Abstract
Short-interfering RNAs (siRNAs), the molecular markers of posttranscriptional gene silencing (PTGS), are powerful tools that interfere with gene expression and counter virus infection both in plants and animals. Here, we report the effect of temperature on geminivirus-induced gene silencing by quantifying virus-derived siRNAs and by evaluating their distribution along the virus genome for isolates of five species of cassava geminiviruses in cassava (Manihot esculenta, Crantz) and Nicotiana benthamiana. Cassava geminivirus-induced RNA silencing increased by raising the temperature from 25°C to 30°C, with the appearance of less symptomatic newly developed leaves, irrespective of the nature of the virus. Consequently, nonrecovery-type geminiviruses behaved like recovery-type viruses under high temperature. Next, we evaluated the distribution of virus-derived siRNAs on the respective virus genome at three temperatures (25°C, 25°C–30°C, and 30°C). For recovery-type viruses, siRNAs accumulated at moderately higher levels during virus-induced PTGS at higher temperatures, and there was no change in the distribution of the siRNA population along the virus genome. For nonrecovery-type viruses, siRNAs accumulated at strikingly higher levels than those observed for infections with recovery-type viruses at high temperature. As determined for an RNA virus, temperature influences gene silencing for single-stranded DNA geminiviruses. It is possible that other mechanisms besides gene silencing also control geminivirus accumulation at high temperatures. The findings presented here should be taken into consideration when implementing PTGS-based strategies to control plant virus accumulation.
Posttranscriptional gene silencing (PTGS) involves sequence-specific suppression of gene expression in diverse eukaryotes. It was first discovered in plants (Napoli et al., 1990). A similar RNA-silencing phenomenon was observed in fungi, termed quelling (Cogoni and Macino, 1997), and in animals, termed RNA interference (Fire et al., 1998). In plants, PTGS serves as a natural antiviral defense response (Waterhouse et al., 2001). As a counter defense, viruses have evolved to encode a protein(s) that suppresses the host PTGS to establish infection in plants (Vance and Vaucheret, 2001). Occasionally, certain viruses become targets of the induced PTGS, and, consequently, infected plants recover from virus infection (Ratcliff et al., 1997; Chellappan et al., 2004a). There are at least three different pathways in the gene-silencing mechanism: the cytoplasmic siRNA silencing, the endogenous mRNA silencing by microRNAs (miRNAs), and the transcriptional gene silencing by DNA methylation (Baulcombe, 2004). A unifying feature of RNA silencing is the cleavage of long double-stranded RNA (dsRNA) into short-interfering (21–24 nt) RNAs (siRNAs; Hamilton and Baulcombe, 1999) by a ribonuclease III-like enzyme termed DICER (Bernstein et al., 2001). Among the four Dicer-like (DCL) enzymes reported in plants (Schauer et al., 2002), DCL1 is involved in miRNA biogenesis, DCL3 in chromatin silencing (Finnegan et al., 2003), and DCL2 has been implicated in viral siRNA production (Xie et al., 2004). The role of DCL4 is not known. The siRNAs must unwind into their component strands prior to being incorporated into RNA-induced silencing complex (RISC), most likely in a RISC-loading complex as reported in Drosophila (Tomari et al., 2004). Thus, the selected guide strand serves as the specificity determinant for the sequence-specific degradation of complementary mRNAs (Khvorova et al., 2003) and interferes with gene expression.
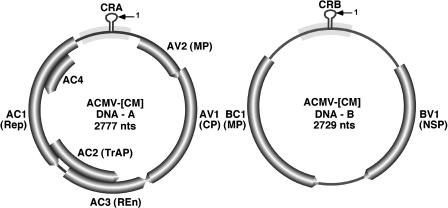
Geminiviruses infect a wide range of economically important crops worldwide (Mansoor et al., 2003). Cassava mosaic disease is caused by single or dual infections of whitefly-transmitted begomoviruses (Geminiviridae) belonging to eight distinct species of geminiviruses (Fauquet and Stanley, 2003). Cassava mosaic disease is a major threat to cassava (Manihot esculenta, Crantz) production, an important food staple for 700 million people in tropical Africa, Asia, and South America (Legg and Fauquet, 2004). Cassava geminiviruses have a genome composed of two circular single-stranded DNA (ssDNA) components termed DNA-A and DNA-B (Fig. 1). DNA-A encodes replication-associated protein (AC1 or Rep), transcriptional activator protein (AC2 or TrAP), replication enhancer protein (AC3 or REn), and coat protein (AV1 or CP). DNA-B encodes nuclear-shuttle protein (BV1 or NSP) and movement protein (BC1 or MP) involved in cell-to-cell and systemic movement of the virus. The nucleotide sequences of DNA-A and DNA-B are different except for a region of approximately 200 nts that shares >90% nucleotide sequence identity, defined as the common region. The common region carries regulatory sequences essential for viral DNA replication and transcription (Timmermans et al., 1994). Among all gene products, Rep is indispensable for viral DNA replication. Virus-host interactions are highly specific in the sense that each virus elicits a different type of symptom in the same host. In a previous study, we reported that African cassava mosaic virus Cameroon isolate (ACMV-[CM]) in Nicotiana benthamiana and cassava and Sri-Lankan cassava mosaic virus (SLCMV) in cassava are able to induce symptoms within approximately 5 d postinoculation (dpi); however, at a later stage, the infected plants recover from virus symptoms and accumulate high levels of virus-derived siRNAs (Chellappan et al., 2004a). Geminiviruses replicate in the nuclei of infected plants via a dsDNA intermediate by a rolling circle mechanism and encounter no dsRNA phase in their replication cycle (Laufs et al., 1995); however, they have the capacity to trigger PTGS with the production of virus-derived siRNAs in infected plants (Lucioli et al., 2003; Chellappan et al., 2004a). Although the exact mechanism by which geminiviruses trigger PTGS is unclear, it is possible that the transcripts of opposite polarity (Townsend et al., 1985) that overlap at their 3′ ends, as evidenced by using strand-specific probes (Chellappan et al., 2004a), could be further extended by the host RNA-dependent RNA polymerase (RdRP) to form long dsRNA, a potential inducer of RNA silencing. Alternatively, it has been hypothesized that the abundant early transcripts (also called AC1 and BC1 transcripts from each DNA-A and DNA-B component) might serve as the template for the host RdRP to synthesize long dsRNA, and it is also possible that the highly structured RNA messenger could serve as a direct template for the DICER to produce siRNAs (Vanitharani et al., 2005). In addition, the introduction of siRNAs designed to target the AC1 gene of ACMV-[CM] inhibited AC1-mRNA and viral DNA accumulation by 90% and 66%, respectively (Vanitharani et al., 2003). By contrast, the symptoms induced by East African cassava mosaic Cameroon virus (EACMCV), EACMV from Uganda (EACMV-[Ug]), and the Indian cassava mosaic virus (ICMV) persisted throughout the plant's life span with low levels of virus-specific siRNA accumulation (Chellappan et al., 2004a). The identification of the AC4 gene of recovery-type viruses (ACMV-[CM] and SLCMV) and the AC2 gene of nonrecovery-type viruses (EACMCV and ICMV) as the RNA-silencing suppressors further supports the fact that each geminivirus is different in eliciting disease symptoms in plants (Vanitharani et al., 2004). In addition, we have found that AC4 of ACMV-[CM] binds to microRNAs and induces developmental defects in Arabidopsis (Chellappan et al., 2005).
Figure 1.
