Abstract
Molecular genetic studies of plant dwarf mutants have indicated that gibberellin (GA) and brassinosteroid (BR) are two major factors that determine plant height; dwarf mutants that are caused by other defects are relatively rare, especially in monocot species. Here, we report a rice (Oryza sativa) dwarf mutant, dwarf and gladius leaf 1 (dgl1), which exhibits only minimal response to GA and BR. In addition to the dwarf phenotype, dgl1 produces leaves with abnormally rounded tip regions. Positional cloning of DGL1 revealed that it encodes a 60-kD microtubule-severing katanin-like protein. The protein was found to be important in cell elongation and division, based on the observed cell phenotypes. GA biosynthetic genes are up-regulated in dgl1, but the expression of BR biosynthetic genes is not enhanced. The enhanced expression of GA biosynthetic genes in dgl1 is not caused by inappropriate GA signaling because the expression of these genes was repressed by GA3 treatment, and degradation of the rice DELLA protein SLR1 was triggered by GA3 in this mutant. Instead, aberrant microtubule organization caused by the loss of the microtubule-severing function of DGL1 may result in enhanced expression of GA biosynthetic genes in that enhanced expression was also observed in a BR-deficient mutant with aberrant microtubule organization. These results suggest that the function of DGL1 is important for cell and organ elongation in rice, and aberrant DGL1-mediated microtubule organization causes up-regulation of gibberellin biosynthetic genes independently of gibberellin signaling.
Plant dwarfism is one of the most important phenotypes used in plant breeding, and more than 60 rice (Oryza sativa) dwarf mutants have been identified (Matsuo et al., 1997). Dwarfism arises from various types of defect, but two major factors responsible for dwarfism, gibberellin (GA) and brassinosteroid (BR), have been the most intensely studied. Numerous dwarf mutants are deficient in the biosynthesis or perception of these phytohormones (Mandava, 1988; Clouse and Sasse, 1998; Taiz and Zeiger, 2002; Fujioka and Yokota, 2003). For several years, our group has studied rice mutants defective in GA and BR biosynthesis or perception and cataloged the typical phenotypes of rice GA- and BR-related mutants. For example, rice GA-related mutants are typically dwarfs with deep green, rough leaves, but exhibit no other abnormal morphology (Sakamoto et al., 2004). In contrast, rice BR-deficient mutants are typically dwarfs with other abnormal morphologies, including malformed leaves with twisted, stiff blades (Hong et al., 2004).
In contrast to the extensive characterization of dwarf mutants related to GA and BR, dwarf mutants with phenotypes that differ from the typical GA- or BR-related phenotypes have not been well characterized. These uncharacterized dwarf mutants may be caused by various defects related to the elongation and/or division of stem cells. For example, analysis of the rice dwarf mutant d3, which exhibits dwarfism and increased tiller numbers, revealed that D3 encodes an F-box Leu-rich-repeat protein orthologous to the Arabidopsis (Arabidopsis thaliana) MAX2/ORE9 (Ishikawa et al., 2005). This suggests that a novel F-box Leu-rich-repeat protein is involved in stem elongation in rice.
In an effort to further understand the molecular mechanisms of stem elongation in rice, we have collected dwarf mutants that show phenotypes that are different from the typical GA- or BR-related dwarf mutants. These mutants have been categorized into several groups according to their characteristics. For example, some mutants exhibit dwarfism with narrow leaves and increased tiller numbers; others show poor growth with erect, narrow leaves; and one dwarf group has leaves with a unique rounded tip. We further examined the latter group of dwarf mutants, dwarf and gladius leaf 1 (dgl1), because these mutants also showed defects in root and flower elongation (see “Results”), suggesting that DGL1 may be involved in a fundamental mechanism in cell elongation in rice. The DGL1 gene was found to encode a microtubule-severing katanin-like protein that is important in cell elongation and division in plants. We analyzed the relationship between DGL1 and GA and BR signaling and found that GA biosynthesis genes are up-regulated in dgl1, although the GA signaling is normal in this mutant. We discuss the possibility that the up-regulation of GA biosynthetic genes occurs due to aberrant microtubule organization, independently of GA feedback regulation, via GA signaling.
RESULTS
Characterization of the dgl1 Mutant
The dgl1 mutants show two characteristic phenotypes: dwarfism and abnormal leaf blade morphology (Fig. 1). We screened for these characteristics to isolate three alleles with different severities (dgl1-1, dgl1-2, and dgl1-3). The dgl1 leaves were shorter and the edges of the leaf tips were more rounded than in wild-type leaves (Fig. 1, B and C). This mutant showed inhibited elongation of the seminal and crown roots and reduced numbers of crown roots (Fig. 1D). The development of dgl1 floral organs was also impaired. The rice flower is composed of one pair of glumes, one lemma, one palea, two lodicules, six stamens, and two stigmas arranged from the peripheral to the central direction (Fig. 1F, far left). The dgl1 flowers were stunted and the lemma and palea were rounded (Fig. 1E). The dgl1 flowers also developed short anthers and filaments (Fig. 1G) and a short, shrunken stigma (Fig. 1H). The severity of each of these abnormal leaf and flower phenotypes correlated with the level of dwarfism. The fertility of the mutants, even those with relatively mild phenotypes, was significantly lower than that of the wild type. These observations indicate that the loss of function of the DGL1 gene causes multiple pleiotropic defects in various organs.
Figure 1.
Morphological characteristics of the dgl1 mutants. A, Gross morphology of dgl1 at 1 month after sowing. From left to right, Wild type, dgl1-2 (mild allele), and dgl1-3 (strong allele). Bar = 30 cm. B, Leaf blade morphology of wild type (left) and dgl1-3 (right). Bar = 5 cm. C, Leaf tip morphology of wild type (left) and dgl1-3 (right). Bar = 1 cm. D, Root morphology of wild type (left) and dgl1-3 (right). Bar = 5 cm. E, Morphology of flower exteriors. From left to right, Wild type, dgl1-2, and dgl1-3. Bar = 5 mm. F, Morphology of flower interiors. From left to right, Wild type, dgl1-2, and dgl1-3. Gl, Glume; Le, lemma; Pl, palea; Lo, lodicule; Sg, stigma; St, stamen. Bar = 5 mm. G, Stamens. From left to right, Wild type, dgl1-2, and dgl1-3. An, Anther; Fl, filament. Bar = 1 mm. H, Pistils. From left to right, Wild type, dgl1-2, and dgl1-3. Sg, Stigma. Bar = 1 mm.
We also examined the plastochron of the dgl1 mutants (Table I). The plastochron of the severe mutant (dgl1-3) was longer than that of the wild type, although the heading times of these two plants were similar. Consequently, the leaf number of the mutant was lower than that of the wild type. This suggests the possibility that the cell proliferation rate of the mutant is reduced (see below).
Table I.
