Abstract
We describe a novel and noninvasive, microscopy-based method for visualizing the structure and dynamics of microbial biofilms, individual fluorescent microbial cells, and inorganic colloids within a model porous medium. Biofilms growing in flow cells packed with granules of an amorphous fluoropolymer could be visualized as a consequence of refractive index matching between the solid fluoropolymer grains and the aqueous immersion medium. In conjunction with the capabilities of confocal microscopy for nondestructive optical sectioning, the use of amorphous fluoropolymers as a solid matrix permits observation of organisms and dynamic processes to a depth of 2 to 3 mm, whereas sediment biofilms growing in sand-filled flow cells can only be visualized in the region adjacent to the flow cell wall. This method differs fundamentally from other refractive index-matching applications in that optical transparency was achieved by matching a solid phase to water (and not vice versa), thereby permitting real-time microscopic studies of particulate-containing, low-refractive-index media such as biological and chromatographic systems.
Most microorganisms in aquatic ecosystems are associated with solid-liquid interfaces, where they form structured, three-dimensional consortia known as biofilms. In biotechnological applications, artificially generated biofilms known as “immobilized-cell systems” are of considerable economical significance (39), and the role of biofilms as reservoirs for pathogenic microorganisms and in the resistance of many pathogenic microorganisms to chemotherapeutic agents is also well recognized (9). A detailed understanding of biofilms is therefore essential for understanding and controlling many phenomena. Most sensitive analytical and imaging methods are unable to provide spatial or compositional information on biofilms, since they are incompatible with water and its natural components, such as salts (44). Hence, there exists a great demand for techniques which permit the dynamic, three-dimensional microscopic observation of hydrated biological objects (7). Ideally, these methods would provide high spatial resolution and capabilities for real-time observation. The observation of dynamic processes in real time, although not especially demanding in terms of resolution, also requires the maintenance of liquid water. In general, cells with a refractive index of approximately 1.36 are surrounded by an aqueous medium with a refractive index of 1.34. Nondestructive visualization techniques such as phase-contrast microscopy serve to emphasize these small differences; however, such techniques are essentially two dimensional, i.e., limited to monolayers of microorganisms, and cannot be applied to biofilms other than during the initial phase of surface colonization.
Much of the recent progress in biofilm research has been possible through the application of the confocal laser scanning microscope (CLSM) (38), which is now used routinely to visualize three-dimensional biofilm architecture and dynamics according to the principle of nondestructive, optical sectioning (28). For convenient observation, monospecies or mixed-species biofilms are grown in custom-built flow cells, whereby an optically transparent window such as a glass coverslip provides the substratum for microbial adhesion, biofilm formation, and subsequent real-time observation. A salient feature of natural sediment biofilms, however, is the significant quantity of particulate inorganic material that restricts microscopic observation to near-surface regions. Such features must be included in laboratory-scale flow cells to simulate, for example, the effects of a porous matrix on microbial and colloidal transport. An important feature which distinguishes microorganisms from inanimate colloids is that they have the capability to multiply within the porous medium under favorable circumstances, thus becoming an integral and dynamic part of the solid matrix. Since biofilms occur to varying extents in all natural systems, the influence of this living phase and its capability for altering hydrodynamic parameters (10), colloid transport (25, 30), and substrate dynamics (biodegradation) must also be considered in new generation column studies. Whereas microelectrodes have been used with success to monitor the concentrations and spatial profiles of numerous chemical species within sediment pores and even within the biofilm matrix (11), visualization of planktonic or biofilm microorganisms is generally not possible, due to strong absorption-attenuation and optical incompatibility between the solid particles and the surrounding medium, a problem which is best described as “refractive index-induced mismatch” (48). Studies of chromatographic processes could also benefit from optically transparent support materials; methods described to date have manipulated the immersion medium rather than the solid phase (31, 32). Using the principle of refractive index matching, we demonstrate the application of a three-dimensional sediment analog which allows us to monitor the dynamics of individual microbial cells and biofilms. This method has broad applicability for nondestructive microscopy of biological and other aqueous systems.
MATERIALS AND METHODS
Transparent porous media.
Several candidate materials which fulfill the requirements of refractive index matching in predominantly aqueous systems were identified. The criteria were that they must possess a refractive index similar to that of water (5) and an amorphous structure, implying high optical transmission over a broad range of the electromagnetic spectrum (Appendix A). We tested Teflon AF and Nafion (DuPont Fluoroproducts, Fayetteville, NC). Teflon FEP and CYTOP (DuPont and Asahi Glass Co., Japan, respectively) should also be well suited for similar applications, bearing in mind that these polymers, unlike Nafion, are very hydrophobic. Ion-exchange-grade Nafion (Sigma-Aldrich 495786) was chosen for biofilm experiments because it is readily available in a form which resembles the morphology of sand grains used in conventional colloid transport experiments.
Measurement of refractive index and polymer film thickness.
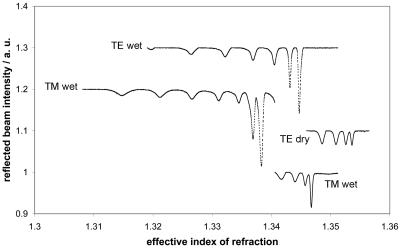
Substances that can be obtained in dissolved form (in this case, by means of perfluorinated solvents) and subsequently deposited as a thin film can be investigated by attenuated total reflectance-leaky mode spectroscopy (37), generating refractive index and film thickness values with high precision (40). Measurements were performed with dry and water-immersed Nafion films, in both cases with transverse electric (TE) and transverse magnetic (TM) polarization directions, where TE represents the in-plane polarization, parallel to the film, while TM is perpendicular to the film plane. With the aid of the Rsim (for reflectivity of a multilayer system) program (29), the measured data were compared to simulated curves according to the so-called transfer matrix method (52).
