Abstract
Escherichia coli strains that produce the K1 polysaccharide capsule have long been associated with pathogenesis. This capsule is believed to increase the cell's invasiveness, allowing the bacteria to avoid phagocytosis and inactivation by complement. It is also recognized as a receptor by some phages, such as K1F and K1-5, which have virion-associated enzymes that degrade the polysaccharide. In this report we show that expression of the K1 capsule in E. coli physically blocks infection by T7, a phage that recognizes lipopolysaccharide as the primary receptor. Enzymatic removal of the K1 antigen from the cell allows T7 to adsorb and replicate. This observation suggests that the capsule plays an important role as a defense against some phages that recognize structures beneath it and that the K1-specific phages evolved to counter this physical barrier.
Many strains of Escherichia coli produce extracellular polysaccharide capsules (for a review see reference 23). Some capsules have been correlated with pathogenic strains of E. coli, and those that produce K1 antigen (polysialic acid) are often associated with septicemia, urinary tract infections, and meningitis (16). The K1 antigen is, however, just one specific type of surface polysaccharide that can be found among E. coli strains. There is a tremendous amount of structural variability among E. coli capsules, and at least 80 different K antigens are known to date. Only a few of these capsule structures have been implicated in pathogenesis, and it is not clear why this diversity exists (23).
It has been known for some time that certain phages have evolved to recognize specific polysaccharide capsules (15). Typically, these phages have enzymatic activities associated with the tail structure of the virions that degrade the capsules, thereby permitting the phage access to the surface of the outer membrane, where they probably bind to a secondary receptor. Capsule-specific phages are also highly diverse. There are phages that recognize and depolymerize many types of surface polysaccharide structures, including the K1 capsule. It appears that this diversity is due in part to phages that acquire different tail enzymes through horizontal gene transfer (14). As well as being receptors for some phages, capsules may serve as a defense against other phages that recognize structures underneath the capsule by acting as a physical barrier. We propose that the persistence of these capsule types in nature is largely due to their ability to protect the cells from phages and that the chemical diversity of bacterial capsules and the diversity of capsule-degrading phages are due to an evolutionary arms race.
The argument described above assumes that capsule-specific phages evolved depolymerization activities to overcome physical polysaccharide barriers; however, there have been relatively few studies that have addressed the possibility that surface polysaccharide structures can directly block phage adsorption (2, 21, 24). In this work we show that the K1 capsule of E. coli directly interferes with phage T7 adsorption and likely plays a major role in the bacterium's ability to defend itself against certain phages.
K1 capsule blocks adsorption of T7.
Phage T7 encodes a tail fiber protein that specifically recognizes and binds to lipopolysaccharide (17) and recognizes E. coli B and many E. coli K-12 strains. E. coli strain EV36 is a K-12/K1 hybrid produced by conjugation with an Hfr kps+ strain and produces a polysialic acid capsule identical to that of natural K1 strains (22). EV36 is susceptible to K1-specific phages, such as K1F and K1-5, that encode virion-associated endosialidases that can hydrolyze the K1 polysialic acid structure.
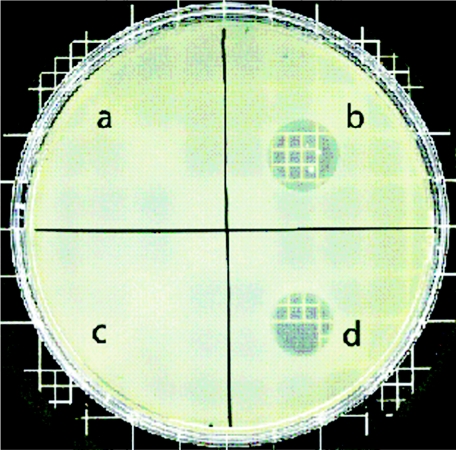
We found that EV36 is resistant to T7 infection. T7 is unable to form plaques on lawns of this strain, and no phage growth is seen in liquid cultures. However, enzymatic removal of the EV36 capsule with purified endosialidase restores T7 susceptibility. Briefly, the gene for K1-5 endosialidase (13) was amplified by PCR and cloned into pET28b (Novagen) as an N-terminal His6 fusion. Expressed protein from this construct was purified by passage on an Ni-nitrilotriacetic acid column. Thrombin-cleaved enzyme was then purified by S-200 size exclusion chromatography. Activity was determined as described by Leggate et al. (5). When purified endosialidase was spotted along with T7 on a lawn of EV36, phage growth was observed as a zone of lysis (Fig. 1). Spotting endosialidase on the plate had no visible effect on the growth of the lawn, and T7 alone could not produce any visible clearing.