Genome structure of ACMV-[CM], a typical bipartite cassava geminivirus. Genome is split into two components termed DNA-A (left) and DNA-B (right). DNA-A comprises six open reading frames (ORFs), and each ORF encodes a specific protein. AC1, Replication-associated protein (Rep); AC2, transcriptional activator protein (TrAP); AC3, replication enhancer protein (REn); AC4, RNA-silencing suppressor; AV1, coat protein (CP); AV2, precoat. DNA-B has two ORFs; BV1 encodes nuclear-shuttle protein (NSP), and BC1 encodes movement protein (MP). V, Virion-sense ORFs; C, complementary-sense ORFs.
It has long been recognized that environmental factors such as temperature greatly influence plant-virus interactions. In virus-infected plants, high temperature is frequently associated with attenuated symptoms (heat masking) and with low virus content (Johnson, 1922). By contrast, low temperature is often associated with rapid spread of virus diseases and the development of severe symptoms (Hine et al., 1970; Gerik et al., 1990). Thermotherapy has been a method of choice to free vegetative material from infected viruses (Manganaris et al., 2003). Half a century ago, Harrison (1956) speculated that the virus content of a plant represents an equilibrium between replication and degradation of the virus by the host system and that the activity of the virus-degrading system increases with temperature. The underlying molecular mechanism behind the effect of temperature on Cymbidium ring spot virus (CymRSV; an RNA virus)-induced symptom severity was found to be associated with gene silencing, and increasing temperature dramatically elevated virus-derived siRNA accumulation, resulting in less symptom development (Szittya et al., 2003). Recently, the ability of defective interfering RNA (DI-RNA) to protect plants from CymRSV was shown to be more efficient at high temperature with elevated virus-specific siRNAs (Havelda et al., 2005). RNA viruses and ssDNA geminiviruses follow fundamentally different mechanisms for their genome replication and gene transcription. Here, we report that ssDNA geminivirus-induced RNA silencing is altered by temperature. We tested the effect of temperature on representatives of five distinct species of cassava geminivirus-induced RNA silencing, ACMV-[CM], SLCMV, EACMCV, ICMV, and EACMV-[Ug], in two plant hosts, N. benthamiana and cassava, in terms of their ability to accumulate virus-specific siRNAs and the level of symptom severity. Furthermore, the effect of temperature on the distribution of virus-derived siRNAs along the virus genome was evaluated.
RESULTS
Effect of Temperature on ACMV-[CM]-Induced Symptom Severity, and Distribution of Virus-Derived siRNAs in Infected Plants
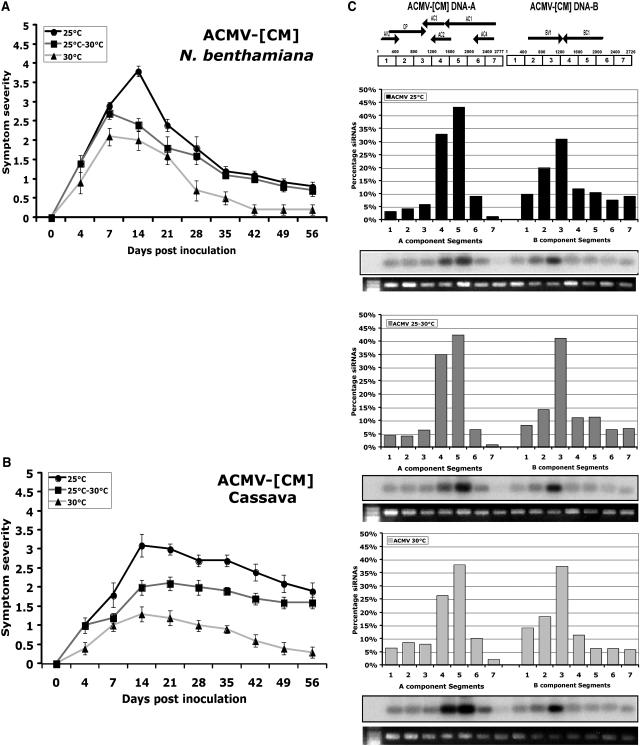
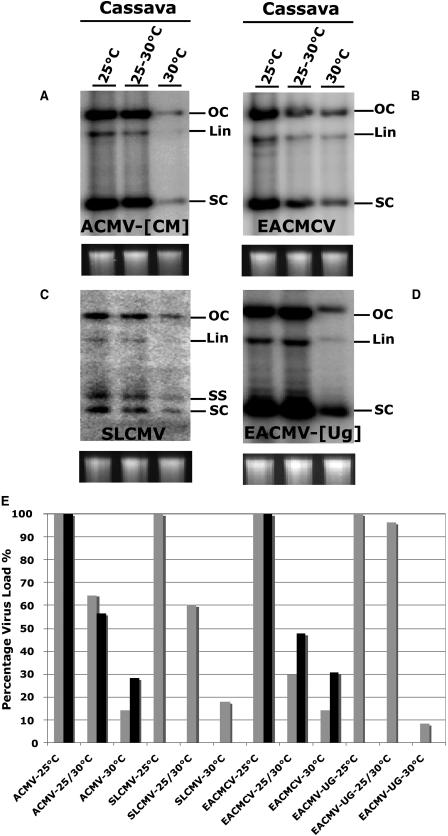
ACMV-[CM] develops disease symptoms within approximately 5 dpi in N. benthamiana and cassava, and reaches maximum severity with leaf distortion, curling, and yellow mosaic in about 2 to 3 weeks postinoculation (wpi). Later, the newly emerged leaves of the infected plants showed recovery from symptoms, which correlated with the abundant virus-derived siRNAs in both N. benthamiana and cassava (Chellappan et al., 2004a). To test the effect of temperature on ACMV-[CM]-induced PTGS in this and subsequent studies with other viruses, seedling of N. benthamiana and/or cassava was inoculated with virus by particle bombardment with a mixture of DNA-A and DNA-B. The N. benthamiana inoculated with ACMV-[CM] developed symptoms approximately 4 dpi at all three temperatures tested: 25°C, 25°C to 30°C, and 30°C. The maximum level of symptoms was reached at 14 dpi at 25°C, and 7 dpi at 25°C to 30°C and 30°C (Fig. 2A). However, the severity of symptoms was greater at 25°C compared with those at 30°C, and it was moderate at 25°C to 30°C. In particular, at 30°C the newly emerged leaves of the infected plants showed recovery from symptom appearance after 7 dpi, but, after 28 dpi, the newly developed leaves were almost free from virus symptoms (Fig. 2A). Although the new growth of the infected plants started to show recovery phenotype after 14 dpi at 25°C, the extent of recovery from symptom appearance was much less compared to the level at 30°C, indicating that high temperature indeed modified the interaction of ACMV-[CM] with N. benthamiana. At 25°C to 30°C, even though the recovery phase started after 7 dpi similar to that of 30°C, after 28 dpi the curve mostly resembled that of 25°C (Fig. 2A). Similar to N. benthamiana, ACMV-[CM]-induced symptoms were severe at 25°C compared with the results obtained at the higher temperatures of 25°C to 30°C and 30°C in cassava. The maximum level of symptoms was reached at 14 dpi in all three temperature conditions tested. The recovery phase from symptom appearance started after 14 and 21 dpi in all three temperatures tested, but the extent of recovery was more dramatic at high temperature (30°C) than plants grown at low temperatures 25°C to 30°C and 25°C (Fig. 2B). The level of ACMV-[CM] DNA accumulation in cassava plants at different temperatures was estimated by Southern-blot hybridization, and we found that viral DNA accumulation was maximum at 25°C, which was considered as 100%, and the virus load was decreased with raising the temperature to 60% at 25°C to 30°C and 12% at 30°C (Fig. 3, A and E). Although ACMV-[CM] behaved in a similar manner in both plant species, with an early increase in symptom severity followed by a recovery from symptoms at the later stage of the infection period, the extent of recovery from symptoms was much higher in N. benthamiana (Fig. 2, A and B). Furthermore, a striking difference in recovery from symptom appearance was noted in plants grown at 30°C compared to 25°C, suggesting that temperature plays a major role in decreasing the virus load. This conclusion was supported by the decrease in virus accumulation observed at 14 dpi (Fig. 3A).
