of most common cancers in 2022. Source: GLOBOCAN 2022 [1, 10]. Created with Canva.
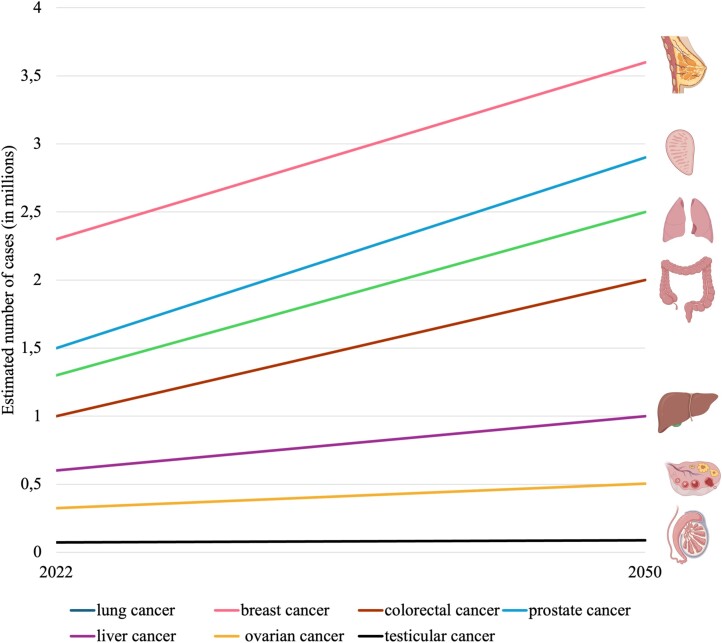
Year by year, there is an observed increase in cancer incidence rates [11]. It is estimated that by 2050, the incidence of various types of cancers will nearly double among both women and men [10]. For example, it is estimated that from 2022 to 2045, the number of cancer cases globally for both genders will increase, including lung cancer (up by about 65.0%), breast cancer (+36.9%), colorectal cancer (+60.0%), PCa (+65.0%), liver cancer (+66.0%), ovarian cancer (OC; +40.0%), and testicular cancer (TC; +7.0%) (Figure 2) [10]. It is projected that by 2040, over 27 million new cancer cases will be diagnosed globally, presenting a substantial public health challenge as the population ages [6, 8].
Figure 2.
Estimated cancer cases (2022–2050). Source: GLOBOCAN 2022. [1, 10]. Created with MS Excel and BioRender.com.
Moreover, the prevalence of tumors, including those affecting the genitourinary system, escalates with age [12]. With life expectancy on the rise, the impact of cancer spans all ages, making aging a primary risk factor for cancerous diseases [13, 14]. By 2050, it is estimated that over 20% of the global population will be aged 60 or older, posing economic, health, financial, and social threats [8, 15]. The relationship between cellular aging and the development of cancerous lesions, as well as the molecular changes that occur, are crucial areas of focus for cancer research and monitoring, leading to potential alterations in entire cellular systems [15].
Molecular research is currently focused on developing methods for the precise elimination of cancerous structures. Accurate knowledge of cellular relationships at multiple levels can be crucial [16–18]. Preclinical animal studies primarily use young organisms, i.e., relatively fresh cells, which may constitute an obstacle to obtaining objective data that can be translated into clinical cases [15]. Another important problem in cancer treatment is the knowledge of people participating in diagnostic procedures. Until recently, information came mainly from research on male bodies and male cell lines. Until 1993, the female sex was excluded from clinical trials, and it was assumed that male and female cells were biologically identical. This resulted in the need to withdraw a large number of drugs already in the first decade of the 21st century, as their adverse effect on the female sex was demonstrated [9, 19]. For the proper development of medicine and the success of clinically developed therapies, it seems necessary to characterize studies in terms of gender, due to differences in epidemiology, mortality, survival, pathophysiology, symptoms, response to treatment, and psychological effects [20, 21]. It is also absolutely necessary to know intracellular and intercellular relationships. The processes of aging, secretion, absorption, and communication at the molecular level have an indisputable impact on the development of most diseases. The formation and movement of certain structures, such as extracellular vesicles, is now such valuable knowledge that scientists devote entire research to them. Seeing their enormous potential, researchers emphasize the need to expand our knowledge of these particles [22–25].
Extracellular vesicles (EVs) have been the subject of extensive research over the past two decades and continue to be a significant area of interest for many researchers [26–28]. It has been shown that EVs are mediators during pathological conditions including cardiovascular diseases [29], neurodegenerative diseases such as Alzheimer disease [30], retinal degeneration [31], endometriosis [32], and uterine inflammation [33], as well as prostate dysfunctions [34]. Additionally, these vesicles contribute to dysfunctions in the reproductive systems of both genders and are linked to reproductive cancers [35].
The aim of this review was to discuss the importance of exosomes, in the carcinogenesis process and cancer progression in the female, as well as male, reproductive system.
Extracellular vesicles
Extracellular vesicles are small lipid-bilayer enclosed particles released from cells into the extracellular matrix (ECM) [36]. They can be found in body fluids, such as blood, follicular fluid, semen, breast milk, saliva, tears, and urine [27, 37, 38]. EVs contribute to cell-to-cell communication; they act like messengers to the target cell [39]. They also contribute to the maintenance of homeostasis [29]. Characterized by their diverse cargo, EVs transport nucleic acids such as DNA and RNA, including miRNA, and messenger RNA (mRNA), as well as proteins, for example signaling proteins, enzymes, transcription factors, and also lipids [26]. This group includes distinct subtypes—exosomes (EXOs), micro-vesicles (MVs), and apoptotic bodies (ABs; also referred to as apoptotic vesicles) [40], which differ from each other in size, biogenesis mechanism, cargo, or even function [39]. EXOs are nano-sized vesicles with a restricted size of 30–150 nm, MVs have a diameter between 100 and 1000 nm, and ABs have a diameter between 1 and 5 μm [41, 42]. These differences are detailed in Table 1.
Table 1.
Differences between subtypes of EVs
| Exosomes | Micro-vesicles | Apoptotic bodies | References | |
|---|---|---|---|---|
| Origin | Exocytosis from multivesicular body (MVB) | Plasma membrane | Apoptotic cell | [43, 44] |
| Size | 30–150 nm | 100–1000 nm | 1–5 μm | [41, 42] |
| Cargo | Nucleic acids, proteins, lipids, enzymes, metabolites | Cytosolic contents—lipids, nucleic acids, metabolites | Different fragments of organelles, chromatin, histones, degraded proteins | [43, 45, 46] |
| Role/Function | Cell-to-cell communication, transportation functions, biomarkers of diseases, modulation of immunological response, tissue regeneration, cancer progression, neurodegenerative diseases | Modulation of immunological response, blood coagulation, angiogenesis, cancer progression, interaction between distant or neighboring cell | Generated during apoptosis, cleaned by phagocytotic cells | [40, 44, 46, 47] |
| Markers | Tetraspanins (CD63, CD81, CD82, CD9), heat shock proteins (HSP60, HSP70, HSP90) | Integrins, selectins, CD40, Annexin A1 | Annexin V, phosphatidylserine | [43, 44, 48] |
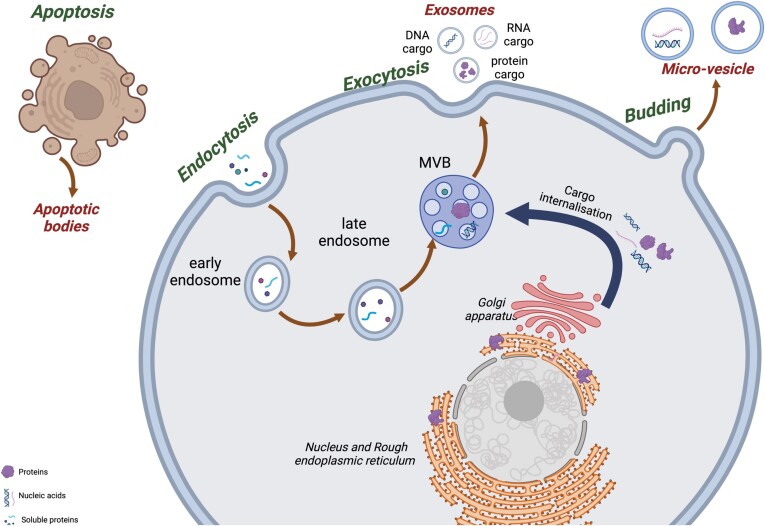
Each subtype of EVs has a different mechanism of biogenesis (Figure 3). Exosomes originate from the fusion of the multivesicular body (MVB) with the membrane during exocytosis. Micro-vesicles are formed from cell membranes through budding, whereas apoptotic bodies are released during apoptosis [43, 49].
Figure 3.
Biogenesis of EVs. Created with BioRender.com.
Exosomes
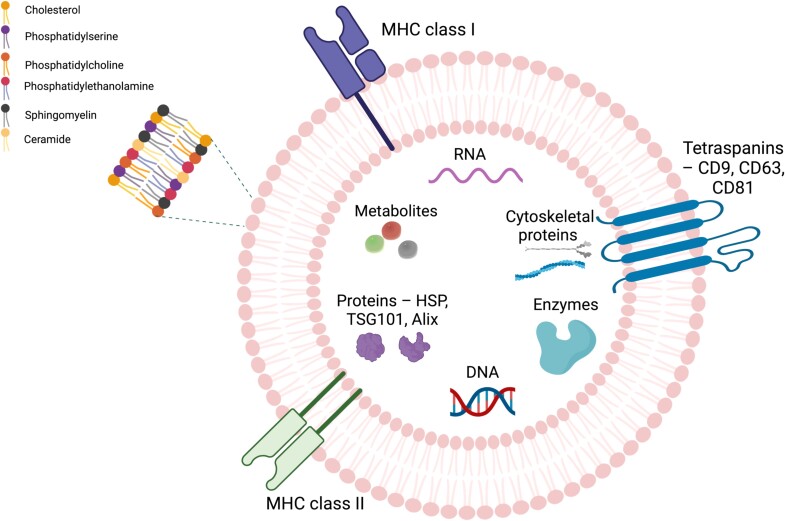
Exosomes are nanoscale vesicles, typically ranging from 30 to 150 nm in diameter. They are formed through a process that begins with endocytosis of the plasma membrane, leading to the development of early endosomes. These early endosomes eventually mature into MVBs [50]. Exosomes are derived from cellular membranes and thus contain a rich mixture of lipids such as phosphatidylserine (PS), phosphatidylcholines (PCs), phosphatidylethanolamine (PE), ceramide, sphingomyelin, and cholesterol (Figure 4) [51, 52]. The lipid bilayer of these vesicles serves as a protective barrier, shielding the cargo from enzymatic degradation during their transit from donor to recipient cells [53]. Moreover, their surface contains extracellular transmembrane proteins, such as Major Histocompatibility Complex (MHC) classes I and II, integrins, and tetraspanins (CD9, CD63, CD81). Exosomes also contain cytoskeletal proteins (e.g., actin, tubulin, myosin), enzymes (proteases), intracellular proteins, including integrins, and acetylcholonesterases, as well as MVB formation proteins like Alix and TSG101 [51, 52, 54, 55]. These proteins are recognized as markers for EVs enabling their identification [52]. Additionally, tetraspanins facilitate cell penetration and fusion, cytoskeletal proteins are involved in exosome release, and exosomes are enriched with heat shock proteins (HSPs), predominantly HSP70 and HSP90, which play a crucial role in stress response [51]. EXOs contain nucleic acids—DNA (single-stranded DNA, double-stranded DNA, mitochondrial DNA), and RNA, inducing messenger RNA (mRNA), microRNA (miRNA), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), and long non-coding RNAs (lncRNAs) [56].
Figure 4.
Structure and composition of exosomes. Created with BioRender.com.
The release of extracellular vesicles depends on specific conditions and can differ in both physiological and pathological states, including during diseases and the process of cancer development [26]. Therefore, they are potential biomarkers of a broad spectrum of diseases and conditions [49]. EXOs are secreted by various types of cells, such as endothelial cells and immune cells (lymphocytes, macrophages, dendritic cells [DCs], natural killer [NK] cells, fibroblasts, and epithelial cells [48]). EVs are also utilized for drug delivery purposes, including gene therapy applications. Exosomes are noted for their excellent biocompatibility, minimal immunogenicity, the capability to cross the blood–brain barrier, and the precision of direct drug delivery to the targeted therapy site [52].
Exosomes can activate a range of bioactivities in recipient cells through the binding of ligands on their membranes. This can occur through internalization, membrane fusion, or receptor–ligand binding. Internalization takes place via endocytic pathways, and fusion of the exosome with the plasma membrane occurs with the aid of Rab and Soluble NSF attachment protein receptors (SNAREs) proteins, which are necessary for this process. On the other hand, receptor binding on the cell surface occurs through ligands on the exosome surface, which carry MHC–peptide complexes and tumor necrosis factor (TNF), participating in the transmission of the immune response signal [57]. Receptor binding occurs in the case of exosomes released from DCs, which contain TNF and TRAIL (TNF-related apoptosis-inducing ligand). They bind to receptors on recipient cells and lead to the activation of T cells, triggering an immune response and inducing apoptosis [58]. Exosomes absorbed through processes such as endocytosis, phagocytosis, or fusion with the plasma membrane of the recipient cell allow for the direct release of their cargo into the cell and bypass lysosomal degradation [54]. There is also the possibility for exosome cargoes to diffuse into the cytoplasm, which may facilitate RNA transport [54]. The cytoplasmic reticulum is a deposit site for mRNA and miRNA [59].