Plastochron and leaf numbers of wild-type and dgl1-3 plants
sd in parentheses; n = 35.
| Wild Type | dgl1-3 | |
|---|---|---|
| Plastochron days | 6.2 | 8.7 |
| Maximum leaf numbers | 13.87 (0.52) | 11.55 (0.69) |
Cell Morphology and Organization in dgl1
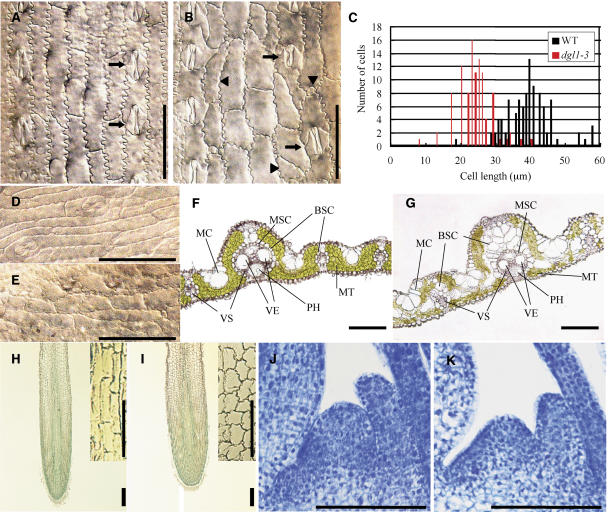
We suspected that the abnormal development of the leaves, roots, and flowers of dgl1 was caused by a defect in cell elongation and/or division. We therefore investigated the microscopic structure of dgl1 leaves. In wild-type plants, the elongated epidermal cells of the leaf blades were arranged in a longitudinal manner and formed well-organized cell files (Fig. 2A). In contrast, the longitudinally arranged cells of the mutant were not well elongated and the cells became bulky and distorted, leading to a disorganized cell file (Fig. 2, B and C). It is noteworthy that the shapes and sizes of the abnormal cells differed, with crescent-, triangle-, trapezoid-, or circular-shaped cells observed, in contrast to the wild type, which developed only rectangular cells (Fig. 2B, arrowheads). In the wild type, the stomatal cells alternated with ordinary epidermal cells to form a linear arrangement, but the equivalent line in dgl1 was disturbed (Fig. 2, A and B, arrows). The elongated, narrow epidermal cells were arranged in a gentle curve at the tip of the wild-type leaf blade (Fig. 2D). In contrast, in dgl1, the epidermal cells at the tip of the leaf blade, in the abnormally rounded region, were not well elongated; additionally, the cell file was not well organized (Fig. 2E). These abnormal cell shapes and disorganized cell arrangements may be caused by a defect in synchronous division and elongation in these cells. In fact, the transverse division of cells in the mutant was often slanted, whereas this abnormal division pattern was not observed in the wild type.
Figure 2.
Structures of cells in various dgl1 tissues. A and B, Epidermal cell morphology of wild-type and dgl1-3 leaf blades, respectively. Arrows indicate stomatal cells. Cells with triangular, crescent, or round and small shapes are indicated in B by arrowheads. Bars = 50 μm. C, Distribution of the lengths of leaf blade surface cells of wild-type and dgl1-3 plants. D and E, Epidermal cell morphology in the tip regions of wild-type and dgl1-3 leaf blades, respectively. Bars = 50 μm. F and G, Cross sections of wild-type and dgl1-3 leaf blades, respectively. In dgl1, the bundle sheath cells (BSC) are vacuolated and arranged in a disordered fashion in double or triple layers. The mother cells (MC) are also enlarged. VS, Vascular bundles; MSC, mestome sheath cells; VE, vessels; PH, phloem; MT, mesophyll tissue. Bars = 100 μm. H and I, Longitudinal sections of wild-type and dgl1-3 roots stained with toluidine blue, respectively. Bars = 100 μm. Close-up views of the elongation zones are superimposed at the top right in each image. Bars = 50 μm. J and K, Longitudinal sections of the SAMs of wild-type and dgl1-3 plants stained with toluidine blue, respectively. Bars = 100 μm.
We also observed the internal structure of wild-type and dgl1 leaf blades using transverse sections. Figure 2, F and G, show cross sections of large vascular bundles in the wild type and dgl1, respectively. In the wild type, the large vascular bundle is located within the high ridge and the small vascular bundles are located within the low ridge (Fig. 2F). The valleys between the two ridges contain several motor cells. Mesophyll tissue is present between the vascular bundles. Each large and small vascular bundle is encircled by bundle sheath cells that contain a few chloroplasts with low chlorophyll content. Inside the bundle sheath cells, there are smaller cells also arranged in a ring, which forms the mestome sheath. The xylem is located on the upper side of the vascular bundles, and the two large vessels are well defined in the large vascular bundles. The phloem is located on the lower side of the vascular bundles. In the small vascular bundles, the vessels are thin and there are few phloem sieve tube elements.
The fundamental structure composed of the vascular bundles, motor cells, mesophyll tissues, and other tissues was present in dgl1, although the bundle sheath cells were enlarged and vacuolated (Fig. 2G). The single-layer structure of the bundle sheath cells was also disordered in dgl1, and the enlarged and vacuolated cells occurred in piles between the vascular and mesophyll cells. In contrast, decreased numbers of mesophyll cells were present in dgl1 (Fig. 2G). The development of the small vascular bundles was also disturbed in the mutant, and the mutant contained increased numbers of motor cells (Fig. 2G).
The internal structure of the seminal root was also studied. In the wild type, cells of the elongation zone were well elongated and organized into cell files (Fig. 2H). In contrast, the elongation zone cells in dgl1 were not elongated or well organized and were abnormally shaped (Fig. 2I). In the dgl1 shoot apical meristem (SAM; Fig. 2, J and K), the size of the SAM dome was smaller than that of the wild type, probably because cell division might not proceed smoothly in dgl1, or cell size of the mutant is smaller than the wild type.
The dgl1 Mutant Exhibits Only a Minimal Response to GA and BR
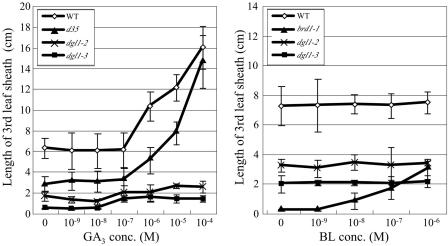
Phenotypic analyses of dgl1 suggested that, in this mutant, cell division and elongation were ubiquitously inhibited. Because treatment with GA or BR rescues the dwarf phenotype of certain rice dwarf mutants, we treated the mutant with GA3 or brassinolide (BL). As controls, the wild type, the GA-deficient d35/Tan-Ginbozu mutant (Itoh et al., 2004), and the brd1-1 BR-deficient mutant (Hong et al., 2002) were also treated. Treatment of the third-leaf sheaths of these plants with GA3 concentrations higher than 10−7 m triggered elongation of the wild-type and d35 leaf sheaths, whereas no response was observed in the dgl1 mutants dgl1-2 and dgl1-3 (Fig. 3A). Wild-type and d35 leaf sheaths both elongated after treatment with 10−4 m GA3, whereas the dgl1 mutants did not respond to any GA3 concentration.
Figure 3.
Elongation of the third-leaf sheath in response to GA3 or BL treatment. The sheath length was measured at 3 d after treatment. Wild type, d35 (GA-deficient mutant), and brd1-1 (BR-deficient mutant) plants were used as controls. Error bars = the sd from the mean (n = 10).