Figure 2.
Effect of temperature on ACMV-[CM]-induced symptom severity in N. benthamiana and cassava, and the distribution of siRNAs in infected cassava. A and B, ACMV-[CM]-induced symptom severity trend in N. benthamiana (A) and in cassava (B) at three temperatures: 25°C, 25°C to 30°C, and 30°C. Ten cassava and N. benthamiana plants were inoculated as five plants in two experiments. Bars in symptom severity curve indicate se values of 10 plants. C, Effect of temperature on the level and distribution of ACMV-[CM] siRNA accumulation at three temperatures. ACMV-[CM] has a split genome; the total length of DNA-A is 2,777 bp, and DNA-B is 2,726 bp. PCR-amplified approximately 400-bp DNA segments from 1 through 7 for DNA-A and segments 1 through 7 for DNA-B of ACMV-[CM] separated in an ethidium bromide-stained 1% agarose gel were blotted and hybridized with 5′-end-labeled small 21- to 26-nt RNAs purified from ACMV-[CM]-infected cassava plants grown at 25°C, 25°C to 30°C, and 30°C. The blots were quantified and values were plotted. Values on the y axis represent the percentage of siRNAs derived from either DNA-A or DNA-B of ACMV-[CM]. Each bar represents the amount of siRNAs for respective DNA segments in comparison to the total amount of siRNAs either for DNA-A or DNA-B. Blots were quantified using image quant (IqMacV1.2) software. Numbers on the x axis indicate the PCR-amplified DNA fragments representing various regions of the ACMV-[CM] genome. The graph following each blot represents the distribution and intensity of ACMV-[CM]-derived siRNAs at 25°C (top), at 25°C to 30°C (middle), and at 30°C (bottom).
Figure 3.
The levels of viral DNA accumulation in infected cassava plants in three temperatures conditions: 25°C, 25°C to 30°C, and 30°C. Southern blots show the levels of viral DNA accumulation: A, ACMV-[CM]; B, EACMCV; C, SLCMV; D, EACMV-[Ug]. Total genomic DNA at the bottom of each blot serves as the loading control. Various forms of viral DNAs are shown: OC, open circular; Lin, linear; SS, single stranded; SC, supercoiled. E, Comparison of the virus loads in two host plants. Recovery-type viruses, ACMV-[CM] and SLCMV; nonrecovery-type viruses, EACMCV and EACMV-[Ug]. Shown are the levels of ACMV-[CM] in cassava (light gray) and N. benthamiana (black); SLCMV in cassava; EACMCV in cassava (light gray) and N. benthamiana (black); and EACMV-[Ug] in cassava. The y axis represents virus load in percentage (%), and the x axis represents different viruses as indicated.
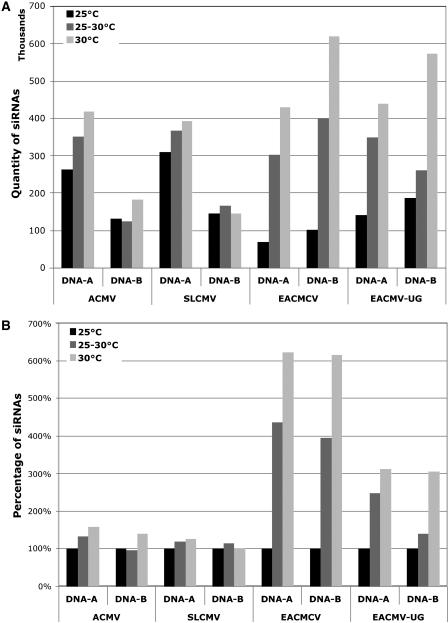
Next, we determined whether temperature changes the ACMV-[CM] sequence targeted during PTGS. Toward that goal, we compared the composition of ACMV-[CM]-derived siRNAs from cassava plants infected with ACMV-[CM] that were grown at three different temperatures: 25°C, 25°C to 30°C, and 30°C. We chose cassava plants in this and subsequent analyses with other viruses because it is the natural host for these viruses. The experiment was performed as follows. Low molecular mass RNA was isolated from leaf tissue obtained from five infected plants. The peak of infection based on symptom scoring, 2 wpi for ACMV-[CM], was chosen as a test point for low molecular mass RNA isolation from infected cassava plants. Gel blots containing segments (approximately 400 bp) of the virus genome were hybridized with ACMV-[CM]-specific siRNAs purified from the virus-infected cassava plants. The results revealed that the majority of siRNAs were derived from the DNA-A component, particularly segments 5 (approximately 40%) and 4 (approximately 30% of the total [100%] siRNAs derived from the DNA-A component), and in DNA-B segment 3 accumulated 40% of the total (100%) siRNAs derived from the DNA-B component at all three temperatures (Fig. 2C). The amount of siRNA accumulation increased with rising temperature. In DNA-A, segments 4 and 5 represent the C terminus of AC1 gene, and the AC2 and AC3 genes that are important for virus replication and gene transcription. In DNA-B, segment 3 corresponds to the C terminus of the BV1 gene essential for virus movement (Fig. 2C). The fact that the total amount of siRNAs derived from the DNA-A and the DNA-B components increased by 58% and 39%, respectively, from 25°C to 30°C (Fig. 4, A and B) indicates that temperature has a major role in virus-derived siRNA generation.
Figure 4.
A, Comparison of virus-derived siRNA accumulation for each of the DNA-A and DNA-B components of ACMV, SLCMV, EACMCV, and EACMV-[Ug] at 25°C, 25°C to 30°C, and 30°C temperatures. Values on the y axis represent the sum of intensities of signals (103). The x axis represents the DNA-A and the DNA-B components of ACMV, SLCMV, EACMCV, and EACMV-[Ug] at three temperatures: 25°C, 25°C to 30°C, and 30°C. B, Comparison of virus-derived siRNA accumulation for each of the DNA-A and DNA-B components of ACMV, SLCMV, EACMCV, and EACMV-[Ug] at 25°C, 25°C to 30°C, and 30°C temperatures, relative to the values at 25°C (100%). Values on the y axis represent the percentage of siRNAs relative to the value at 25°C for each DNA component and for each virus. The x axis represents the DNA-A and the DNA-B components of ACMV, SLCMV, EACMCV, and EACMV-[Ug] at three temperatures: 25°C, 25°C to 30°C, and 30°C.
From these results, we conclude that ACMV-[CM]-induced symptom development is temperature dependent. Either the plant defense response is highly active at higher temperature, or the virus itself becomes a better target for the virus-induced PTGS. Furthermore, the role of virus-encoded PTGS suppressors might become less efficient and/or unavailable at high temperature to counter defend and to continue the infection.
