Abstract
The exocyst, a complex of eight proteins, contributes to the morphogenesis of polarized cells in a broad range of eukaryotes. In these organisms, the exocyst appears to facilitate vesicle docking at the plasma membrane during exocytosis. Although we had identified orthologs for each of the eight exocyst components in Arabidopsis (Arabidopsis thaliana), no function has been demonstrated for any of them in plants. The gene encoding one exocyst component ortholog, AtSEC8, is expressed in pollen and vegetative tissues of Arabidopsis. Genetic studies utilizing an allelic series of six independent T-DNA mutations reveal a role for SEC8 in male gametophyte function. Three T-DNA insertions in SEC8 cause an absolute, male-specific transmission defect that can be complemented by expression of SEC8 from the LAT52 pollen promoter. Microscopic analysis shows no obvious abnormalities in the microgametogenesis of the SEC8 mutants, and the mutant pollen grains appear to respond to the signals that initiate germination. However, in vivo assays indicate that these mutant pollen grains are unable to germinate a pollen tube. The other three T-DNA insertions are associated with a partial transmission defect, such that the mutant allele is transmitted through the pollen at a reduced frequency. The partial transmission defect is only evident when mutant gametophytes must compete with wild-type gametophytes, and arises in part from a reduced pollen tube growth rate. These data support the hypothesis that one function of the putative plant exocyst is to facilitate the initiation and maintenance of the polarized growth of pollen tubes.
Asymmetrical growth and development in eukaryotic cells is established through localized cell expansion driven by polarized exocytosis/endocytosis. Golgi-generated secretory vesicles are delivered to a specific region of the plasma membrane, where their fusion with the plasma membrane results in the incorporation of new membrane lipids and proteins to that region. In many organisms, polarized exocytosis has been found to require the involvement of a protein complex known as the exocyst (for review, see Hsu et al., 2004). The exocyst (first described in TerBush et al., 1996) is composed of eight proteins, Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84, most of which were initially identified as temperature-sensitive secretory mutants of the budding yeast (Saccharomyces cerevisiae; for review, see Schekman and Novick, 2004). In yeast, mutation of any one of the exocyst components leads to arrested formation of the polarly growing bud and the accumulation of secretory vesicles (TerBush et al., 1996; Guo et al., 1999). Orthologs for genes encoding all eight of the exocyst components have subsequently been identified in a growing number of eukaryotes and implicated in a variety of cellular processes involving polarized exocytosis. For example, the exocyst is associated with neurite outgrowth in neurons from Drosophila melanogaster (Murthy et al., 2003) and in differentiated PC12 cells (Vega and Hsu, 2001). In cultured Swiss 3T3 fibroblasts, the exocyst mediates filopodia formation induced by the GTPase RalA and tumor necrosis factor-α (Sugihara et al., 2002). In adipocytes, the exocyst is implicated in the targeted exocytosis of the Glc transporter Glut4 to the plasma membrane in response to insulin (Inoue et al., 2003). In general, the exocyst appears to be involved in polarized exocytosis to regions of the plasma membrane experiencing rapid polarized growth or requiring tightly controlled delivery of specific membrane components. On the basis of subcellular localization and molecular associations, it has been proposed that the exocyst functions as a tethering protein complex, targeting incoming secretory vesicles to specific sites on the plasma membrane prior to the docking and fusion events mediated by SNARES (Pfeffer, 1999; Whyte and Munro, 2002; Hsu et al., 2004).
Orthologs for genes encoding all components of the exocyst have been identified in Arabidopsis (Arabidopsis thaliana; Jurgens and Geldner, 2002; Elias et al., 2003) and rice (Oryza sativa; R.A. Cole, J.E. Fowler, L. Synek, and V. Zarsky, unpublished data), suggesting the existence of a plant exocyst. The genome of Arabidopsis contains single copies of genes for Sec6, Sec8, and Sec10, two copies each of Sec3, Sec5, and Sec15, three copies of Exo84, and a remarkable 23 copies of Exo70. However, a demonstrated functional role for the exocyst or any of its components in plants has not been reported. The most dramatic example of polarized growth in plants is the tip growth observed in root hairs and pollen tubes (for review, see Fowler and Quatrano, 1997; Hepler et al., 2001; Feijó et al., 2004), making these likely sites for exocyst activity. Tip growth requires the rapid delivery of secretory vesicles to the specific region on the membrane where growth of new membrane is focused. Pollen tube tip growth also involves the localization and recycling of membrane components, such as ion channels and possibly receptors, to the tip via endocytosis and exocytosis. The precise location of growth at the tip is essential to the guidance of the pollen tube from the stigma, through the transmitting tract of the style, and ultimately to the ovule where fertilization occurs. Such directed tip growth involves a complex interplay of signaling pathways, most notably involving Rho-related protein from plants (ROP) GTPases and calcium (Li et al., 1999; Camacho and Malhó, 2003; for review, see Yang, 2002; Feijó et al., 2004; Gu et al., 2004). The exocyst could conceivably provide a target for regulation of this directed growth. It was therefore noteworthy when we identified a T-DNA mutant of the Arabidopsis SEC8 homolog (Supplemental Fig. 1, protein alignment) with a defect affecting transmission of the mutant allele through the pollen.
The study of Sec8 orthologs has played a key role in establishing functional roles for the exocyst in nonplant species. In MDCK epithelial cells, Sec8 localization studies (Yeaman et al., 2004), combined with studies in which anti-Sec8 antibodies disrupt protein targeting (Grindstaff et al., 1998), have supported a role for the exocyst in the targeting of proteins to the basolateral, but not the apical, plasma membrane. In heterologous cells and neurons, overexpression of a mutant Sec8 resulted in decreased N-methyl-d-Asp receptor-mediated current and surface expression, indicating that the exocyst may be involved in the delivery of these neurotransmitter receptors to the plasma membrane (Sans et al., 2003). Finally, in cultures of embryonic hippocampal neurons, immunofluorescent labeling of Sec8 and Sec6 have implicated the exocyst in the process of neurite outgrowth (Hazuka et al., 1999), supporting the conclusion reached by the study of other exocyst components (Vega and Hsu, 2001; Murthy et al., 2003). Thus, there is a precedent for focusing on Sec8 as an experimental handle for investigating the function of the entire complex. In addition, the examples highlighting the role of Sec8 in neurons are of particular interest because of the many parallels between the targeted growth of pollen tubes to the ovule and the directed growth of axons to neural synapses (Palanivelu and Preuss, 2000). A demonstrated function for SEC8 in Arabidopsis would provide a first step in exploring the role of the exocyst in plants.
We therefore used T-DNA insertional mutations of AtSEC8 to test the hypothesis that the exocyst is involved in tip growth in plants. We focused our attention on pollen, i.e. the male gametophyte. The germination of the pollen grain to form a pollen tube and the growth of the pollen tube are both processes that could in theory involve the exocyst. Additionally, we considered the possibility that the putative plant exocyst could be involved in cytokinesis during microgametogenesis. Ultimately, our genetic and microscopic studies revealed that AtSEC8 is required for both pollen germination and competitive pollen tube growth, supporting our hypothesis.