Next, we treated the third-leaf sheaths of plants with various concentrations of BL. In the BR-deficient mutant brd1-1, leaf sheath elongation was triggered by treatment with 10−9 m BL. In contrast, wild-type plants did not respond to this BL concentration, probably because the wild type contains a sufficient amount of endogenous bioactive BR (Fig. 3B). The mutants did not respond to treatment with BL at any concentration. The lack of response of the dgl1 mutants to exogenous GA or BR suggests that the abnormal phenotypes of the mutants are not caused by defects in GA or BR biosynthesis.
Positional Cloning of the DGL1 Gene
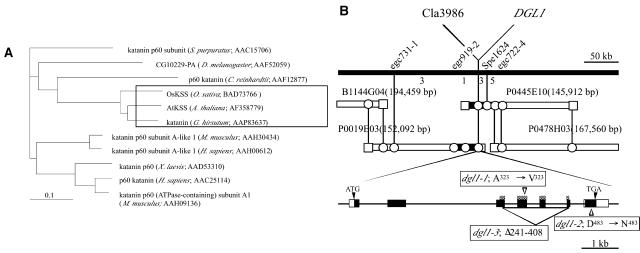
Genetic analysis indicated that the dgl1 mutation is recessive and is at a single locus (data not shown). We positionally cloned the DGL1 gene to investigate the molecular nature of the encoded protein, using F2 plants of a cross between dgl1-1 (japonica) and Kasalath (indica). The F2 plants segregated into two groups, with a 3:1 ratio of the normal and dwarf phenotypes. A total of 1,150 F2 mutant plants were used for positional mapping of the DGL1 locus. One derived cleaved amplified polymorphic sequence marker on chromosome 1, Cla3986, was found to be linked to the dgl1-1 mutation (Fig. 4B). Fine mapping using several molecular markers designed around this position revealed that the DGL1 locus is located in a 44.5-kb region between markers egr919-2 and Spe1624.
Figure 4.
Phylogenic relationship between DGL1 and katanin-like proteins in other species and physical map of the DGL1 gene. A, Phylogenetic relationship between DGL1 and katanin-like proteins in other organisms. The plant subgroup is boxed. The structural relationship was determined using ClustalW (http://www.ddbj.nig.ac.jp/search/clustalw-j.html) and illustrated using TreeView (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html). B, High-resolution linkage and physical maps of the DGL1 locus. The top line is a physical map around the DGL1 locus of chromosome 1, near 121 cM. The vertical bars represent the molecular markers, and the numbers of recombinant plants are indicated between the markers. The DGL1 mutation is tightly linked with the marker Cla3986. The DGL1 gene consists of seven exons and six introns. Black and white boxes indicate the coding and noncoding regions in exons, and ATG and TGA indicate the sites of translation initiation and stop, respectively. The mutations identified in dgl1-1, dgl1-2, and dgl1-3 are indicated. Checkered boxes indicate the ATP-binding domain.
Five putative genes annotated by the Rice Genome Project are present in the 44.5-kb region. One of the genes was of special interest because it shows similarity to a 60-kD microtubule-associated ATPase katanin-like protein (KTN1). The loss of function of Arabidopsis KTN1 causes a dwarf phenotype (Bouquin et al., 2003). The deduced amino acid sequence of the rice DGL1 gene is highly similar, over almost the entire sequence, to those of all of the katanin proteins of known sequence in other organisms (Fig. 4A), including KTN1 (81% identity), which is encoded by FRA2/BOT1/AtKSS/ERH3/LUE1 (Bichet et al., 2001; Burk et al., 2001; McClinton et al., 2001; Webb et al., 2002; Bouquin et al., 2003). We sequenced the dgl1 genes of the three dgl1 mutants. The two mild alleles contained 1-bp substitutions that generated a 1-amino acid substitution from Ala to Val in exon 4 in the putative ATP-binding domain (dgl1-1), and a 1-amino acid substitution from Asp to Asn in exon 7 in the C terminus (dgl1-2; Fig. 4B). The severe allele, dgl1-3, contained a large deletion (1,788 bp) between exons 3 and 6, which corresponds to the entire ATP-binding domain, suggesting that dgl1-3 is a null mutant. These results indicate that DGL1 encodes a rice katanin-like protein.
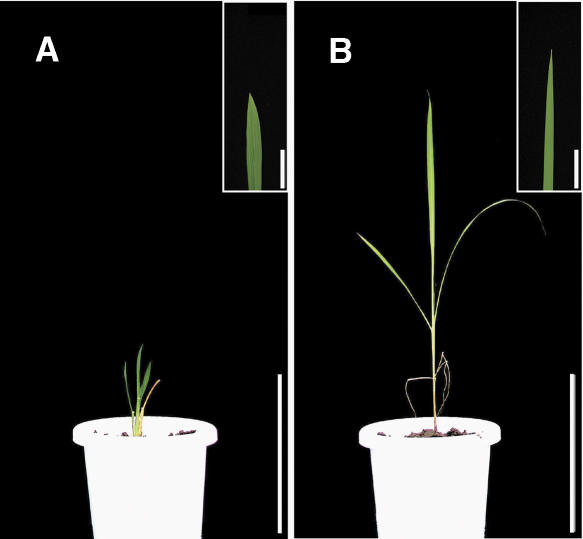
To confirm that the gene corresponds to the dgl1 locus, we performed a complementation experiment. A 9.5-kb DNA fragment containing the entire DGL1 sequence was introduced into dgl1-3 by Agrobacterium tumefaciens-mediated transformation. The dwarf phenotype of dgl1-3 was rescued in all the plants that were resistant to hygromycin, the selection marker used for transformation (Fig. 5). These results confirm that the dgl1 phenotype is caused by the loss of function of a rice katanin-like protein.
Figure 5.
Phenotypic complementation by the introduction of DGL1. A, Regenerated dgl1-3 plant transformed with the empty vector. Bar = 15 cm. B, A dgl1-3 plant transformed with a DNA fragment encompassing the entire DGL1 gene. Bar = 15 cm. The images superimposed at the top right are close-up views of the tip of leaf blade. Bar = 5 cm.
Disorganized Cortical Microtubule Arrays in dgl1 Cells
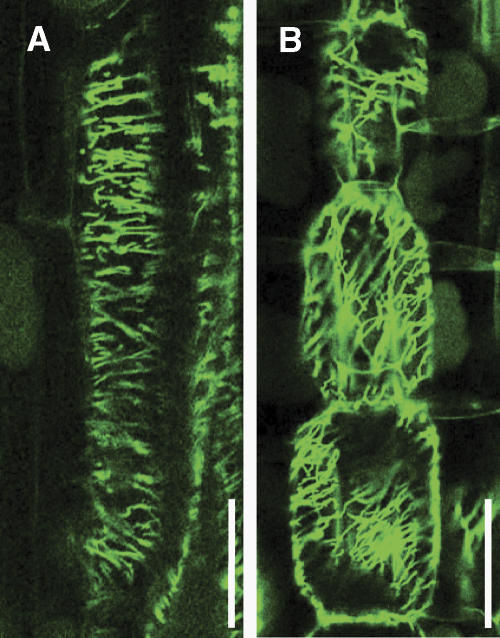
The cells of the Arabidopsis fra2 mutant, which is defective in KTN1, show abnormal microtubule distribution (Burk et al., 2001; Burk and Ye, 2002). Therefore, we examined the microtubule organization in dgl1 cells. Elongating wild-type internode cells exhibited well-organized cortical microtubules that formed parallel arrays (Fig. 6A). In contrast, in elongating dgl1 internode cells, the cortical microtubules were disorganized and arranged obliquely instead of in a parallel manner (Fig. 6B). This observation suggests that the dgl1 mutation disrupts the function of the microtubule-severing katanin-like protein, resulting in the dwarf phenotype and other abnormal morphologies, because of the aberrant organization of cortical microtubules. The observation that KTN1 has in vitro microtubule-severing activity (Stoppin-Mellet et al., 2002) supports this idea (see below).
