Abstract
Nitrosomonas europaea strain Schmidt produces at least three acyl homoserine lactone (AHL) signal molecules: C6-homoserine lactone (HSL), C8-HSL, and C10-HSL. These compounds were identified in extracts of chemostat culture effluent by three independent methods. The concentrations of AHL in effluent were low (0.4 to 2.2 nM) but within the range known to induce AHL-responsive systems. The absence of LuxI and LuxM homologs from the genome of N. europaea strain Schmidt suggested that AHL synthesis occurs by an alternate pathway, possibly mediated by an HdtS homolog. To the best of our knowledge, the present report is the first to document the types and levels of AHLs produced by N. europaea.
Nitrosomonas europaea and other ammonia-oxidizing bacteria (AOB) play a pivotal role in affecting the fate and behavior of nitrogen in the environment. Terrestrial and freshwater AOB grow predominantly in biofilms (1, 7, 13, 18), but little is known about how conditions unique to the biofilm environment affect their biology and, consequently, nitrogen cycling. In other members of the class Proteobacteria, a major process affecting cellular structure and function in biofilms is quorum sensing, which is mediated by acyl-homoserine lactone (AHL) signal molecules (6). Much is known about AHL production by a diversity of heterotrophic proteobacteria, but to date, AHL production by the chemolithotrophic AOB has not been conclusively documented. The objectives of this study were to determine if N. europaea strain Schmidt produces AHL and, if so, to identify the types and levels of these molecules.
N. europaea strain Schmidt was obtained from the American Type Culture Collection (ATCC strain 19718). Batch cultures were grown in ATCC medium 2265 (2) at 25°C in light-shielded flasks to which aliquots of sterile 30% (wt/vol) K2CO3 were periodically added. Continuous culture of N. europaea was done in a BioFlo 110 modular benchtop fermentor (New Brunswick Scientific Co., Edison, NJ). Cells were grown in ATCC medium 2265 (lacking cresol red) in a light-shielded vessel at a specific growth rate of 0.025/h (71% of the maximum specific growth rate reported for N. europaea [9]). The temperature was held at 25°C, the dissolved oxygen concentration was maintained at 5 mg/liter (60% of the saturation level at 25°C), and the pH was maintained at 7.1 ± 0.1. A near-neutral pH was used to minimize the potential for AHL degradation by lactonolysis (20). At steady state, the culture optical density at 600 nm was 0.11 (6 × 106 cells/ml, 45 μg biomass dry weight/ml). Aliquots (100 μl) of the culture were regularly plated on 1/10 nutrient broth to verify the absence of heterotrophic contaminants.
Extracts were prepared from supernatants of N. europaea batch cultures (5 liters total) and chemostat effluent. Batch cultures were harvested when the cultures had twice acidified their medium, after which accumulation of nitrite-limited growth (final optical density at 600 nm = 0.08). For the chemostat culture, 7 liters of effluent was collected, the cells were removed by centrifugation (20 min, 5,500 × g) and the clarified supernatant acidified to pH <2 with HCl. After incubating overnight at room temperature, the culture fluid was extracted as described by Shaw et al. (19). Dried extracts were reconstituted in ethyl acetate and then stored at −20°C. Noninoculated medium 2265 was extracted as a negative control.
Reporter strains used for AHL detection were Chromobacterium violaceum CV026 (12) and Agrobacterium tumefaciens NTLR/pCF372/pCF218 (11) (Table 1). Extracts and AHL standards (Sigma-Aldrich, St. Louis, MO) were spotted (AHL standards, 1 to 4 μg; extracts, 5 to 50 μl) onto reversed-phase octadecyl thin-layer chromatography (TLC) plates (200-μm layer; Baker) that were developed and analyzed by agar overlays with A. tumefaciens NTLR as described elsewhere (19). For preparative TLC, extracts were applied in a line (20 to 30 μl/cm, 300- to 400-μl total volume) and the A. tumefaciens NTLR overlay was poured over part of the developed plate to locate the AHL. Corresponding areas in the overlay-free area were excised and extracted as described by Shaw et al. (19).