RESULTS
AtSEC8 Is Expressed in Pollen and Can Be Investigated Using Reverse Genetics
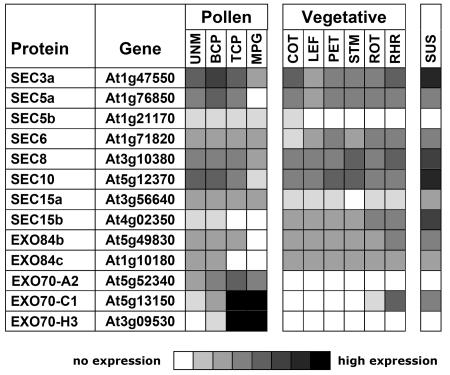
Recent microarray expression analyses have explored the pollen transcriptome in Arabidopsis at various stages of pollen development (Honys and Twell, 2004). Transcripts for all components of the putative exocyst, including AtSEC8, were found in pollen or the developing male gametophyte (Fig. 1), a requisite condition for exocyst function in pollen. Further analyses of microarray data from additional experiments demonstrated that transcripts for all eight components were widely distributed in vegetative tissues, raising the possibility that the putative plant exocyst functions in diverse developmental contexts. Intriguingly, some of the highest expression levels for these genes were in cells in suspension culture, an actively dividing state. Exocyst components encoded by multiple genes were expressed differentially, with certain gene family members predominating in pollen (e.g. AtSEC15a, three of the AtEXO70 genes). Notably, the AtSEC8 transcript was detectable throughout pollen development in uninucleate microspores following meiosis, in bicellular and tricellular pollen following the subsequent two mitotic divisions, and, finally, in the mature pollen grain.
Figure 1.
Microarray data for putative exocyst components in Arabidopsis indicate that transcripts for all eight subunits are expressed widely. In particular, the experiments of Honys and Twell (2004) show that AtSEC8, as well as other putative exocyst components, are expressed in developing pollen. Pollen sources of RNA for the expression analysis included uninucleate microspores (UNM), bicellular pollen (BCP), immature tricellular pollen (TCP), and mature pollen grains (MPG). Additional sources of RNA for the analysis included cotyledons (COT), leaves (LEF), petioles (PET), stems (STM), roots (ROT), root hairs (RHR), and cell suspensions (SUS). White (no expression) indicates that expression was not consistently detected among replicates. For brevity, the data shown for AtEXO70 represent only three of the 23 homologs of this exocyst component in Arabidopsis; these are the homologs highly expressed in the pollen. Nomenclature is based upon that of Elias et al. (2003).
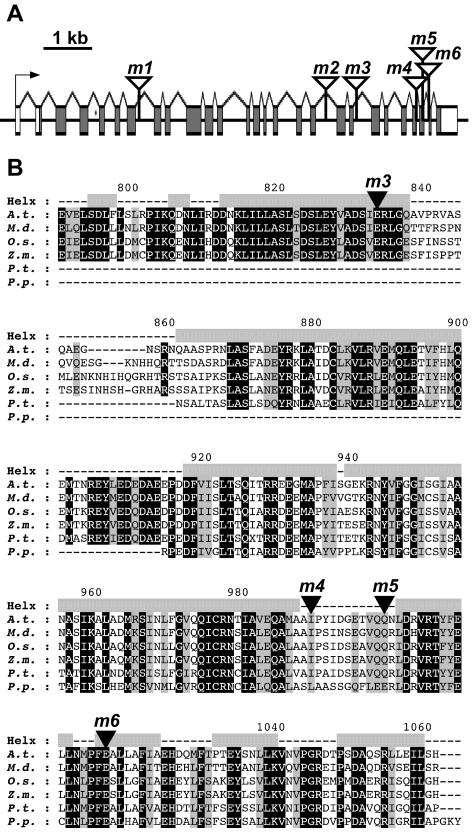
We focused our attention on the large, single-copy AtSEC8 gene (hereafter referred to simply as SEC8) to begin testing the hypothesis that the plant exocyst is crucial for polarized plant cell growth, specifically the growth of the pollen tube. We employed a reverse genetics approach, searching the Salk collection (Alonso et al., 2003) to obtain six T-DNA insertional mutant lines in the gene. The location and insertional nature of each T-DNA mutation was verified by sequencing from both T-DNA borders (Fig. 2A). Amino acid sequences predicted from assembled expressed sequence tag (EST) contigs for a number of plant species show a high degree of conservation in a region likely to form a series of α-helices, which were also predicted to be interrupted by the insertion mutations (Fig. 2B). Conservation fell off in the short region between -m3 and the other three insertions (Supplemental Fig. 2).
Figure 2.
T-DNA insertional mutations interrupt the coding region of SEC8 (At3g10380) at multiple sites. A, Six independent insertions (designated m1 through m6) interrupt the approximately 8.5-kb gene. Based on the sequence of a full-length 3.7-kb cDNA (accession no. AY059763), the gene contains 27 exons (boxed) and is predicted to encode a 1,053-residue protein. B, Stretches of primary sequence at the C-terminal end of plant SEC8 orthologs are conserved in widely divergent species, as shown by alignment of predicted protein sequences. Identical residues are shaded black; similar residues, gray. Insertion sites for four of the mutant alleles are designated by a black triangle. Arabidopsis (A.t.) and rice (O.s.) sequences are based on genomic data; all others are based on a consensus of assembled ESTs. Other species are as follows: M.d., Malus domestica (apple); Z.m., Zea mays (maize); P.t., Pinus taeda (loblolly pine); P.p., Physcomitrella patens (moss). Helx, Regions of predicted α-helical structure (gray boxes).
Mutations in SEC8 Cause a Male-Specific Transmission Defect
A phenotype for the SEC8 mutants was revealed when genetic studies showed that the mutant alleles were not transmitted to progeny in the expected Mendelian ratios (Table I). Genotyping for individual plants in the progeny populations was achieved by PCR (Fig. 3E), using sets of primers annealing to DNA within the insert (i.e. the T-DNA sequence) and on either side of the insertion site. Self-crosses of plants heterozygous for the sec8-m1, -m2, or -m3 alleles failed to produce homozygous mutant progeny (Table I). To determine whether the defect was due to a problem in male and/or female gametogenesis, reciprocal outcrosses were performed between plants heterozygous for each mutant SEC8 allele and wild-type Columbia-0 plants. When wild-type pollen was used to pollinate the stigma of a SEC8 heterozygote, the progeny demonstrated the expected 1:1 Mendelian ratio of heterozygotes to wild-type homozygotes for all six mutant alleles (Table I). Thus, no detectable defect was associated with mutation of SEC8 in the female gametophyte.
Table I.
Inheritance of mutant SEC8 alleles
| Natural Self-Cross of SEC8 Heterozygotes
|
SEC8 Allele
|
No. of Crosses
|
No. of Progeny
|
Genotypes of Progeny
|
χ2
|
P
|
||
|---|---|---|---|---|---|---|---|---|
| +/+ | +/ma | m/m | ||||||
| 25% | 50% | 25% | Expected | |||||
| m1 | 3 | 180 | 52% | 48% | 0% | 127.5 | ≤0.001 | |
| m2 | 4 | 216 | 50% | 50% | 0% | 106.0 | ≤0.001 | |
| m3 | 6 | 267 | 47% | 53% | 0% | 119.8 | ≤0.001 | |
| m4 | 8 | 260 | 28% | 49% | 23% | 1.472 | NSb | |
| m5 | 3 | 85 | 24% | 47% | 29% | 0.832 | NS | |
| m6 | 2 | 78 | 19% | 58% | 23% | 3.178 | NS | |
| Outcross of SEC8 Heterozygotes
|
SEC8 Allele
|
No. of Crosses
|
No. of Progeny
|
Genotypes of Progeny
|
χ2
|
P
|
||
| +/+
|
+/ma
|
m/m
|
||||||
| 50% | 50% | – | Expected | |||||
| Pollen source: +/+; pollen recipient: +/m | m1 | 4 | 157 | 47% | 53% | 0.516 | NS | |
| m2 | 3 | 90 | 54% | 46% | 0.711 | NS | ||
| m3 | 3 | 101 | 50% | 50% | 0.010 | NS | ||
| m4 | 5 | 157 | 51% | 49% | 0.057 | NS | ||
| m5 | 4 | 147 | 52% | 48% | 0.333 | NS | ||
| m6 | 6 | 159 | 52% | 48% | 0.308 | NS | ||
| Pollen source: +/m; pollen recipient: +/+ | m1 | 4 | 150 | 100% | 0% | 150.0 | ≤0.001 | |
| m2 | 3 | 71 | 100% | 0% | 71.0 | ≤0.001 | ||
| m3 | 3 | 105 | 100% | 0% | 105.0 | ≤0.001 | ||
| m4 | 10 | 577 | 82% | 18% | 238.5 | ≤0.001 | ||
| m5 | 6 | 235 | 78% | 22% | 75.27 | ≤0.001 | ||
| m6 | 6 | 157 | 69% | 31% | 22.17 | ≤0.001 | ||
Mutant sec8 allele.