Figure 6.
Arrangement of cortical microtubules in elongating internodal parenchyma cells. Internodal parenchyma cells were subjected to immunofluorescent staining for α-tubulin. The elongating cells in the wild type (A) contain transversely oriented cortical microtubules, whereas dgl1-3 cells (B) contain aberrantly oriented cortical microtubules. Bars = 100 μm.
DGL1 Expression Analysis
The pleiotropic effect of the dgl1 mutation in various organs indicates that the DGL1 gene functions in all of these organs. Therefore, we examined the expression of DGL1 in various rice organs with RNA gel-blot analysis using, as a probe, the entire length of the 3′-untranslated region of its cDNA. As expected, hybridizing bands were observed in RNAs extracted from each of the organs (Fig. 7A). RNAs extracted from the elongating stem and the SAM produced the strongest bands, and a band of intermediate intensity was produced using RNA from flowers, whereas RNAs from expanded leaf blades and sheaths and elongated roots produced relatively low-intensity bands. The preferential expression of DGL1 in the stems and the SAM, and the low expression in expanded leaves and elongated roots, may correspond to normal levels of cell division and/or elongation in these organs.
Figure 7.
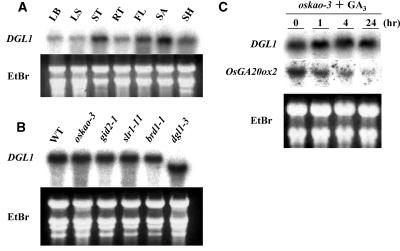
Expression of DGL1. A, Expression of DGL1 in various organs of wild-type rice. Total RNA was isolated from leaf blade (LB), leaf sheath (LS), stem (ST), root (RT), young flower before heading (FL), shoot apex (SA), and entire young seedlings (SH). Five micrograms of total RNA were loaded in each lane. The gel was stained with ethidium bromide (EtBr) as a control. B, Expression of DGL1 in GA- and BR-related mutants. Total RNAs were extracted from 1-month-old seedlings of the mutants and the RNAs were analyzed as in A. oskao-3, Severe-GA-deficient dwarf mutant; gid2-1, severe-GA-insensitive dwarf mutant; slr1-11, constitutive-GA-response slender mutant; brd1-1, severe-BR-deficient dwarf mutant; dgl1-3, severe allele of dgl1. C, DGL1 expression in the GA-deficient mutant oskao-3 after GA3 treatment. The experiments were performed as in A. The expression of OsGA20ox2/SD1 was also examined as a control for the GA3 treatment. The down-regulation of OsGA20ox2/SD1 indicates the functioning of the negative feedback regulation of GA signaling.
The steady-state level of the DGL1 mRNA was studied in various GA-related mutants (Fig. 7B), as it has been reported that the GA level positively regulates KTN1 expression (Bouquin et al., 2003). We used one GA-deficient mutant that shows a severe dwarf phenotype caused by a defect in the kaurenoic acid oxidase (KAO) activity, oskao-3 (Sakamoto et al., 2004), as well as the GA-signaling-related mutants gid2, which shows a GA-insensitive dwarf phenotype caused by a defect in the GID2/F-box protein (Sasaki et al., 2003), and slr1, which shows a constitutive-GA-response slender phenotype caused by a defect in the SLR1/DELLA protein (Ikeda et al., 2001; Itoh et al., 2002). In these GA-related mutants, DGL1 expression was very similar to that in the wild type (Fig. 7B), demonstrating that the transcriptional expression of DGL1 is not regulated by GA signaling.
In the BR-deficient mutant brd1 and in dgl1, an internal deletion allele, DGL1, was expressed at levels similar to those in the wild type (Fig. 7B). Therefore, BR does not regulate the expression of DGL1. Bouquin et al. (2003) discussed the possibility that KTN1 is involved in a feed-forward regulation of the transcription of its own gene, based on the markedly reduced KTN1 mRNA levels in the KTN1 mutant lue1. The similar levels of DGL1 mRNA in the wild type and dgl1-3 demonstrate that DGL1 expression is not regulated by the functioning of DGL1, at least in rice.
To confirm the lack of involvement of GA in DGL1 expression, we also examined the expression of the gene in GA-treated seedlings of the GA-deficient mutant oskao-3 (Fig. 7C). The levels of DGL1 expression were similar before and after GA3 treatment, whereas the OsGA20ox2/SD1 gene, which is negatively regulated by GA, was down-regulated by GA3. This result clearly shows that DGL1 expression is independent of GA.
GA Signaling in dgl1
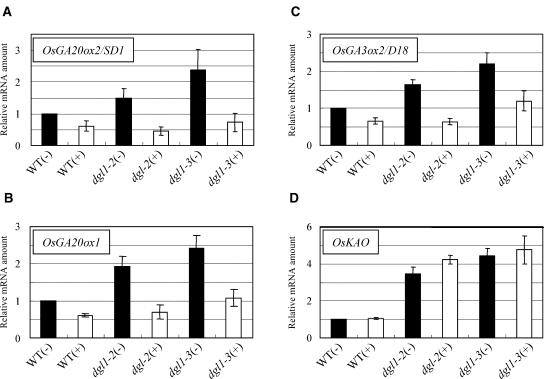
As mentioned above, dgl1 plants showed almost no response to GA3 (Fig. 3). Bouquin et al. (2003) reported that the loss of katanin function results in inappropriate feedback regulation of the GA biosynthetic AtGA20ox1 gene, as well as partial GA insensitivity. Therefore, we investigated whether GA signaling is defective in dgl1. Using quantitative reverse transcription (RT)-PCR, we first examined the expression of three rice genes involved in GA biosynthesis, OsGA20ox2/SD1, OsGA20ox1, and OsGA3ox2/D18, the expression of all of which is negatively regulated by the level of GA in a feedback manner (Itoh et al., 2001, 2002). As expected, the expression of each of these genes was negatively regulated by GA treatment in the wild type in a feedback manner through the GA-signaling pathway (Fig. 8, A–C). In the dgl1 mutants, the levels of OsGA20ox2/SD1, OsGA20ox1, and OsGA3ox2/D18 transcripts were increased relative to those in the wild type, with the levels correlated to the severity of each allele (Fig. 8, A–C). In the severe allele dgl1-3, the levels of these transcripts were 2 or more times those in the wild type. In each of the dgl1 alleles, the increased expression of these genes was suppressed by application of GA3. The down-regulation of the GA biosynthetic genes in dgl1 by GA3 treatment indicates that the normal feedback regulation of these genes occurs in the dgl1 mutants, despite their increased expression in the mutants.
Figure 8.