Confocal laser scanning microscopy.
Examinations of porous media analogs and biofilm samples were performed using a LSM 510 confocal laser scanning microscope (Zeiss, Jena, Germany). The system consisted of a laser-scanning module mounted on an Axiovert 100 M BP inverted microscope (Zeiss), an argon laser (458 nm, 488 nm, and 514 nm), and two helium-neon lasers (543 nm and 633 nm). In this study, we principally used Plan-Neofluar 20×/0.5 NA and LD Achroplan 40×/0.60 NA corrected lenses. The pinhole size was adjusted to 1.0 Airy unit. Digital image acquisition and analysis of the CLSM optical sections were performed with Zeiss LSM software, version 3.2.
Optical characterization of porous media analogs.
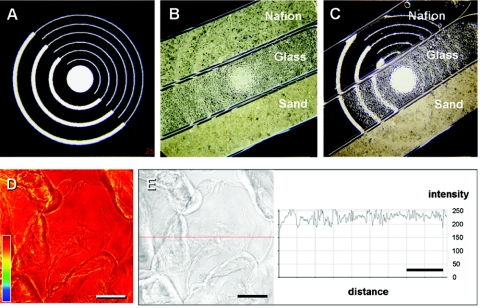
The optical properties of sand and glass beads as common porous media analogs, as well as of Nafion grains, were investigated by stereo microscopy and confocal microscopy. Accurate optical characterizations were simplified by packing rectangular glass capillaries (cross-sectional dimensions of 0.5 by 5 mm; Microslides; VitroCom, Inc., NJ) with granular Nafion. Capillaries either filled with the dry materials or immersed in water were placed over a test grid (see Fig. 2A to C). Images were taken with a camera system connected to an MZ6 stereo microscope (Leica, Bensheim, Germany). CLSM images of the packed and water-immersed capillaries were recorded by using the transmitted light detector. The images were recorded in false color (representing intensity profiles) and as optical images. Transmission profiles were added to the optical images by using image analysis software (Zeiss LSM software).
FIG. 2.
Optical properties of commonly used porous medium materials and Nafion. (A) Photographic target measuring 2 by 2 cm; (B) dry porous media in rectangular glass capillaries; (C) porous media immersed in distilled water. Note that the optical target behind the Nafion-filled capillary becomes visible after filling with water but remains essentially free from distortion. (D) Preservation of signal intensity observed for multiple layers of Nafion grains (generally limited to three layers by the geometry of the rectangular capillary used for microscopy). The color profile refers to an arbitrary intensity scale, whereby similar colors represent similar transmission properties. (E) Optical image and transmission profile corresponding to data shown in panel D. Gain settings were increased to make the Nafion visible. Scale bars (D and E), 200 μm.
Organism and culture conditions.
Prior to flow cell experiments, Pseudomonas aeruginosa SG81 (23), a model organism used for bacterial biofilm formation, was grown under batch conditions in 1/5-strength tryptic soy broth for 24 h at 36°C in a rotary water bath. Cells were washed three times by centrifugation at 4,000 × g for 10 min in phosphate-buffered saline. A 10-ml inoculum with a cell density of 5 × 107 cells ml−1 was introduced to the Nafion-packed flow cell with a syringe, and the inoculated flow cell was left stagnant for 30 min before commencement of flow. A peristaltic pump was used to feed the flow cell biofilm with 1/5-strength tryptic soy broth at a flow rate of 0.1 ml min−1. The porosity of the sterile, Nafion-filled flow cell was determined to be 0.44. Biofilms were visible macroscopically after approximately 48 h, as evidenced by discoloration of the flow cell material. This is a qualitative indication of excellent biofilm growth on the hydrophilic and positively charged Nafion granules.
Staining procedure and in situ visualization of microorganisms.
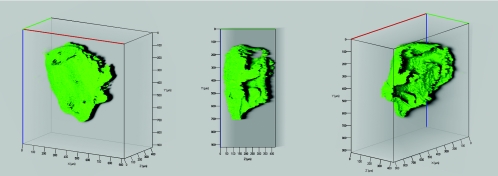
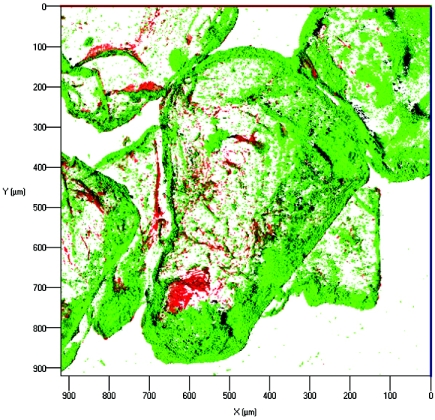
Microorganisms within the packed flow cell were stained with the nucleic acid binding fluorochrome SYTO 9 (Molecular Probes) as shown previously (30). In brief, 5 ml of a staining solution containing 1.5 μl SYTO 9 stock solution (L-7012; Molecular Probes, Oregon) in 0.14 M saline was pumped through the flow cell at a flow rate of 0.1 ml min−1. Three-dimensional confocal image stacks were recorded at an excitation wavelength of 488 nm using a 505-nm long-pass filter for detection. Three-dimensional shadow projections (see Fig. 5) were calculated by image processing after automatic thresholding. Alternatively, cells were stained with the Live/Dead BacLight membrane permeability kit (Molecular Probes, Leiden, The Netherlands) and examined according to the manufacturer's instructions (see Fig. 6).