NS, Not significantly different.
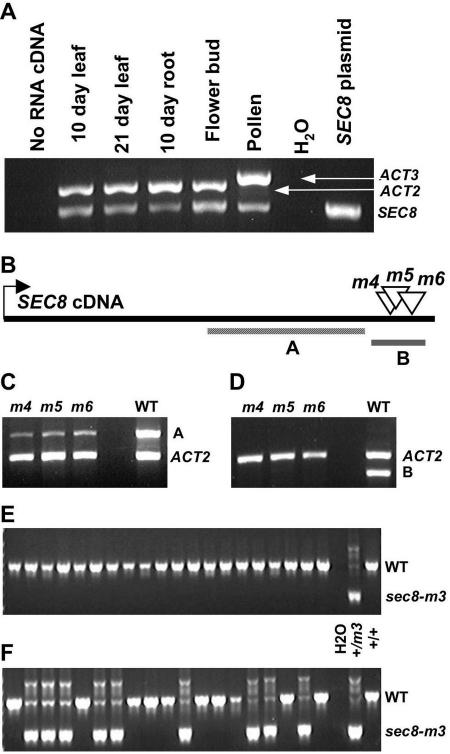
Figure 3.
Insertional mutations affect the SEC8 transcript and transmission through the male gametophyte. A, The SEC8 transcript is detectable in several wild-type tissues, including mature pollen, by RT-PCR. Primers against ACTIN2 (sporophyte) and ACTIN3 (gametophyte) were included as internal controls. B, Schematic of the SEC8 transcript and the strategy to test for aberrant transcripts generated by the -m4, -m5, and -m6 alleles. RT-PCR product A is located 5′ to the insertion sites, whereas product B spans all three insertion sites. C and D, In RNA made from immature floral tissue, product A is detectable in the wild type and all three mutant homozygotes (C), whereas product B is only detectable in the wild type (D). E and F, Twenty outcross progeny were genotyped by PCR using a set of three primers (see “Materials and Methods”) to produce distinct wild-type (WT) and sec8-m3 heterozygote banding patterns. E, No heterozygous progeny were produced in an outcross to a wild-type homozygote using pollen from a sec8-m3 heterozygote. F, Heterozygous progeny were produced in an outcross using pollen from a sec8-m3 heterozygote also carrying a LAT52::SEC8 construct, demonstrating male transmission of the mutant allele, and complementation.
In contrast, a strong male-specific transmission defect was evident in the reciprocal outcrosses. When the pollen of a mutant heterozygote was used on a wild-type stigma, there were significantly fewer heterozygotes in the progeny than predicted by Mendelian genetics (Table I). For the -m1, -m2, and -m3 alleles, the transmission defect was absolute, and the mutant allele never appeared in the progeny (combined n = 326). Interestingly, even though we had not detected a transmission defect in the self-crossed progeny, the -m4, -m5, and -m6 alleles all demonstrated a partial male-specific transmission defect when outcrossed, with the mutant allele appearing at frequencies of 18%, 22%, and 31%, respectively. The transmission defect was maintained through at least three generations for each allele. These data argued strongly for an important functional role for SEC8 in the male gametophyte.
When the six mutant alleles were compared, it was notable that the transmission defect became less severe as the site of the insertion got closer to the 3′-end of the gene. The differences in phenotypic severity among the six alleles raised the possibility that the three 5′-most insertions generated null alleles, whereas the three other insertions, located in a relatively small region in the 3′-end of the gene, were associated with partially functional (hypomorphic) alleles. In this scenario, truncated transcripts from the hypomorphic alleles should be detectable in plants homozygous for the sec8-m4, -m5, and -m6 alleles. To test this and to confirm the microarray expression data, we initially conducted reverse transcription (RT)-PCR on RNA samples from several wild-type tissues. SEC8 transcript was detectable in all samples tested, including RNA from pollen (Fig. 3A). We then utilized two different primer pairs, one located at the extreme 3′-end and another further 5′, producing products designated B and A, respectively (Fig. 3B), to evaluate the presence of truncated transcripts in plants homozygous for the sec8-m4, -m5, or -m6 allele. As predicted, RT-PCR using the 5′-most primer pair on immature floral tissue samples from the mutants produced a PCR product of the same size as that obtained for wild-type plants. However, no PCR product was detected when primers located on opposite sides of the insertion sites were used on the same samples, as might be expected for truncated transcripts (Fig. 3, C and D). To confirm the presence of the truncated transcripts, PCR products were amplified from each hypomorph, using a 5′-SEC8 primer along with a T-DNA-annealing primer, and sequenced. Each allele produced a transcript composed of truncated SEC8 sequence fused to a portion of the T-DNA sequence at the 3′-end. Using these sequences, we predicted that the -m4, -m5, and -m6 mutations alter the C terminus of the wild-type protein by truncating it by 70, 63, or 45 amino acids, respectively, and by adding 16, 15, or 25 amino acids translated from the T-DNA sequence (Fig. 2A; Supplemental Fig. 3).
We could not definitively test whether sec8-m1, -m2, and -m3 produced transcripts because we could not generate homozygotes for these three putative null SEC8 alleles. However, no PCR products were detected in attempts to isolate truncated transcripts containing T-DNA sequences from plants heterozygous for these alleles (data not shown). Additional data supporting the idea that the three 5′-most insertions were null alleles were obtained from male outcrosses of a sec8-m3/sec8-m4 trans-heterozygote. Even when in the environment of reduced pollen competition provided by the partial -m4 allele (see below), we never saw transmission of the -m3 allele through the pollen (n = 98 progeny).
To verify that the transmission defect was indeed due to mutation of SEC8, and not a tightly linked but unrelated mutation, a complementation experiment was performed. A construct containing the wild-type SEC8 coding sequence driven by the pollen-specific LAT52 promoter (Twell et al., 1991), as well as a promoter-only control, was transformed into sec8-m3 heterozygotes (associated with an absolute transmission defect; Fig. 3E). Six independent transformants were obtained, and pollen from these plants was outcrossed to wild type. Five of the six LAT52::SEC8 transformants demonstrated complete complementation, with sec8-m3 heterozygotes appearing in the hygromycin-resistant progeny at the expected Mendelian ratio (n = 121 plants, χ2 = 1.86, P < 0.25; Fig. 3F). As an internal control, 26 sibling progeny lacking the construct were also genotyped and, as expected, revealed no heterozygotes. Additionally, transformation with a promoter-only control did not complement the transmission defect in sec8-m3 heterozygotes (two transformant lines, 37 plants). Thus, we concluded that the transmission defect associated with the sec8-m3 allele was complemented by a pollen-expressed SEC8, confirming that the transmission defect was caused by loss of SEC8 function.