FIG. 1.
Infection of EV36 by T7 is blocked by capsule. A lawn of EV36 was spotted with 0.3 mg/ml of endosialidase, which had little effect on growth (a); 0.3 mg/ml of endosialidase plus 104 T7 particles (growth is indicated by clearing); T7 alone, which could not adsorb (c); and 104 phage K1-5 particles (K1 specific) (d).
Experiments with liquid cultures also confirmed that addition of free endosialidase allowed T7 to infect EV36. EV36 was grown in LB medium at 37°C with shaking at 200 rpm. When the optical density at 600 nm of a culture reached 0.2, phage T7 was added at a multiplicity of infection of 0.01 (phage/bacteria). Endosialidase was added along with the phage to a final concentration of 0.03 mg/ml. The controls were T7 alone without enzyme, enzyme alone, and nothing added. At 5-min intervals, samples were taken and phage titers were determined by a plaque assay using BL21 as the host. At 30 min a burst of T7 equivalent to a burst size of 80 PFU/cell was seen in the endosialidase-treated tube; after 80 min the culture had cleared. In the sample that was not treated with endosialidase no T7 replication was revealed by the plaque assay, and neither T7 alone nor endosialidase had an effect on the growth of BL21 as measured by the optical density.
In another experiment, we designed a T7 construct that produced free endosialidase that was released into the lysate after infection and lysis. The K1-5 endosialidase gene was amplified by PCR and cloned downstream of the major capsid protein of the T7 select 415 vector (Novagen). The construct (T7endo) was designed with a stop codon for the capsid protein followed by a ribosome binding site and start codon for the entire endosialidase gene, creating a polycistronic transcript. T7endo was thus designed to express endosialidase late in phage development under the strong capsid promoter. This construct was packaged into T7 heads and plaqued on a mixed lawn of BL21 (a noncapsulated host for T7) and EV36. Wild-type T7 formed turbid plaques on such a lawn due to its inability to replicate on EV36. However, T7endo produced clear plaques due to the production of endosialidase that was released into the lysate, which could strip EV36 cells of the capsule, allowing the phage to infect these cells as well. T7endo could also be propagated in a liquid culture with a mixture of BL21 and EV36 with complete clearing of both strains due to the release of free enzyme. This is the first example in which the effective host range of a phage has been extended by expression of a free enzyme that modifies a host phenotype.
We discovered that T7endo could, in fact, form plaques on a lawn of just EV36; however, the plaquing efficiency was 103- to 104-fold lower than the plaquing efficiency when BL21 was the host. It appears that T7endo (and probably wild-type T7) can infect EV36 at a low level, probably by adsorbing at some point on the cell where the capsule does not completely cover the surface, allowing the phage access to the lipopolysaccharide. This event is too rare for wild-type T7 to develop plaques on a lawn of EV36 or to measure any phage growth in liquid culture, but since T7endo expresses endosialidase, the enzyme can diffuse to its close neighbors and remove the capsule from the cells, allowing the infection to spread and a plaque to form.
Conclusions.
In addition to other functions, including protecting the cell from mammalian immune systems, the K1 capsule can act as a defense against certain types of phages. T7 is an aggressively virulent phage that has overcome many other bacterial defenses. For instance, it encodes an early protein that blocks type I restriction enzymes (20) and avoids many type II restriction enzymes by simply lacking the sites in the genome (12). However, even a phage such as T7 with such robust infection mechanisms can be sterically blocked by the K1 surface polysaccharide. In theory, this feature could give the bacteria a distinct survival advantage in an environment where T7 is present. In fact, protection from phages could be among the greatest selective pressures for maintenance of polysaccharide capsules in bacteria.