Effect of Temperature on SLCMV-Induced Symptom Severity, and Distribution of Virus-Derived siRNAs in Infected Plants
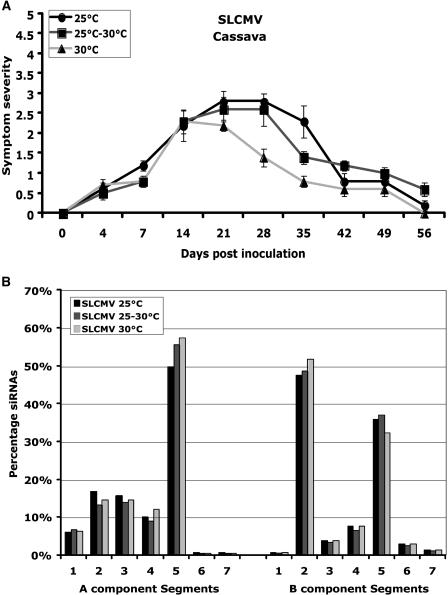
In cassava, SLCMV infection caused symptoms within approximately 4 dpi at all three temperatures tested: 25°C, 25°C to 30°C, and 30°C (Fig. 5A). The maximum level of symptoms was reached between 21 and 28 dpi at both 25°C and 25°C to 30°C. At 30°C, the maximum level of symptoms was reached on 14 dpi. The severity of symptoms was similar at this time on plants at the different temperatures, but with time plants grown at 30°C showed a greater recovery from symptoms (Fig. 5A). Similar to ACMV-[CM], SLCMV induced severe symptoms during the early stage of infection, and later the newly emerged leaves showed recovery from symptom development. However, the extent of recovery from symptoms was higher in SLCMV compared with ACMV-[CM] infections (Fig. 5A). SLCMV infection in N. benthamiana leads to plant death; hence, no analysis was performed. Southern-blot analysis revealed that viral DNA accumulation decreased from 100% at 25°C to 20% at 30°C (Fig. 3, C and E), which correlated with the reduced level of symptoms at high temperature.
Figure 5.
Effect of temperature on symptom severity of SLCMV on cassava, and the level and distribution of virus genome siRNAs in infected cassava plants. A, SLCMV symptom severity trend in cassava in three temperatures conditions: 25°C, 25°C to 30°C, and 30°C. Ten cassava plants were inoculated as five plants in two experiments. Bars in symptom severity curve indicate se values of 10 plants. B, Gel blots (first seven segments for DNA-A and second seven segments for DNA-B) were subjected to gel-blot hybridization using 5′-labeled virus-derived siRNAs purified from SLCMV-infected plants grown at 25°C, 25°C to 30°C, and 30°C. The intensity of signals in the blots was quantified and plotted into graphs. Values on the y axis represent the percentage of siRNAs derived from either DNA-A or DNA-B of SLCMV. Each bar represents the amount of siRNAs for respective DNA segments in comparison to the total amount of siRNAs either for DNA-A or DNA-B. Blots were quantified using image quant (IqMacV1.2) software. Numbers in the x axis indicate the PCR-amplified DNA fragments representing various regions of the SLCMV genome.
Then, the influence of temperature on the target viral sequence on SLCMV-induced PTGS was analyzed by comparing the distribution of siRNAs from cassava plants infected with SLCMV at three temperatures (25°C, 25°C–30°C, and 30°C). The results revealed that the majority of the siRNAs were derived from the DNA-A component, in particular segment 5 occupying approximately 60% of the total siRNAs of the DNA-A component (Fig. 5B). This segment corresponds to the C terminus of AC1 and N terminus of AC2 genes at all three temperatures tested (25°C, 25°C–30°C, and 30°C). Segments from 1 to 4 were almost uniformly targeted, but the level of siRNA accumulation was below 20% (Fig. 5B). In DNA-B, segment 2 held approximately 50% and segment 5 approximately 35% of the total siRNAs derived from the DNA-B component (Fig. 5B). Nevertheless, the quantity of siRNAs showed a slight increase (approximately 27%) with rising temperatures for DNA-A of SLCMV (Fig. 4, A and B). These results revealed that SLCMV-induced PTGS preferentially targeted a region in the DNA-A component of the genome that encodes the replication-associated protein (AC1) and transcriptional activator protein (AC2), essential proteins for viral DNA replication and gene expression, respectively. In DNA-B, the targeted region corresponds to BV1 and BC1 genes, both essential for cell-to-cell and systemic movement of the virus (Fig. 5B). The fact that SLCMV, like ACMV-[CM], targets functional genes such as replication-associated and movement proteins correlated well with the subsequent reduction in virus DNA accumulation as revealed by Southern hybridization and resulting recovery from symptom development.
Effect of Temperature on EACMCV-Induced Symptom Severity, and Distribution of Virus-Derived siRNAs in Infected Plants
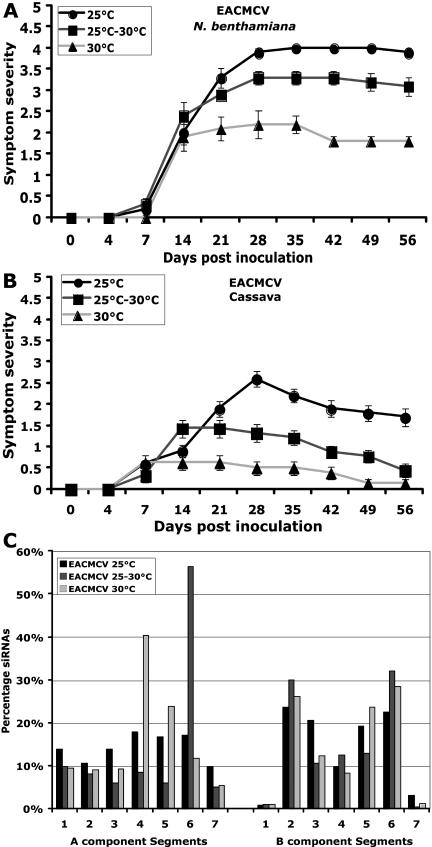
To investigate whether temperature has any effect on EACMCV-induced disease development, EACMCV-inoculated cassava and N. benthamiana plants were grown at three temperatures: 25°C, 25°C to 30°C, and 30°C. EACMCV-infected N. benthamiana plants developed symptoms between 7 and 14 dpi, and infection persisted throughout the life span of the plants at 25°C and 25°C to 30°C (Fig. 6A). But at 30°C, the overall symptom severity was low with the infected N. benthamiana plants showing mild symptoms (Fig. 6A). In cassava, EACMCV infection induced symptoms from 7 to 14 dpi, but the level of symptom severity was mild compared to N. benthamiana at 25°C (Fig. 6B). At 25°C to 30°C, the maximum symptom severity was one-half the level of that observed at 25°C. Surprisingly, at 30°C the symptoms were very mild, and the newly emerged leaves were almost free from symptoms at the later stage of infection (Fig. 6B). In EACMCV-infected cassava, Southern-blot analysis revealed that virus DNA accumulation was about 75% at 25°C to 30°C compared to 100% at 25°C, and decreased to 20% by raising the temperature to 30°C (Fig. 3, B and E). The extent of symptom severity was much lower at 30°C compared to 25°C, which correlated with the reduced levels of virus DNA accumulation both in EACMCV-infected cassava and N. benthamiana (Fig. 3E), indicating that temperature modified the effect of virus infection in both plant species.
Figure 6.
Effect of temperature on symptom severity of EACMCV on N. benthamiana and cassava, and the level and distribution of virus genome siRNAs in infected cassava plants. A and B, EACMCV symptom severity trend in N. benthamiana (A) and in cassava (B) at three ranges of temperature: 25°C, 25°C to 30°C, and 30°C. Ten N. benthamiana and cassava plants were inoculated as five plants in two experiments. Bars in symptom severity curve indicate se values of 10 plants. C, Effect of temperature on the level and distribution of EACMCV genome siRNA accumulation at three different temperatures. EACMCV has a split genome; the total length of DNA-A is 2,802 bp, and DNA-B has 2,741 bp. Gel blots (first seven segments for DNA-A and second seven segments for DNA-B) were subjected to hybridization using 5′-labeled virus-derived siRNAs purified from EACMCV-infected plants grown at 25°C, 25°C to 30°C, and 30°C. The intensity of signals in the blots was quantified and plotted into graphs. Values on the y axis represent the percentage of siRNAs derived from either DNA-A or DNA-B of EACMCV. Each bar represents the amount of siRNAs for respective DNA segments in comparison to the total amount of siRNAs either for DNA-A or DNA-B. Blots were quantified using image quant (IqMacV1.2) software. Numbers in the x axis indicate the PCR-amplified DNA fragments representing various regions of the EACMCV genome.