Microgametogenesis Appears Normal in sec8-m3 Heterozygotes
Male-specific transmission defects can arise at any of several stages of male gametophytic development, including microgametogenesis, pollen germination, or pollen tube growth (for review, see McCormick, 2004). Recent electron tomographic studies have revealed an exocyst-like structure attached to vesicles that are assembling at the Arabidopsis cell plate during cytokinesis, including the postmeiotic plate during tetrad formation(Otegui and Staehelin, 2004; Segui-Simarro et al., 2004). In addition, SEC8 was expressed during the microspore, bicellular, and tricellular stages, as well as in mature pollen grains (Fig. 1). We therefore hypothesized that SEC8 and, more generally, the putative plant exocyst might be involved in cytokinesis during microgametogenesis.
To evaluate early male gametophyte development, the immature pollen of sec8-m3 heterozygotes and of homozygous wild-type siblings was compared. We used aniline blue staining of callose to detect the cytokinetic cell plate in developing pollen (Park and Twell, 2001) and 4′,6-diamino-phenylindole (DAPI) staining to determine whether mature pollen had successfully become trinuclear. Finally, transmission electron microscopy (TEM) was used to examine the ultrastructure of mature pollen. If the sec8-m3 allele transmission defect were due to an abnormality in microgametogenesis, one-half of the pollen from the heterozygote would exhibit a phenotype distinct from wild type. As shown in Supplemental Figure 4, developing and mature pollen from sec8-m3 heterozygotes appeared identical to pollen from wild-type siblings at all stages of development evaluated using light microscopy. In agreement with these observations, TEM micrographs showed no gross differences between mature pollen grains from heterozygotes and those from wild-type plants (n = 30 for each; e.g. Fig. 5I). In summary, gametophytes from sec8-m3 heterozygotes demonstrated no obvious defect in microgametogenesis.
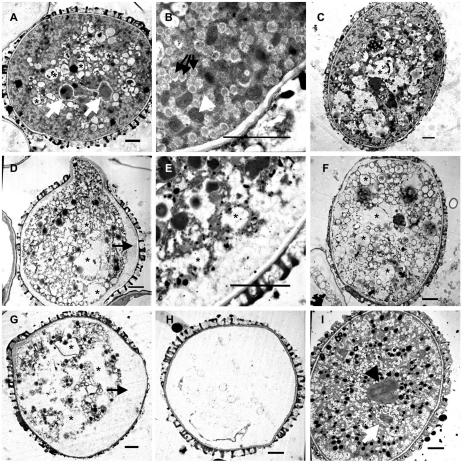
Figure 5.
TEM images indicate that ungerminated pollen from sec8-m3 heterozygotes responds to signals to germinate. A, D, G, and H, Wild-type pollen grains in vivo, illustrating (respectively) a likely progression of intracellular morphologies during germination. D and G, Vacuolar space generated adjacent to the pollen cell wall (black arrow). H, An apparently emptied coat. Larger, apparently coalescing, vacuoles (examples marked by asterisks) are also characteristic of germinating grains and are not present in mature pollen (I). B, Detail of A, adjacent to the pollen cell wall. Mitochondria (white arrowhead) and small membrane-bound structures of unknown identity (black arrows) are apparent. E, Detail of a pollen grain similar to D, showing vacuoles (asterisks) apparently fusing with the coat-associated vacuolar space and a few of the apparent small membrane-bound structures seen in B. C and F, Representative ungerminated grains (i.e. no separation of the cytoplasm from the coat) from a sec8-m3 heterozygote of class I and II, respectively (see text). Class I and II grains show certain characteristics similar to A and D, respectively. Both contain larger vacuoles (examples marked by asterisks); class I grains (C) contain small membrane-bound structures, whereas class II grains do not. Bar = 2 μm. White arrows, Sperm cells (A and I); black arrowhead, vegetative nucleus (I).
The Absolute Transmission Defect in Plants Carrying sec8-m3 Is Associated with a Pollen Germination Defect
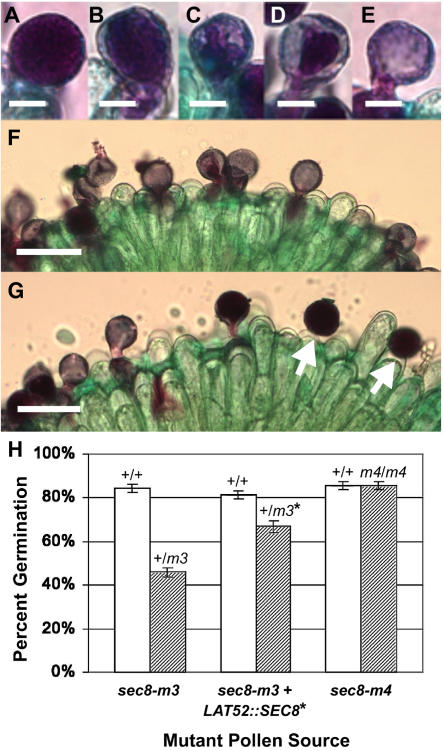
The absence of a sec8 mutant phenotype in mature pollen led us to evaluate the ability of mutant pollen to germinate and grow pollen tubes postpollination. In vitro techniques have been widely employed to evaluate pollen germination (Johnson-Brousseau and McCormick, 2004), but we were unable to achieve consistent results in several different media. As an alternative, we utilized an in vivo pollen germination assay (Lalanne et al., 2004a) employing Alexander staining (Alexander, 1969), which stains cytoplasm deep purple. Thus, ungerminated pollen grains appear dark, whereas in germinated grains, the movement of cytoplasm into the growing pollen tube leaves a vacuolated pollen grain, which appears pale blue. Pollen germination is then scored by the appearance of an empty, or partially empty, light blue pollen grain or a purple pollen tube growing out of the grain (Fig. 4, A–G).
Figure 4.
Alexander staining reveals that a SEC8 allele with an absolute transmission defect is associated with a pollen germination defect. A to E, Staining phenotypes of wild-type pollen grains in vivo, illustrating (from left to right, ungerminated to empty grain) a likely progression of cytoplasm (stained purple) out of the grain and into the growing pollen tube. F and G, Representative fields of stained pollen grains from a wild-type homozygote (F) and a sibling sec8-m3 heterozygote (G). White arrows, Ungerminated grains; stigma cells stain green. H, Quantitation of in vivo germination rates from three different pairs of lines, assayed blindly 4 h after pollination. Hatched bars, Mutants; white bars, wild-type siblings.
Stigmas were pollinated with pollen from either a sec8-m3 heterozygote or its wild-type sibling (n = 18 of each) and then stained after 4 h. In this assay, 84% (se 1.8%) of pollen grains from a homozygous wild-type sibling plant germinated compared with only 46% (se 2.0%) of the pollen from the sec8-m3 heterozygote (Fig. 4H). This implies that the ungerminated half of the pollen from the heterozygote consisted primarily of the mutant gametophytes. Similar experiments using aniline blue staining to score for germinated pollen tubes also showed a significant decrease in germination in mutant heterozygotes (data not shown).
To verify that the pollen germination defect was due to the sec8-m3 mutation, we also scored for germination in the complemented LAT52::SEC8 line. Due to independent assortment, only one-half of the sec8-m3 pollen from the transgenic heterozygote should contain the LAT52::SEC8 construct. Thus, complementation should increase the rate of germination to a level midway between that of the sec8-m3 heterozygote and wild type. As predicted, 66% (se 2.5%) of pollen grains from the transgenic heterozygote germinated (Fig. 4H). These results confirmed that the absolute transmission defect observed for the sec8-m3 mutant allele is due to a defect in pollen germination.