Exosomes significantly contribute to intercellular communication and are produced by both healthy and defective cells [60]. Research has demonstrated that exosomes are crucial in immunological regulation, tissue repair, angiogenesis, and cellular signaling, and they also play roles in reproductive processes [61, 62]. In our previous paper, we extensively reviewed the involvement of exosomes in the reproductive systems of both genders, covering aspects such as oogenesis, embryo implantation, fertilization process, sperm maturation, capacitation, and acrosomal reaction [63].
Exosomes in cancer growth
Besides participating in physiological functions, exosomes also play a crucial role in cancer development [64]. The vesicles released from tumors are referred to as tumor-derived exosomes (TEXs) [65]. Tumor-derived exosomes are characterized by generating immunosuppression, uncontrolled cell growth, angiogenesis, and metastasis. Moreover, these particles can be used as cancer biomarkers [66]. The cargoes carried by exosomes released from healthy cells and those from the tumor microenvironment differ [65]. Moreover, cancer cells secrete more exosomes than other cells [67].
The release of exosomes and the nature of their cargo vary depending on the cell type, growth status, or receptor stimulation [68]. Research has shown that exosomes play a critical role in mediating communication between cancer cells and the surrounding stromal cells by delivering non-coding RNAs (ncRNAs) [53]. Despite the fact that ncRNAs do not encode proteins, these molecules participate in various physiological and pathological processes, including the life activities of cells and the development of diseases [12]. They are pivotal in evaluating the efficacy of cancer treatments, offering a method for non-invasive early detection and diagnosis of cancer through their role as biomarkers [69]. Additionally, they are key in developing therapeutic strategies to halt cancer progression and support the immune system’s strength [70].
These ncRNAs are implicated in the onset and development of various cancers, including those affecting the reproductive organs [12, 71]. The ncRNA group includes microRNAs (miRNAs), lncRNAs, and circular RNAs (circRNAs), each playing distinct regulatory roles [72]. MiRNAs are involved in regulating gene expression at the post-translational level and cell proliferation and influence genes and signaling pathways [73]. In turn, lncRNAs contribute to the establishment of disease states, and circRNAs, which are cell-specific, often show altered expression patterns during the process of carcinogenesis, potentially affecting the spread of cancer. Exosomes, enriched with ncRNA, can traverse through bodily fluids from donor cells, such as cancer cells, to distant recipient cells, significantly impacting tumor growth, proliferation, progression, and metastasis [74–76]. This exosomal pathway helps cancer metastases evade the immune system [75]. Numerous studies have indicated that exosomal ncRNA can be employed as a biomarker for various cancers, including breast cancer [77], lung cancer [53], gastric cancer [78], and colorectal cancer [79]. There is increasing research proving the role of exosomes and ncRNA, especially miRNA, in genitourinary tumors [12, 80].
The tumor microenvironment (TME) provides a unique setting for communication between cancerous and non-cancerous cells through messengers like proteins, lipids, and ncRNAs, which are transported within exosomes as their cargo [81]. Moreover, the TME is involved in cancer growth [12]. The TME comprises cancer cells, fibroblasts, immune cells, and products of secretion like cytokines and chemokines, as well as metabolites (e.g., lactic acid) and blood vessels [50, 82, 83]. This environment is characterized by low oxygen levels and high acidosis due to lactate content [84]. The TME also includes immune cells, notably tumor-associated macrophages (TAMs), which are the most abundant [85]. Macrophages are categorized into two types—M1 and M2. M1 macrophages, which secrete pro-inflammatory cytokines like lipopolysaccharide (LPS) and interferon-gamma (IFN-γ), combat cancer, whereas M2 macrophages produce cytokines that support anti-inflammatory responses and aid in tumor progression [86, 87]. Moreover, M2 macrophages play a role in angiogenesis, tissue repair, and tumor progression [86]. Cytokines like IL-4 and IL13 promote M2 macrophage polarization within the TME [88]. It has been proven that macrophages within the TME can spontaneously transform from the M1 to M2 phenotype [86]. Exosomes have been shown to influence the polarization of these macrophages and promote tumor growth [86, 87]. Tumor-associated macrophages are known to promote tumorigenesis and are associated with poor prognosis [85]. Cancer cells may encourage TAMs to adopt an M2 phenotype, while TAMs can infiltrate tumors utilizing exosomes laden with miRNAs that are essential for cancer progression [85].
The functions of exosomes vary depending on the tissue or cell from which the vesicles originate [89]. It has been proven that cells that are linked to the cancers secrete more extracellular vesicles than other, non-cancer, cells. This occurs to meets the need for nutrient supply and transfer of information. Cancer patients have almost twice the content of EXOs in their blood compared to healthy individuals [90]. Exosomes and their bioactive cargo can be involved in the regulation of intercellular communication between tumor cells and the TME and participate in angiogenesis [12]. Moreover, exosomes released by tumors are important in immune modulation because of their suppressive cargo [91]. Exosomes contribute to promoting tumor growth, angiogenesis, and preparation of pre-metastasis niches.
Promoting tumor growth
Exosomes released by cells retain the properties of the donor cells and can thus modulate the recipient cells, as is the case in cancers [92]. Cancer EVs have been shown to carry oncogenic proteins, RNA, and DNA [93]. Additionally, they can carry bioactive tumor molecules that, when taken up by recipient cells in the TME, can contribute to immune tolerance or unresponsiveness. In contrast, EVs derived from immune cells can inhibit tumor growth and proliferation [93]. It is also possible to activate signaling pathways that promote cell proliferation, such as the PI3K/Akt and mitogen-activated protein kinase (MAPK) pathways [94]. Activation of the phosphoinositide 3-kinase (PI3K/AKT) pathway occurs mainly in cancers and translates into the promotion of tumor cell growth. In turn, the MAPK pathway has been shown to promote tumor cell migration [94]. Research indicates that the disruption of signaling pathways such as Ras/Raf/MEK/ERK, p53, mammalian Target of Rapamycin (mTOR), and STAT3 in cancer may impact exosome release and subsequent proliferation. MEK and ERK kinases, frequently altered by the Ras and Raf kinases through small G proteins, play significant roles in cancer proliferation, invasion, and metastatic spread [95]. The mTOR
signal pathway is important in maintaining cell proliferation, growth, and survival [96]. Similarly, the STAT3 pathway contributes to cancer growth, survival, metastasis, and angiogenesis during tumor development [97]. Additionally, mutations in the tumor suppressor protein p53, present within exosomes, are linked to increased metastatic potential and resistance to cancer therapies [98].
Angiogenesis
Exosomes can promote the formation of new blood vessels (angiogenesis) by carrying pro-angiogenic factors such as VEGF (vascular endothelial growth factor), TNF-α (tumor necrosis factor alpha), and miRNAs that regulate angiogenic pathways. This provides increased blood supply to the tumor, facilitating its growth and expansion [99, 100]. Exosomes released by fibroblasts enhance tumor activity, linked to increased cancer cell invasiveness and the facilitation of metastasis [12]. Furthermore, hypoxia in the tumor environment induces the expression of hypoxia-inducible factors (HIFs), which subsequently modulate the process of blood vessel formation [101]. Pro-angiogenic factors act on endothelial cells, which promotes proliferation. It has been shown that tumor-derived exosomes carry pro-angiogenic factors, and, when taken up by endothelial cells, they can stimulate the process of blood vessel formation, which may be important for tumor development [99].
Epithelial–mesenchymal transition
Exosomes contribute to cancer metastasis by participating in the regulation of epithelial–mesenchymal transition (EMT) and remodeling of the ECM, a component of the tumor environment [93, 102]. Extracellular matrix remodeling is a process in which epithelial cells lose their cellular polarity and adhesive properties and gain migratory and invasive properties. During this process, tumor epithelial cells, under the influence of cancer-associated fibroblasts (CAFs) in the tumor matrix, transform and acquire mesenchymal characteristics [93]. Cancer-associated fibroblasts have been shown to promote cancer progression and indicate drug resistance properties by reshaping the ECM into a physical barrier that impedes drug penetration [102]. Interactions between CAF, tumor, and mesenchymal cells take place with the participation of exosomes, and the stimulants are transforming growth factor beta (TGF-β), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), interleukin-1 (IL-1), and interleukin-6 (IL-6) [103]. It has also been shown that a group of Wnt glycoproteins, which are important in the development of neoplastic diseases, is important in the context of communication between stromal and epithelial cells [104]. The transport of these hydrophobic molecules is facilitated by exosomes [104]. Wnt then activates the Wnt/β-catenin pathway, which allows the preservation of tumor stemness [104].
Preparation of pre-metastatic niches
Tumor-derived exosomes can travel to distant organs and modify the local environment to create a pre-metastatic niche [105]. Furthermore, in the bone marrow, exosomes can contribute to cell–cell interactions due to their regular migration through the circulatory system and promote metastasis by altering the ECM and modulating local immune cells, making the distant site more conducive to cancer cell colonization [105, 106].
Integrin-dependent organotropism
Cancer metastasis is not random but depends on a series of controlling cells that have the ability to colonize and metastasize [107]. Exosomes have specific integrins in their structure, which define their target site in the body and can contribute to exosome biogenesis [108]. Exosomal integrins are involved in the process of carcinogenesis, particularly in preparing metastatic niches or modulating angiogenesis [108]. For example, exosomal integrins α6β4 and α6β1 are associated with lung metastasis, while those with αvβ5 integrins tend to direct metastases to specific organs, including the liver [107]. This specificity helps to direct metastases to specific organs. Integrins α6β1 in exosomes derived from pancreatic tumors can result in lung metastasis [109]. Integrin β1 is associated with PCa. In addition, α2β1 has been shown to reduce proliferation and increase invasion of this type of cancer [110]. Also, in cervical cancer (CC), increased expression of integrin β1 has been reported [111].
Another pathway of exosome’s action during tumor growth is their impact on DCs, which are important in immune responses against cancer and in maintaining immune tolerance. It has been demonstrated that TEX inhibits DC differentiation from bone marrow progenitors, thus weakening anti-tumor reactions. Moreover, components of the tumor’s exosomes, such as IL-6, can participate in the reduction of maturation of T cells. Tumor-derived exosomes can also disturb TNF-α and IL-12 production, which is crucial for T-cell activation [86].
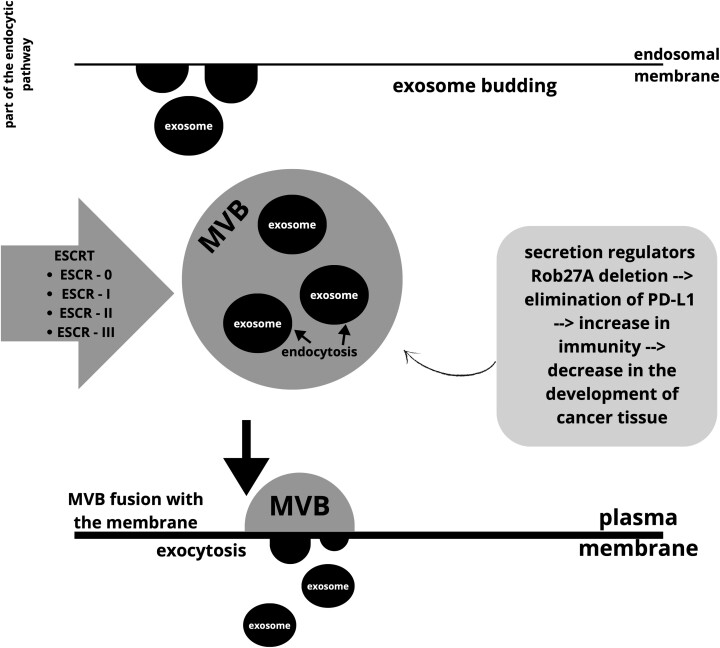
According to data from the exosome database, there are currently almost 10 000 proteins, over 3400 mRNA structures, over 2800 miRNAs, and over 1100 lipids [112]. The process of exosome formation (Figure 5) involves budding into the endosomal membrane, leading to the accumulation of vesicles in larger multivesicular bodies. For this reason, exosomes are classified as part of the endocytic pathway. Transmembrane-type proteins, such as exosomes, must initially be endocytosed and transferred to the early stages of endosomes. After the MVB fuses with the plasma membrane, the particle in the form of an exosome is released outside the cell through exocytosis. The entire process is influenced by factors influencing the formation of MVB structures but also the maturation of the endosomes themselves. One of the best-known factors of this type is the endosomal sorting complex required for transport (ESCRT). The structure of ESCRT is composed of several complexes: ESRT-0, ESRT-I, ESRT-II, and ESRT-III. The mechanism is designed to sort ubiquitinated cargoes that will be used for the lysosomal degradation process. Regulators of exosome secretion may affect MVB fusion with the membrane. Additionally, it has been shown that deletion of one of such regulators—Rab27A, leading to the elimination of exosomal programmed death-ligand 1 (PD-L1), can block the development of cancer tissue by stimulating anti-tumor immunity [26, 59, 113, 114].
Figure 5.
The process of exosome formation. Created with Canva.com.
Tumor progression modulators associated with exosomes
Exosomes transport a variety of cargo, including bioactive molecules such as proteins, lipids, RNA, and DNA that can influence various aspects of tumor biology, such as tumor growth, metastasis, immune evasion, and drug resistance [83, 115]. Modulators associated with cancer progression and exosomes may include a wide group of factors [116].
MicroRNA
Extracellular vesicles transporting miRNAs are associated with drug resistance. Administering it to drug-sensitive cancer cells complicates the treatment because it causes them to become desensitized to the administered medicinal substances. It is noted that the transfer of MDR-1/P-glycoprotein by this type of exosomes resulted in increased resistance to the administered drug. At the same time, exosomes have the ability to bind to drugs, which inhibits the reaction to the administered substance [116]. In addition, exosomal miRNAs are associated with the preparation of niches and components of the TME. They also participate in cell–cell communication and can be markers of cancer diseases [117].