Next, we determined the effect of temperature on the target viral sequence on EACMCV-induced PTGS by comparing the distribution of siRNAs from cassava plants infected with EACMCV at three temperatures (25°C, 25°C–30°C, and 30°C). The results revealed that DNA-B fragments were heterogeneously targeted at all three temperatures tested; however, the level of siRNA accumulation was found to be higher in segments 2 and 6 (approximately 30% of the total siRNAs derived from the DNA-B component; Fig. 6C). In the DNA-A component, variations in target region occurred with changing temperature, in particular targeting segments 6 and 4 at 25°C to 30°C and 30°C, respectively (Fig. 6C), but the significance of this finding will require additional study. Interestingly, the EACMCV-derived siRNAs obtained from plants grown at 30°C originated mainly from segment 4 (40%) and segment 5 (25%) of the DNA-A, which correspond to the C terminus of AC1 gene and the AC2 and AC3 genes that have major roles in virus replication and gene transcription (Fig. 6C). But at temperature 25°C to 30°C, segment 6 (58%), which corresponds to the AC1 gene, was preferentially targeted. Although EACMCV is a nonrecovery-type virus, at higher temperature (30°C) the newly emerged leaves from the virus-infected plants showed a less symptomatic phenotype that correlated with an increasing amount of virus-derived siRNA accumulation targeting both DNA-A and DNA-B components compared to the levels obtained at low (25°C) temperature. The total amount of siRNA accumulation increased with increasing temperature by more than 600% for both components of the genome (Fig. 4). In conclusion, EACMCV-induced PTGS was much more efficient at higher temperature, and this could explain, at least partially, the attenuated symptoms displayed by plants at these temperatures (Fig. 6, A and B).
Effect of Temperature on ICMV-Induced Symptom Severity and on EACMV-[Ug]-Induced Symptom Severity, and Distribution of Virus-Derived siRNAs in Infected Plants
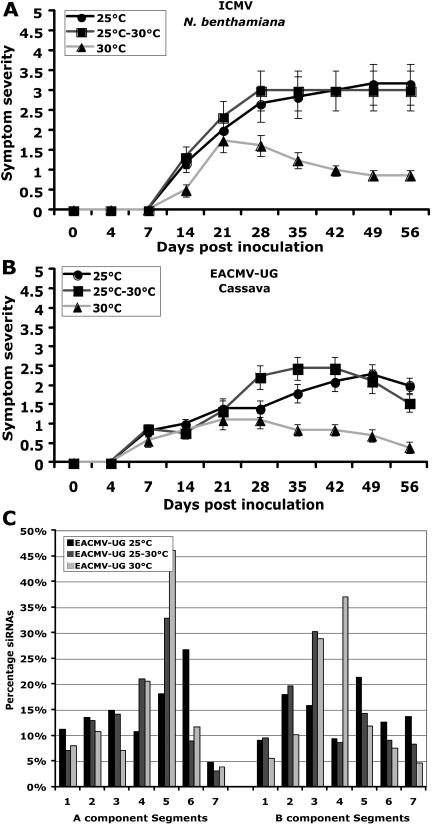
Infectious clones of ICMV have the ability to infect N. benthamiana but not cassava (Saunders et al., 2002). To check the effect of temperature on this virus-induced disease severity, the ICMV-inoculated N. benthamiana plants were grown in three temperature conditions (25°C, 25°C–30°C, and 30°C). In general, the level of symptom development in N. benthamiana was reduced with increasing temperature (Fig. 7A). At 25°C and 25°C to 30°C, the severity of symptoms was almost similar, but at 30°C a reduction in symptom severity was accompanied by the emergence of less symptomatic new leaves at the later stage of the infection period (Fig. 7A), indicating that temperature in fact altered the ICMV-induced symptom development.
Figure 7.
Effect of temperature on symptom severity of ICMV in N. benthamiana and on EACMV-[Ug] infection in cassava, and the level and distribution of EACMV-[Ug] genome siRNAs in infected cassava plants. A and B, ICMV symptom severity trend in N. benthamiana (A) and EACMV-[Ug] in cassava (B) in three ranges of temperature: 25°C, 25°C to 30°C, and 30°C. Ten plants were inoculated as five plants in two experiments. Bars in symptom severity curve indicate se values of 10 plants. C, The level and distribution of virus-derived siRNA accumulation were assessed only in virus infection in cassava. Gel blots (first seven segments for DNA-A and second seven segments for DNA-B) were subjected to hybridization using 5′-labeled virus-derived siRNAs purified from EACMV-[Ug]-infected plants grown at 25°C, 25°C to 30°C, and 30°C. The intensity of signals in the blots was quantified and plotted into graphs. Values on the y axis represent the percentage of siRNAs derived from either DNA-A or DNA-B of EACMV-[Ug]. Each bar represents the amount of siRNAs for respective DNA segments in comparison to the total amount of siRNAs either for DNA-A or DNA-B. Blots were quantified using image quant (IqMacV1.2) software. Numbers in the x axis indicate the PCR-amplified DNA fragments representing various regions of the SLCMV genome.
The effect of temperature on EACMV-[Ug]-induced disease development was also studied. Symptom severity was much higher in EACMV-[Ug]-infected plants that were grown under 25°C; however, severity gradually decreased by increasing the temperature to 30°C. Indeed, the newly emerged leaves of the infected plants grown at 30°C showed recovery from symptom appearance (Fig. 7B). Southern-blot analysis revealed a 90% virus DNA accumulation at 25°C to 30°C compared to 30% at 30°C (Fig. 3, D and E).
Next, we tested the effect of temperature on EACMV-[Ug]-induced PTGS. The distribution of siRNAs on the virus genome was analyzed by probing gel blots carrying segments of the DNA-A and DNA-B. At 25°C, segments were mostly randomly targeted; however, at 25°C to 30°C and 30°C, segments 5 of DNA-A and 3 of DNA-B were more targeted (Fig. 7C). This nonrandom targeting of segments was similar to what was observed for EACMCV and requires further study. The total amount of siRNA accumulation from DNA-A and DNA-B components increased with raising the temperature by more than 300% (Fig. 4), indicating that EACMV-[Ug]-induced PTGS is temperature dependent. Both in EACMCV and EACMV-[Ug], the level of virus-derived siRNAs increased 3- to 6-fold for both DNA-A and DNA-B with increasing growth temperatures, to levels similar or higher than those observed for the recovery-type viruses.
DISCUSSION
In plants, PTGS serves as a natural antiviral defense response (Waterhouse et al., 2001). Viruses have the ability to induce (Hamilton and Baulcombe, 1999) and suppress plant PTGS (Anandalakshmi et al., 1998). Indeed, it is evident that certain viruses become targets of the induced PTGS; as a consequence, the newly emerged leaves of the infected plants show recovery from virus symptoms at a later stage of the infection period (Ratcliff et al., 1997; Chellappan et al., 2004a). Environmental factors have been shown to modify plant-virus interactions so much that virus-induced symptoms are in fact attenuated at high temperature (Harrison, 1956). In this study, we investigated five distinct species of cassava geminiviruses. We report that symptom severity attenuated with raising the temperature of virus-infected plants, irrespective of the nature of the virus. Geminiviruses have ssDNA genome, and replication is accomplished via dsDNA by a rolling circle mechanism in the nuclei of infected plant cells (Laufs et al., 1995). However, these viruses have the ability to trigger host PTGS with the production of virus-derived siRNAs. Indeed, ACMV-[CM]- and SLCMV-induced recovery phenotype correlated with high levels of virus-derived siRNA accumulation. On the other hand, in nonrecovery-type cassava-infecting geminiviruses such as EACMCV, EACMV-[Ug] and ICMV, although inducing PTGS, the amount of virus-derived siRNAs was very low compared to the amount accumulated in ACMV-[CM]-infected plants (Chellappan et al., 2004a).