Pollen Grain Ultrastructure Suggests That the sec8-m3 Pollen Responds to Signals to Germinate
We were curious to know whether the sec8-m3 mutant pollen was unable to germinate due to an inability to generate tip growth or, alternatively, due to an inability to perceive stigma-originating signals initiating germination. These alternatives might be distinguishable by examining the ultrastructure of mutant pollen that failed to germinate after placement on a stigma. TEM was, therefore, employed to compare pollen from sec8-m3 heterozygotes and wild-type siblings after germination in vivo (Fig. 5).
The TEM images, combined with the Alexander staining results (Fig. 4, A–E), indicated a likely progression of ultrastructural changes that occur during wild-type pollen germination. Following placement of the pollen on the stigma, vacuoles within the pollen grains appeared to enlarge, to become prominent electron-transparent regions. Initially, organelles and small membrane-bound structures were readily observable (Fig. 5, A and B), but, as the vacuoles continued to enlarge, the small membrane-bound structures became much less apparent. The growing vacuoles appeared to coalesce to form a space next to the pollen cell wall, often more prominent on one side of the germinating pollen grain and coincident with the movement of the cytoplasm into the growing pollen tube (Fig. 5, D, E, and G). Ultimately, a large vacuole entirely filled the pollen grain (Fig. 5H). The enlargement of the vacuolar space, ultimately separating the cytoplasm from the pollen wall, was observed with both TEM and Alexander staining (Fig. 4, A–E), suggesting that this change was not an artifact of the chemical fixation used to prepare the pollen for TEM.
The initial TEM examination of pollen from a sec8-m3 heterozygote following pollination revealed no obvious novel phenotypes specific to the mutant. Nongerminating pollen grains were more prevalent in the pollen from a mutant heterozygote, as expected, but they exhibited stages of vacuolar development similar to the wild-type grains. To be more quantitative, we subsequently used TEM to categorize pollen from a sec8-m3 heterozygote (n = 59) and from a wild-type sibling (n = 65) into various phenotypic classes displayed at 2 h after pollination (Table II). Pollen grains showing an enlarged vacuolar space next to the pollen cell wall were considered to have germinated. Based upon this criterion, only 11% percent of the wild-type pollen grains failed to germinate (i.e. lacked a large, cell wall-associated vacuole), whereas 32% from the sec8-m3 heterozygote had not germinated, a statistically significant difference (χ2 = 8.57, P ≤ 0.01). There were no examples of putative autolysis (i.e. osmiophilic globoids or putative lysosomes; Yamamoto et al., 2003) in this set of pollen grains from the sec8-m3 heterozygote. Only one pollen grain from each source failed to demonstrate a response to being placed on the stigma. The remaining 18 ungerminated pollen grains from the mutant appeared to be undergoing changes normally associated with germinating wild-type pollen. Most of these, designated class I, showed enlarged vacuoles but no separation from the pollen cell wall (as illustrated in Fig. 5C). In five grains, designated class II, these changes included both extensive vacuolar development, as well as a decreased prevalence of small membrane-bound structures, typical of a late stage of pollen tube germination (Fig. 5F). The increased prevalence of class I and II phenotypes in heterozygous pollen, compared to homozygous wild type (Table II), strongly suggests that these are the mutant gametophytes. These observations are consistent with a defect in which the mutant was responding to the signals to germinate, but was unable to extrude its cytoplasmic contents into a growing pollen tube.
Table II.
Categorization of pollen grain TEM phenotypes 2 h after pollination
Comparison of pollen from sec8-m3 heterozygotes and wild-type siblings.
| Pollen Source
|
Germinated Pollen Grains
|
Nongerminated Pollen Grainsa
|
Total Grains Evaluated
|
|||
|---|---|---|---|---|---|---|
| Class Ib | Class IIc | No Responsed | Putative Autolysise | |||
| +/+ | 58 (89.2%) | 3 (4.6%) | 0 (0.0%) | 1 (1.5%) | 3 (4.6%) | 65 |
| m3/+ | 40 (67.8%) | 13 (22.0%) | 5 (8.5%) | 1 (1.7%) | 0 (0.0%) | 59 |
Lacking a large vacuole separating the cytoplasm from the pollen cell wall (see text, Fig. 5).
Class I, Larger vacuoles, small membrane-bound structures, no separation of cytoplasm from coat (e.g. Fig. 5C).
Class II, Larger vacuoles, no small membrane-bound structures, no separation of cytoplasm from coat (e.g. Fig. 5F).
No Response, no larger vacuoles, similar to mature pollen grain (Fig. 5I).
Putative Autolysis, Contains osmiophilic globoids, a characteristic of entry into a hypothesized autolysis pathway (Yamamoto et al., 2003).
The Partial Transmission Defect in sec8-m4 Heterozygotes Results from Reduced Competitive Ability and Is Associated with a Reduced Pollen Tube Growth Rate
The partial transmission defect evident in the sec8-m4, -m5, and -m6 mutants allowed the generation of plants homozygous for these mutant alleles. Based on comparisons of silique length, seed count, incidence of seed gaps, and incidence of deformed seeds (Supplemental Table I), the siliques produced by plants homozygous for each of the three hypomorphic alleles were indistinguishable from those of their wild-type siblings. Evidently, the mutant pollen from homozygous plants is fully capable of performing its functions, leading to a fully fertilized ovary. Given that the defect was only revealed in an outcross using pollen from a heterozygote, we hypothesized that pollen carrying these alleles expressed a competitive defect, i.e. one that made it less likely to accomplish fertilization in the presence of wild-type pollen.
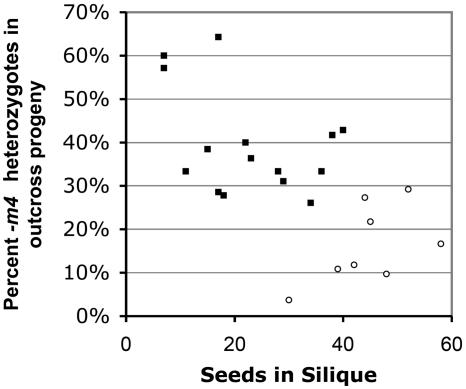
The importance of competition in the sec8-m4 transmission phenotype was demonstrated in two experiments. First, competition in a set of outcrosses was reduced by applying only a sparse quantity of pollen from a sec8-m4 heterozygote to a wild-type stigma. In the standard outcross, competition is accentuated by the application of a large quantity of pollen simultaneously to the stigma. Reducing competition by applying only a sparse quantity of pollen was therefore predicted to increase the percentage of heterozygotes in the outcross progeny. However, it is difficult to assess the exact quantity of pollen applied to the stigma in an outcross. We made the assumption that, when the number of pollen grains applied is limiting (i.e. less than the number of available ovules), then the number of seeds ultimately produced is an indirect indicator of the amount of pollen applied. Figure 6 is a plot of the percentage of heterozygotes present in the outcross progeny of a set of sparse pollen and excess pollen crosses as a function of seed count in the silique. The graph indicates that the reduced competition associated with sparse pollination was associated with an increase in the transmission of the mutant allele through the pollen.
Figure 6.
Transmission of the sec8-m4 allele increases in incompletely filled siliques. Pollinations (using sec8-m4/+ as the male) were done with either sparse (black squares) or excess (white circles) pollen placed on each wild-type stigma. Genotypes of progeny were determined by PCR.