TABLE 1.
Reporter strain sensitivities to common AHLs based on data from Cha et al. (4) and Shaw et al. (19)a
| Reporter strain | 3-Oxo- HSL
|
3-Unsubstituted HSL
|
3-Hydroxy- HSL
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C6 | C8 | C10 | C12 | C4 | C6 | C8 | C10 | C12 | C6 | C8 | C10 | |
| Agrobacterium tumefaciens NTLR/pCR372/pCF218 | + | + | + | + | − | + | + | + | + | + | + | + |
| Chromobacterium violaceum CV026 | − | − | − | − | + | + | + | + | + | − | − | + |
Plus and minus signs indicate detection or lack of detection, respectively, of a given AHL by the indicated reporter.
Extracts from preparative TLC and AHL standards were analyzed by gas chromatography-mass spectrometry (GC-MS) by using a Hewlett-Packard 6890 series gas chromatograph equipped with a 6890 series injector and a model 5972 mass selective detector. The instrument was fitted with a Varian VF-1MS column (12 m by 0.2 mm; film thickness, 0.33 μm; Varian Scientific, Palo Alto, CA), and the operation parameters were as follows: injector, 275°C; He carrier gas, 1-ml/min constant flow; initial oven temperature, 85°C, hold 1 min; 5°C/min to 250°C, hold 2 min; splitless injection (1 μl); 1-min purge. The MS transfer line temperature was 280°C, and the MS source temperature was 170°C. Ionization was by electron ionization at 70 eV.
In plate assays, only A. tumefaciens NTLR detected AHL in culture extracts and detected AHL in both the batch and chemostat cultures. However, stronger signals were obtained with the latter and all subsequent analysis focused on these samples. A volume of extract equivalent to at least 4 ml of N. europaea continuous-culture effluent was necessary for routine detection of AHL in the extract by A. tumefaciens NTLR.
Separation of the extract by TLC yielded three well-resolved spots. The largest spot had a relative migration factor (Rf) of 0.23, which was similar to that of the C8-homoserine lactone (HSL) standard. The next largest spot had an Rf of 0.09, matching that of C10-HSL, while an Rf of 0.47 for the smallest spot corresponded to C6-HSL. Standards of 3-oxo-substituted AHL were also analyzed by TLC, including (Rf): 3-oxo-C12 (0.07), 3-oxo-C10 (0.18), 3-oxo-C8 (0.41), and 3-oxo-C6 (0.68). We could tentatively rule out identities of the unknowns as oxo-substituted AHLs based on dissimilar Rf values compared to 3-oxo-substituted AHL standards. Additionally, the TLC migration of 3-oxo-substituted AHLs produces a characteristic tailing (19), which the unknowns lacked. No AHL were detected in the extract from noninoculated medium.