A recent report revealed that the RNA silencing-mediated defense response is temperature dependent by the fact that CymRSV (an RNA virus)-induced symptom severity was found to be higher at low temperature, and decreased with rising temperature and elevated levels of virus-derived siRNAs (Szittya et al., 2003). Furthermore, the role of gene silencing in virus protection was evidenced by the fact that defective RNA (DI-RNA) associated with CymRSV enhanced the virus-specific siRNAs at high temperature (Havelda et al., 2005). In our case, we found that cassava geminivirus-induced RNA silencing also increased with rising temperature in all cases, irrespective of the recovery-type or the nonrecovery-type viruses. Normally, ACMV-[CM] and SLCMV infection leads to severe symptoms during the initial stage of infection, but the infected plants show recovery from symptom appearance at a later stage with high levels of virus-derived siRNAs. Raising the temperature indeed altered the ACMV-[CM] infection by decreasing the overall symptom severity, and, in addition, recovery from symptoms appeared much earlier at 30°C compared to plants grown at 25°C. However, the total amount of virus-derived siRNA accumulation was increased only by 20% to 30%, and the targeted region in the virus genome remained the same in all three temperatures tested. Interestingly, raising the temperature from 25°C to 30°C dramatically altered the capacity of virus-induced PTGS of nonrecovery-type cassava geminiviruses with elevated levels of virus-derived siRNAs. The overall level of virus-derived siRNA accumulation was less abundant in nonrecovery-type viruses (EACMCV, EACMV-[Ug], and ICMV) compared to the level accumulated by recovery-type viruses (ACMV-[CM] and SLCMV) at 25°C. But in EACMCV and EACMV-[Ug], the level of virus-derived siRNAs increased by a factor between 3- and 6-fold from lower (25°C) to higher (30°C) temperatures. These results revealed that the effect of RNA silencing, induced by temperature, seems to be much more efficient in nonrecovery-type viruses than in recovery-type viruses (Fig. 4). The target virus sequences generally remained unchanged in recovery-type viruses but appear to be different for nonrecovery-type viruses at higher temperatures. This result requires further experimentation to be confident in these findings but point to the potential that small temperature changes may affect the selection of targets for PTGS. The target sequences of ACMV-[CM]-induced PTGS were the same between N. benthamiana (Chellappan et al., 2004a) and cassava. However, EACMCV-induced PTGS was found to target segment 4 of the DNA-B in N. benthamiana but segments 2 and 6 in cassava, indicating that the silencing system or the virus behaved differently between these two host plants. In general, N. benthamiana is a susceptible host for many plant viruses, a condition due perhaps to its defective RdRP (Yang et al., 2004). But no information is available about the gene-silencing machinery in cassava; therefore, it is difficult to pinpoint the exact cause for this case.
During the past decade, considerable evidence has been obtained supporting the idea that PTGS suppression by viruses is often required to establish infection in plants. Virus-encoded silencing suppressors are structurally and functionally diverse. Although 33 silencing suppressor proteins have been identified both in plant and animal viruses, the exact mode of action was demonstrated only for a few proteins (Roth et al., 2004). The p19 of tombusviruses and the p21 of beet yellows virus have been shown to bind to the duplex forms of siRNAs and miRNAs, indicating that these silencing suppressor proteins might target the small RNA duplex unwinding process of the PTGS pathway (Silhavy et al., 2002; Chapman et al., 2004). CymRSV-induced symptom severity was higher at low temperature, but the severity decreased with rising temperature and elevated levels of siRNAs (Szittya et al., 2003). Although CymRSV p19 protein has the capacity to bind duplex siRNAs, the presence of DI-RNA in fact protected the plants from CymRSV at higher temperature with the production of elevated levels of virus-specific siRNAs (Havelda et al., 2005). The temperature-dependent virus protection in CymRSV was proposed to be due to enhanced DICER activity to generate enormous amounts of siRNAs (Szittya et al., 2003). In cassava geminiviruses, the AC4 gene of ACMV-[CM] and SLCMV (recovery type) and the AC2 gene of EACMCV and ICMV (nonrecovery type) were identified as PTGS suppressors, indicating differential roles of AC2 and AC4 genes in different viruses (Vanitharani et al., 2004). In this study, we report that the recovery from virus infection was enhanced by raising the temperature from 25°C to 30°C regardless of the virus type (recovery versus nonrecovery). The recovery phenotype correlated with decreasing virus levels in the plant (Fig. 3); however, the correlation was not perfect (e.g. the levels of SLCMV were less correlated with siRNA levels and disease symptoms; Fig. 5). More detailed time-course studies will better resolve this issue. At high temperatures, a large increase in siRNAs occurred during infection with nonrecovery-type viruses, but only a modest increase occurred during infection with recovery-type viruses (Fig. 4). In addition, although requiring further study, it appeared that the target sequence was altered at high temperature during infection with nonrecovery- versus recovery-type viruses (Figs. 4, 6, and 7).
We propose the following hypotheses to explain the scenario of the influence of temperature on RNA silencing of cassava geminiviruses. (1) It is possible that RdRP and Dicer might be more active at higher temperatures. Cellular RdRP is involved in the signal amplification process to generate transitive siRNAs (Dalmay et al., 2000). The fact that geminivirus-based virus vectors require host RdRP to facilitate silencing of host genes suggests that host RdRP might play a role in geminivirus-induced PTGS (Muangsan et al., 2004). Specifically, the RdRP may create more substrates for the Dicer. It is also possible that the Dicer directly involved in the production of siRNAs from long dsRNAs is directly affected by temperature, leading to a dramatic increase of the siRNAs. (2) Another host factor(s) involved in geminivirus replication and transcription may be less effective with rising temperature; as a result, there would be a reduction in transcript levels and, consequently, fewer PTGS suppressor proteins, which in turn would enhance the gene-silencing system. (3) It is also possible that the interaction of the viral suppressors with their host counterpart(s) may be altered by temperature changes, resulting in much higher gene-silencing activity at high temperature.
In conclusion, geminiviruses, in spite of having ssDNA genome and no dsRNA phase during their replication cycle, are able to trigger PTGS with the generation of virus-derived siRNAs (Chellappan et al., 2004a). Indeed, virus-derived siRNA accumulation correlated with recovery from symptoms in ACMV-[CM]-infected plants. Nonrecovery-type cassava geminiviruses also induce PTGS with the production of low levels of virus-derived siRNAs (Chellappan et al., 2004a). Our results revealed that cassava geminivirus-induced PTGS is modified by temperature, regardless of the virus type (recovery or nonrecovery). There is a general trend of decreased symptom and virus load with higher temperature, indicating that ssDNA viruses follow the same trend as RNA viruses. However, our experiments clearly demonstrate a differential effect of temperature on the level of virus-derived siRNAs between recovery and nonrecovery types of viruses, with approximately 6-fold more virus-derived siRNAs for the latter category. There must be a critical difference between the two types of viruses, and we have already determined that these viruses use different types of viral proteins for PTGS suppression (Vanitharani et al., 2004). Nonrecovery-type EACMCV and ICMV use the AC2 gene as PTGS suppressor. On the other hand, the recovery-type ACMV-[CM] and SLCMV use the AC4 gene for PTGS suppression, suggesting that the two viruses are different at least in that aspect. Therefore, it is possible that the differential effect of temperature on these viruses could be due to the direct influence of temperature on their PTGS suppressors. The effect of temperature on these different types of viral silencing suppressor proteins is under investigation.