In a second experiment, competition was increased in self-crosses by manually applying a large excess of pollen to the stigma of sec8-m4 heterozygotes. We hypothesized that a naturally occurring self-cross creates a less competitive situation in which there is a more gradual and lighter deposition of pollen onto the stigma. This reduced competition in a natural cross would explain the 23% frequency of mutant homozygotes from our initial self-crosses (Table I), which is far greater than would be predicted (approximately 9%) based on the outcross transmission rate. We predicted that the increased competition created by applying a large excess of pollen would favor the wild-type pollen over the sec8-m4 pollen and thereby result in decreased transmission of the mutant allele when compared with natural self-pollination of the same plant. The observed genotypes of progeny of natural self-pollination (n = 90) were in the expected Mendelian ratio (Table III). The manual self-pollination (n = 156), however, showed decreased transmission of the mutant allele and produced progeny in a genotypic ratio of approximately 2 (+/+) to 3 (+/m4) to 1 (m4/m4), significantly different from the Mendelian ratio (χ2 = 9.37, P < 0.01). Thus, two lines of evidence support the hypothesis that the partial transmission defect of the sec8-m4 allele is due to a decreased ability to compete with wild-type pollen.
Table III.
Natural versus manual (excess pollen applied to stigma) self-crosses of sec8-m4 heterozygotes
| Type of Self-Cross
|
No. of Crosses
|
No. of Progeny
|
Genotypes of Progeny
|
χ2
|
P
|
||
|---|---|---|---|---|---|---|---|
| +/+ | +/ma | m/m | |||||
| Natural | 3 | 90 | 23 | 44 | 23 | 0.044 | NSb |
| 25.6% | 48.9% | 25.6% | |||||
| Manual | 3 | 156 | 52 | 79 | 25 | 9.372 | ≤0.01 |
| 33.3% | 50.6% | 16.0% | |||||
| Expected Mendelian ratio | 25% | 50% | 25% | ||||
Mutant sec8 allele.
NS, Not significantly different.
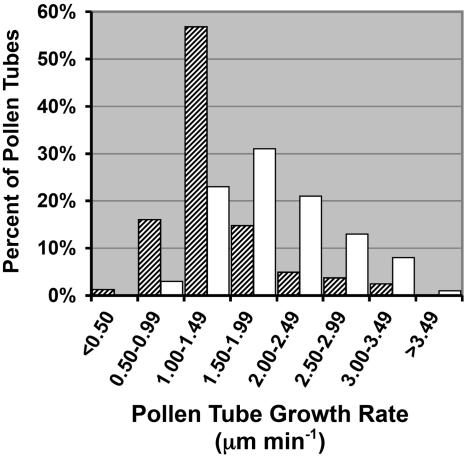
The reduced ability of pollen carrying the sec8-m4 allele to compete with wild-type pollen could conceivably arise from defects in pollen germination, pollen tube growth, or pollen tube guidance. However, there was no defect in the pollen germination of sec8-m4 pollen detected in in vivo pollen germination assays 4 h after pollination (Fig. 4H), or using aniline blue staining 2 h after pollination (data not shown). Therefore, we turned our attention to evaluating postgermination characteristics of the sec8-m4 gametophytes. To evaluate the possibility that the pollen tube growth rate of sec8-m4 gametophytes was slower than wild type, we compared the growth rate of pollen tubes from wild-type and homozygous mutant siblings (Fig. 7). A semi in vivo method was employed in which pollen was germinated on a stigma, the stigma was placed on a slide coated with growth media, and pollen tubes were subsequently visualized as they grew out from the stigma onto the media. Wild-type pollen (n = 100 pollen tubes) demonstrated a mean growth rate of 2.0 μm min−1 (se, 0.06 μm min−1), compared to sec8-m4 pollen (n = 81 pollen tubes), which had a mean growth rate of 1.4 μm min−1 (se, 0.04 μm min−1). Thus the mutant pollen has a significantly lower growth rate (P = 5 × 10−11, t test) compared to pollen of a wild-type sibling.
Figure 7.
The sec8-m4 mutation decreases pollen tube growth rate in culture. A histogram of the rates for individual pollen tubes (n = 81 mutant, 100 wild type) from five different homozygous mutant plants (cross-hatched bars) and five different sibling wild-type plants (white bars) shows a significantly different distribution in the two genotypes. Rates were determined by imaging germinated, growing pollen tubes at 15- to 30-min intervals.
In vivo differences in pollen tube growth rates between the wild-type and mutant pollen of a heterozygote can be evidenced by a nonrandom distribution of mutant seeds within the resultant silique (e.g. Goubet et al., 2003). Slower growing pollen tubes from mutant gametophytes result in the mutant allele being more prevalent in seeds from the top half (stigma end) of the silique, relative to the bottom half. Therefore, to determine whether sec8-m4 mutants show a reduced pollen tube growth rate in vivo, a top-to-bottom comparison was performed on 14 siliques harvested from heterozygous outcrosses. In this population, a significantly higher proportion of mutant heterozygotes arose from seeds in the top half of the siliques (22.9%, n = 306), compared to the bottom half (13.8%, n = 283; χ2 = 8.064, P ≤ 0.01). These results support the hypothesis that the competitive disadvantage of sec8-m4 mutants is due, at least in part, to a slower pollen tube growth rate than wild type. SEC8 function is thereby implicated in pollen tube growth in addition to pollen germination.
In the course of this study, we observed that plants homozygous for the sec8-m4 allele appeared identical to their wild-type siblings with respect to such gross morphological characteristics as leaf shape and size, appearance of inflorescences, trichome structure, and root hair shape and development. The appearance of root hairs was of particular interest because, like pollen tubes, they demonstrate tip growth, and because another exocyst component, SEC3, is associated with a mutant root hair phenotype in maize (Wen et al., 2005). However, maximum root hair lengths measured in 5-d-old seedlings revealed no significant difference (P = 0.182, t test) between sec8-m4 homozygotes (mean = 0.85 mm; se, 0.02 mm; n = 63) and their wild-type siblings (mean = 0.82 mm; se, 0.02 mm; n = 63). We were unable to assess the effect of stronger alleles (i.e. sec8-m1, -m2, or -m3) on sporophytic characteristics due to our inability to obtain plants homozygous for these alleles.
DISCUSSION
The polarized growth associated with the germination of the pollen tube from a specific site on the pollen grain and the subsequent tip growth of the pollen tube involve the rapid and localized exocytosis of secretory vesicles. In a wide range of eukaryotes, a protein complex known as the exocyst has been shown to facilitate such localized exocytosis (for review, see Hsu et al., 2004). The Arabidopsis SEC8 is hypothesized, on the basis of sequence homology, to be a component of the putative plant exocyst (Jurgens and Geldner, 2002; Elias et al., 2003). We have shown that the SEC8 gene is required for pollen germination and competitive pollen tube growth. The most severe mutant alleles of SEC8 hinder germination of the pollen grain, resulting in an absolute male-specific transmission defect. Less severe mutant alleles of SEC8 lead to a decline in the ability of mutant gametophytes to compete with wild-type gametophytes. This decreased competitiveness is at least in part due to a reduced pollen tube growth rate and results in a partial male-specific transmission defect. These results, along with those of Wen et al. (2005), provide the first demonstrated functional role for a putative exocyst component in plants.