Long non-coding RNA
Long non-coding RNAs play an important role in the process of oncogenesis. Long non-coding RNAs are involved in the regulation of many biological processes, such as apoptosis and proliferation, cell cycle, and cell proliferation [118]. At the same time, however, they may be expressed incorrectly during tumor formation. As a result, some lncRNAs act as tumor promoters or act as tumor suppressors. The functional role of lncRNAs is not fully understood, especially their involvement in carcinogenic processes [119].
DNA
Oncogenes such as C-myc exhibit specific mechanisms of cellular deregulation: patterns of amplification, mutation, and translocation. These reactions and changes in the genetic material characterize malignant tumors. The C-myc-related translocation process is occasionally combined with a second process—amplification or mutations. This type of deregulation caused by C-myc may influence clinical changes in cancer cells. As a result, it is necessary to modify therapeutic strategies [120].
Proteins
Exosomes released from cancer cells contain TGF-β, PD-L1, and epidermal growth factor receptor (EGFR) [121]. The concentration of TGF-β3 protein in EVs is a strong predictor of response to chemoradiotherapy. Exosomes isolated from the plasma of cancer patients contained, among others, significant amounts of TGF-β, OX40 (CD134), OX40L (CD134L), and HSP70. Particles of this type induce the process of apoptosis. As a result of this reaction, they can regulate the immune response. In the case of cancer patients, this leads to fueling the carcinogenesis process. Anti-PGE2 and TGF-β antibodies do not allow the induction of Myeloid-Derived Suppressor Cells (MDSCs) and, as a result, weaken the capacity of MDSCs. Knowledge of these molecular relationships may prove helpful in developing targeted tumor treatment strategies [122].
Lipids
Sphingolipids are involved in apoptosis and the development of cancer cells. This happens because these particles control the transmission of signals by cancer cells [123]. In tumor tissues, the recruitment of non-malignant cells, e.g., immunosuppressive cells, is induced by lipid mediators such as prostaglandin E2 (PGE2). Prostaglandins themselves are also synthesized in this type of cells. This may lead to the development of an immunosuppressive TME [124].
Cytokines
Cytokines such as IL-6 can induce mRNA expression in cancer cells. Wei et al. [125], in their study on mice, showed that IL-6 promoted the growth and development of CC due to angiogenesis. It has also been shown that colorectal cancer cells are able to produce IL-6 on their own. Stimulation of these cells with another type of cytokine (IL-17) also causes these cells to produce IL-6, which further stimulates angiogenesis. This process is directly related to the development of cancer tissue. Some CXC chemokines also show this type of activity, but, additionally, they are based on the ELR (Glu-Leu-Arg) motif. The presence of this motif in the particle promotes angiogenesis, while its absence inhibits it. Thus, the chemokines CXCL1, CXCL6, CXCL8, and CXCLR5 are ELR-positive and promote angiogenesis, while CXCL4, CXCL10, and CXCL14 are ELR-negative and thus inhibit angiogenesis. The chemokine CXCL12 is an exceptional case in this situation because it is characterized by an ELR-negative model and nevertheless induces the angiogenesis process [126].
Integrins
Cell adhesion receptors are integrins; their function depends on biochemical reactions inside the cell. As signalers, integrins bind extracellular ligands. In the case of signals coming from the extracellular environment, integrins collect information and transmit it inside. In healthy tissue, these particles act as checkpoints and monitor the effect on proliferation. In the case of cancer tissue, this effect changes. A change in the internal environment of the cell may cause disruption of metabolic processes, including oxygen. Lack of sufficient amounts of these particles increases the production of certain integrins. This may be related primarily to cancer metastases and a reduction in the patient’s prognosis. Changes in the processes related to signaling between the internal and external environment of the cell may cause changes in integrin, e.g., the transformation of α3β1-integrin to α6β4, which indirectly leads to the differentiation of cancer cells. Also, changes in αvβ3 surface integrins may increase the migration of cancer cells [127].
Signal transduction molecules
Signal transduction molecules, in the case of their mutations or overactivity, forms of EGFR are noticed in cancer tissues. Biochemical mechanisms involving EGFR may promote the development of cancer tissue in the event of such an abnormality. In cancer treatment, some substances specifically block EGFR reaction pathways. However, some patients do not respond to therapies based on anti-EGFR drugs. Some patients also become tolerant to these types of substances to the point that they stop reacting to them [128].
Mutation in Mitochondrial DNA (mtDNA)
Mutations in mtDNA affect cancerous tissues and often result in a deterioration of the patient’s health. Mitochondria play a key role in the biophysical state of the cells examined. At the same time, mtDNA may be a potential target for anticancer therapies [129].
Matrix metalloproteinases
Matrix metalloproteinases (MMPs) MMP-2 and MMP-9 are involved in the degradation of the ECM. This leads to simplified invasion of cancer cells and, consequently, tumor metastasis. Effects on these metalloproteinases can block tumor growth and cell invasion. Inhibiting the transformation of these particles seems to be a potential therapeutic strategy [130].
The role of exosomes in male reproductive cancers
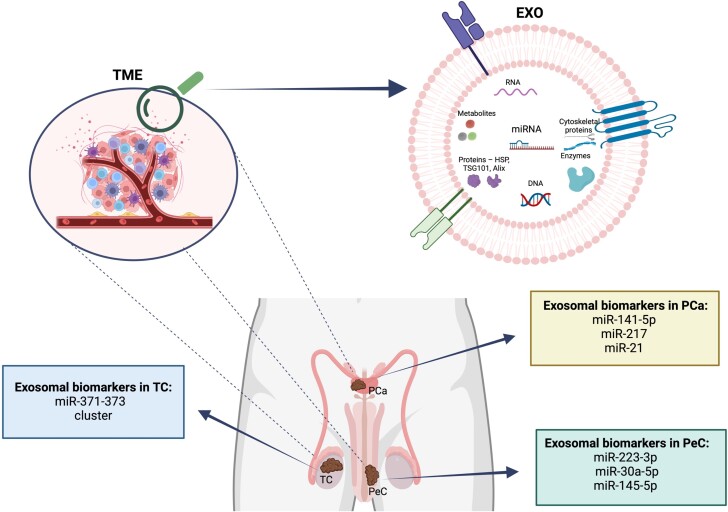
Semen is abundant with exosomes that enhance sperm motility [131] and capacitation [132] and also help inhibit premature acrosome reactions [133, 134]. Prostasomes are the exosomes derived from the prostate, while exosomes from epididymal fluid are referred to as epididymosomes [63]. Exosomes, particularly prostasomes, play a protective role in the female reproductive system by modulating sperm activities. These prostasomes enhance sperm motility and capacitation, manage acrosomal reactions, and prevent premature capacitation by delivering cholesterol and sphingomyelin. Additionally, epididymosomes from the epididymis are crucial for increasing sperm’s fertilization capabilities, protecting them from oxidative stress, and controlling their movement [63]. They also can affect spermatogenesis due to their ability to transfer the molecules that can improve communication within the testes structures such as Sertoli cells and Leydig cells [61, 135]. These vesicles can indicate dysfunctions and conditions around male reproductive system [134]. In 2022, the most abundant cancer among men was lung cancer; the second was PCa [10]. Cancers within the male reproductive tract (Figure 6) can lead to infertility, overall health issues, and reduced semen quality in men [136].
Figure 6.
Male reproductive cancers and their exosomal potential biomarkers. Created with BioRender.com. Legend: PCa—prostate cancer, PeC—penile cancer, TC—testicular cancer, TME—tumor microenvironment, EXO—exosomes.
Prostate cancer
Prostate cancer ranks as the second most common cancer in men [137] and stands fifth in global cancer mortality rates [138]. Risk factors include age, obesity, smoking, and genetic predispositions [139]. This is the most commonly detected cancer in men over the age of 50 [140]. Prostate-specific antigen testing, currently used for diagnosis, shows limited specificity, leading to potential false positives [141]. The development of liquid biopsy techniques, which utilize the isolation of EVs as potential biomarkers, shows promise for more accurate diagnostics [142].
To better identify markers for disease, researchers are studying exosomal miRNAs. In their study, Li et al. [143] collected blood from 31 PCa patients and 19 healthy men, isolating exosomes to analyze for miRNA-125a-5p and miR-141-5p. They found elevated levels of miRNA-141-5p in patients with PCa compared to healthy individuals, whereas miRNA-125a-5p levels were significantly lower in patients than in healthy controls. Moreover, the ratio of miR-125a-5p to miR-141-5p was higher in men with PCa than in those from the control group [143]. It has also been shown that exosomal miR-141-3p from prostate cancer modulates the cancer microenvironment and contributes to the development of PCa bone metastasis [144]. The researchers suggest that high levels of exosomal miR-141-3p and low levels of miR-125a-5p could serve as biomarkers for this type of tumor [143]. In the study conducted by Xu et al. [145], differences in miRNA-141 expression from EVs collected from urine and blood serum were analyzed in benign prostatic hyperplasia (BPH) and PCa. The authors did not detect significant differences [145].
In a study conducted by Zhou et al. [146], exosomes isolated from the blood of 10 prostate cancer patients and 10 healthy individuals revealed that levels of miR-217 were elevated in the patient group compared to the healthy group. The research indicated that higher levels of miR-217 contribute to the proliferation and invasion of cells [146].
Reports indicate that miR-21 and miR-375 show varied expressions in cases of PCa [147]. In studies conducted by Joković et al. [148], blood was collected from 35 prostate cancer patients and 34 men with BPH, and exosomes from the patients’ plasma were analyzed for the expression of miR-21 and miR-375 in aggressive and non-aggressive forms of the tumor. Nearly three times higher expression levels of exosomal miR-21 were found in patients with aggressive forms of the tumor compared to less aggressive PCa. No differences were observed for miR-375. The researchers suggest the potential use of miR-21 as a prognostic marker in PCa [148]. It has been proven that miR-21 can participate in proliferation and tumor cell apoptosis by signaling pathway PTEN/PI3K/AKT, which can be a key target for chemotherapy [149]. Overexpression of miR-21 may be associated with EMT and lead to tumor malignancy. miR-21 can be successfully used as a biomarker of this type of cancer, but its molecular regulatory pathways need to be explored [150].
Testicular cancer
According to 2022 data, TC ranked 27th in the incidence of cancers [10]. However, it is one of the most common malignant diseases among young men aged 20–40 [140, 151]. Ninety-five percent of TC diagnoses involve germ cell tumors (GCTs), while the other 5% comprise sex cord–stromal tumors that typically develop from Leydig or Sertoli cells [152, 153]. However, GCTs are the most commonly encountered type [152]. Furthermore, within GCTs, there are classifications of seminomas and non-seminomas [153].
The miR-371-373 cluster is considered a potential marker for testicular cancer, as it shows higher expression in patients with this condition [154]. In the research conducted by Alonso-Crisostomo et al. [80], the significance of exosomes in the context of testicular GCTs (TGCTs) and their TME was explored. This involved employing five distinct cell lines that represent various levels of TGCT malignancy, along with cultures of human testicular fibroblasts (symbolizing healthy tissue), human umbilical vein endothelial cells, and human macrophages to define the tumor’s environment. Exosomes were subsequently extracted. The investigation pinpointed crucial miRNAs, particularly those in the miR-371 ~ 373 and miR-302/367 clusters, which displayed significant elevation in TGCT-derived EVs compared to control samples [80]. These miRNAs appeared prevalently across different TGCT subtypes, highlighting their possible involvement in tumor dynamics and interactions with the TME. Experiments involving co-culturing and further analysis demonstrated that miRNAs transmitted through EVs from TGCT cells could notably modify miRNA profiles in TME cells such as fibroblasts and endothelial cells. These alterations were shown to impact critical cellular functions and activate pathways within the recipient cells, showcasing the significant effects of EV-mediated miRNA transport. Moreover, the overexpression system of the miRNA (miR-371a-OE) also induced angiogenesis in endothelial cells, suggesting that exosomes released from testicular cancer could facilitate tumor progression [80]. Studies [155] within this cluster have shown that the dynamics of expression in tumors were higher for miR-371a-3p compared to miR-372-3p and miR-373-3p.
Penile cancer
Penile cancer (PeC) is not a commonly diagnosed tumor, ranking 30th in cancer prevalence in 2022 [10]. It predominantly affects men in developing regions [156]. The majority of these cancers are squamous cell carcinomas (SCCs), typically occurring on the glans or the foreskin [140]. Characterized by a gradual spread to the inguinal lymph node metastasis (LNM), this cancer often progresses to more extensive metastasis [157]. Metastatic presence in lymph nodes serves as a prognostic factor for patient outcomes [158]. Key risk factors include smoking, inadequate hygiene, and chronic inflammation, although most SCC cases are associated with the human papillomavirus (HPV) [156].
Research by Ayoubian et al. [159] aimed to identify miRNAs associated with the development of SCC and their connection to HPV infection. In this study, 27 cancer tissue samples and 18 normal tissues as controls were collected from patients for RNA extraction. Of the cancer samples analyzed, 12 were HPV-negative and 10 were HPV-positive. Significant differences in the expression of 876 miRNAs (P ≤ 0.01) were found in HPV-positive SCC tissues compared to HPV-negative SCC tissues. Furthermore, variations in the expression of 118 miRNAs (P ≤ 0.01) were observed when comparing SCCs with metastasis to those without metastasis. The lowest expression levels in metastatic cancer tissues compared to non-metastatic ones were noted for miR-137 and miR-328-3p. These results suggest that HPV infection may influence miRNA expression in PeC [159].
A research initiative examined the relationship between miRNA levels and LNMs using 50 formalin-fixed, paraffin-embedded SCC samples [160]. This study measured the expression of miR-223-3p and compared it to non-cancerous cells within the same samples. Significantly higher levels of miR-223-3p (P < 0.001) were found in primary tumor samples from patients with LNMs than in non-cancerous tissues. Additionally, miR-223-3p levels were notably higher in samples from metastatic lymph nodes than in the primary tumors of the same patients [160]. The authors suggest that miR-223-3p levels may be a marker for LNM [160]. Additionally, higher levels of elements at metastatic sites of cancer enable faster and non-invasive detection of subsequent changes in individual exposed to them, which is a potential new method of monitoring patient health [161].