FIG. 5.
Three-dimensional reconstruction of a P. aeruginosa SG81 biofilm growing on a Nafion grain in a flow cell. Unlike the case for conventional sand columns, the biofilm is visible in its entirety on individual grains, as shown within the depths of the flow cell. The surrounding grains were removed electronically for clarity.
FIG. 6.
Visualization of P. aeruginosa SG81 biofilm formed on Nafion grains after being stained with the Live/Dead cell membrane permeability kit (see Materials and Methods for details). Red cells are stained with the cell-impermeant dye propidium iodide and therefore have compromised membranes, implying lack of viability.
RESULTS
Refractive index match and mismatch as observed by confocal laser scanning microscopy.
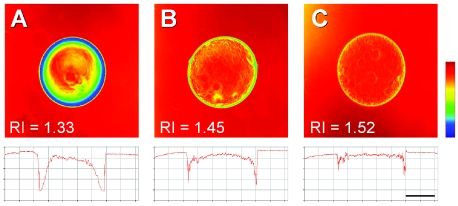
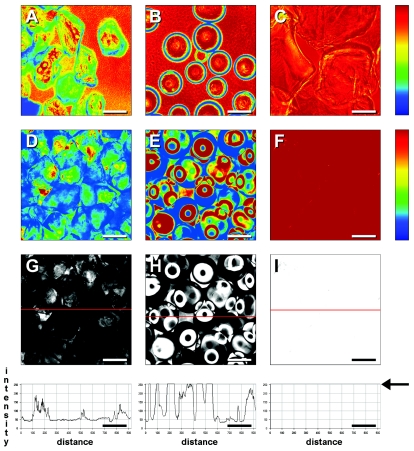
Since glass beads and geological media typically have a refractive index markedly higher than that of water, the only possibility for refractive index matching when confined to these materials as a biofilm growth matrix is to choose an appropriate immersion medium. This was demonstrated convincingly by confocal images of a glass bead in various common immersion media (Fig. 1). In contrast to the central axis of the bead, the edges showed pronounced refraction, especially when the refractive index of the immersion medium was markedly different from that of the solid (see especially Fig. 1A). Since the nominal refractive index is a composite of the entire field of view (19), regions of pronounced mismatch can be corrected selectively by varying the immersion medium, whereas regions showing a good initial match remain transparent (48). Refractive index matching is therefore a convenient and effective method for suppressing spherical aberrations. Unfortunately, alternative immersion media such as glycerol are not compatible with physiological requirements and absorb much of the incident radiation, as do typical porous media. Sand and glass are generally impermeable to water, and their refractive index values are sufficiently high so as to preclude optical transmission at laser intensities used for biological imaging (Fig. 2, and 3).
FIG. 1.
Refractive index matching as demonstrated with a constant solid phase (glass bead) and various common immersion media: water (A), 80% glycerol (B), and microscopy immersion oil (C), with refractive indices (RI) as indicated. The bead has a refractive index of approximately 1.55 and therefore is almost completely transparent in immersion oil (as shown by color similarity); note, however, that light transmission is attenuated significantly by the immersion medium (implying higher absorption), as indicated by the lighter background color compared to that in panel A (for example). Scale bar, 200 μm.
FIG. 3.
Comparison of refractive index matching and intensity profiles for water-immersed conventional porous media and Nafion by confocal laser scanning microscopy. (A and B) Individual sand grains and glass beads, respectively, demonstrating considerable refractive index mismatch with the surrounding medium; (C) multiple layers of Nafion grains. Data shown in panels A to C were recorded at a lower detector gain setting. (D to F) Multiple layers of sand, glass beads, and Nafion, respectively, visualized at typical gain settings; (G to I) optical images corresponding to data shown in panels D to F and their intensity profiles for the regions indicated by red lines; the intensity for panel I is at the arbitrary maximum over the entire line profile, as indicated by the arrow in the graph at the bottom right of the panel. Note also the fine material present in the backgrounds of panels A and B. All scale bars, 200 μm.
Unlike conventional porous media, amorphous fluoropolymers such as Nafion have refractive index values very similar to that of water, thus rendering these materials transparent at macroscopic and microscopic levels of observation. Granules of the amorphous fluoropolymer Nafion showed excellent optical transmission, even throughout multiple layers of the material (Fig. 2). This was also the case for confocal images, where transmission was shown to be close to 100%. We investigated sand, glass beads, and Nafion under conditions typical for visualization of microorganisms in packed flow cells. As expected, sand grains, either in isolation or in multiple layers, showed poor optical matching with water. Although glass beads show excellent matching in their central regions (analogous to looking through the planar walls of the flow cell), the edges resulted in severe diffraction, which was compounded by packing in random layers (Fig. 3). Using detector gain settings typical for visualization of biofilms in the absence of particulate material, Nafion layers were essentially transparent.