Male-specific transmission defects can result from developmental abnormalities that occur at any of a number of stages during microsporogenesis or gametogenesis (Grini et al., 1999; Procissi et al., 2001; Johnson et al., 2004; Lalanne et al., 2004b). We initially suspected that mutation of SEC8 might affect early pollen development because of the exocyst's hypothesized role in plant cytokinesis (Otegui and Staehelin, 2004; Segui-Simarro et al., 2004), which can be envisioned as a polarization of the exocytic pathway toward the division plane (for review, see Bednarek and Falbel, 2002). Other mutations affecting early pollen development (e.g. those in SCP1 [Chen and McCormick, 1996]; GEM1 [Park et al., 1998]; and MAD1 [Grini et al., 1999]) display aberrant cell plate formation or abnormal cell division that is readily evident under microscopic examination. In the case of SEC8 mutants, however, microscopic examination revealed no characteristics that would distinguish mutant pollen from wild type prior to the time of germination, arguing against participation of SEC8 in gametophytic cytokinesis. It is possible that the mitotic divisions of male gametogenesis use an alternative form of cytokinesis that does not require SEC8 or the exocyst. It is also possible that residual wild-type SEC8 transcripts or protein from the heterozygous microsporocytes are present in functional quantities after meiosis, compensating for the mutation in the haploid microspores. Regardless of these possibilities, our evidence strongly argues against a link between cytokinetic defects and the reduced transmission of the SEC8 mutants.
In contrast to the apparent wild-type development of the pollen grain in the mutant, in vivo assays and a complementation experiment definitively demonstrated that the transmission defect in sec8-m3 gametophytes is due to a defect in pollen germination. Mutations associated with germination defects can be assigned to two broad categories: those that affect the reception of stigma-originating signals initiating germination and those that affect the response to these signals, i.e. the generation of polar growth. Germination signals are likely to involve adhesion and hydration, mediated by molecules in the pollen coat and its interaction with the stigma (for review, see Edlund et al., 2004). Other possible cellular components involved in signal reception include apyrases (Steinebrunner et al., 2003); receptor-like kinases and their ligands (Tang et al., 2002; Wengier et al., 2003); as well as calcium (Iwano et al., 2004), calmodulin, and calmodulin-binding proteins (Golovkin and Reddy, 2003). Mutations that appear to affect response (e.g. in SETH1 and SETH2, affecting GPI-anchored proteins; Lalanne et al., 2004a) often have distinct phenotypes, such as short pollen tubes. Obviously aberrant pollen tubes from the sec8-m3 heterozygote were not evident in the in vivo pollen germination assay, leaving an important question unanswered. Were the mutant pollen grains unable to sense the signals to germinate or were they simply unable to elicit the proper response?
To explore this question, we utilized TEM to evaluate the ultrastructure of pollen grains from a sec8-m3 heterozygote after placement on a stigma. In wild-type grains, polarized accumulation of vesicles can be seen in the grain within 10 min after pollination (Kandasamy et al., 1994). Then, coincident with pollen tube germination, vacuoles enlarge and coalesce dynamically, eventually filling the pollen grain as the cytoplasm moves into the growing pollen tube (Hicks et al., 2004). Unfortunately, we were unable to capture any TEM images revealing the localization of secretory vesicles during germination. However, the ungerminated pollen from a sec8-m3 heterozygote, which most likely is primarily made up of mutant gametophytes, did respond to its placement on a stigma with the development of prominent vacuoles. In some cases, the development of vacuoles was quite extensive, typical of wild-type pollen in an advanced stage of pollen tube development. This occurred without the development of a vacuolar space near the pollen cell wall characteristic of a pollen grain emptying its cytoplasmic contents into a growing pollen tube. Our TEM investigation was admittedly limited in several respects. First, the chemical fixation used in this study is known to be less optimal for preserving fine ultrastructure than the cryofixation/freeze substitution techniques used by several other groups (e.g. Yamamoto et al., 2003). Second, any unique ultrastructural differences in the mutant pollen may be spatially and temporally limited to the site of pollen tube formation at the time of germination and thereby missed in our collection of samples. Third, the relatively low frequency of ungerminated pollen found from the heterozygote, compared to the Alexander staining results, suggests that some grains may have been lost during sample preparation. In spite of these limitations, the TEM study identified clear differences between pollen from mutant heterozygotes and pollen from wild types, strongly suggesting that sec8-m3 mutant pollen grains do respond to the signals initiating germination, but are simply not able to generate a pollen tube. The observations are consistent with the hypothesis that SEC8, as part of the plant exocyst, is required for localized exocytosis at the initial site of polarized growth, leading to germination and the emergence of a pollen tube.
Further insight into the role of SEC8 in the male gametophyte was gained from an evaluation of the three alleles demonstrating a partial transmission defect, sec8-m4, -m5, and -m6. We hypothesized that this transmission defect was due to a competitive disadvantage for the mutant gametophyte compared to wild type, similar to previously isolated mutations in Arabidopsis (seth8, seth9, and seth10; Lalanne et al., 2004b) and maize (rop2; Arthur et al., 2003). To test this hypothesis, we reduced the competition in outcrosses by applying only sparse sec8-m4/+ pollen to the stigma and observed a resultant increase in transmission of the mutant allele. In a second, converse test of the competition hypothesis, we increased pollen competition in a self-cross of heterozygous plants by manually applying an excess of pollen. We found that the transmission of the mutant allele was significantly reduced in the manual self-cross, compared to the natural self-cross, as was predicted. These results not only support our hypothesis but also have implications for self-pollination in Arabidopsis. Apparently, a more gradual deposition of pollen onto the stigma during natural self-crosses can allow gametophytes with certain competitive defects to successfully fertilize ovules at a frequency on par with the wild type. These results suggest that mutant screens relying on segregation distortion to identify gametophytically important genes (e.g. those of Grini et al., 1999; Johnson et al., 2004; Lalanne et al., 2004b) will miss mutant alleles with subtle, but reproducible, effects on pollen competitive ability.
The competitive disadvantage of the SEC8 partial mutants could arise from defects in pollen germination, pollen tube growth, or pollen tube guidance from the stigma to the ovule. However, we found that sec8-m4 pollen was able to germinate at the same frequency as that of wild-type siblings when evaluated at 2 (aniline blue staining) or 4 h (Alexander staining) after pollination. The data did not rule out the possibility that the mutant pollen has a delayed germination of 2 h or less, but they did point us toward later stages of gametophytic development. The role of SEC8 in postgermination pollen tube growth was explored by monitoring the growth rates of individual pollen tubes over several hours. We discovered that the growth rate of sec8-m4 pollen tubes (1.4 μm min−1) is significantly less than that of wild-type pollen tubes (2.0 μm min−1). The slightly reduced growth rate of the mutant pollen tubes does not prevent a self-crossed sec8-m4 homozygote from producing full-length siliques filled with viable seed, but apparently becomes a detriment when the mutant pollen must compete with wild-type pollen. In an outcross using pollen from a heterozygote, this growth rate competition results in a nonuniform distribution of seeds with the mutant allele in the resultant silique, i.e. the mutant allele is less prevalent at the end of the silique farthest from the stigma. These results clearly demonstrate that SEC8 plays a role not only in pollen germination, but also in the subsequent growth of the pollen tube in vivo.
We were interested in calculating whether the growth rate defect was sufficient to explain the severity of the sec8-m4 transmission disadvantage. In a typical outcross, we observed that approximately 300 pollen grains are applied to the stigma. Those with the fastest growing pollen tubes can be hypothesized to result in fertilization and the formation of the typical 45 seeds in a silique. The growth rate distributions (Fig. 7) predict that the 45 fastest pollen tubes in a heterozygote outcross will be composed of approximately 18% mutant gametophytes. This proportion is the same as the observed outcross results for the sec8-m4 allele (i.e. 18% of the outcross progeny were heterozygotes), suggesting that the growth rate defect is sufficient to account for the partial transmission defect. Caution must be taken, however, as in vitro measurements of pollen tube growth rates are notoriously much lower than those measured in vivo (4–16 μm min−1; Kandasamy et al., 1994; Iwano et al., 2004), and our result may not represent the growth rate distribution in vivo. In addition, we have not ruled out the possibility that sec8-m4 causes additional defects (e.g. in guidance) that also contribute to the mutant's competitive disadvantage in vivo. Nevertheless, this analysis argues that a growth rate defect is the predominant cause of the competitive disadvantage observed in the sec8-m4 mutant gametophytes.