In a study aimed at determining the role of miRNAs in the carcinogenesis and growth of penile cancer, Furuya et al. [162] collected tissue samples from 24 cancerous and 24 non-cancerous sources. During the research, 83 miRNA transcripts were profiled, and differential expression was found in 8 of them. Specifically, miR-31-5p exhibited increased levels, whereas miR-30a-5p, miR-432-5p, miR-487b-3p, and miR-145-5p showed decreased expression in cancerous tissues compared to non-cancerous ones. The study highlights that the expression disparities in miRNAs such as miR-30a-5p, miR-432-5p, miR-487b-3p, and miR-145-5p could help distinguish between cancerous and non-cancerous conditions. The authors suggest the potential use of these miRNAs as biomarkers for diagnosing and understanding the progression of PeC [162].
The role of exosomes in female reproductive tract cancers
Exosomes play a pivotal role in the reproductive processes within the female reproductive tract. Originating from cells of this system, they are involved in regulating transcriptional activities, enhancing cellular growth and differentiation, and supporting oogenesis and oocyte maturation [63]. The follicular fluid contains numerous molecules and EXOs. These structures have been shown to play a significant role in the process of follicle regulation and maturation [118, 163]. Furthermore, these vesicles are crucial for embryo implantation and help sustain pregnancy through hormonal regulation. Due to their origin-specific nature, EXOs also function as potential biomarkers for detecting disorders such as polycystic ovary syndrome (PCOS) and various reproductive cancers [63].
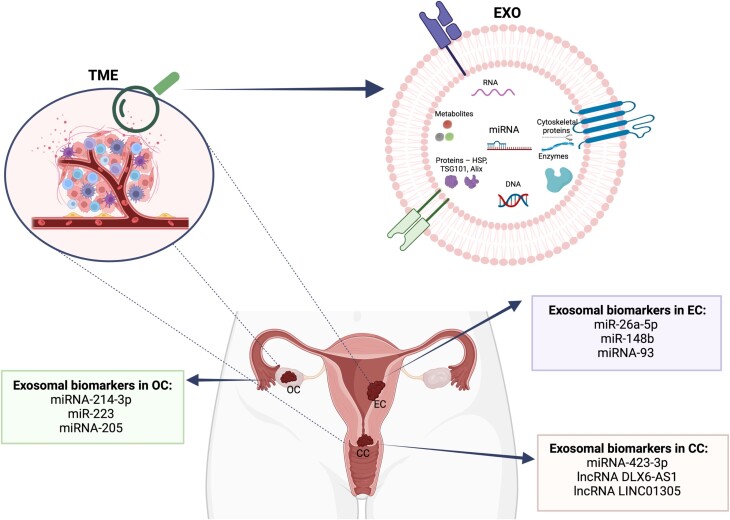
In 2022, breast, lung, and colorectal cancers were the most common among women, with CC ranking as the fourth most prevalent [10]. Gynecological cancers pose a significant threat to women’s health, impacting reproductive organs like the uterus, ovaries, and cervix (Figure 7) [75, 164]. These cancers are highly lethal and present diagnostic challenges. While cancer metastasis is typically thought to occur via the lymphatic or circulatory systems, there is growing evidence that exosomes also play a role in this process [164]. Among gynecological malignancies, OC is the most lethal of all conditions in women; in the second place ranks CC [165].
Figure 7.
Gynecological cancers and their exosomal potential biomarkers. Created with BioRender.com
Endometrial cancer
Endometrial cancer (EC) is a very common malignancy of the female reproductive tract [166]. The risk factors of this type of condition include PCOS, infertility, estrogen exposure, and obesity and usually occur after menopause [167, 168]. The majority of EC cases occur in women between the ages of 65 and 75 [169]. The prognosis is poorer in cases with lymphatic metastasis [170]. Exosomes carrying miRNA contribute to the pathophysiology of EC by originating from tumor cells [169]. These oncogenic miRNA serves as a biomarker for EC [171].
Endometrial cancer arising in the lining of the uterus is divided into the more common endometrial EC (80% of patients) and the rarer non-endometrial EC (20% of patients). Exosomal miRNAs as biomarkers may play a key role not only in detecting the appearance of cancerous tissue but also the occurrence of metastases to other places in the body. This potential biomarker is also a valuable indicator in screening, diagnostic, and immune studies [169, 171, 172].
In research conducted by Wang et al. [170], it was found that the level of exosomal miR-26a-5p was lower in patients with EC, especially those with LNM human lymphatic endothelial cells (HLECs), compared to healthy individuals. The researchers also observed that transferring miR-26a-5p to HLECs could promote lymphangiogenesis in vitro. Furthermore, in a mouse model, the application of exosomal miR-26a-5p was shown to inhibit the proliferation of EC tumors and their metastasis to lymphatic vessels [170].
Research has shown that the development and migration of endometrial cancer may occur through CAFs, which produce exosomes carrying miRNAs [173]. In studies, 23 samples of EC were obtained from which CAFs were isolated, and, from a control group without cancer changes, normal fibroblasts (NFs) were isolated. An in vivo study was conducted on immunodeficient BALB/C mice to observe cancer progression. Throughout the study, the expression of miR-148b, a key regulator of EC progression, was assessed. It was found that miR-148b levels were lower in the culture of tumor-associated fibroblasts and EC compared to normal fibroblasts. Researchers argue that reduced levels of this miRNA in EC cells and the TME may indicate disease progression and a poor prognosis. In mice injected with NF, CAF, or CAF overexpressing miR-148b, after 4 weeks, CAFs significantly promoted the formation of EC metastases to the lungs, while overexpression of miR-148b limited the ability of CAFs to promote proliferation. Exosomes obtained from CAFs enhance cancer proliferation [173]. Li et al. [173] demonstrated that overexpression of miR-148b in endometrial tumor cells could result in inhibition of tumor progression.
In additional studies, the impact of exosomal miRNA-93 on EC was explored by Zheng et al. [174]. For this research, blood samples were collected from 100 patients with confirmed EC. These patients exhibited higher levels of exosomal miRNA-93 compared to healthy subjects, and, notably, higher levels were also observed in patients who smoked. This suggests that exosomal miRNA-93 levels could serve as prognostic indicators for the condition [174].
Ovarian cancer
Ovarian cancer ranks as the eighth most common cause of cancer deaths among women globally [175]. It primarily manifests in epithelial and mesenchymal forms, with the mesenchymal type accounting for over 90% of OC cases [176]. Almost half of the high-grade ovarian tumors are associated with mutations in the BRCA1 and BRCA2 genes [176]. Exosomes released from OC cells carry crucial proteins such as EpCAM, CD24, and cancer antigen 125 (CA125), which are significant for the development of this cancer type. [177, 178].
A study conducted by Yang et al. [76] analyzed miRNA-214-3p levels in OC tissues and serum from 29 patients. They found higher expressions of miR-21-5p, miR-141-3p, miR-200a-3p, miR-200b-3p, miR-203-3p, miR-205-5p, and miR-214-3p in both low- and high-malignancy OC tissues compared to benign tumors, particularly noting the highest expression of miRNA-214-3p in high-grade epithelial OC (EOC) tissues and exosomes [76]. The miR-200 family (including miR-200a, miR-200b, and miR-141) plays an important role in the EMT [179] and may be associated with a poorer prognosis in patients with EOC [180].
MiR-223 plays a critical role in regulating immune cell functions, including macrophage polarization and inflammatory responses [181]. This miRNA is also involved in the development and invasion of breast cancer and its resistance to treatment [182]. In the research conducted by Yang et al. [183], miR-223 levels in plasma exosomes from 78 patients with EOC, 40 patients with benign ovarian tumors, and 52 healthy controls were analyzed. Significantly higher levels of exosomal miR-223 were found in patients with EOC compared to healthy individuals and those with benign tumors. Higher expression of miR-223 was also observed in patients with metastases, and a correlation between CA125 and miR-223 levels suggested a link with disease progression [183]. Similar results of increased miR-223-3p expression were reported in the study by Fang et al. [184].
Further research focused on exosomal miRNA-205 [185], which is linked to the growth and progression of OC [176, 186]. A study involving 99 participants, including 36 with OC, 31 with benign tumors, and 32 healthy controls, showed higher expressions of miR-205 and CA125 in the OC groups compared to the others [185]. Higher levels of miR-205 were also noted in patients with LNMs, indicating that miR-205 is associated with OC and metastasis and may serve as a prognostic marker in patients with OC [185].
Cervical cancer
The primary factor implicated in CC is the HPV [165]. Despite the availability of vaccines, the disease remains a significant health threat [187]. The progression of cervical cancer may be influenced by the dysregulation of exosomal miRNA [87]. miRNA-423-3p, known for its role in the development of cancers such as colorectal and lung cancer, also affects the malignancy of CC. Exosomes can affect the polarization of macrophages; TAMs are induced to become either M1 or M2 within the TME. M1 macrophages secrete pro-inflammatory cytokines to counteract cancer, while M2 macrophages produce cytokines that support cancer development [87].
In research conducted by Yan et al. [87] on CC cell lines HeLa, CaSki, and SiHa, as well as normal human CC lines, showed that patients with CC exhibited lower plasma exosomal miRNA expression (including miR-328–3p, miR-423–3p, miR-323a-3p, miR-10b-5p, miR-10a-5p, miR-125a-5p, miR-99b-5p, and miR-139–5p). There was a noted reduction in the expression of miRNA-423-3p in CC cell lines. It was also confirmed that exosomal miRNA-423-3p inhibits the M2 polarization of macrophages, leading to reduced malignancy of CC cells and tumor growth. This suggests that this miRNA could serve as a biomarker for CC treatment [87].
Other researchers have shown that the oncogenic lncRNA DLX6-AS1 plays a role in various cancers, including breast [188], bladder [189], and gastric cancer [190]. In additional studies involving 114 patients with CC, 60 individuals with cervical intraepithelial neoplasia (CIN), and 110 healthy controls, the role of exosomal lncRNAs in the progression and initiation of cervical cancer was examined [191]. Elevated levels of exosomal lncRNA DLX6-AS1 were found in the serum of CC patients compared to those with CIN and healthy individuals. A correlation between disease metastasis to lymph nodes and high expression of lncRNA DLX6-AS1 was also demonstrated. The researchers identify lncRNA DLX6-AS1 as a biomarker in the diagnosis of CC [191].
Other studies also confirm the role of lncRNA in the progression of CC [192]. Research on the role of lncRNA LINC01305 in the development of this cancer was conducted using 114 tissue samples from patients with CC. It was demonstrated that exosomes are the primary distributors of this RNA, which enhances cancer progression. Furthermore, a correlation was established between the level of expression and the survival rate of the patients studied. Higher expression in exosomes is associated with the progression of the disease [192].
Vulvar cancer
Vulvar cancer (VC), which accounts for only 5% of gynecological cancers, occurs in four different histological types: squamous cell, basal cell, extramammary Paget disease, and melanoma. In the case of this cancer, the patient may not show symptoms for a long time, which results in delayed diagnosis and initiation of treatment procedures [193]. If the disease is detected quickly and treated effectively, patient survival can reach 88% [194].
Researchers’ attention is once again focusing on exosomal miRNAs due to the possibility of using them as biomarkers allowing the identification of tissues with cancer lesions. Changes in the expression of miRNAs in exosomal form (miR-21, miR-141, miR-200a, miR200b, miR-200c, miR-203, miR-205, miR-214) have been proven, regardless of their origin. This shows that the mentioned exosomes can be used identically to the miRNA profile in cancer tissue [180, 195].
Exosomal secretion is observed between tumor cells and the tumor environment. Rapid identification of this type of molecular transmitter can potentially be used to improve the diagnosis of gynecological cancers [196, 197]. However, the impact of exosomal particles is much broader due to their impact on the immune system, which actually affects the body’s homeostasis during cancer [196, 198]. It should be remembered that the described relationships do not occur individually but constitute a certain part of larger gene and protein relationships. The observed changes are therefore not the result of one factor but a complex process. For this reason, the rapid identification of exosomes as biomarkers specific to a given ailment may allow not only the early detection of cancer but also the determination of its stage, which is crucial when starting treatment of the patient [199].
Limitations
Exosomal ncRNAs, including microRNAs, lncRNAs, and circular RNAs, have become key players in the regulation of gene expression and cellular processes [200]. In the case of reproductive system cancers, such as OC, EC, CC, and PCa, and exosomal ncRNAs have gained considerable attention as potential biomarkers and prognostic indicators due to their stability in body fluids and their ability to reflect the molecular state of tumors [201–204]. This area of research holds promise for improving cancer diagnosis, prognosis, and treatment strategies.
The tumor environment is rich in a variety of cell types that secrete exosomes. Cancer cells secrete exosomes rich in oncogenic factors, exosomes derived from cancer-associated fibroblasts remodel the ECM and enhance progression, and immune cells carry exosomes that stimulate immune responses. In turn, platelet-derived exosomes have been shown to promote cancer cell survival [83]. Extracellular vesicles can be secreted by every cell in the body [205], which may implicate research due to the chaotic mix of exosomes in the blood. Exosomes carry a molecular fingerprint of the cell from which they were released [206]. A significant challenge in exosome identification is their internal cargo heterogeneity. This makes analysis in body fluids difficult, and the detection of a single exosome carrying a specific cargo may contribute to better diagnostics and faster cancer detection [207].