Gene silencing has a potential application in generating virus-resistant transgenic plants, and the fact that low temperature affects gene silencing is a major constraint for implementing gene silencing-based strategies for virus resistance. Therefore, understanding the molecular switch or factor(s) involved in stimulating RNA silencing at high temperature is of prime importance; it would pave the way to develop strategies to overcome the influence of temperature on PTGS in the application of PTGS-based virus resistance in the field.
MATERIALS AND METHODS
Multiplication of Cassava and Nicotiana benthamiana Plants
Cassava (Manihot esculenta, Crantz) plants were multiplied in vitro by micropropagation. Nodal cuttings of the cassava cv TMS 60444 from 6-week-old regenerated plants were obtained. They were surface-sterilized for 30 min in 15% bleach solution containing 100 μL of Tween 20 and washed thoroughly in sterilized water. These sterilized cuttings were cultured in petri dishes containing MS2 medium (4.31 g Murashige and Skoog salt + 20 g Suc + 1 mL of vitamins [1,000×] + 0.5 mL naphthylacetic acid [10−3], pH 5.8, solidified by phytagel) and incubated at 28°C under 16 h light for 3 to 4 weeks. Rooted plantlets were transplanted into pots (7-cm diameter×6-cm depth) containing growth medium 702 Metro-Mix (Scotts, Marysville, OH). The plants were maintained in a greenhouse for 3 to 4 weeks before they were inoculated with virus. Seeds of N. benthamiana were sown in a pot and kept in humidifying chambers. One week later, seedlings were transplanted into individual pots containing a mix of Scotts 360 with Coir. The plants were maintained in a greenhouse for 3 to 4 weeks before they were inoculated with virus.
Virus Inoculation
Construction of the infectious clones of DNA-A and DNA-B of ACMV-[CM] and EACMCV (Fondong et al., 2000) and EACMV-[UG] (Pita et al., 2001) has been described previously. Infectious clones of DNA-A and DNA-B of SLCMV and ICMV (Saunders et al., 2002) were the kind gift of Dr. John Stanley (John Innes Centre, Norwich, UK). For virus inoculation, gold particles (0.6 μ) were coated separately with a mixture of 10 ng each of DNA-A and DNA-B of infectious clones of ACMV-[CM], SLCMV, EACMCV, EACMV-[UG], and ICMV. Five plants were inoculated for each virus using a particle delivery system at 1,100 psi (Bio-Rad model PDS-1000/He; Bio-Rad Laboratories, Hercules, CA), and the experiment was repeated two times. To ensure 100% infection, the youngest unfolded leaf was targeted on the adaxial side at the point where the leaflets are attached to the petiole. Virus-inoculated plants were grown in growth chambers set at three temperatures: 25°C (constant), 25°C to 30°C (11 h at 25°C, 4 h at 30°C, and ramp 9 h), and 30°C (constant) with 14 h light and 10 h dark in all three temperatures. The light setting uses a combination of light sources (fluorescent and incandescent bulbs) at 300 μE m−2 s−1.
Assessment of Symptoms and Southern-Blot Analysis
Plants were maintained in three temperature conditions: 25°C, 25°C to 30°C, and 30°C. Systemically infected leaves were scored every other day after inoculation for 8 weeks. A leaf was regarded as systemically infected if there was no scar on the leaf surface as a result of inoculation and symptoms developed first at the joint of the petiole and the leaflets. Symptom severity score was rated on a six-point scale: 0, no symptoms; 1, mild chlorosis without leaf deformation; 2, clear mosaic with or without slight leaf deformation; 3, strong mosaic all over the leaflets with leaf deformation; 4, as in 3 but with severe leaf deformation; and 5, severe mosaic and severe reduction of leaf size (Chellappan et al., 2004a).
The symptomatic young leaves were collected during the peak of infection to determine viral DNA accumulation by Southern-blot hybridization and virus-derived siRNA distribution by northern-blot analysis (ACMV-[CM] 2 wpi, SLCMV 3 wpi, EACMCV 4 wpi, and EACMV-[Ug] 5 wpi). Total DNA (8 μg) isolated from a pool of leaf tissue collected from five infected cassava and N. benthamiana plants grown in three temperature conditions (25°C, 25°C–30°C, and 30°C) was blotted and hybridized to specific probes as described previously (Chellappan et al., 2004b). Blots were scanned and quantified using IQMacV1.2 software and a PhosphorImager (Molecular Dynamics/Amersham, Piscataway, NJ).
Isolation, Labeling, and Assessment of 21- to 26-Nucleotide RNAs
The low Mr RNAs were isolated from a pool of leaves collected from five infected plants for each virus as described (Chellappan et al., 2004a). Low Mr RNA was subjected to electrophoresis through a 15% denaturing polyacrylamide gel in 0.5× Tris-borate/EDTA buffer followed by staining in ethidium bromide solution (0.5 μg/mL) for about 30 min. The 21- to 26-nt RNAs were visualized by UV light and excised from gel. The gel slice was crushed and covered with 0.3 m NaCl and incubated overnight at 4°C. The gel residue was pelleted down by centrifugation, and the RNA was precipitated with ethanol (Chellappan et al., 2004a). The 21- to 26-nt RNAs (approximately 1–2 μg) were dephosphorylated (Alkaline Phosphatase, Calf Intestinal; New England Biolabs, Beverly, MA) and labeled subsequently in a 30-μL reaction in the presence of [γ-32P]ATP using T4-polynucleotide kinase (New England Biolabs). The labeled ACMV-[CM], EACMCV, EACMV-[Ug], SLCMV, and ICMV-specific 21- to 26-nt RNAs were used for hybridization of blots containing PCR-amplified approximately 400-bp DNA segments (using specific primers) of DNA-A and DNA-B of ACMV-[CM], EACMCV, EACMV-[Ug], and SLCMV. Hybridization was carried out at 42°C overnight in 5× SSC (0.75 m NaCl, 0.075 m trisodium citrate, pH 7.0), 1× Denhardt's solution (0.1% each Ficoll, polyvinylpyrrolidone, and bovine serum albumin), and 0.5% SDS with competitor DNA (Herring sperm DNA; Sigma, St. Louis). Posthybridization washes were done sequentially with 2× SSC, 0.5× SSC, and 0.2× SSC along with 0.1% SDS, each wash for 30 min. Blots were scanned and quantified using IQMacV1.2 software and PhosphorImager (Molecular Dynamics/Amersham).
Acknowledgments
We thank Dr. John Stanley (John Innes Institute, Norwich, UK) for providing SLCMV and ICMV clones. We thank Patricia Cosgrove for careful reading of the manuscript. We acknowledge our greenhouse staff, Edward Fischer and his team, for good care of the plants.
This work was supported by the Donald Danforth Plant Science Center.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.066563.