Characterization of Nafion film thickness and refractive index.
Nafion is an atypical fluoropolymer in that it is highly hydrophilic. In fact, it swells to some extent in water, thus accounting for its macroscopically altered refractive index upon immersion in water. Our measurements show that Nafion deposited as a film and measured in a dry state has a refractive index of 1.35 (Table 1); however, the refractive index of the hydrated film was even more similar to that of water (Fig. 4). Nafion is therefore transparent or “isorefractive” in aqueous media. Although small pores may allow water to penetrate a porous structure, as in the case of some types of glass beads, for example, the refractive index of the solid part of the structure remains unchanged. Our measurements of Nafion films suggest a refractive index of 1.34 for the hydrated film (Table 1), which is optimal for matching with physiological buffers and defined nutrient media. Differences between TE and TM (see Materials and Methods for details) imply that the polymer is optically anisotropic when produced as a thin film. The films used to determine refractive index were between 7 μm and 11 μm thick, as measured by the same attenuated total reflectance technique.
TABLE 1.
Optical properties of fluoropolymer films
| State of Nafion film | Refractive indexa
|
|
|---|---|---|
| TE | TM | |
| Dry | 1.3539 | 1.3472 |
| Wet | 1.3433 | 1.3366 |
See Materials and Methods for details.
FIG. 4.
Refractive index values obtained for dry and hydrated polymer films.
Visualization of microbial biofilms in porous media.
Whereas microbial biofilms in sand-packed flow cells are visible only on the outermost surfaces of sand grains and to the limits of the adjacent pores, Nafion-packed flow cells allowed three-dimensional visualization of all regions of the biofilm. We selected one grain at random and rotated it by using the visualization features of the microscope software to show that the morphology of the biofilm can be recognized in areas that are normally invisible (Fig. 5). The test organism, Pseudomonas aeruginosa SG81, grew rapidly on Nafion, to such an extent that the entire flow cell was markedly discolored within 48 to 72 h (not shown); however, the large amount of biomass did not prevent imaging of deeper regions of the biofilm. Similar results were obtained for P. aeruginosa visualized with the Live/Dead kit, demonstrating the potential of the method to provide information concerning the integrity of cell membranes (Fig. 6).
DISCUSSION
Studies of porous medium dynamics are generally performed with the aim of monitoring planktonic microorganisms, inorganic colloids, and pollutant species, which can be broadly categorized as (i) colloids which also constitute pollutants (e.g., radionuclides), (ii) colloids such as clay minerals or oxides of iron and manganese which are in themselves relatively innocuous but can facilitate the transport of sorbed pollutants, and (iii) animate and inanimate “biocolloids,” which include most prokaryotic microorganisms and viruses, respectively. Proteins in chromatographic media constitute a further example of inanimate biocolloids (31, 32). A major remaining obstacle to studies of natural biofilm systems has been the prevalence of inorganic matter, which is opaque to microscopic observation. In many cases, this material represents a dominant component of the microbial habitat. Examples include sediment biofilms containing sand and various other mineral particles, and carbonate-containing microbial mats.
Without refractive index matching, particulate-containing samples must be analyzed offline after chemical fixation, dehydration, and thin sectioning prior to microscopy and pseudo-three-dimensional reconstruction (13, 14). The solid porous medium component and associated transport parameters can be characterized in isolation using CLSM. This approach (20, 21) relies on impregnation of the porous structure with a fluorochrome-doped epoxy resin applied under vacuum, which is in some ways analogous to traditional biological sample preparation techniques that may not be representative of native structure. Furthermore, such methods preclude observations of dynamic processes and microscale chemical features. Further attempts to address this problem have been made in the field of soil science (33, 42). These approaches utilized the refractive index-matching concept, but the liquid media used in these experiments were not compatible with biological systems.
Model systems designed to investigate colloidal and bacterial transport typically comprise chromatography columns packed with quartz sand, in which the influent and effluent levels of chosen parameters can be monitored online and in real time. This setup does not allow any type of visualization within the porous medium, and it is therefore not surprising that such studies typically neglect bacterial growth and behavior (27). The size of conventional columns, however, can be scaled to minimize any influence associated with the walls of the columns. Various groups have adapted the column approach to conventional transmitted light microscopy or confocal microscopy by packing microscope flow cells with quartz sand and mounting the chambers horizontally on a microscope stage (24, 30, 46). This configuration allows particle velocimetry, as well as morphological analysis. Recently, we demonstrated the utility of CLSM in conjunction with image analysis for measuring transport patterns of fluorescently labeled bacteria and clay colloids within a sand-filled microscope flow cell (30). The advantages of this method are that it is relatively insensitive to detection errors arising from similar influent (C0) and effluent (C) concentrations, provides some level of comparability to conventional column studies, and allows simultaneous observation of static features and dynamic processes which, by definition, take place within the porous medium. Without an optically transparent sediment analog, however, much of the confocal advantage is lost. In sand-filled flow cells, hydrated biofilms and the contents of interstitial spaces can only be visualized until a refractive index-induced mismatch is encountered (17). This technical limitation has two fundamental consequences. First, gradients that develop throughout the porous medium and result in a spatially discrete population of metabolic groups of microorganisms cannot be visualized by optical techniques. These gradients exist at the level of the biofilm (tens of micrometers) and the porous medium (millimeter scale), a fact which has been confirmed by microelectrode studies (11). Long working distance objectives are necessary to exploit the potential of the transparent porous medium system, and in the case of oil immersion lenses, working distance will obviously be restricted. Nevertheless, 40× objective lenses should suffice for most applications concerning attached microorganisms and biofilms. Second, hydrodynamic effects can be altered by the presence of the adjacent wall material, commonly represented by a glass coverslip. So-called glass micromodels (15, 36, 51) comprising hand-drawn templates etched into glass slides are convenient in that they are amenable to conventional transmitted light microscopy. Unfortunately, these systems are not analogous to conventional column experiments. Micromodels are essentially two dimensional. The resulting “wall effects” arise since there is a radial distribution of interstitial porosity, i.e., void spaces increase closer to the wall (geometrical wall effect), and because the porous medium particles and the flow cell wall have different zeta potentials (electrokinetic wall effect) (49). The restricted size of flow cells that can be used for microscopy represents a fundamental limitation to the goal of measuring transport parameters. Certainly, the wall effects experienced with the system described here will be far less problematic than with etched-glass flow cells, but these artifacts should not be regarded as nonexistent.