Future research directions should focus on (a) developing diagnostic biomarkers aimed at early detection of cancer and assessment of disease stage, as well as predicting treatment response; (b) elucidating the possible mechanism of their impact on the tumor microenvironment through in-depth studies on the ability to tumorigenesis and inhibit immune response; and (c) the therapeutic potential of exosomal ncRNAs. Currently, the possibility of using exosomes to assess the response to treatment in cancer diseases is being investigated. The challenge is how to determine the origin of exosomes because cells from the TME can secrete them and which can differ in size and cargo. In addition, the use of exosomes to transport specific contents may be an undoubtedly promising technique in cancer treatment [208]. From this perspective, further research is needed.
Conclusion
Exosomes significantly influence the development and progression of cancer by facilitating communication between cancer cells and the microenvironment, as well as changing immune responses and increasing the possibility of metastasis. The review highlights the importance of exosomes as carriers of genetic markers that have the potential to be used in cancer diagnosis and treatment.
Moreover, the involvement of exosomes in reproductive system cancers highlights their role in both promoting and potentially inhibiting cancer through their complex interactions with cellular processes. As the incidence of cancer continues to increase worldwide, understanding the multifaceted role of exosomes offers promising avenues for developing new therapeutic strategies and improving patient outcomes. Future research should aim to elucidate the detailed mechanisms by which exosomes influence cancer progression and explore their potential for clinical applications, including more precise biomarkers and targeted delivery systems for cancer therapy.
Contributor Information
Alicja Kowalczyk, Department of Environment Hygiene and Animal Welfare, Wrocław University of Environmental and Life Sciences, Wrocław, Poland.
Marcjanna Wrzecińska, Department of Ruminant Science, West Pomeranian University of Technology in Szczecin, Szczecin, Poland.
Elżbieta Gałęska, Department of Environment Hygiene and Animal Welfare, Wrocław University of Environmental and Life Sciences, Wrocław, Poland.
Ewa Czerniawska-Piątkowska, Department of Ruminant Science, West Pomeranian University of Technology in Szczecin, Szczecin, Poland.
Mercedes Camiña, Department of Physiology, University of Santiago de Compostela, Santiago de Compostela, Spain.
Jose P Araujo, Mountain Research Centre (CIMO), Instituto Politécnico de Viana do Castelo, Ponte de Lima, Portugal.
Zbigniew Dobrzański, Department of Environment Hygiene and Animal Welfare, Wrocław University of Environmental and Life Sciences, Wrocław, Poland.
Authorship contribution
All mentioned authors contributed equally to this work. All authors have seen and approved the submitted manuscript and all related materials, agreed to be listed as an author, and agreed to the submitted order of authorship.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clinicians 2024; 74:229–263. [DOI] [PubMed] [Google Scholar]
- 2. Almalki WH, Almujri SS. The dual roles of circRNAs in Wnt/β-catenin signaling and cancer progression. Pathol Res Pract 2024; 255:155132. [DOI] [PubMed] [Google Scholar]
- 3. Nevola R, Tortorella G, Rosato V, Rinaldi L, Imbriani S, Perillo P, Mastrocinque D, La Montagna M, Russo A, Di Lorenzo, Alfano M, Rocco M, et al. Gender differences in the pathogenesis and risk factors of hepatocellular carcinoma. Biology 2023; 12:984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lakkis NA, Abdallah RM, Musharrafieh UM, Issa HG, Osman MH. Epidemiology of breast, corpus uteri, and ovarian cancers in Lebanon with emphasis on breast cancer incidence trends and risk factors compared to regional and global rates. Cancer Control 2024; 31. 10.1177/10732748241236266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teschendorff AE. On epigenetic stochasticity, entropy and cancer risk. Philos Trans R Soc B 2024; 379:20230054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai YK, Ye JF, Ran Q, Ao HS. Internet-based eHealth technology for emotional well-being among the older adults with a family cancer history: full mediation effects of health information self-efficacy and cancer fatalism. BMC Psychol 2024; 12:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramamoorthy T, Nath A, Singh S, Mathew S, Pant A, Sheela S, Kaur G, Sathishkumar K, Mathur P. Assessing the global impact of ambient air pollution on cancer incidence and mortality: a comprehensive meta-analysis. JCO Glob Oncol 2024; 10:e2300427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roos E, Heikkinen S, Seppä K, Pietiläinen O, Ryynänen H, Laaksonen M, Roos T, Knekt P, Männistö S, Härkänen T, Jousilahti P, Koskinen S, et al. Pairwise association of key lifestyle factors and risk of solid cancers - a prospective pooled multi-cohort register study. Prev Med Rep 2024; 38:102607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero J-J, DeMeo DL, De Vries GJ, Epperson CN, Govindan R, Klein SL, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet 2020; 396:565–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today (version 1.1). International Agency for Research Cancer, France. Available online: https://gco.iarc.who.int/today (accessed on May 22, 2024).
- 11. Wang Q, Shao X, Zhang Y, Zhu M, Wang FXC, Mu J, Li J, Yao H, Chen K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med 2023; 12:11149–11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu S, Mu C, Sun J, Hu X, Yao Y. Role of Exosomal non-coding RNA in the tumour microenvironment of genitourinary system tumours. Technol Cancer Res Treat 2023; 22:15330338231198348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2022. J National Cancer Cent 2024; 4:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Montégut L, López-Otín C, Kroemer G. Aging and cancer. Mol Cancer 2024; 23:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer 2020; 20:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chakraborty S, Sharma G, Karmakar S, Banerjee S. Multi-OMICS approaches in cancer biology: new era in cancer therapy. Biochim Biophys Acta (BBA) - Mol Basis Dis 2024; 1870:167120. [DOI] [PubMed] [Google Scholar]
- 17. Haupt S, Caramia F, Klein SL, Rubin JB, Haupt Y. Sex disparities matter in cancer development and therapy. Nat Rev Cancer 2021; 21:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Srivastava S, Jayaswal N, Kumar S, Sharma PK, Behl T, Khalid A, Mohan S, Najmi A, Zoghebi K, Alhazmi HA. Unveiling the potential of proteomic and genetic signatures for precision therapeutics in lung cancer management. Cell Signal 2024; 113:110932. [DOI] [PubMed] [Google Scholar]
- 19. Albert H. Breaking down sex and gender barriers in search of precision medicine. Inside Precis Med 2023; 10:6–10. [Google Scholar]
- 20. Bernstein SR, Kelleher C, Khalil RA. Gender-based research underscores sex differences in biological processes, clinical disorders and pharmacological interventions. Biochem Pharmacol 2023; 215:115737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Merdji H, Long MT, Ostermann M, Herridge M, Myatra SN, De Rosa S, Metaxa V, Kotfis K, Robba C, De Jong A, Helms J, Gebhard CE. Sex and gender differences in intensive care medicine. Intensive Care Med 2023; 49:1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bamankar S, Londhe VY. The rise of extracellular vesicles as new age biomarkers in cancer diagnosis: promises and pitfalls. Technol Cancer Res Treat 2023; 22:153303382211492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dwivedi M, Ghosh D, Saha A, Hasan S, Jindal D, Yadav H, Yadava A, Dwivedi M. Biochemistry of exosomes and their theranostic potential in human diseases. Life Sci 2023; 315:121369. [DOI] [PubMed] [Google Scholar]
- 24. Salvioli S, Basile MS, Bencivenga L, Carrino S, Conte M, Damanti S, De Lorenzo R, Fiorenzato E, Gialluisi A, Ingannato A, Antonini A, Baldini N, et al. Biomarkers of aging in frailty and age-associated disorders: state of the art and future perspective. Ageing Res Rev 2023; 91:102044. [DOI] [PubMed] [Google Scholar]
- 25. Wu Q, Fu S, Xiao H, Du J, Cheng F, Wan S, Zhu H, Li D, Peng F, Ding X, Wang L. Advances in extracellular vesicle nanotechnology for precision Theranostics. Adv Sci 2023; 10:e2204814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kumar MA, Baba SK, Sadida HQ, Marzooqi SA, Jerobin J, Altemani FH, Algehainy N, Alanazi MA, Abou-Samra A-B, Kumar R, al-Shabeeb Akil AS, Macha MA, et al. Extracellular vesicles as tools and targets in therapy for diseases. Sig Transduct Target Ther 2024; 9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manai F, Smedowski A, Kaarniranta K, Comincini S, Amadio M. Extracellular vesicles in degenerative retinal diseases: a new therapeutic paradigm. J Control Release 2024; 365:448–468. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y, Tang Y, Chen X, Sun X, Zhao M, Chen Q. Therapeutic potential of miRNAs in placental extracellular vesicles in ovarian and endometrial cancer. Hum Cell 2024; 37:285–296. [DOI] [PubMed] [Google Scholar]
- 29. Caño-Carrillo S, Castillo-Casas JM, Franco D, Lozano-Velasco E. Unraveling the Signaling dynamics of small extracellular vesicles in cardiac diseases. Cells 2024; 13:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cyr B, Cabrera Ranaldi EDLRM, Hadad R, Dietrich WD, Keane RW, De Rivero Vaccari JP. Extracellular vesicles mediate inflammasome signaling in the brain and heart of Alzheimer’s disease mice. Front Mol Neurosci 2024; 17:1369781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martins B, Pires M, Ambrósio AF, Girão H, Fernandes R. Contribution of extracellular vesicles for the pathogenesis of retinal diseases: shedding light on blood-retinal barrier dysfunction. J Biomed Sci 2024; 31:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chu X, Hou M, Li Y, Zhang Q, Wang S, Ma J. Extracellular vesicles in endometriosis: role and potential. Front Endocrinol 2024; 15:1365327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abeysinghe P, Turner N, Mosaad E, Logan J, Mitchell MD. Dynamics of inflammatory cytokine expression in bovine endometrial cells exposed to cow blood plasma small extracellular vesicles (sEV) may reflect high fertility. Sci Rep 2023; 13:5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Troisi A, Schrank M, Bellezza I, Fallarino F, Pastore S, Verstegen JP, Pieramati C, Di Michele A, Talesa VN, Martìnez Barbitta M, Orlandi R, Polisca A. Expression of CD13 and CD26 on extracellular vesicles in canine seminal plasma: preliminary results. Vet Res Commun 2024; 48:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gurunathan S, Kang M-H, Song H, Kim NH, Kim J-H. The role of extracellular vesicles in animal reproduction and diseases. J Animal Sci Biotechnol 2022; 13:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xue VW, Wong SCC, Zhao H, Cho WCS. Proteomic characterization of extracellular vesicles in programmed cell death. Proteomics 2024; 24:e2300024. [DOI] [PubMed] [Google Scholar]
- 37. Muraoka A, Yokoi A, Yoshida K, Kitagawa M, Asano-Inami E, Murakami M, Bayasula, Miyake N, Nakanishi N, Nakamura T, Osuka S, Iwase A, et al. Small extracellular vesicles in follicular fluids for predicting reproductive outcomes in assisted reproductive technology. Commun Med 2024; 4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xiong Y, Lou P, Xu C, Han B, Liu J, Gao J. Emerging role of extracellular vesicles in veterinary practice: novel opportunities and potential challenges. Front Vet Sci 2024; 11:1335107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barranco I, Alvarez-Barrientos A, Parra A, Martínez-Díaz P, Lucas X, Roca J. Immunophenotype profile by flow cytometry reveals different subtypes of extracellular vesicles in porcine seminal plasma. Cell Commun Signal 2024; 22:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Uddin MJ, Mohite P, Munde S, Ade N, Oladosu TA, Chidrawar VR, Patel R, Bhattacharya S, Paliwal H, Singh S. Extracellular vesicles: the future of therapeutics and drug delivery systems. Intell Pharm 2024; 2:312–328. [Google Scholar]
- 41. Ramezani A, Tafazoli A, Salimi F, Ghavami M, Arjmandi H, Khalesi B, Hashemi ZS, Khalili S. Current knowledge on therapeutic, diagnostic, and prognostics applications of exosomes in multiple myeloma: opportunities and challenges. Arch Biochem Biophys 2024; 756:109994. [DOI] [PubMed] [Google Scholar]
- 42. Zou X, Lei Q, Luo X, Yin J, Chen S, Hao C, Shiyu L, Ma D. Advances in biological functions and applications of apoptotic vesicles. Cell Commun Signal 2023; 21:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Afridi S, Sharma P, Choudhary F, Rizwan A, Nizam A, Parvez A, Farooqi H. Extracellular vesicles: a new approach to study the brain’s neural system and its diseases. Cell Biochem Biophys 2024; 82:521–534. [DOI] [PubMed] [Google Scholar]