Besides the K1 capsule, many other K antigens (which differ in chemical composition) and other “surface polysaccharides,” such as O antigens and exopolysaccharide, are also known to be present in various strains of E. coli. It is difficult to envision an ecological niche for all these bacterial strains from the standpoint of pathogenicity, as most of the strains have not been correlated with any disease. However, their role in phage defense helps to provide a model for why this diversity exists. Since they are potentially such effective barriers against phage attack, the capsules provide a selective advantage for phages to overcome these obstacles by evolution of the ability to degrade them. In fact, workers have discovered many such phages that have the ability to counter the polysaccharide barriers with tail proteins that possess depolymerization activity. The capsule-degrading phages with specificities for E. coli K antigens include K26, K29, K30, K31, K32, K39, K42 (18), K28-1, K28-2, K38 (19), K1 (3, 7, 11), K3, K7, K12 (8), K5 (4), K20 (1, 10), K36 (10), and K95 (9), and it is likely that many more exist. Pressure from these phages as predators could then provide selection for bacteria to evolve different polysaccharide structures that are not susceptible to polysaccharide-degrading phages but still protect the cell from phages (such as T7) that recognize structures underneath the capsule. This, in turn, would provide a selective advantage for new phages to acquire the appropriate enzymatic activity to degrade the new structures. This arms race between phages and bacteria at the level of adsorption could have generated the diversity seen in both bacterial capsules and bacteriophages that can degrade them. Capsule types such as the K1 antigen that confer pathogenicity to a given strain may not have evolved entirely because of the advantages that they give bacteria in invasion of a mammalian host, but they could have arisen due to pressure from phages, and their capacity to aid in pathogenesis is a secondary effect.
The idea of an arms race between bacteria and phages is not new. Lenski proposed theoretical models based on bacteria developing receptor mutations that confer phage resistance and phages countering with mutations in the receptor-recognizing organelle (6). However, the case of bacterial capsules and capsule-degrading phages is more complex and must be considered in light of the natural environment where horizontal gene transfer between other bacteria and phages is a dominant factor in the evolution of both. Phages that have the ability to degrade polysaccharide capsules appear to have evolved by the acquisition of enzymes by recombination, probably from host cells. It is highly unlikely that a phage such as T7 could evolve the ability to degrade the K1 capsule by random mutations in the tail fiber gene. In fact, the K1-specific phage K1F appears to have arisen from a T7 ancestor that has a K1-5-like endosialidase fused to the tail fiber protein (11; Scholl et al., unpublished results for the K1F genome sequence). Likewise, capsule production in bacteria requires large clusters of genes, and horizontal gene transfer likely played a major role in the evolution of new capsules.
Other types of surface polysaccharides have also been shown to interfere with phage adsorption, although the data are limited and not well appreciated. E. coli lipopolysaccharide O-antigen chains have been shown to block both colicins and phages that bind to outer membrane proteins (21), and both Staphylococcus aureus and Streptococcus pneumoniae capsules can also prevent phage infection (2, 24). The present study is the first study to show that a K antigen (in this case, the very well studied K1 antigen) plays a similar role. We also show that physical removal of the capsule with an enzyme restores phage susceptibility, suggesting that the mechanism of resistance is simply steric blocking of phage adsorption.
REFERENCES
- 1.Altmann, F., B. Kwiatkowski, and S. Stirm. 1986. A bacteriophage-associated glycanase cleaving β-pyranosidic linkages of 3-deoxy-d-mannose-2-octulosonic acid. Biochem. Biophys. Res. Commun. 136:329-335. [DOI] [PubMed] [Google Scholar]
- 2.Bernheimer, H. P., and J.-G. Tiraby. 1976. Inhibition of phage infection by Pneumococcus capsule. Virology 73:308-309. [DOI] [PubMed] [Google Scholar]