Replacement of the sand with Nafion provides a solid yet optically transparent phase for biofilm growth and monitoring (Fig. 5). Clearly, particle-filled columns are more realistic than glass micromodels for determinations of transport parameters. Unlike previous studies that matched the solid and liquid phases by altering the composition of the liquid phase, we deliberately altered only the solid phase such that the physiological properties of the biofilm were maintained. In the case of geological materials, penetration depths using maximum available laser intensities are limited to ca. 150 to 250 μm (20, 21). Since Nafion is essentially transparent at typical experimental settings, low laser intensities can be employed to porous medium biofilms with maximum effect. The newly described technique can be applied to almost the entire depth of conventional 3-mm-deep microscope flow cells (limited only by microscope optics), allowing the acquisition of three-dimensional, real-time data sets throughout the porous medium. An additional benefit is the ability to detect characteristics of the fluid phase, such as preferential flow. We suggest caution in applying this method for flow experiments, since the working distance of objective lenses typically used for biofilm studies may not be sufficiently large to exclude the possibility of wall effects.
Manipulation of refractive index to achieve desirable optical effects is exemplified by the use of immersion oils in conjunction with microscope objective lenses that have a high numerical aperture and short working distance. The concept of refractive index matching has also been recently applied with success to the investigation of dynamic molecular-scale phenomena (48). A near-perfect match between solid and liquid phases was achieved using porous, borosilicate glass beads and a solution of 90% dimethyl sulfoxide and 10% water, thereby enabling the study of electrokinetic phenomena in real time and with excellent spatial resolution. Requisite for biological studies, however, is the use of nontoxic, predominantly aqueous systems. In the case of liposomes, a compromise was reached by the addition of 50% sucrose (3). In this case, refractive index matching was necessary to measure peptide orientation by using flow dichroism, a problem which differs fundamentally from the challenge of maintaining biofilm physiology. Solid media with a refractive index equal to or slightly higher than that of water are most desirable for visualization applications, since dissolved salts and nutrient components of typical buffers and growth media raise the refractive index of the solution marginally at typical experimental concentration; 5% solutions of many common sugars and salts have a refractive index of 1.34 (2). There is also scope to match solid and liquid phases with high accuracy, since the refractive index of water is proportional to salt concentration at constant temperature (18). This is also the case for hydrogels formed by biomacromolecules. By definition, collapse of these gels upon drying results in dense, compact layers with high refractive index (ca. 1.53) (50). Such gels do not fulfill the requirements of solid sediment experiments, since they are by definition semisolid and since they are biologically unstable over long periods of time.
Amorphous fluoropolymers such as Teflon AF, cyclic transparent optical polymer (CYTOP), and Nafion are good candidates for refractive index matching in biological systems because they have a number of advantageous and unique material properties. These properties include exceptional optical clarity throughout the range from UV to infrared wavelengths (1) and, in the case of Teflon AF1600, the lowest refractive index of any polymeric material (Table 1), which is in fact lower than that of water. These properties are closely related to polymer structure. Crystalline fluoropolymers such as polytetrafluoroethylene (Teflon) are opaque, despite having a refractive index similar to that of the amorphous fluoropolymers; the latter have a transmittance of approximately 95% in the 200- to 2,000-nm range (12). In practice, the use of such fluoropolymers in flow cell experiments does not restrict the choice of fluorochrome for cell- or colloid-labeling experiments any more than quartz sand, both of which autofluoresce under UV excitation. The system is therefore unsuitable for monitoring phototrophs by means of chlorophyll autofluorescence or UV-excitable DNA stains such as DAPI (4′,6′-diamidino-2-phenylindole), since the matrix fluorescence will mask the sample fluorescence.