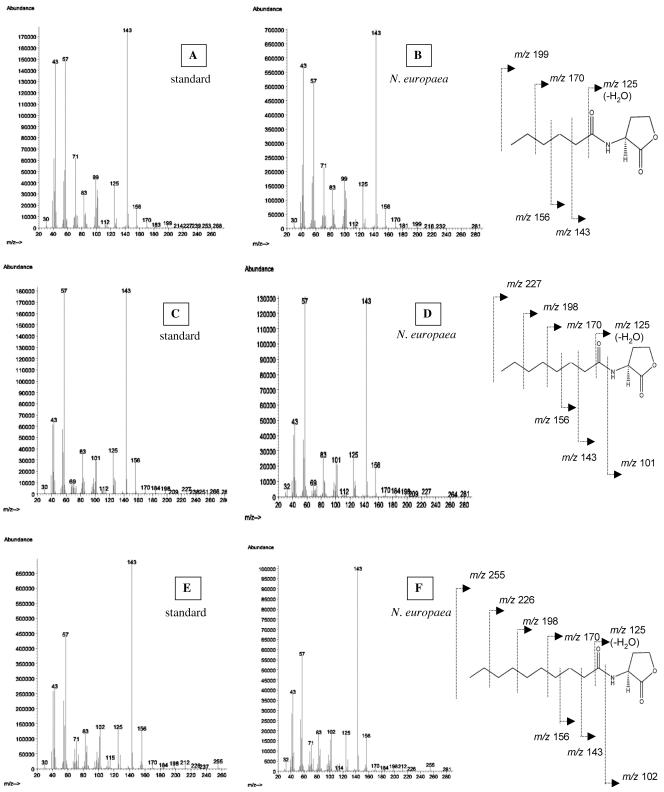
In each of the three spot extracts, an AHL was identified by GC-MS that matched the AHL predicted by TLC in GC retention time (C6-HSL, 14.6 min; C8-HSL, 18.7 min; C10-HSL, 22.6 min) and mass spectra (Fig. 1A to F). From GC-MS analysis, the total amounts of AHL extracted from 7 liters of chemostat effluent were estimated to be 0.5 μg, 1.2 μg, and 3.8 μg for C6-, C8-, and C10-HSL, respectively. Thus, concentrations in the culture of the individual AHLs ranged from 0.4 nM to 2.2 nM; these levels were within the range known to induce AHL-responsive systems in heterotrophic bacteria (8, 14, 16, 17, 21). These results show that while the amounts of AHL produced by chemolithotrophically grown AOB may be low, they could be sufficient to affect the organisms' physiology. Low levels of AHL production by AOB likely reflect the energetic constraints under which these organisms grow.
FIG. 1.
Mass spectra of standard AHLs and a putative AHL produced by N. europaea. Panels: A and B, C6-HSL; C and D, C8-HSL; E and F, C10-HSL.
To the best of our knowledge, the present report is the first to document the production of multiple AHLs by N. europaea and to provide identification of these molecules (6). Batchelor et al. (3) detected an AHL(s) in a fractionated culture extract of an N. europaea soil isolate, but the putative AHL(s) was not identified. Batchelor et al. (3) also demonstrated an apparent response by N. europaea to 46 nM 3-oxo-C6, an AHL not detected in the present study. Transcription factor recognition of nonnative AHL has been demonstrated in other bacteria (4, 15) and could explain the response of N. europaea to 3-oxo-C6 observed by Batchelor et al. (3). Alternatively, strains of N. europaea may vary in AHL biosynthetic pathways and 3-oxo-C6 might be a native AHL produced by the N. europaea soil isolate tested by Batchelor et al. (3).
The genome of N. europaea strain Schmidt lacks homologs to AHL synthases of either the LuxI or the LuxM family (5), and the absence of LuxI and LuxM homologs suggests that the AHL(s) identified in the present study was synthesized by an alternate pathway. As noted by Chain et al. (5), one such route could be mediated by an HtdS homolog (NE1184) present in the N. europaea strain Schmidt genome. HdtS-like proteins have been proposed to constitute a third family of AHL synthases, and an HdtS homolog was shown to catalyze production of C6-, C10-, and 3-OH,C14:1-HSL (10). Further studies are warranted to confirm the biosynthetic activity that is proposed to be encoded by NE1184.
Acknowledgments
We thank Jo Handelsman and Neelawan Pongsilp for providing cultures of the AHL bioreporter strains.
This project was supported by National Research Initiative competitive grant 2001-35107-11046 (to W.J.H.) from the USDA Cooperative State Research, Education, and Extension Service and by funding from the UW—Madison Biotechnology Training Program (traineeship to E.O.B.).
Footnotes
Contribution 364 from the University of Wisconsin—Madison Molecular and Environmental Toxicology Center
REFERENCES
- 1.Aakra, A., M. Hesselsoe, and L. R. Bakken. 2000. Surface attachment of ammonia-oxidizing bacteria in soil. Microb. Ecol. 39:222-235. [DOI] [PubMed] [Google Scholar]
- 2.American Type Culture Collection. http://www.atcc.org/mediapdfs/2265.pdf.