Enhanced expression of GA biosynthetic genes in dgl1 and suppression by GA3 treatment. Quantitative RT-PCR was performed using primers specific for each GA biosynthetic gene (see “Materials and Methods”). The plants were treated with (+) 10−4 m GA3 or control solution (−). The expression of OsGA20ox2/SD1 (A), OsGA20ox1 (B), and OsGA3ox2/D18 (C) was enhanced in dgl1 and suppressed by GA3 treatment, whereas OsKAO expression (D) was enhanced in dgl1 but not suppressed by GA.
These results suggested that the increased expression of these GA biosynthetic genes is due to a mechanism other than a defect in GA signaling. To examine this possibility, we examined the expression of GA biosynthetic genes for which the expression is not regulated by the GA level or GA signaling. Interestingly, the expression of the OsKAO gene, the product of which catalyzes the oxidation of ent-kaurenoic acid, was increased about 4 times in the mutants, as compared to the wild type, whereas GA3 treatment did not affect the expression of this gene in wild-type or mutant plants (Fig. 8D). This observation demonstrates that the increased expression of the GA biosynthetic genes is not limited to genes regulated by GA signaling but that it also occurs in genes that are not affected by GA and suggests, consequently, that the increased expression of GA biosynthetic genes in dgl1 is not related to GA signaling but is due, instead, to an unknown mechanism. We examined the expression of another GA biosynthetic gene, OsKO2, the product of which catalyzes the oxidation of ent-kaurene, and found that its expression in dgl1 was very similar to that in the wild type (data not shown), indicating that not all GA biosynthetic genes are up-regulated in dgl1.
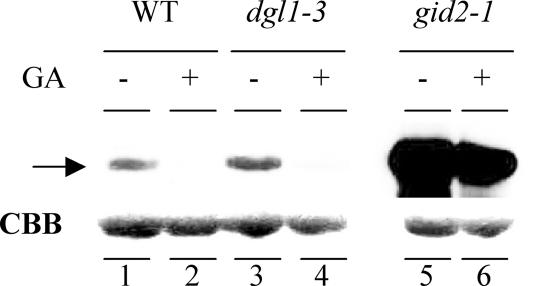
To confirm that normal GA signaling takes place in dgl1, we also examined the degradation of the SLR1 protein in the mutant using immunoblot analysis. SLR1, the sole DELLA protein in rice, functions as a repressor of GA signaling (Ikeda et al., 2001; Itoh et al., 2002; Fleet and Sun, 2005). The degradation of SLR1 is triggered by GA and is essential for the downstream action of GA (Sasaki et al., 2003). As previously reported, the GA-dependent degradation of SLR1 triggered by GA3 occurred within 1 h in both wild-type and dgl1-3 plants (Fig. 9, lanes 1–4), whereas no degradation was observed in the GA-insensitive mutant gid2 (lanes 5 and 6). Furthermore, the level of SLR1 protein was similar in wild-type and dgl1 plants not treated with GA3, but was high in gid2 (lanes 1, 3, and 5). The GA-dependent degradation of SLR1 and the similar levels of the protein in wild-type and dgl1 plants confirm that normal GA signaling takes place in dgl1.
Figure 9.
Degradation of SLR1 triggered by GA3 treatment of dgl1. Protein gel-blot analysis of SLR1 in young seedlings of the wild type, dgl1-3, and the GA-insensitive mutant gid2-1. The plants were treated with (+) 10−4 m GA3 or control solution (−), as described in “Materials and Methods.” The arrow indicates the protein bands corresponding to endogenous SLR1. Each lane contains 10 μg of total protein. The loading control of Coomassie Brilliant Blue staining is shown.
The up-regulation of GA biosynthetic genes in dgl1 suggested that BR biosynthetic genes are also up-regulated in the mutant. However, increased expression of BR biosynthetic genes was not observed in the mutants, including the genes corresponding to OsCPD (a homolog of the Arabidopsis CPD), D11 (Tanabe et al., 2005), and OsDWARF (a BR-C6 oxidase; Hong et al., 2002; data not shown). These observations indicate that, in contrast to GA biosynthesis, BR biosynthesis is not activated in the mutants.
Increased GA Biosynthetic Gene Expression Also Occurs in the BR-Deficient Mutant brd1
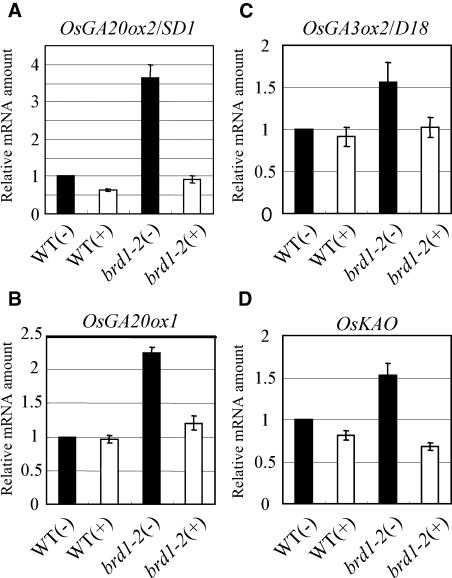
In both rice and Arabidopsis mutants defective in KTN1, GA biosynthetic genes are up-regulated. We questioned whether the lack of KTN1 activity or aberrant microtubule organization caused the up-regulation of these genes. Therefore, we measured the levels of the OsGA20ox2/SD1, OsGA20ox1, OsGA3ox2/D18, and KAO transcripts in the BR-deficient mutant brd1-2, in which the microtubule organization is thought to be aberrant (Yamamuro et al., 2000; Hong et al., 2002). The levels of transcripts of these genes in brd1-2 were higher than in the wild type, and the high expression was suppressed by BL treatment (Fig. 10). These results strongly suggest that the up-regulation of these GA biosynthetic genes is caused by aberrant microtubule organization and is not a direct effect of the loss of KTN1 activity (see “Discussion”).
Figure 10.
Enhanced expression of GA biosynthetic genes in the BR-deficient mutant brd1-2 and suppression by BL treatment. Quantitative RT-PCR was performed using primers specific for each GA biosynthetic gene, as described in “Materials and Methods.” Plants were treated with (+) 10−6 m BL or control solution (−), as described in “Materials and Methods.” The expression levels of OsGA20ox2/SD1 (A), OsGA20ox1 (B), OsGA3ox2/D18 (C), and OsKAO (D) were enhanced in brd1-2 and suppressed by BL treatment.
DISCUSSION
DGL1 Encodes a Rice KTN1 Protein
The positional cloning of the dgl1 locus revealed that DGL1 encodes a rice KTN1, a 60-kD microtubule-associated ATPase katanin-like protein. Katanin is a microtubule-severing protein that disassembles microtubules into tubulin subunits while hydrolyzing ATP (Vale, 1991; McNally and Vale, 1993; McNally et al., 1996; Hartman et al., 1998; McNally and Thomas, 1998; Hartman and Vale, 1999). Katanin is a major microtubule-severing factor in animal cells and its activity was first identified in Xenopus laevis mitotic egg extracts (Vale, 1991). In sea urchin oocytes, katanin forms a heterodimer composed of 60- and 80-kD subunits that are a catalytic protein and a regulatory protein, respectively. The 60-kD subunit is a member of the AAA (ATPases associated with various cellular activities) protein family, and the animal katanin 80-kD subunit contains WD40 repeats. A recent model proposes that the dimerization of these subunits is transient, and a ring-shaped p60 hexamer binds to and severs microtubules (Wasteneys, 2002). The hydrolysis of ATP by the 60-kD subunit is sufficient to sever microtubules in vitro (McNally et al., 2000), and the 80-kD subunit is involved in targeting the enzyme to the centrosome (Hartman et al., 1998). Thus, DGL1 may also sever microtubules in vivo.