In addition to the numerous desirable properties afforded by fluoropolymers (resistance to biodegradation, lack of toxicity, and no leaching of additives), Nafion and conventional fluoropolymer resins such as Teflon FEP and CYTOP offer possibilities for studies of biofilm dynamics at hydrophilic or hydrophobic surfaces, respectively (Table 1), in analogy to detailed studies of laminar flow systems (4, 22). Nafion can be obtained in nanoporous forms that may be interesting for investigations of substrate transport phenomena. The functionalization of the fluoropolymer surface offers yet another range of possibilities, in terms of surface free energies intermediate between that of Nafion (contact angle, 0°) and Teflon FEP (contact angle, 105°) and surface charge. Judicious near-surface modifications by (for example) radiofrequency plasmas are unlikely to disturb the bulk optical properties of the base fluoropolymers. The variety of fluorochromes available to probe membrane integrity and cell vitality, for example, can be applied in such systems. Enhanced resolution and depth penetration can be achieved by using commercial two-photon instruments. Compositional information will be obtainable using CLSM-based hybrid techniques such as CLSM-nuclear magnetic resonance (34) and CLSM-Raman spectroscopy (6). Finally, the use of transparent porous media offers new perspectives for three-dimensional, quantitative confocal fluorescence microscopy, which has previously been impossible due to refractive index mismatch-induced aberrations or loss in image brightness associated with the use of nonaqueous immersion media (35).
TABLE A1.
Refractive index values
| Immersion, growth, or transport medium | Refractive indexa (reference or source) |
|---|---|
| Air | 1.000 (2) |
| Water | 1.333 (2) |
| Methanol | 1.329 (48) |
| Acetonitrile | 1.344 (48) |
| Dimethyl sulfoxide | 1.479 (48) |
| Glycerol (80% [vol/vol] aqueousb; 100%) | 1.451 (35), 1.472 (35, 48) |
| Mineral oilb | 1.518 (manufacturer) |
| Polyvinyl alcohol | 1.38-1.40 (35) |
| HEPES buffer, 10 mM (pH 7.4) | 1.332 (50) |
| Defined aqueous bufferc | 1.337 (45) |
| Citric acid (1% [wt/vol] aqueous) | 1.33 (2) |
| Sucrose solution (1%, 20%, 50% [wt/vol] | |
| aqueous) | 1.33, 1.36, 1.42 (2) |
| Sodium chloride solution (1%, 5% | |
| [wt/vol] aqueous) | 1.33, 1.34 (2) |
| Biological structures (hydrated) | |
| Liposomes, vesicles, lipid bilayersd | 1.42-1.45 (3) |
| Viruses, bacteria, diatoms | 1.36-1.38 (47) |
| Carbon-storage polymerse | 1.4-1.5 (48) |
| Tissue culture cells | 1.33-1.37 (35) |
| Porous media (dry) | |
| Mesoporous silica | 1.14 (43) |
| Silica microspheres | 1.37-1.46 |
| Glass microspheres | |
| Soda lime | 1.474 (48) |
| Borosilicate | 1.465 (48), 1.52 (2) |
| Porous borosilicate | 1.37 (48) |
| Poly(methylmethacrylate) | 1.49 (41) |
| Polyethyleneg | 1.50 (8), 1.58 (8) |
| Polystyrene microspheresf | 1.565 (16), 1.592 (16) |
| Amorphous fluoropolymers (dry) | |
| Nafion | 1.37 (manufacturer) |
| 1.35 (this study) | |
| Teflon AF | 1.29 (1), 1.31 (1)h |
| Teflon FEP | 1.34 (1) |
| CYTOPi | 1.34 (12) |
| Fluorinated polystyrene microspheresf | 1.366 (26) |
Relative to air; conditions were 589 nm and 25°C unless stated otherwise; values are quoted to a maximum of three decimal places, as available.
Microscopy immersion medium (80% glycerol or synthetic immersion oil). Source of mineral oil: Carl Zeiss, Oberkochen, Germany.
Buffer A (100 mM Tricine/NaOH, pH 7.4, 75 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaN3, and 0.1 mM ouabain).
Estimated values.
Polyesters, e.g., poly(hydroxyalkanoates).
Latex beads; value 1 is valid for 55-nm and 144-nm beads, and value 2 is valid for 1.70-μm beads.
Values are for planar films and refer to high-density and low-density polyethylene, respectively.
Two separate values refer to structurally different forms according to molecular weight: Teflon AF 1600 and Teflon AF 2400, respectively.
CYTOP was from Asahi Glass Company, Japan.
Acknowledgments
Permission to use Teflon AF as a cell growth support was provided by the DuPont Corporation. We thank Peter A. Wilderer for helpful discussions; Amos Gottlieb, Random Technologies, for the generous gift of Teflon AF microbore tubing; and two anonymous reviewers for their useful comments and suggestions.
APPENDIX A
Refractive index values for common biological and inanimate components of porous media and common liquid phases are shown below in Table A1.
REFERENCES
- 1.Altkorn, R., I. Koev, and A. Gottlieb. 1997. Waveguide capillary cell for low-refractive-index liquids. Appl. Spectrosc. 51:1554-1558. [Google Scholar]
- 2.Anonymous. 1991. CRC handbook of chemistry and physics, 72nd ed. CRC Press, Boca Raton, Fla.
- 3.Ardhammar, M., P. Lincoln, and B. Nordén. 2002. Invisible liposomes: refractive index matching with sucrose enables flow dichroism assessment of peptide orientation in lipid vesicle membrane. Proc. Natl. Acad. Sci. USA 99:15313-15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos, R., H. C. van der Mei, and H. J. Busscher. 1999. Physico-chemistry of initial microbial adhesive interactions—its mechanisms and methods for study. FEMS Microbiol. Rev. 23:179-230. [DOI] [PubMed] [Google Scholar]
- 5.Brandrup, J., E. H. Imergut, and E. A. Grulke. 1998. Handbook of polymers. Wiley-Interscience, New York, N.Y.