- 3.Batchelor, S. E., M. Cooper, S. R. Chhabra, L. A. Glover, G. Stewart, P. Williams, and J. I. Prosser. 1997. Cell density-regulated recovery of starved biofilm populations of ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 63:2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cha, C., P. Gao, Y. C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 5.Chain, P., J. Lamerdin, F. Larimer, W. Regala, V. Lao, M. Land, L. Hauser, A. Hooper, M. Klotz, J. Norton, L. Sayavedra-Soto, D. Arciero, N. Hommes, M. Whittaker, and D. Arp. 2003. Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J. Bacteriol. 185:2759-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 7.Gieseke, A., U. Purkhold, M. Wagner, R. Amann, and A. Schramm. 2001. Community structure and activity dynamics of nitrifying bacteria in a phosphate-removing biofilm. Appl. Environ. Microbiol. 67:1351-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keen, G. A., and J. I. Prosser. 1987. Steady state transient growth of autotrophic nitrifying bacteria. Arch. Microbiol. 159:73-79. [Google Scholar]
- 10.Laue, R. E., Y. Jiang, S. R. Chhabra, S. Jacob, G. S. A. B. Stewart, A. Hardman, J. A. Downie, F. O'Gara, and P. Williams. 2000. The biocontrol strain Pseudomonas fluorescens F113 produces the Rhizobium small bacteriocin, N-(3-hydroxy-7-cis-tetradecenoyl)homoserine lactone, via HdtS, a putative novel N-acylhomoserine lactone synthase. Microbiology 146:2469-2480. [DOI] [PubMed] [Google Scholar]
- 11.Luo, Z. Q., T. E. Clemente, and S. K. Farrand. 2001. Construction of a derivative of Agrobacterium tumefaciens C58 that does not mutate to tetracycline resistance. Mol. Plant-Microbe Interact. 14:98-103. [DOI] [PubMed] [Google Scholar]
- 12.McClean, K., M. Winson, L. Fish, A. Taylor, S. Chhabra, M. Camara, M. Daykin, J. Lamb, S. Swift, B. Bycroft, G. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 13.Okabe, S., and Y. Watanabe. 2000. Structure and function of nitrifying biofilms as determined by in situ hybridization and the use of microelectrodes. Water Sci. Technol. 42:21-32. [PubMed] [Google Scholar]
- 14.Pearson, J. P., K. M. Gray, L. Passador, K. D. Tucker, A. Eberhard, B. H. Iglewski, and E. P. Greenberg. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 91:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaefer, A., B. Hanzelka, A. Eberhard, and E. Greenberg. 1996. Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. J. Bacteriol. 178:2897-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaefer, A. L., B. L. Hanzelka, M. R. Parsek, and E. P. Greenberg. 2000. Detection, purification, and structural elucidation of the acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol. 305:288-301. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer, A. L., T. A. Taylor, J. T. Beatty, and E. P. Greenberg. 2002. Long-chain acyl-homoserine lactone quorum-sensing regulation of Rhodobacter capsulatus gene transfer agent production. J. Bacteriol. 184:6515-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schramm, A., L. H. Larsen, N. P. Revsbech, N. B. Ramsing, R. Amann, and K. H. Schleifer. 1996. Structure and function of a nitrifying biofilm as determined by in situ hybridization and the use of microelectrodes. Appl. Environ. Microbiol. 62:4641-4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yates, E. A., B. Philipp, C. Buckley, S. Atkinson, S. R. Chhabra, R. E. Sockett, M. Goldner, Y. Dessaux, M. Camara, H. Smith, and P. Williams. 2002. N-Acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 70:5635-5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang, L. H., P. J. Murphy, A. Kerr, and M. E. Tate. 1993. Agrobacterium conjugation and gene-regulation by N-acyl-l-homoserine lactones. Nature 362:446-448. [DOI] [PubMed] [Google Scholar]