Recently, a plant katanin-like protein was identified through analyses of Arabidopsis mutants. The inflorescence stems of the fragile fiber 2 (fra2) mutant of Arabidopsis contain interfascicular fibers with defective mechanical strength (Burk and Ye, 2002). The fra2 mutation causes a remarkable reduction in cell length and an increase in cell width in all organs, resulting in a global alteration in the morphology of the plant. The FRA2 gene encodes KTN1. Recombinant KTN1 interacts with microtubules in cosedimentation assays and severs microtubules in vitro in the presence of ATP (Burk and Ye, 2002; Stoppin-Mellet et al., 2002). These results demonstrate that the plant KTN1 functions similarly to the animal katanin. The strong similarity of the primary structures of DGL1 and KTN1, as well as the phenotypic resemblance of dgl1 and fra2, suggests that DGL1 has a function similar to that of KTN1. dgl1 exhibited an unorganized cortical microtubule arrangement in elongating internodal parenchyma (Fig. 6). Burk and Ye (2002) proposed that the aberrant microtubule orientation caused by the mutation in KTN1 results in an altered deposition of cellulose microfibrils that leads to defective cell elongation. As defective cell elongation and aberrant microtubule orientation were observed in the rice dgl1 mutants, the above model may explain the abnormal phenotype of dgl1, including its abnormal morphology, impaired root elongation, and stunted flowers.
We also found that the plastochron of the mutant (dgl1-3) is longer than that of the wild type (Table I), even though the size of the SAM in dgl1 was smaller than that of the wild type (Fig. 2, J and K). The longer plastochron and smaller SAM indicate that the possibility that the mutant SAM exhibits reduced cell division frequency is not excluded and, therefore, that DGL1 activity may be involved not only in cell elongation but also in cell division. It is possible that the dwarf phenotype of dgl1 is caused by disturbances in both cell elongation and cell division.
The Up-Regulation of GA Biosynthetic Genes in dgl1 Is Not Caused by Inadequate GA Signaling But by Aberrant Cortical Microtubule Arrangement
Mundy and colleagues have reported that AtGA20ox1/GA5, the expression of which is negatively regulated by bioactive GA in a feedback manner, is up-regulated in the KTN1-defective mutant lue1, and its expression in the mutant is further increased by GA3 (Meier et al., 2001; Bouquin et al., 2003). This observation indicates that the loss of KTN1 function results in inappropriate feedback regulation of AtGA20ox1/GA5 expression by GA, consequently inducing GA insensitivity. They also showed that the KTN1 mRNA level is positively regulated by the level of GA (Bouquin et al., 2003). These observations suggested that microtubules and/or KTN1 activity are involved in the feedback control of GA synthesis, and that the GA-dependent modulation of the functioning of KTN1 might affect microtubule organization (Foster et al., 2003).
We examined the possibility that this model applies to the dgl1 mutant. In dgl1 plants, as in lue1 plants, certain GA biosynthetic genes, such as OsGA20ox2/SD1, OsGA20ox1, and OsGA3ox2/D18, are up-regulated. However, the up-regulation of these GA biosynthetic genes in dgl1 may not be caused by inadequate feedback regulation of the genes because the increased levels of OsGA20ox2/SD1, OsGA20ox1, and OsGA3ox2/D18 mRNAs that are normally observed in this mutant were prevented by GA3 treatment, and the degradation of the rice DELLA protein SLR1 was triggered by GA. Moreover, a GA biosynthetic gene, OsKAO, whose expression is independent of the level of GA, is also up-regulated in dgl1. These observations indicate that the loss of DGL1 activity does not diminish GA signaling in rice.
Furthermore, the expression of the rice DGL1 gene was found not to be regulated by GA (Fig. 7). Taken together, these results suggest that the aberrant arrangement of microtubules does not affect GA signaling, and GA does not affect DGL1 expression, at least in rice. However, the aberrant microtubule organization may be related to the GA biosynthesis activity. In fact, up-regulation of GA biosynthetic genes was observed in both rice and Arabidopsis KTN1 mutants. Interestingly, up-regulation of GA biosynthetic genes is not specific to DGL1-deficient mutants but is also observed in the BR-deficient mutant brd1 (Fig. 10). This suggests that the loss of DGL1 function does not directly cause the enhanced GA biosynthetic gene expression. It is noteworthy that the aberrant microtubule organization does not enhance the expression of BR biosynthetic genes in contrast to GA biosynthetic genes. This suggests that an unknown specific mechanism connects the aberrant microtubule organization and the expression of the GA biosynthetic genes. Further studies will be necessary to reveal this mechanism.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Three dgl1 mutant lines derived from rice (Oryza sativa), dgl1-1, dgl1-2, and dgl1-3, were used in this study. The dgl1-1 and dgl1-2 alleles were identified in a mutant library generated using N-methyl-N-nitrosourea. The dgl1-3 allele, which was kindly donated by Dr. Hirochika (National Institute of Agrobiological Sciences, Tsukuba, Ibaraki, Japan), was derived from a rice plant (cv Nipponbare) regenerated from a cell suspension that had been in culture for several months. Wild-type rice plants, four dgl1 alleles, the GA-deficient mutant oskao-3, the GA-insensitive mutant gid2-2, the constitutive-GA-response mutant slr1-11, and the BR-deficient mutant brd1-1 were grown in a greenhouse at 30°C (day) and 24°C (night).
GA and BR Induction in Shoot Elongation
Seeds of wild-type and mutant rice lines were sterilized with 2.5% NaClO for 30 min and washed five times in sterile distilled water. The seeds were then placed on 1% agar plates, grown under fluorescent light at 30°C for 4 d, and then transferred to 1% agar plates containing various concentrations of GA3 or BL. The length of the third-leaf sheath was measured at 4 d after application of GA3 or 2 weeks after application of BL.
Mapping, Isolation, and Sequencing of DGL1
To map DGL1, F2 plants from a cross between dgl1-1 and the indica strain Kasalath were used. Approximately 1,150 F2 plants of this cross were used for the positional cloning of DGL1. To identify the mutation sites in the dgl1 alleles, DGL1 was amplified using genomic DNA extracted from the three alleles. The amplified DNA fragments were sequenced directly using appropriate primers. A DGL1 genomic DNA fragment was isolated from the PAC clone P0019E03, which is derived from the DNA bank of the Ministry of Agriculture, Forestry and Fisheries GenBank project. The clone contains the entire coding region and the 5′- and 3′-flanking regions of DGL1.