- 44. Kim HI, Park J, Zhu Y, Wang X, Han Y, Zhang D. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp Mol Med 2024; 56:836–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen Y, Li X, Yang M, Liu S-B. Research progress on morphology and mechanism of programmed cell death. Cell Death Dis 2024; 15:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee YJ, Shin KJ, Chae YC. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp Mol Med 2024; 56:877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Potrich C, Pedrotti A, Pederzolli C, Lunelli L. Functional surfaces for exosomes capturing and exosomal microRNAs analysis. Colloids Surf B Biointerfaces 2024; 233:113627. [DOI] [PubMed] [Google Scholar]
- 48. Moeinzadeh L, Razeghian-Jahromi I, Zarei-Behjani Z, Bagheri Z, Razmkhah M. Composition, biogenesis, and role of exosomes in tumor development. Stem Cells Int 2022; 2022:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yu J, Sane S, Kim J-E, Yun S, Kim H-J, Jo KB, Wright JP, Khoshdoozmasouleh N, Lee K, Oh HT, Thiel K, Parvin A, et al. Biogenesis and delivery of extracellular vesicles: harnessing the power of EVs for diagnostics and therapeutics. Front Mol Biosci 2024; 10:1330400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen S, Sun J, Zhou H, Lei H, Zang D, Chen J. Department of Oncology, the second Hospital of Dalian Medical University, Dalian 116023, China; Department of Radiotherapy, the Second Hospital of Dalian Medical University, Dalian 116023, China new roles of tumor-derived exosomes in tumor microenvironment. Chinese. J Cancer Res 2024; 36:151–166. 10.21147/j.issn.1000-9604.2024.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palomar-Alonso N, Lee M, Kim M. Exosomes: membrane-associated proteins, challenges and perspectives. Biochem Biophys Rep 2024; 37:101599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun M, Zhang H, Liu J, Chen J, Cui Y, Wang S, Zhang X, Yang Z. Extracellular vesicles: a new star for gene drug delivery. IJN 2024; 19:2241–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lai X, Zhong J, Zhang B, Zhu T, Liao R. Exosomal non-coding RNAs: novel regulators of macrophage-linked intercellular communication in lung cancer and inflammatory lung diseases. Biomolecules 2023; 13:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wandrey M, Jablonska J, Stauber RH, Gül D. Exosomes in cancer progression and therapy resistance: molecular insights and therapeutic opportunities. Life 2023; 13:2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hánělová K, Raudenská M, Masařík M, Balvan J. Protein cargo in extracellular vesicles as the key mediator in the progression of cancer. Cell Commun Signal 2024; 22:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abdelsalam M, Ahmed M, Osaid Z, Hamoudi R, Harati R. Insights into exosome transport through the blood–brain barrier and the potential Therapeutical applications in brain diseases. Pharmaceuticals 2023; 16:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lau NCH, Yam JWP. From exosome biogenesis to absorption: key takeaways for cancer research. Cancer 1992; 15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Han W, Zhang H, Feng L, Dang R, Wang J, Cui C, Jiang P. The emerging role of exosomes in communication between the periphery and the central nervous system. MedComm 2023; 4:e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal 2021; 19:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gangadaran P, Madhyastha H, Madhyastha R, Rajendran RL, Nakajima Y, Watanabe N, Velikkakath AKG, Hong CM, Gopi RV, Muthukalianan GK, Valsala Gopalakrishnan A, Jeyaraman M, et al. The emerging role of exosomes in innate immunity, diagnosis and therapy. Front Immunol 2023; 13:1085057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Izadpanah M, Yalameha B, Sani MZ, Cheragh PK, Mahdipour M, Rezabakhsh A, Rahbarghazi R. Exosomes as Theranostic agents in reproduction system. Adv Biol 2024; 8:e2300258. [DOI] [PubMed] [Google Scholar]
- 62. Wan R, Liu S, Feng X, Luo W, Zhang H, Wu Y, Chen S, Shang X. The revolution of exosomes: from biological functions to therapeutic applications in skeletal muscle diseases. J Orthop Transl 2024; 45:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kowalczyk A, Wrzecińska M, Czerniawska-Piątkowska E, Kupczyński R. Exosomes – spectacular role in reproduction. Biomed Pharmacother 2022; 148:112752. 10.1016/j.biopha.2022.112752. [DOI] [PubMed] [Google Scholar]
- 64. Lyu C, Sun H, Sun Z, Liu Y, Wang Q. Roles of exosomes in immunotherapy for solid cancers. Cell Death Dis 2024; 15:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Andre M, Caobi A, Miles JS, Vashist A, Ruiz MA, Raymond AD. Diagnostic potential of exosomal extracellular vesicles in oncology. BMC Cancer 2024; 24:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kumar S, Dhar R, Kumar LBSS, Shivji GG, Jayaraj R, Devi A. Theranostic signature of tumor-derived exosomes in cancer. Med Oncol 2023; 40:321. [DOI] [PubMed] [Google Scholar]
- 67. Gong X, Chi H, Strohmer DF, Teichmann AT, Xia Z, Wang Q. Exosomes: a potential tool for immunotherapy of ovarian cancer. Front Immunol 2023; 13:1089410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xiong M, Chen Z, Tian J, Peng Y, Song D, Zhang L, Jin Y. Exosomes derived from programmed cell death: mechanism and biological significance. Cell Commun Signal 2024; 22:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aqil F, Gupta RC. Exosomes in cancer therapy. Cancer 2022; 14:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yang Q, Li S, Ou H, Zhang Y, Zhu G, Li S, Lei L. Exosome-based delivery strategies for tumor therapy: an update on modification, loading, and clinical application. J Nanobiotechnol 2024; 22:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hashemipour M, Boroumand H, Mollazadeh S, Tajiknia V, Nourollahzadeh Z, Rohani Borj M, Pourghadamyari H, Rahimian N, Hamblin MR, Mirzaei H. Exosomal microRNAs and exosomal long non-coding RNAs in gynecologic cancers. Gynecol Oncol 2021; 161:314–327. [DOI] [PubMed] [Google Scholar]
- 72. Ma B, Wang S, Wu W, Shan P, Chen Y, Meng J, Xing L, Yun J, Hao L, Wang X, Li S, Guo Y. Mechanisms of circRNA/lncRNA-miRNA interactions and applications in disease and drug research. Biomed Pharmacother 2023; 162:114672. 10.1016/j.biopha.2023.114672. [DOI] [PubMed] [Google Scholar]
- 73. Do Nascimento Medeiros, Sarmento ACA, Bernardes-Oliveira E, De Oliveira R, Lima MEGB, Gonçalves AK, De Souza Dantas D, De Oliveira Crispim JC. Evaluation of Exosomal miRNA as potential biomarkers in cervical cancer. Epigenomes 2023; 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jasim SA, Al-Hawary SIS, Kaur I, Ahmad I, Hjazi A, Petkov I, Ali SHJ, Redhee AH, Shuhata Alubiady MH, Al-Ani AM. Critical role of exosome, exosomal non-coding RNAs and non-coding RNAs in head and neck cancer angiogenesis. Pathol Res Pract 2024; 256:155238. [DOI] [PubMed] [Google Scholar]
- 75. Xu C, Xu P, Zhang J, He S, Hua T, Huang A. Exosomal noncoding RNAs in gynecological cancers: implications for therapy resistance and biomarkers. Front Oncol 2024; 14:1349474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yang C, Kim HS, Park SJ, Lee EJ, Kim SI, Song G, Lim W. Inhibition of miR-214-3p aids in preventing epithelial ovarian cancer malignancy by increasing the expression of LHX6. Cancer 11, 11:1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ashekyan O, Abdallah S, Shoukari AA, Chamandi G, Choubassy H, Itani ARS, Alwan N, Nasr R. Spotlight on Exosomal non-coding RNAs in breast cancer: An In Silico analysis to identify potential lncRNA/circRNA-miRNA-target Axis. IJMS 2022; 23:8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jiang C, Zhang J, Wang W, Shan Z, Sun F, Tan Y, Tong Y, Qiu Y. Extracellular vesicles in gastric cancer: role of exosomal lncRNA and microRNA as diagnostic and therapeutic targets. Front Physiol 2023; 14:1158839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yu X, Bu C, Yang X, Jiang W, He X, Sun R, Guo H, Shang L, Ou C. Exosomal non-coding RNAs in colorectal cancer metastasis. Clin Chim Acta 2024; 556:117849. [DOI] [PubMed] [Google Scholar]
- 80. Alonso-Crisostomo L, Trendell J, Ferraresso M, Bailey S, Ward D, Scurlock ZGL, Wenlock SC, Bastos CAP, Jugdaohsingh R, Faria NJ, Enright AJ, Scarpini CG, et al. Testicular germ cell tumour cells release microRNA -containing extracellular vesicles that induce phenotypic and genotypic changes in cells of the tumour microenvironment. Int J Cancer 2024; 154:372–388. [DOI] [PubMed] [Google Scholar]
- 81. Da Costa VR, Araldi RP, Vigerelli H, D’Ámelio F, Mendes TB, Gonzaga V, Policíquio B, Colozza-Gama GA, Valverde CW, Kerkis I. Exosomes in the tumor microenvironment: from biology to clinical applications. Cells 2021; 10:2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tan S, Yang Y, Yang W, Han Y, Huang L, Yang R, Hu Z, Tao Y, Liu L, Li Y, Oyang L, Lin J, et al. Exosomal cargos-mediated metabolic reprogramming in tumor microenvironment. J Exp Clin Cancer Res 2023; 42:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Panda SS, Sahoo RK, Patra SK, Biswal S, Biswal BK. Molecular insights to therapeutic in cancer: role of exosomes in tumor microenvironment, metastatic progression and drug resistance. Drug Discov Today 2024; 29:104061. [DOI] [PubMed] [Google Scholar]
- 84. Paskeh MDA, Entezari M, Mirzaei S, Zabolian A, Saleki H, Naghdi MJ, Sabet S, Khoshbakht MA, Hashemi M, Hushmandi K, Sethi G, Zarrabi A, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol 2022; 15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang Y, Ma H, Li Y, Su R. MiR-192-5p-modified tumor-associated macrophages-derived exosome suppressed endometrial cancer progression through targeting IRAK1/NF-κB Signaling. Reprod Sci 2022; 29:436–447. [DOI] [PubMed] [Google Scholar]
- 86. Wang H, Liu S, Zhan J, Liang Y, Zeng X. Shaping the immune-suppressive microenvironment on tumor-associated myeloid cells through tumor-derived exosomes. Int J Cancer 2024; 154:2031–2042. [DOI] [PubMed] [Google Scholar]
- 87. Yan, Zhang S, Jia J, Yang J, Song Y, Duan H. Exosomal MiR-423–3p inhibits macrophage M2 polarization to suppress the malignant progression of cervical cancer. Pathol Res Pract 2022; 235:153882. [DOI] [PubMed] [Google Scholar]
- 88. Zhang Y, Ma S, Li T, Tian Y, Zhou H, Wang H, Huang L. ILC1-derived IFN-γ regulates macrophage activation in colon cancer. Biol Direct 2023; 18:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Qiu Y, Lu G, Li N, Hu Y, Tan H, Jiang C. Exosome-mediated communication between gastric cancer cells and macrophages: implications for tumor microenvironment. Front Immunol 2024; 15:1327281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. He G, Peng X, Wei S, Yang S, Li X, Huang M, Tang S, Jin H, Liu J, Zhang S, Zheng H, Fan Q, et al. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol Cancer 2022; 21:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Safaei S, Fadaee M, Farzam OR, Yari A, Poursaei E, Aslan C, Samemaleki S, Shanehbandi D, Baradaran B, Kazemi T. Exploring the dynamic interplay between exosomes and the immune tumor microenvironment: implications for breast cancer progression and therapeutic strategies. Breast Cancer Res 2024; 26:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pan J, Sheng S, Ye L, Xu X, Ma Y, Feng X, Qiu L, Fan Z, Wang Y, Xia X, Zheng JC. Extracellular vesicles derived from glioblastoma promote proliferation and migration of neural progenitor cells via PI3K-Akt pathway. Cell Commun Signal 2022; 20:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, Fu W, Yi J, Wang J, Du G. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B 2021; 11:2783–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wu Y, Niu D, Deng S, Lei X, Xie Z, Yang X. Tumor-derived or non-tumor-derived exosomal noncodingRNAs and signaling pathways in tumor microenvironment. Int Immunopharmacol 2022; 106:108626. [DOI] [PubMed] [Google Scholar]
- 95. Hikita T, Uehara R, Itoh RE, Mitani F, Miyata M, Yoshida T, Yamaguchi R, Oneyama C. MEK/ERK-mediated oncogenic signals promote secretion of extracellular vesicles by controlling lysosome function. Cancer Sci 2022; 113:1264–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. You M, Xie Z, Zhang N, Zhang Y, Xiao D, Liu S, Zhuang W, Li L, Tao Y. Signaling pathways in cancer metabolism: mechanisms and therapeutic targets. Sig Transduct Target Ther 2023; 8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Almouh M, Pakravan K, Ghazimoradi MH, Motamed R, Bakhshinejad B, Hassan ZM, Babashah S. Exosomes released by oxidative stress-induced mesenchymal stem cells promote murine mammary tumor progression through activating the STAT3 signaling pathway. Mol Cell Biochem 2024; 479:3375–3391. [DOI] [PubMed] [Google Scholar]
- 98. Pavlakis E, Neumann M, Stiewe T. Extracellular vesicles: messengers of p53 in tumor–stroma communication and cancer metastasis. IJMS 2020; 21:9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ghosh S, Mahajan AA, Dey A, Rajendran RL, Chowdhury A, Sen S, Paul S, Majhi S, Hong CM, Gangadaran P, Ahn BC, Krishnan A. Exosomes in bone cancer: unveiling their vital role in diagnosis, prognosis, and therapeutic advancements. J Cancer 2024; 15:4128–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Uslu D, Abas BI, Demirbolat GM, Cevik O. Effect of platelet exosomes loaded with doxorubicin as a targeted therapy on triple-negative breast cancer cells. Mol Divers 2024; 28:449–460. [DOI] [PubMed] [Google Scholar]