- 3.Gross, R. J., T. Cheasty, and B. Rowe. 1977. Isolation of bacteriophages specific for the K1 polysaccharide antigen of E. coli. J. Clin. Microbiol. 6:548-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta, D. S., B. Jann, and K. Jann. 1983. Enzymatic degradation of the capsular K5-antigen of E. coli by coliphage K5. FEMS Microbiol. Lett. 16:13-17. [Google Scholar]
- 5.Leggate, D. R., J. M. Bryant, M. B. Redpath, D. Head, P. W. Taylor, and J. P. Luzio. 2002. Expression, mutagenesis, and kinetic analysis of recombinant K1E endosialidase to define the site of proteolytic processing and requirements for catalysis. Mol. Microbiol. 44:749-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenski, R. E. 1984. Coevolution of bacteria and phage: are there endless cycles of bacterial defenses and phage counterdefenses? J. Theor. Biol. 108:319-325. [DOI] [PubMed] [Google Scholar]
- 7.Machida, Y., K. Miyake, K. Hattori, S. Yamamoto, M. Kawase, and S. Iijima. 2000. Structure and function of a novel coliphage-associated sialidase. FEMS Microbiol. Lett. 182:333-337. [DOI] [PubMed] [Google Scholar]
- 8.Nimmich, W., U. Krallmann-Wenzel, B. Muller, and G. Schmidt. 1992. Isolation and characterization of bacteriophages specific for capsular antigens K3, K7, K12, and K13 of E. coli. Zentbl. Bakteriol. 276:213-220. [DOI] [PubMed] [Google Scholar]
- 9.Nimmich, W. 1994. Detection of E. coli K95 strains by bacteriophages. J. Clin. Microbiol. 32:2843-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimmich, W. 1997. Degradation studies on Escherichia coli capsular polysaccharides. FEMS Microbiol. Lett. 153:105-110. [DOI] [PubMed] [Google Scholar]
- 11.Petter, J. G., and E. R. Vimr. 1993. Complete nucleotide sequence of the bacteriophage K1F tail gene encoding endo-N-acylneuraminidase (endo-N) and comparison to an endo-N homolog in bacteriophage PK1E. J. Bacteriol. 175:4354-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg, A. H., M. N. Simon, F. W. Studier, and R. J. Roberts. 1979. Survey and mapping of restriction endonuclease sites in bacteriophage T7 DNA. J. Mol. Biol. 135:907-915. [DOI] [PubMed] [Google Scholar]
- 13.Scholl, D., S. Rogers, S. Adhya, and C. Merril. 2001. Bacteriophage K1-5 encodes two different tail fiber proteins, allowing it to infect and replicate on both K1 and K5 strains of Escherichia coli. J. Virol. 75:2509-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholl, D., J. Kieleczawa, P. Kemp, J. Rush, C. C. Richardson, C. Merril, S. Adhya, and I. J. Molineux. 2004. Genomic analysis of bacteriophages SP6 and K1-5, an estranged subgroup of the T7 supergroup. J. Mol. Biol. 335:1151-1171. [DOI] [PubMed] [Google Scholar]
- 15.Scholl, D., and C. Merril. Polysaccharide-degrading phages. In Phage: role in bacterial pathogenesis and biotechnology, in press. ASM Press, Washington, D.C.
- 16.Silver, R. P., and E. R. Vimr. 1990. Polysialic acid capsule of E. coli K1, p. 39-60. In The bacteria, vol. 11. Molecular basis of bacterial pathogenesis. Academic Press, Inc., New York, N.Y.
- 17.Steven, A. C., B. L. Trus, J. V. Maizel, M. Unser, D. A. Parry, J. S. Wall, J. F. Hainfeld, and F. W. Studier. 1988. Molecular substructure of a viral receptor-recognition protein. The gp17 tail-fiber of bacteriophage T7. J. Mol. Biol. 200:351-365. [DOI] [PubMed] [Google Scholar]
- 18.Stirm, S. 1968. E. coli K bacteriophages. I. Isolation and introductory characterization of five E. coli K bacteriophages. Virology 2:1107-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stirm, S., and E. Freund-Molbert. 1971. E. coli capsule bacteriophages. II. Morphology. J. Virol. 8:330-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Studier, F. W. 1975. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of its host. J. Mol. Biol. 94:283-295. [DOI] [PubMed] [Google Scholar]
- 21.van der Ley, P., P. De Graaff, and J. Tommassen. 1986. Shielding of Escherichia coli outer membrane proteins as receptors for bacteriophages and colicins by O-antigenic chains of lipopolysaccharide. J. Bacteriol. 168:449-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vimr, E. R., and F. A. Troy. 1985. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J. Bacteriol. 164:845-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly, and regulation of expression of capsules in E. coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson, B. J., and K. M. Holmes. 1979. Staphylococcus aureus cell surface: capsule as a barrier to bacteriophage adsorption. Infect. Immun. 23:549-552. [DOI] [PMC free article] [PubMed] [Google Scholar]