Complementation of the dgl1 Mutant
A construct containing the DGL1 gene was introduced into dgl1-3. The DGL1 gene in the PAC clone P0019E03 (provided by the National Institute of Agrobiological Sciences) was digested with NaeI and BamHI and inserted into the blunted XbaI site of a pBluescript vector. The clone was redigested with BamHI and NaeI, after which the ends were filled in. The fragment of approximately 9.5 kb was fused into the SmaI site of the binary vector pBI-Hm12, which contains a hygromycin-resistance gene, kindly provided by Dr. Hiroyuki Hirano (Tokyo University, Tokyo). The binary vector was introduced into Agrobacterium tumefaciens strain EHA101 (Hood et al. 1986) by electroporation, and rice plants were transformed with this strain as described by Hiei et al. (1994). Control plants were generated by introducing the empty vector into dgl1-3 mutant callus.
RNA Isolation and RNA Gel-Blot Analysis
Total RNAs (5 μg) isolated from various rice tissues using the RNeasy kit (Qiagen, Hilden, Germany) with the addition of an RNase-free DNase I treatment (TaKaRa Shuzo, Tokyo), and from seedlings of wild-type, oskao-3, gid2-1, slr1-11, brd1-1, and dgl1-3 using the aurintricarboxylic acid method (Gonzalez et al. 1980), were separated on a 1% agarose gel and transferred to Hybond N+ membrane (Amersham-Pharmacia, Piscataway, NJ). The membrane was hybridized with a 32P-labeled partial DGL1 cDNA fragment of 560 bp, which was amplified using the primers 5′-TGAAGTTGCACGGAGGACAG-3′ and 5′-CCAGCTTCCATGTTTCGCAC-3′, which correspond to regions in exon 7 and the 3′-untranslated region, respectively. To investigate the effects of GA3 on DGL1 expression, oskao-3 seeds were grown on 1% agar plates under fluorescent light at 30°C for 7 d, sprayed with 10−4 m GA3, and then harvested after 1 or 4 h. Total RNAs were isolated using the aurintricarboxylic acid method. Five and 10 μg of total RNAs were electrophoresed on 1% agarose gels and then transferred to membranes. An OsGA20ox2-specific probe of 420 bp was amplified from the cDNA and fused into pCRII (Invitrogen, Carlsbad, CA), and the resulting plasmid was digested with EcoRI. A DGL1-specific probe was prepared as described above. The membrane was hybridized at 65°C in a hybridization solution of 5× SSC (1× SSC = 0.15 m NaCl and 0.015 m sodium citrate), 5× Denhardt's solution (1×Denhardt's solution = 0.02% Ficoll, 0.02% polyvinylpyrrolidone, and 0.02% bovine serum albumin), 0.5% SDS, 10% dextran sulfate, and 0.1 mg mL−1 denatured salmon sperm DNA. DNA probes were labeled with 32P-dCTP. The membrane was washed twice with 2× SSC and 0.1% SDS at 65°C for 20 min and once with 0.2× SSC and 0.1% SDS at 65°C for 10 min. The membranes were exposed to a Fuji imaging plate and the image was visualized using a BAS2000 imaging analyzer (Fuji Photo Film, Tokyo).
Observation of Microtubule Arrangement
Internodal parenchyma tissue was prefixed for 45 min at room temperature in 3.7% (w/v) paraformaldehyde in microtubule-stabilizing buffer (0.1 m piperazine-diethanolsulfonic acid, 1 mm MgCl2, 5 mm EGTA, 0.2% (v/v) Triton X-100, 1% (w/v) glycerol, pH 6.9). Longitudinal sections were cut with a fresh razor blade, incubated in the above buffer for 40 min, and washed in microtubule-stabilizing buffer. The sections were then incubated for 1 h at 37°C with an α-tubulin rabbit monoclonal antibody (kindly donated by Dr. Mizuno, Osaka University, Osaka) diluted 1:500 in phosphate-buffered saline containing 0.1% (v/v) Triton X-100 (PBST). The sections were washed three times in PBST and then incubated for 2 h at 37°C with anti-rabbit IgG fluorescein-linked whole antibody from donkey (Amersham-Pharmacia) diluted 1:25 in PBST. After three washes in this buffer, the sections were mounted in antifading solution (FluoroGuard antifade reagent; Bio-Rad Laboratories, Hercules, CA) and viewed under a confocal fluorescence microscope (Fluoview FV500 version 4.0; Olympus, Tokyo).
Microscopic Analysis
For light microscopy, tissues were fixed in FAA (5% formaldehyde, 5% glacial acetic acid, 63% ethanol) and dehydrated in a graded ethanol series. The samples were embedded in Paraplast Plus (Sherwood Medical, St. Louis). Microtome sections of 10 μm in thickness were applied to silane-coated glass slides (Matsunami Glass, Osaka). The sections were deparaffinized in xylene, dehydrated through a graded ethanol series, and dried overnight before staining with toluidine blue. To investigate the morphology of the epidermal cells of the leaf blade, the samples were cleared in benzyl-benzoate-four-and-a-half fluid (Herr, 1982).
Protein Extraction and Immunoblot Analysis
Proteins were extracted from seedlings by grinding in liquid nitrogen and resuspending the resulting powder in extraction buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 0.5% Tween 20) containing 0.5× Complete protease inhibitor solution (Roche, Mannheim, Germany). The extracts were cleared with two centrifugations of 20 min in a microcentrifuge. The protein concentrations of the soluble fractions were determined using Bradford reagent (Bio-Rad Laboratories) with bovine serum albumin as the standard. For immunoblot analysis, the proteins were separated by SDS-PAGE and transferred to Hybond ECL membrane (Amersham-Pharmacia Biotech, Uppsala) by semidry blotting. Immunoblot analysis was carried out with a 1:10,000 dilution of a rabbit anti-SLR1 serum (Itoh et al., 2002) as the primary antibody and a horseradish peroxidase-conjugated goat anti-rabbit IgG (Medical and Biological Laboratories, Nagoya, Japan) as the secondary antibody. Peroxidase activity was detected according to the method of the manufacturer (Pierce, Rockford, IL).
Reverse-Transcription Quantitative PCR
Wild-type and dgl1 seeds were grown on 1% agar plates and sprayed with 10−4 m GA3 or control solution as described above. Wild-type and brd1-2 seeds were grown on 1% agar plates containing or lacking 10−6 m BL. Total RNAs were extracted as described above. First-strand cDNA was synthesized in a RT reaction with 2 μg of total RNA using the Omniscript reverse transcription kit (Qiagen). Quantitative PCR was performed using a Roche LightCycler (Roche) and the QuantiTect SYBR Green PCR kit, according to the manufacturer's instructions, at the appropriate annealing temperature for each gene (OsGA20ox2/SD1, 60°C; OsGA20ox1, 60°C; OsGA3ox2/D18, 72°C; OsKAO, 55°C; OsCPD, 55°C; D11, 57°C; OsDWARF, 57°C; and ACTIN1, 62°C). The 5′ and 3′ primers for each gene were as follows: OsGA20ox2/SD1, 5′-CAGCACTACCCGGACTTCAC-3′ and 5′-GCTTCTGTTCGTTCCGTTTC-3′; OsGA20ox1, 5′-TCGGCTGGAGATGAAGAGG-3′ and 5′-AAGAATCGCCGGAAGTAGTG-3′; OsGA3ox2/D18, 5′-TCTCCAAGCTCATGTGGTCCGAGGGCTA-3′ and 5′-TGGAGCACGAAGGTGAAGAAGCCCGAGT-3′; OsKAO, 5′-GAGATCGTCGACGTCCTCATCATGTACC-3′ and 5′-AGATGTTGACGCAGCGAAGTGTCTCGTC-3′; OsCPD, 5′-TTCTTCTCCATCCCCTTTCCTCTCGCCA-3′ and 5′-CACCCTCCGCCTCAAGAAGCTCCTCAA-3′; D11, 5′-TTGGGTCATGGCATGGCAAGAGCAAGGA-3′ and 5′-TTGTTGCTGGAGCCAGCATTCCTCCTCT-3′; OsDWARF, 5′-ATGGTGTTGGTGGCGATTGGGGTGGTTG-3′ and 5′-ATGTTGTTCCGCCCCAGGATGTCCAGCA-3′; and ACTIN1, 5′-CATCTTGGCATCTCTCAGCAC-3′ and 5′-AACTTTGTCCACGCTAATGAA-3′.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.062968.