References
- Anandalakshmi R, Pruss GJ, Ge X, Marathe R, Mallory AC, Smith TH, Vance VB (1998) A viral suppressor of gene silencing in plants. Proc Natl Acad Sci USA 95: 13079–13084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe D (2004) RNA silencing in plants. Nature 431: 356–363 [DOI] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409: 363–366 [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Prokhnevsky AI, Gopinath K, Dolja VV, Carrington JC (2004) Viral RNA silencing suppressors inhibit the microRNA pathway at an intermediate step. Genes Dev 18: 1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan P, Masona MV, Vanitharani R, Taylor NJ, Fauquet CM (2004. b) Broad spectrum resistance to ssDNA viruses associated with transgene-induced gene silencing in cassava. Plant Mol Biol 56: 601–611 [DOI] [PubMed] [Google Scholar]
- Chellappan P, Vanitharani R, Fauquet CM (2004. a) Short-interfering RNA accumulation correlates with host recovery in DNA virus infected hosts and gene silencing targets specific viral sequences. J Virol 78: 7465–7477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan P, Vanitharani R, Fauquet CM (2005) MicroRNA-binding viral protein interferes with Arabidopsis development. Proc Natl Acad Sci USA 102: 10381–10386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogoni C, Macino G (1997) Isolation of quelling-defective (qde) mutants impaired in posttranscriptional transgene-induced gene silencing in Neurospora crassa. Proc Natl Acad Sci USA 94: 10233–10238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe DC (2000) An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101: 543–553 [DOI] [PubMed] [Google Scholar]
- Fauquet CM, Stanley J (2003) Geminivirus classification and nomenclature: progress and problems. Ann Appl Biol 142: 165–189 [Google Scholar]
- Finnegan EJ, Margis R, Waterhouse PM (2003) Posttranscriptional gene silencing is not compromised in the Arabidopsis CARPEL FACTORY (DICER-LIKE1) mutant, a homolog of Dicer-1 from Drosophila. Curr Biol 13: 236–240 [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806–811 [DOI] [PubMed] [Google Scholar]
- Fondong VN, Pita JS, Rey ME, de Kochko A, Beachy RN, Fauquet CM (2000) Evidence of synergism between African cassava mosaic virus and a new double-recombinant geminivirus infecting cassava in Cameroon. J Gen Virol 81: 287–297 [DOI] [PubMed] [Google Scholar]
- Gerik JS, Duffus JE, Perry R, Stenger DC, Van Maren AF (1990) Etiology of tomato plant decline in the California desert. Phytopathology 80: 1352–1356 [Google Scholar]
- Hamilton AJ, Baulcombe DC (1999) A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286: 950–952 [DOI] [PubMed] [Google Scholar]
- Harrison BD (1956) Studies on the effect of temperature on virus multiplication in inoculated leaves. Ann Appl Biol 44: 215–226 [Google Scholar]
- Havelda Z, Hornyik C, Valoczi A, Burgyan J (2005) Defective interfering RNA hinders the activity of a tombusvirus-encoded posttranscriptional gene silencing suppressor. J Virol 79: 450–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine RB, Osborne WE, Dennis RE (1970) Elevation and temperature effects on severity of maize virus in sorghum in Arizona. Plant Dis Rep 54: 1064–1068 [Google Scholar]
- Johnson J (1922) The relation of air temperature to the mosaic disease of potatoes and other plants. Phytopathology 12: 438–440 [Google Scholar]
- Khvorova A, Reynolds A, Jayasena SD (2003) Functional siRNAs and miRNAs exhibit strand bias. Cell 115: 209–216 [DOI] [PubMed] [Google Scholar]
- Laufs J, Traut W, Heyraud F, Matzeit V, Rogers SG, Schell J, Gronenborn B (1995) In vitro cleavage and joining at the viral origin of replication by the replication initiator protein of tomato yellow leaf curl virus. Proc Natl Acad Sci USA 92: 3879–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legg JP, Fauquet CM (2004) Cassava mosaic geminiviruses in Africa. Plant Mol Biol 56: 585–599 [DOI] [PubMed] [Google Scholar]
- Lucioli A, Noris E, Brunetti A, Tavazza R, Ruzza V, Castillo AG, Bejarano ER, Accotto GP, Tavazza M (2003) Tomato yellow leaf curl Sardinia virus rep-derived resistance to homologous and heterologous geminiviruses occurs by different mechanisms and is overcome if virus-mediated transgene silencing is activated. J Virol 77: 6785–6798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganaris GA, Economou AS, Boubourakas IN, Katis NI (2003) Elimination of PPV and PNRSV through thermotherapy and meristem-tip culture in nectarine. Plant Cell Rep 22: 195–200 [DOI] [PubMed] [Google Scholar]
- Mansoor M, Briddon RW, Zafar Y, Stanley J (2003) Geminivirus disease complexes: an emerging threat. Trends Plant Sci 8: 128–134 [DOI] [PubMed] [Google Scholar]
- Muangsan N, Beclin C, Vaucheret H, Robertson D (2004) Geminivirus VIGS of endogenous genes requires SGS2/SDE1 and SGS3 and defines a new branch in the genetic pathway for silencing in plants. Plant J 38: 1004–1014 [DOI] [PubMed] [Google Scholar]
- Napoli C, Lemieux C, Jorgensen R (1990) Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2: 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pita JS, Fondong VN, Sangare A, Otim-Nape GW, Ogwal S, Fauquet CM (2001) Recombination, pseudorecombination and synergism of geminiviruses are determinant keys to the epidemic of severe cassava mosaic disease in Uganda. J Gen Virol 82: 655–665 [DOI] [PubMed] [Google Scholar]
- Ratcliff F, Harrison BD, Baulcombe DC (1997) A similarity between viral defense and gene silencing in plants. Science 276: 1558–1560 [DOI] [PubMed] [Google Scholar]
- Roth BM, Pruss GJ, Vance VB (2004) Plant viral suppressors of RNA silencing. Virus Res 102: 97–108 [DOI] [PubMed] [Google Scholar]
- Saunders K, Salim N, Mali VR, Malathi VG, Briddon R, Markham PG, Stanley J (2002) Characterisation of Sri Lankan cassava mosaic virus and Indian cassava mosaic virus: evidence for acquisition of a DNA B component by a monopartite begomovirus. Virology 293: 63–74 [DOI] [PubMed] [Google Scholar]
- Schauer SE, Jacobsen SE, Meinke DW, Ray A (2002) DICER-LIKE1: blind men and elephants in Arabidopsis development. Trends Plant Sci 7: 487–491 [DOI] [PubMed] [Google Scholar]
- Silhavy D, Molnar A, Lucioli A, Szittya G, Hornyik C, Tavazza M, Burgyan J (2002) A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J 21: 3070–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szittya G, Silhavy D, Molnar A, Havelda Z, Lovas A, Lakatos L, Banfalvi Z, Burgyan J (2003) Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J 22: 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans MCP, Das OP, Messing J (1994) Geminiviruses and their uses as extrachromosomal replicons. Annu Rev Plant Physiol Plant Mol Biol 45: 79–112 [Google Scholar]
- Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD (2004) A protein sensor for siRNA asymmetry. Science 306: 1377–1380 [DOI] [PubMed] [Google Scholar]
- Townsend R, Stanley J, Curson SJ, Short MN (1985) Major polyadenylated transcripts of cassava latent virus and location of the gene encoding coat protein. EMBO J 4: 33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance V, Vaucheret H (2001) RNA silencing in plants—defense and counterdefense. Science 292: 2277–2280 [DOI] [PubMed] [Google Scholar]
- Vanitharani R, Chellappan P, Fauquet CM (2003) Short interfering RNA-mediated interference of gene expression and viral DNA accumulation in cultured plant cells. Proc Natl Acad Sci USA 100: 9632–9636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanitharani R, Chellappan P, Fauquet CM (2005) Geminiviruses and RNA silencing. Trends Plant Sci 10: 144–151 [DOI] [PubMed] [Google Scholar]
- Vanitharani R, Chellappan P, Pita JS, Fauquet CM (2004) Differential roles of AC2 and AC4 of cassava geminiviruses in mediating synergism and posttranscriptional gene silencing suppression. J Virol 78: 9487–9498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse PM, Wang M, Lough T (2001) Gene silencing as an adaptive defence against viruses. Nature 411: 834–842 [DOI] [PubMed] [Google Scholar]
- Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC (2004) Genetic and functional diversification of small RNA pathways in plants. PLoS Biol 2: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SJ, Carter SA, Cole AB, Cheng NH, Nelson RS (2004) A natural variant of a host RNA-dependent RNA polymerase is associated with increased susceptibility to viruses by Nicotiana benthamiana. Proc Natl Acad Sci USA 101: 6297–6302 [DOI] [PMC free article] [PubMed] [Google Scholar]