- 6.Brenan, C. J. H., I. W. Hunter, and J. M. Brenan. 1997. Noninvasive confocal Raman imaging of immiscible liquids in a porous medium. Anal. Chem. 69:45-50. [Google Scholar]
- 7.Cody, S. H., and D. A. Williams. 1997. A novel organ bath design enables the use of saline-immersible lenses on inverted microscopes. J. Microsc. 185:94-97. [Google Scholar]
- 8.Coelho, J. M. P., M. A. Abreu, and F. C. Rodrigues. 2004. Methodologies for determining thermoplastic films optical parameters at 10.6 μm laser wavelength. Polym. Test. 23:307-312. [Google Scholar]
- 9.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham, A. B., W. G. Characklis, F. Abedeen, and D. Crawford. 1991. Influence of biofilm accumulation on porous media hydrodynamics. Environ. Sci. Technol. 25:1305-1311. [Google Scholar]
- 11.de Beer, D., and A. Schramm. 1999. Micro-environments and mass transfer phenomena in biofilms studied with microsensors. Water Sci. Technol. 39:173-178. [Google Scholar]
- 12.Degenaar, P., B. Le Pioufle, L. Griscom, A. Tixier, Y. Akagi, Y. Morita, Y. Murakami, K. Yokoyama, H. Fujita, and E. Tamiya. 2001. A method for micrometer resolution patterning of primary culture neurons for SPM analysis. J. Biochem. 130:367-376. [DOI] [PubMed] [Google Scholar]
- 13.DeLeo, P. C., and P. Baveye. 1997. Factors affecting protozoan predation of bacteria clogging laboratory aquifer microcosms. Geomicrobiol. J. 14:127-149. [Google Scholar]
- 14.DeLeo, P. C., P. Baveye, and W. C. Ghiorse. 1997. Use of confocal laser scanning microscopy on soil thin-sections for improved characterization of microbial growth in unconsolidated soils and aquifer materials. J. Microbiol. Methods 30:193-203. [Google Scholar]
- 15.Dunsmore, B. C., C. J. Bass, and H. M. Lappin-Scott. 2004. A novel approach to investigate biofilm accumulation and bacterial transport in porous matrices. Environ. Microbiol. 6:183-187. [DOI] [PubMed] [Google Scholar]
- 16.Dushkin, C. D., G. S. Lazarov, S. N. Kotsev, H. Yoshimura, and K. Nagayama. 1999. Effect of growth conditions on the structure of two-dimensional latex crystals: experiment. Colloid Polym. Sci. 277:914-930. [Google Scholar]
- 17.Ebihara, T., and P. L. Bishop. 1999. Biofilm structural forms utilized in bioremediation of organic compounds. Water Sci. Technol. 39:203-210. [Google Scholar]
- 18.Esteban, O., M. Cruz-Navarrete, A. Gonzalez-Cano, and E. Bernabeu. 1999. Measurement of the degree of salinity of water with a fiber-optic sensor. Appl. Optics 38:5267-5271. [DOI] [PubMed] [Google Scholar]
- 19.Franklin, J., and Z.-Y. Wang. 2002. Refractive index matching: A general method for enhancing the optical clarity of a hydrogel matrix. Chem. Mater. 14:4487-4489. [Google Scholar]
- 20.Fredrich, J. T. 1999. 3D imaging of porous media using laser scanning confocal microscopy with application to microscale transport processes. Phys. Chem. Earth A Solid Earth Geod. 24:551-561. [Google Scholar]
- 21.Fredrich, J. T., B. Menendez, and T. F. Wong. 1995. Imaging the pore structure of geomaterials. Science 268:276-279. [DOI] [PubMed] [Google Scholar]
- 22.Gottenbos, B., H. C. van der Mei, and H. J. Busscher. 1999. Models for studying initial adhesion and surface growth in biofilm formation on surfaces. Biofilms 310:523-534. [DOI] [PubMed] [Google Scholar]
- 23.Grobe, S., J. Wingender, and H. G. Trüper. 1995. Characterization of mucoid Pseudomonas aeruginosa strains isolated from technical water systems. J. Appl. Bacteriol. 79:94-102. [DOI] [PubMed] [Google Scholar]
- 24.Hendry, M. J., J. R. Lawrence, and P. Maloszewski. 1997. The role of sorption in the transport of Klebsiella oxytoca through saturated silica sand. Ground Water 35:574-584. [Google Scholar]
- 25.Kersting, A. B., D. W. Efurd, D. L. Finnegan, D. J. Rokop, D. K. Smith, and J. L. Thompson. 1999. Migration of plutonium in ground water at the Nevada test site. Nature 397:56-59. [Google Scholar]
- 26.Koenderink, G. H., S. Sacanna, C. Pathmamanoharan, M. Rasa, and A. P. Philipse. 2001. Preparation and properties of optically transparent aqueous dispersions of monodisperse fluorinated colloids. Langmuir 17:6086-6093. [Google Scholar]
- 27.Lawrence, J. R., and M. J. Hendry. 1996. Transport of bacteria through geologic media. Can. J. Microbiol. 42:410-422. [Google Scholar]
- 28.Lawrence, J. R., and T. R. Neu. 1999. Confocal laser scanning microscopy for analysis of microbial biofilms. Biofilms 310:131-144. [DOI] [PubMed] [Google Scholar]