References
- Bichet A, Desnos T, Turner S, Grandjean O, Hofte H (2001) BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J 25: 137–148 [DOI] [PubMed] [Google Scholar]
- Bouquin T, Mattsson O, Naested H, Foster R, Mundy J (2003) The Arabidopsis lue1 mutant defines a katanin p60 ortholog involved in hormonal control of microtubule orientation during cell growth. J Cell Sci 116: 791–801 [DOI] [PubMed] [Google Scholar]
- Burk DH, Liu B, Zhong R, Morrison WH, Ye ZH (2001) A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell 13: 807–827 [PMC free article] [PubMed] [Google Scholar]
- Burk DH, Ye ZH (2002) Alteration of oriented deposition of cellulose microfibrils by mutation of a katanin-like microtubule-severing protein. Plant Cell 14: 2145–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49: 427–451 [DOI] [PubMed] [Google Scholar]
- Fleet CM, Sun TP (2005) A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol 8: 77–85 [DOI] [PubMed] [Google Scholar]
- Foster R, Mattsson O, Mundy J (2003) Plants flex their skeletons. Trends Plant Sci 8: 202–204 [DOI] [PubMed] [Google Scholar]
- Fujioka S, Yokota T (2003) Biosynthesis and metabolism of brassinosteroids. Annu Rev Plant Biol 54: 137–164 [DOI] [PubMed] [Google Scholar]
- Gonzalez RG, Haxo RS, Schleich T (1980) Mechanism of action of polymeric aurintricarboxylic acid, a potent inhibitor of protein-nucleic acid interactions. Biochemistry 19: 4299–4303 [DOI] [PubMed] [Google Scholar]
- Hartman JJ, Mahr J, McNally K, Okawa K, Iwamatsu A, Thomas S, Cheesman S, Heuser J, Vale RD, McNally FJ (1998) Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell 93: 277–287 [DOI] [PubMed] [Google Scholar]
- Hartman JJ, Vale RD (1999) Microtubule disassembly by ATP-dependent oligomerization of the AAA enzyme katanin. Science 286: 782–785 [DOI] [PubMed] [Google Scholar]
- Herr JM Jr (1982) An analysis of methods for permanently mounting ovules cleared in four-and-a-half type clearing fluids. Stain Technol 57: 161–169 [DOI] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6: 271–282 [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Matsuoka M (2004) Brassinosteroids and rice architecture. J Pestic Sci 29: 184–188 [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S, Inukai Y, Fujioka S, Shimada Y, Takatsuto S, Agetsuma M, Yoshida S, Watanabe Y, et al (2002) Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J 32: 495–508 [DOI] [PubMed] [Google Scholar]
- Hood EE, Helmer GL, Fraley RT, Chilton MD (1986) The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J Bacteriol 168: 1291–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, Matsuoka M, Yamaguchi J (2001) slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 13: 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J (2005) Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol 46: 79–86 [DOI] [PubMed] [Google Scholar]
- Itoh H, Tatsumi T, Sakamoto T, Otomo K, Toyomasu T, Kitano H, Ashikari M, Ichihara S, Matsuoka M (2004) A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol Biol 54: 533–547 [DOI] [PubMed] [Google Scholar]
- Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M (2002) The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14: 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Ueguchi-Tanaka M, Sentoku N, Kitano H, Matsuoka M (2001) Cloning and functional analysis of two gibberellin 3β-hydroxylase genes that are differently expressed during the growth of rice. Proc Natl Acad Sci USA 98: 8909–8914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandava NB (1988) Plant growth-promoting brassinosteroids. Annu Rev Plant Physiol Plant Mol Biol 39: 23–52 [Google Scholar]
- Matsuo T, Futsuhara Y, Kikuchi F, Yamaguchi H (1997) Science of the Rice Plant, Vol 3. Nobunkyo, Tokyo, pp 302–303
- McClinton RS, Chandler JS, Callis J (2001) cDNA isolation, characterization, and protein intracellular localization of a katanin-like p60 subunit from Arabidopsis thaliana. Protoplasma 216: 181–190 [DOI] [PubMed] [Google Scholar]
- McNally FJ, Okawa K, Iwamatsu A, Vale RD (1996) Katanin, the microtubule-severing ATPase, is concentrated at centrosomes. J Cell Sci 109: 561–567 [DOI] [PubMed] [Google Scholar]
- McNally FJ, Thomas S (1998) Katanin is responsible for the M-phase microtubule-severing activity in Xenopus eggs. Mol Biol Cell 9: 1847–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally FJ, Vale RD (1993) Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell 75: 419–429 [DOI] [PubMed] [Google Scholar]
- McNally KP, Bazirgan OA, McNally FJ (2000) Two domains of p80 katanin regulate microtubule severing and spindle pole targeting by p60 katanin. J Cell Sci 113: 1623–1633 [DOI] [PubMed] [Google Scholar]
- Meier C, Bouquin T, Nielsen ME, Raventos D, Mattsson O, Rocher A, Schomburg F, Amasino RM, Mundy J (2001) Gibberellin response mutants identified by luciferase imaging. Plant J 25: 509–519 [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Agrawal GK, Takeda S, Abe K, et al (2004) An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol 134: 1642–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M, et al (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299: 1896–1898 [DOI] [PubMed] [Google Scholar]
- Stoppin-Mellet V, Gaillard J, Vantard M (2002) Functional evidence for in vitro microtubule severing by the plant katanin homologue. Biochem J 365: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiz L, Zeiger E (2002) Plant Physiology, Ed 3. Sinauer Associates, Sunderland, MA, pp 461–488
- Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, Yano M, Yoshimura A, Kitano H, Matsuoka M, Fujisawa Y, et al (2005) A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17: 776–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD (1991) Severing of stable microtubules by a mitotically activated protein in Xenopus egg extracts. Cell 64: 827–839 [DOI] [PubMed] [Google Scholar]
- Wasteneys GO (2002) Microtubule organization in the green kingdom: chaos or self-order? J Cell Sci 115: 1345–1354 [DOI] [PubMed] [Google Scholar]
- Webb M, Jouannic S, Foreman J, Linstead P, Dolan L (2002) Cell specification in the Arabidopsis root epidermis requires the activity of ECTOPIC ROOT HAIR 3-a katanin-p60 protein. Development 129: 123–131 [DOI] [PubMed] [Google Scholar]
- Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M (2000) Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12: 1591–160511006334 [Google Scholar]