- 101. Niu S, Hu J, Lin S, Hong Y. Research progress on exosomes/microRNAs in the treatment of diabetic retinopathy. Front Endocrinol 2022; 13:935244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Peng Z, Tong Z, Ren Z, Ye M, Hu K. Cancer-associated fibroblasts and its derived exosomes: a new perspective for reshaping the tumor microenvironment. Mol Med 2023; 29:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fang Z, Meng Q, Xu J, Wang W, Zhang B, Liu J, Liang C, Hua J, Zhao Y, Yu X, Shi S. Signaling pathways in cancer-associated fibroblasts: recent advances and future perspectives. Cancer Commun 2023; 43:3–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hu Y-B, Yan C, Mu L, Mi Y-L, Zhao H, Hu H, Li X-L, Tao D-D, Wu Y-Q, Gong J-P, Qin JC. Exosomal Wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene 2019; 38:1951–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bhatia R, Chang J, Munoz JL, Walker ND. Forging new therapeutic targets: efforts of tumor derived exosomes to prepare the pre-metastatic niche for cancer cell dissemination and dormancy. Biomedicine 2023; 11:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Menck K, Sivaloganathan S, Bleckmann A, Binder C. Microvesicles in cancer: small size, large potential. IJMS 2020; 21:5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Si G, Chen X, Li Y, Yuan X. Exosomes promote pre-metastatic niche formation in colorectal cancer. Heliyon 2024; 10:e27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Soe ZY, Park EJ, Shimaoka M. Integrin regulation in immunological and cancerous cells and exosomes. IJMS 2021; 22:2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen Y, Kleeff J, Sunami Y. Pancreatic cancer cell- and cancer-associated fibroblast-derived exosomes in disease progression, metastasis, and therapy. Discov Oncol 2024; 15:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Samaržija I. The potential of extracellular matrix- and integrin adhesion complex-related molecules for prostate cancer biomarker discovery. Biomedicine 2024; 12:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Schettino MT, Preti EP, Vietri V, Agrillo N, Iavazzo N, Fasulo DD, De Franciscis P, Campitiello MR, Vastarella MG, Riemma G, Gardella B, Murina F. The role of β1 integrin/CD29 as a potential prognostic factor for the risk of progression to cervical carcinoma in HPV-associated lesions. Medicina 2024; 60:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Soni N, Jangra J, Chaudhary M, Nandi G, Bissa B. Perspective Chapter: Exosomes – The Surreptitious Intercellular Messengers in the Body. In Exosomes - Recent Advances From Bench to Bedside. IntechOpen; 2023. Available at: 10.5772/intechopen.110779. [DOI] [Google Scholar]
- 113. Chang W-H, Cerione RA, Antonyak MA. Extracellular vesicles and their roles in cancer progrsssion. Cancer Cell Signaling 2020; 2174:143–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Xie, Zhou X, Fang M, Li H, Su P, Tu Y, Zhang L, Zhou F. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv Sci 2019; 6:1901779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yokoi A, Ochiya T. Exosomes and extracellular vesicles: rethinking the essential values in cancer biology. Semin Cancer Biol 2021; 74:79–91. [DOI] [PubMed] [Google Scholar]
- 116. Palazzolo S, Memeo L, Hadla M, Duzagac F, Steffan A, Perin T, Canzonieri V, Tuccinardi T, Caligiuri I, Rizzolio F. Cancer extracellular vesicles: next-generation diagnostic and drug delivery Nanotools. Cancer 2020; 12:3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Li C, Zhou T, Chen J, Li R, Chen H, Luo S, Chen D, Cai C, Li W. The role of Exosomal miRNAs in cancer. J Transl Med 2022; 20:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Marima R, Basera A, Miya T, Damane BP, Kandhavelu J, Mirza S, Penny C, Dlamini Z. Exosomal long non-coding RNAs in cancer: interplay, modulation, and therapeutic avenues. Non-coding RNA Res 2024; 9:887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tan J, Liu L, Zuo Z, Song B, Cai T, Ding D, Lu Y, Ye X. Overexpression of novel long intergenic non-coding RNA LINC02454 is associated with a poor prognosis in papillary thyroid cancer. Oncol Rep 2020; 44:1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Papouliakos S, Chrysovergis A, Papanikolaou V, Spyropoulou D, Papanastasiou G, Asimakopoulos AD, Mastronikoli S, Stathopoulos P, Roukas D, Adamopoulou M, Tsiambas E, Peschos D, et al. Clinical impact of C-myc oncogenic diversity on solid and lymphoid malignancies. Maedica 2024; 19:355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Meng, Zhang C, Yu P. Treating cancer through modulating exosomal protein loading and function: the prospects of natural products and traditional Chinese medicine. Pharmacol Res 2024; 203:107179. [DOI] [PubMed] [Google Scholar]
- 122. Wang X, Guo J, Yu P, Guo L, Mao X, Wang J, Miao S, Sun J. The roles of extracellular vesicles in the development, microenvironment, anticancer drug resistance, and therapy of head and neck squamous cell carcinoma. J Exp Clin Cancer Res 2021; 40:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jianyong Z, Yanruo H, Xiaoju T, Yiping W, Fengming L. Roles of lipid profiles in human non-small cell lung cancer. Technol Cancer Res Treat 2021; 20:153303382110414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Vasseur S, Guillaumond F. Lipids in cancer: a global view of the contribution of lipid pathways to metastatic formation and treatment resistance. Oncogenesis 2022; 11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wei L-H, Kuo M-L, Chen C-A, Chou C-H, Lai K-B, Lee C-N, Hsieh C-Y. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 2003; 22:1517–1527. [DOI] [PubMed] [Google Scholar]
- 126. Geindreau M, Bruchard M, Vegran F. Role of cytokines and chemokines in angiogenesis in a tumor context. Cancer 2022; 14:2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yousefi H, Vatanmakanian M, Mahdiannasser M, Mashouri L, Alahari NV, Monjezi MR, Ilbeigi S, Alahari SK. Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance. Oncogene 2021; 40:1043–1063. [DOI] [PubMed] [Google Scholar]
- 128. Uribe ML, Marrocco I, Yarden Y. EGFR in cancer: Signaling mechanisms, drugs, and acquired resistance. Cancer 2021; 13:2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Xie F, Guo W, Wang X, Zhou K, Guo S, Liu Y, Sun T, Li S, Xu Z, Yuan Q, Zhang H, Gu X, et al. Mutational profiling of mitochondrial DNA reveals an epithelial ovarian cancer-specific evolutionary pattern contributing to high oxidative metabolism. Clin Transl Med 2024; 14:e1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shoari A. Potential of MMP-2 and MMP-9 gelatinase blockade as a therapeutic strategy in Fibrosarcoma treatment: a decadal review. Targets 2024; 2:104–125. [Google Scholar]
- 131. Ali W, Deng K, Bian Y, Liu Z, Zou H. Spectacular role of epididymis and bio-active cargo of nano-scale exosome in sperm maturation: a review. Biomed Pharmacother 2023; 164:114889. [DOI] [PubMed] [Google Scholar]
- 132. Roca J, Rodriguez-Martinez H, Padilla L, Lucas X, Barranco I. Extracellular vesicles in seminal fluid and effects on male reproduction. An overview in farm animals and pets. Anim Reprod Sci 2022; 246:106853. [DOI] [PubMed] [Google Scholar]
- 133. Sun J, Zhao Y, He J, Zhou Q, El-Ashram S, Yuan S, Chi S, Qin J, Huang Z, Ye M, Huang S, Li Z. Small RNA expression patterns in seminal plasma exosomes isolated from semen containing spermatozoa with cytoplasmic droplets versus regular exosomes in boar semen. Theriogenology 2021; 176:233–243. [DOI] [PubMed] [Google Scholar]
- 134. Xu Z, Xie Y, Wu C, Gu T, Zhang X, Yang J, Yang H, Zheng E, Huang S, Xu Z, Li Z, Cai G, et al. The effects of boar seminal plasma extracellular vesicles on sperm fertility. Theriogenology 2024; 213:79–89. [DOI] [PubMed] [Google Scholar]
- 135. Mohammadi A, Shabani R, Bashiri Z, Rafiei S, Asgari H, Koruji M. Therapeutic potential of exosomes in spermatogenesis regulation and male infertility. Biol Cell 2024; 116:e2300127. 10.1111/boc.202300127. [DOI] [PubMed] [Google Scholar]
- 136. Maiolino G, Fernández-Pascual E, Ochoa Arvizo MA, Vishwakarma R, Martínez-Salamanca JI. Male infertility and the risk of developing testicular cancer: a critical contemporary literature review. Medicina 2023; 59:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. James ND, Tannock I, N’Dow J, Feng F, Gillessen S, Ali SA, Trujillo B, Al-Lazikani B, Attard G, Bray F, Compérat E, Eeles R, et al. The lancet commission on prostate cancer: planning for the surge in cases. Lancet 2024; 403:1683–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lavi Arab F, Hoseinzadeh A, Hafezi F, Sadat Mohammadi F, Zeynali F, Hadad Tehran M, Rostami A. Mesenchymal stem cell-derived exosomes for management of prostate cancer: An updated view. Int Immunopharmacol 2024; 134:112171. [DOI] [PubMed] [Google Scholar]
- 139. Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, Bray F, Brawley O, Luckenbaugh AN, Mucci L, Morgan TM, Carlsson SV. 2022 update on prostate cancer epidemiology and risk factors—a systematic review. Eur Urol 2023; 84:191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Crocetto F, Arcaniolo D, Napolitano L, Barone B, La Rocca R, Capece M, Caputo VF, Imbimbo C, De Sio M, Calace FP, Manfredi C. Impact of sexual activity on the risk of male genital Tumors: a systematic review of the literature. IJERPH 2021; 18:8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Merriel SWD, Pocock L, Gilbert E, Creavin S, Walter FM, Spencer A, Hamilton W. Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients. BMC Med 2022; 20:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Alahdal M, Perera RA, Moschovas MC, Patel V, Perera RJ. Current advances of liquid biopsies in prostate cancer: molecular biomarkers. Mol Ther Oncolytics 2023; 30:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Li W, Dong Y, Wang KJ, Deng Z, Zhang W, Shen HF. Plasma exosomal miR-125a-5p and miR-141-5p as non-invasive biomarkers for prostate cancer. neo 2021; 67:1314–1318. [DOI] [PubMed] [Google Scholar]
- 144. Ye Y, Li S-L, Ma Y-Y, Diao Y-J, Yang L, Su M-Q, Li Z, Ji Y, Wang J, Lei L, Fan WX, Li LX, et al. Exosomal miR-141-3p regulates osteoblast activity to promote the osteoblastic metastasis of prostate cancer. Oncotarget 2017; 8:94834–94849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Xu Y, Qin S, An T, Tang Y, Huang Y, Zheng L. MiR-145 detection in urinary extracellular vesicles increase diagnostic efficiency of prostate cancer based on hydrostatic filtration dialysis method. Prostate 2017; 77:1167–1175. [DOI] [PubMed] [Google Scholar]
- 146. Zhou C, Chen Y, He X, Zheng Z, Xue D. Functional implication of Exosomal miR-217 and miR-23b-3p in the progression of prostate cancer. Oncol Targets Ther 2020; Volume 13:11595–11606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Movahedpour A, Khatami SH, Karami N, Vakili O, Naeli P, Jamali Z, Shabaninejad Z, Tazik K, Behrouj H, Ghasemi H. Exosomal noncoding RNAs in prostate cancer. Clin Chim Acta 2022; 537:127–132. [DOI] [PubMed] [Google Scholar]
- 148. Joković SM, Dobrijević Z, Kotarac N, Filipović L, Popović M, Korać A, Vuković I, Savić-Pavićević D, Brajušković G. MiR-375 and miR-21 as potential biomarkers of prostate cancer: comparison of matching samples of plasma and exosomes. Genes 2022; 13:2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Jain G, Das P, Ranjan P, Neha, Valderrama F, Cieza-Borrella C. Urinary extracellular vesicles miRNA—A new era of prostate cancer biomarkers. Front Genet 2023; 14:1065757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hashemi M, Mirdamadi MSA, Talebi Y, Khaniabad N, Banaei G, Daneii P, Gholami S, Ghorbani A, Tavakolpournegari A, Farsani ZM, Zarrabi A, Nabavi N, et al. Pre-clinical and clinical importance of miR-21 in human cancers: tumorigenesis, therapy response, delivery approaches and targeting agents. Pharmacol Res 2023; 187:106568. [DOI] [PubMed] [Google Scholar]
- 151. Faja F, Esteves S, Pallotti F, Cicolani G, Di Chiano S, Delli Paoli E, Lenzi A, Lombardo F, Paoli D. Environmental disruptors and testicular cancer. Endocrine 2022; 78:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sadek KM, AbdEllatief HY, Mahmoud SFE, Alexiou A, Papadakis M, Al-Hajeili M, Saad HM, Batiha GE. New insights on testicular cancer prevalence with novel diagnostic biomarkers and therapeutic approaches. Cancer Rep 2024; 7:e2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Shahrokh S, Tran D, Lines B, Bhuriwala MN. Testicular germ cell tumor composed of seminoma and Teratoma metastasizing as Choriocarcinoma to the lung successfully treated with salvage chemotherapy and bone-marrow transplant: a case report. Cureus 2022; 14:e22885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Regouc M, Belge G, Lorch A, Dieckmann K-P, Pichler M. Non-coding microRNAs as novel potential tumor markers in testicular cancer. Cancer 2020; 12:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Murray MJ, Bell E, Raby KL, Rijlaarsdam MA, Gillis AJM, Looijenga LHJ, Brown H, Destenaves B, Nicholson JC, Coleman N. A pipeline to quantify serum and cerebrospinal fluid microRNAs for diagnosis and detection of relapse in paediatric malignant germ-cell tumours. Br J Cancer 2016; 114:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Fahey CC, Nebhan CA, York S, Davis NB, Hurley PJ, Gordetsky JB, Schaffer KR. Metastatic penile squamous cell carcinoma responsive to Enfortumab Vedotin. IJMS 2023; 24:16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Hu J, Li H, He T, Deng H, Gong G, Cui Y, Liu P, Ren W, Li C, Chen J, Zu X. A nomogram incorporating PD-L1, NLR, and clinicopathologic features to predict inguinal lymph node metastasis in penile squamous cell carcinoma. Urol Oncol Semin Orig Investig 2020; 38:641.e19–641.e29. [DOI] [PubMed] [Google Scholar]