- 29.Leitz, M., R. P. Podgorsek, H. Franke, and J. Woods. 2000. Optimized leaky mode spectroscopy with a single planar film. Appl. Phys. Lett. 77:2674-2676. [Google Scholar]
- 30.Leon-Morales, C. F., A. P. Leis, M. Strathmann, and H.-C. Flemming. 2004. Interactions between laponite and microbial biofilms in porous media: implications for colloid transport and biofilm stability. Water Res. 38:3614-3626. [DOI] [PubMed] [Google Scholar]
- 31.Linden, T., A. Ljunglof, L. Hagel, M. R. Kula, and J. Thommes. 2002. Visualizing patterns of protein uptake to porous media using confocal scanning laser microscopy. Sep. Sci. Technol. 37:1-32. [Google Scholar]
- 32.Linden, T., A. Ljunglof, M. R. Kula, and J. Thommes. 1999. Visualizing two-component protein diffusion in porous adsorbents by confocal scanning laser microscopy. Biotechnol. Bioeng. 65:622-630. [PubMed] [Google Scholar]
- 33.Liu, J. Y., M. G. Iskander, and S. Sadek. 2003. Consolidation and permeability of transparent amorphous silica. Geotech. Test. J. 26:390-401. [Google Scholar]
- 34.Majors, P. D., K. R. Minard, E. J. Ackerman, G. R. Holtom, D. F. Hopkins, C. I. Parkinson, T. J. Weber, and R. A. Wind. 2002. A combined confocal and magnetic resonance microscope for biological studies. Rev. Sci. Instrum. 73:4329-4338. [Google Scholar]
- 35.Martini, N., J. Bewersdorf, and S. W. Hell. 2002. A new high-aperture glycerol immersion objective lens and its application to 3D-fluorescence microscopy. J. Microsc. 206:146-151. [DOI] [PubMed] [Google Scholar]
- 36.Mattax, C. C., and J. R. Kyte. 1961. Ever see a water flood? Oil Gas J. 59:115-128. [Google Scholar]
- 37.Osterfeld, M., H. Franke, and C. Feger. 1993. Optical gas-detection using metal-film enhanced leaky mode spectroscopy. Appl. Phys. Lett. 62:2310-2312. [Google Scholar]
- 38.Pawley, J. B. 1995. Handbook of biological confocal microscopy. Plenum Press, New York, N.Y.
- 39.Pilkington, F. H., A. Margaritis, and N. A. Mensour. 1998. Mass transfer characteristics of immobilized cells used in fermentation processes. Crit. Rev. Biotechnol. 18:237-255. [Google Scholar]
- 40.Podgorsek, R. P., and H. Franke. 2002. Selective optical detection of aromatic vapors. Appl. Optics 41:601-608. [DOI] [PubMed] [Google Scholar]
- 41.Royall, C. P., M. E. Leunissen, and A. van Blaaderen. 2003. A new colloidal model system to study long-range interactions quantitatively in real space. J. Phys. Condens. Matter 15:S3581-S3596. [Google Scholar]
- 42.Sadek, S., M. G. Iskander, and J. Y. Liu. 2002. Geotechnical properties of transparent silica. Can. Geotech. J. 39:111-124. [Google Scholar]
- 43.Schmidt, M., G. Boettger, M. Eich, W. Morgenroth, U. Huebner, R. Boucher, H. G. Meyer, D. Konjhodzic, H. Bretinger, and F. Marlow. 2004. Ultralow refractive index substrates—a base for photonic crystal slab waveguides. Appl. Phys. Lett. 85:16-18. [Google Scholar]
- 44.Schneider, R. P., and A. Leis. 2002. Conditioning films in aquatic environments, p. 928-941. In G. Bitton (ed.), Encyclopedia of environmental microbiology, vol. 2. Wiley, New York, N.Y.
- 45.Shapiro, A. B., and V. Ling. 1998. Transport of LDS-751 from the cytoplasmic leaflet of the plasma membrane by the rhodamine-123-selective site of P-glycoprotein. Eur. J. Biochem. 254:181-188. [DOI] [PubMed] [Google Scholar]
- 46.Stoodley, P., D. Debeer, and Z. Lewandowski. 1994. Liquid flow in biofilm systems. Appl. Environ. Microbiol. 60:2711-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stramski, D., and C. D. Mobley. 1997. Effects of microbial particles on oceanic optics: a database of single-particle optical properties. Limnol. Oceanogr. 42:538-549. [Google Scholar]
- 48.Tallarek, U., E. Rapp, H. Sann, U. Reichl, and A. Seidel-Morgenstern. 2003. Quantitative study of electrokinetic transport in porous media by confocal laser scanning microscopy. Langmuir 19:4527-4531. [Google Scholar]
- 49.Tallarek, U., T. W. J. Scheenen, and H. Van As. 2001. Macroscopic heterogeneities in electroosmotic and pressure-driven flow through fixed beds at low column-to-particle diameter ratio. J. Phys. Chem. B 105:8591-8599. [Google Scholar]
- 50.Vörös, J. 2004. The density and refractive index of adsorbing protein layers. Biophys. J. 87:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan, J., J. L. Wilson, and T. L. Kieft. 1994. Influence of the gas-water interface on transport of microorganisms through unsaturated porous media. Appl. Environ. Microbiol. 60:509-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeh, P. 1988. Optical waves in layered media. Wiley, New York, N.Y.