- 158. Niforatos S, Sandhu M, Siegenthaler A, Bordas J, Loon T, Akhtar K. Dyspnea secondary to mediastinal mass: a rare presentation of metastatic penile squamous cell carcinoma. J Investig Med High Impact Case Rep 2023; 11:23247096231205348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ayoubian H, Heinzelmann J, Hölters S, Khalmurzaev O, Pryalukhin A, Loertzer P, Heinzelbecker J, Lohse S, Geppert C, Loertzer H, Wunderlich H, Bohle RM, et al. miRNA expression characterizes histological subtypes and metastasis in penile squamous cell carcinoma. Cancer 2021; 13:1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pinho JD, Silva GEB, Teixeira Júnior AAL, Belfort MRDC, Mendes JM, Cunha IWD, Quintana LG, Calixto JDRR, Nogueira LR, Coelho RWP, Khayat AS. MIR-107, MIR-223-3P and MIR-21-5P reveals potential biomarkers in penile cancer. Asian Pac J Cancer Prev 2020; 21:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Barbagallo D, Ponti D, Bassani B, Bruno A, Pulze L, Akkihal SA, George-William JN, Gundamaraju R, Campomenosi P. MiR-223-3p in cancer development and cancer drug resistance: same coin, different faces. IJMS 2024; 25:8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Furuya TK, Murta CB, Murillo Carrasco AG, Uno M, Sichero L, Villa LL, Cardilli L, Coelho RF, Guglielmetti GB, Cordeiro MD, Leite KRM, Nahas WC, et al. Disruption of miRNA-mRNA networks defines novel molecular signatures for penile carcinogenesis. Cancer 2021; 13:4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Zhou Q, Liu Z, Liao Z, Zhang Y, Qu M, Wu F, Tian J, Zhao H, Peng Q, Zheng W, Huang M, Yang S. miRNA profiling of granulosa cell-derived exosomes reveals their role in promoting follicle development. J Cell Physiol 2024; 239:e31140, 20–e31135. [DOI] [PubMed] [Google Scholar]
- 164. Roy M, Yang Y-P, Bosquet O, Deo SK, Daunert S. Understanding the role and clinical applications of exosomes in Gynecologic malignancies: a review of the current literature. Cancer 2022; 14:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Xi Y, Guo Y, Qiu S, Lv F, Deng Y, Xie J, Xing Z, Bo Y, Chang C, Zhang F, Ji F, Li M. Trends in gynaecologic cancer mortality and the impact of the COVID-19 pandemic in the United States. Infect Agents Cancer 2024; 19:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ma J, Zhang J, Liu S, Gao S, Xi H, Wang Z. Dual-drug-loaded MSCs-derived exosomal vesicles inhibit endometrial cancer cell proliferation by promoting apoptosis through the migration and invasion of Rac1/NF-κB/MMP2 signalling pathway. Biotechnol Bioproc E 2024; 29:551–563. [Google Scholar]
- 167. Stefanoudakis D, Karopoulou E, Matsas A, Katsampoula GA, Tsarna E, Stamoula E, Christopoulos P. Immunotherapy in cervical and endometrial cancer: current landscape and future directions. Life 2024; 14:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Sykaras AG, Christofidis K, Politi E, Theocharis S. Exosomes on endometrial cancer: a biomarkers treasure trove? Cancer 2022; 14:1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Iavarone I, Molitierno R, Fumiento P, Vastarella MG, Napolitano S, Vietri MT, De Franciscis P, Ronsini C. MicroRNA expression in endometrial cancer: current knowledge and therapeutic implications. Medicina 2024; 60:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Wang J, Gong X, Yang L, Li L, Gao X, Ni T, Yang X, Fan Q, Sun X, Wang Y. Loss of exosomal miR-26a-5p contributes to endometrial cancer lymphangiogenesis and lymphatic metastasis. Clin Transl Med 2022; 12:e846. 10.1002/ctm2.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Yao Y, Shi L, Zhu X. Four differentially expressed exosomal miRNAs as prognostic biomarkers and therapy targets in endometrial cancer: Bioinformatic analysis. Medicine 2023; 102:e34998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Capaci V, Kharrat F, Conti A, Salviati E, Basilicata MG, Campiglia P, Balasan N, Licastro D, Caponnetto F, Beltrami AP, Monasta L, Romano F, et al. The deep proteomics approach identified extracellular vesicular proteins correlated to extracellular matrix in type one and two endometrial cancer. IJMS 2024; 25:4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Li B, Lu W, Qu J, Ye L, Du G, Wan X. Loss of exosomal miR-148b from cancer-associated fibroblasts promotes endometrial cancer cell invasion and cancer metastasis. J Cell Physiol 2019; 234:2943–2953. [DOI] [PubMed] [Google Scholar]
- 174. Zheng W, Yang J, Wang Y, Liu X. Exosomal miRNA-93 and miRNA-205 expression in endometrial cancer. J King Saud Univ Sci 2020; 32:1111–1115. [Google Scholar]
- 175. Liu Y, Li X, Zhang T, Liu G. The roles of exosomes in ovarian cancer chemo-resistance. J Cancer 2023; 14:2128–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Cammarata G, Barraco N, Giusti I, Gristina V, Dolo V, Taverna S. Extracellular vesicles-ceRNAs as ovarian cancer biomarkers: looking into circRNA-miRNA-mRNA code. Cancer 2022; 14:3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Yoshida K, Htike K, Eguchi T, Kawai H, Eain HS, Tran MT, Sogawa C, Umemori K, Ogawa T, Kanemoto H, Ono K, Nagatsuka H, et al. Rab11 suppresses head and neck carcinoma by regulating EGFR and EpCAM exosome secretion. J Oral Biosci 2024; 66:205–216. [DOI] [PubMed] [Google Scholar]
- 178. Zhao M, Li Q, Zhao Y, Zhou H, Yan Y, Kong R-M, Tan Q, Kong W, Qu F. Dual-Aptamer recognition of DNA logic gate sensor-based specific Exosomal proteins for ovarian cancer diagnosis. ACS Sens 2024; 9:2540–2549. [DOI] [PubMed] [Google Scholar]
- 179. Cavallari I, Ciccarese F, Sharova E, Urso L, Raimondi V, Silic-Benussi M, D’Agostino DM, Ciminale V. The miR-200 family of microRNAs: fine tuners of epithelial-mesenchymal transition and circulating cancer biomarkers. Cancer 2021; 13:5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Miśkiewicz J, Mielczarek-Palacz A, Gola JM. MicroRNAs as potential biomarkers in gynecological cancers. Biomedicine 2023; 11:1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Jiao P, Wang X-P, Luoreng Z-M, Yang J, Jia L, Ma Y, Wei D-W. miR-223: An effective regulator of immune cell differentiation and inflammation. Int J Biol Sci 2021; 17:2308–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Citron F, Segatto I, Vinciguerra GLR, Musco L, Russo F, Mungo G, D’Andrea S, Mattevi MC, Perin T, Schiappacassi M, Massarut S, Marchini C, et al. Downregulation of miR-223 expression is an early event during mammary transformation and confers resistance to CDK4/6 inhibitors in luminal breast cancer. Cancer Res 2020; 80:1064–1077. [DOI] [PubMed] [Google Scholar]
- 183. Yang L, Yang Z, Liu Z, Qi N, Tao L. Diagnostic value of plasma-derived exosomal miR-223 for epithelial ovarian cancer. BMC Womens Health 2024; 24:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Fang G, Liu J, Wang Q, Huang X, Yang R, Pang Y, Yang M. MicroRNA-223-3p regulates ovarian cancer cell proliferation and invasion by targeting SOX11 expression. IJMS 2017; 18:1208. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 185. Zhu Z, Chen Z, Wang M, Zhang M, Chen Y, Yang X, Zhou C, Liu Y, Hong L, Zhang L. Detection of plasma exosomal miRNA-205 as a biomarker for early diagnosis and an adjuvant indicator of ovarian cancer staging. J Ovarian Res 2022; 15:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Chen L, Lai L, Zheng L, Wang Y, Lu H, Chen Y. Construction of an exosome-associated miRNA-mRNA regulatory network and validation of FYCO1 and miR-17-5p as potential biomarkers associated with ovarian cancer. Transl Cancer Res 2024; 13:1052–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Bhat A, Yadav J, Thakur K, Aggarwal N, Chhokar A, Tripathi T, Singh T, Jadli M, Veerapandian V, Bharti AC. Transcriptome analysis of cervical cancer exosomes and detection of HPVE6*I transcripts in exosomal RNA. BMC Cancer 2022; 22:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Du C, Wang Y, Zhang Y, Zhang J, Zhang L, Li J. LncRNA DLX6-AS1 contributes to epithelial–mesenchymal transition and cisplatin resistance in triple-negative breast cancer via modulating Mir-199b-5p/Paxillin Axis. Cell Transplant 2020; 29:096368972092998. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 189. Wang H, Niu X, Jiang H, Mao F, Zhong B, Jiang X, Fu G. Long non-coding RNA DLX6-AS1 facilitates bladder cancer progression through modulating miR-195-5p/VEGFA signaling pathway. Aging 2020; 12:16021–16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Liang Y, Zhang C-D, Zhang C, Dai D-Q. DLX6-AS1/miR-204-5p/OCT1 positive feedback loop promotes tumor progression and epithelial–mesenchymal transition in gastric cancer. Gastric Cancer 2020; 23:212–227. [DOI] [PubMed] [Google Scholar]
- 191. Ding X, Zhang S, Deng X, Qiang J. Serum Exosomal lncRNA DLX6-AS1 is a promising biomarker for prognosis prediction of cervical cancer. Technol Cancer Res Treat 2021; 20:153303382199006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Huang X, Liu X, Du B, Liu X, Xue M, Yan Q, Wang X, Wang Q. LncRNA LINC01305 promotes cervical cancer progression through KHSRP and exosome-mediated transfer. Aging 2021; 13:19230–19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Huang J, Chan SC, Fung YC, Pang WS, Mak FY, Lok V, Zhang L, Lin X, Lucero-Prisno DE, Xu W, Zheng ZJ, Elcarte E, et al. Global incidence, risk factors and trends of vulvar cancer: a country-based analysis of cancer registries. Int J Cancer 2023; 153:1734–1745. [DOI] [PubMed] [Google Scholar]
- 194. Abu-Rustum NR, Yashar CM, Arend R, Barber E, Bradley K, Brooks R, Campos SM, Chino J, Chon HS, Crispens MA, Damast S, Fisher CM, et al. Vulvar cancer, version 3.2024, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 2024; 22:117–135. [DOI] [PubMed] [Google Scholar]
- 195. Zandieh MA, Naeini NR, Rajabi R, Salimimoghadam S, Hushmandi K, Eng H, Kumar AP. Chapter 8: Exosomal long non-coding RNAs (lncRNAs) in cancer progression and diagnosis. In: Non-coding RNA Transcripts in Cancer Therapy. 2023: 189–219.
- 196. Masoudi-Khoram N, Soheilifar MH, Ghorbanifar S, Nobari S, Hakimi M, Hassani M. Exosomes derived from cancer-associated fibroblasts mediate response to cancer therapy. Crit Rev Oncol Hematol 2023; 185:103967. [DOI] [PubMed] [Google Scholar]
- 197. Nousiopoulou E, Vrettou K, Damaskos C, Garmpis N, Garmpi A, Tsikouras P, Nikolettos N, Nikolettos K, Psilopatis I. The role of urothelial cancer-associated 1 in gynecological cancers. CIMB 2024; 46:2772–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Wang Y, Cao S, Li J, Zhang Y, Ling X, Zhang L, Zhou Y, Zhong H. The predictive value of plasma exosomal lncRNAs/mRNAs in NSCLC patients receiving immunotherapy. Adv Med Sci 2023; 68:86–93. [DOI] [PubMed] [Google Scholar]
- 199. Mishra A, Mulpuru V, Mishra N. An interaction network driven approach for identifying cervical, endometrial, vulvar Carcinomic biomarkers and their multi-targeted inhibitory agents from few widely available medicinal plants. Appl Biochem Biotechnol 2023; 195:6893–6912. [DOI] [PubMed] [Google Scholar]
- 200. Arcucci V, Stacker SA, Achen MG. Control of gene expression by exosome-derived non-coding RNAs in cancer angiogenesis and lymphangiogenesis. Biomolecules 2021; 11:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Najafi S. Circular RNAs as emerging players in cervical cancer tumorigenesis; a review to roles and biomarker potentials. Int J Biol Macromol 2022; 206:939–953. [DOI] [PubMed] [Google Scholar]
- 202. Zhang Y, Wei Y-J, Zhang Y-F, Liu H-W, Zhang Y-F. Emerging functions and clinical applications of Exosomal ncRNAs in ovarian cancer. Front Oncol 2021; 11:765458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Niebora J, Woźniak S, Domagała D, Data K, Farzaneh M, Zehtabi M, Dari MAG, Pour FK, Bryja A, Kulus M, Mozdziak P, Dzięgiel P, et al. The role of ncRNAs and exosomes in the development and progression of endometrial cancer. Front Oncol 2024; 14:1418005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Li Y, Tang X, Wang B, Chen M, Zheng J, Chang K. Current landscape of exosomal non-coding RNAs in prostate cancer: modulators and biomarkers. Non-coding RNA Res 2024; 9:1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Li G, Zhang S, Zou Y, Ai H, Zheng X, Qian K, Lei C, Fu W. The therapeutic potential of exosomes in immunotherapy. Front Immunol 2024; 15:1424081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Batista IA, Machado JC, Melo SA. Advances in exosomes utilization for clinical applications in cancer. Trends Cancer 2024; 10:947–968. [DOI] [PubMed] [Google Scholar]
- 207. Yu D, Li Y, Wang M, Gu J, Xu W, Cai H, Fang X, Zhang X. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer 2022; 21:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Wu Y, Han W, Dong H, Liu X, Su X. The rising roles of exosomes in the tumor microenvironment reprogramming and cancer immunotherapy. MedComm 2024; 5:e541. 10.1002/mco2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]