The existence of six different alleles of SEC8 has potential for illuminating particular functional domains of the SEC8 protein. The three alleles with inserts closest to the 5′-end of the gene demonstrate an absolute transmission defect, while those closest to the 3′-end of the gene result in only a partial transmission defect. Two-hundred-fifty amino acid residues span the region at the carboxyl end of the protein that is predicted to be affected by the -m3, -m4, -m5, and -m6 mutations. Available EST sequence data show that this region of the protein is well conserved in homologs across a broad range of plant species, which include angiosperms (both monocots and dicots), gymnosperms, and a moss, attesting to its potential significance. In addition, the partial male transmission defects associated with all three of the C-terminal mutant alleles suggests functional importance for the C terminus of AtSEC8. Intriguingly, mammalian homologs of SEC8 contain at their carboxyl termini a PDZ-binding motif (the amino acids TTV) that is essential for the exocyst complex to direct PDZ-containing proteins to the correct membrane locations (Riefler et al., 2003; Sans et al., 2003). This particular motif is absent in nonmammalian homologs of SEC8, including those we identified (Fig. 2B). Nevertheless, the mammalian results raise the possibility that the function of the plant SEC8 C terminus is to interact with particular exocyst-directed molecules. No other hints relating structure to function could be gleaned from a search for functional domains within the primary structure of the protein. It should again be noted that we have not yet ruled out the possibility that the three hypomorphic SEC8 mutations lead to the observed phenotypes by causing a decrease in transcript levels rather than altered protein structure. Generating a set of transgenic lines expressing mutant derivatives of SEC8 will be important to further address these possibilities.
CONCLUSION
This report, along with the report of Wen et al. (2005), provides functional data to test the hypothesis that the exocyst is involved in tip growth in plants. Orthologs of two different exocyst components in two widely divergent plant species, SEC8 in Arabidopsis (this report) and SEC3 in maize (Wen et al., 2005), have been shown to be important for the proper development of the polarly growing pollen tube and root hair, respectively. These observations provide strong circumstantial evidence that a function for the exocyst in polarized exocytosis is conserved across plant, fungal, and mammalian species. In addition, preliminary evidence suggests that other exocyst subunits are active in pollen tube growth as a part of a high Mr complex (L. Synek, M. Quentin, and V. Zarsky, unpublished data). Due to our inability to obtain a homozygous plant carrying a strong SEC8 mutation, we were unable to assess whether SEC8 is important for root hair growth. The lack of an obvious pollen-associated phenotype in the maize rth1 mutant can be explained by the relatively low expression level for rth1 in pollen and the possible presence of genetic redundancy for SEC3 in the maize genome. Regardless, our hypothesis would predict that mutations in additional plant exocyst genes would also affect root hair and/or pollen tube development. Intriguingly, one other class of protein shown to be important for the polarized growth of both root hair and pollen tube is the Rop GTPase, a key regulator of plant cell polarity (for review, see Yang, 2002). Indeed, the exocyst has been mentioned as a possible target for Rop regulation (Fu and Yang, 2001) and, in mammalian and yeast cells, has been shown to be regulated by a number of related GTPases (for review, see Novick and Guo, 2002). As new mutants and molecular tools (e.g. antibodies) become available, additional genetic, cellular, and molecular experiments should be pursued to verify the existence of a plant exocyst complex, determine its localization in both gametophytic and sporophytic cells, and explore its regulation and functional role in plant cell morphological development.
MATERIALS AND METHODS
Sequence and Expression Database Analysis
Plant sequences orthologous to AtSEC8 were identified by Web-based BLAST (Altschul et al., 1997) and downloaded from GenBank for further analysis. All sequencing of PCR products (see below) used standard automated protocols and was done at the Oregon State University (OSU) Center for Gene Research and Biotechnology (CGRB) Central Services Lab. Sequences were analyzed using either the Wisconsin Package, version 10.3 (Accelrys, San Diego), or Web-based tools available at the OSU CGRB Bioinformatics Web site.
Expression data for haploid male gametophyte development was extracted from the work of Honys and Twell (2004). Additional microarray data for sporophytic tissues and cell suspensions from the Nottingham Arabidopsis Stock Centre (NASC) Affymetrix Web site were also available in the Honys and Twell database, which has been subject to normalization and statistical analysis.
Growth and Assessment of Plants, and Genetic Methods
Arabidopsis (Arabidopsis thaliana) lines with T-DNA insertions in the SEC8 gene in a Columbia-0 background were obtained from the SALK Institute: SEC8-m1 (SALK 057409), SEC8-m2 (SALK 016128), SEC8-m3 (SALK 026204), SEC8-m4 (SALK 118129), SEC8-m5 (SALK 039659), and SEC8-m6 (SALK 091118). SEC8-specific primers were used with either left- or right-border T-DNA primers to obtain PCR products on both sides of each insertion, which were sequenced to confirm the insertion site. Seeds were sterilized (1 min in 95% ethanol, 10 min in 20% bleach, and five rinses in sterile water) and cold treated in water for 5 d prior to planting on growth medium (Murashige and Skoog medium supplemented with 0.0001% [w/v] nicotinic acid, 0.0001% [w/v] thiamine-HCl, 0.0001% [w/v] pyroxidine-HCl, 0.01% [w/v] myoinositol, 0.0004% [w/v] Gly) or potting soil (SB40; Sun Gro Horticulture, Bellevue, WA). Plants were grown in a growth chamber at 24°C, 16 h light per day. Plants used for root hair assessment were grown on growth medium in petri dishes, oriented vertically, and the three longest root hairs of each seedling were measured.
To determine plant genotypes, leaf DNA was extracted using a rapid prep method (http://www.agron.missouri.edu/mnl/77/57vejlupkova.html). PCR genotyping was performed using three primers: a primer to the left border of the T-DNA insert (LBb1) and a pair of primers designed to amplify the section of DNA containing the insert site. Primer sequences are listed in Supplemental Table II. Outcrosses were performed by applying pollen from newly dehiscing flowers onto the stigmas of flowers that had been surgically emasculated just prior to dehiscence. Columbia-0 was used as a wild-type tester in outcrosses. Silique evaluations were performed by harvesting mature intact siliques, teasing them open without disturbing the seeds, and examining them under a dissecting microscope.
For complementation, a construct was created by inserting the coding sequence from a full-length cDNA for SEC8 (accession no. AY059763, clone RAFL07-11-G22; Yamada et al., 2003) into a pCAMBIA1300-derived plasmid containing the LAT52 pollen promoter (a gift from the lab of Z. Yang). This binary vector was used to transform Agrobacterium tumefaciens and the transformants were identified by screening for resistance to rifampicin. The transformed Agrobacterium was in turn used to transform Arabidopsis plants carrying the sec8-m3 allele using the floral-dip method (Clough and Bent, 1998). Transformed plants were identified by screening for hygromycin resistance (Nakazawa and Matsui, 2003), and sec8-m3 mutants were identified among this population of transgenics.
Expression Analysis by RT-PCR
Root and immature leaf samples from 10-d-old seedlings and mature leaf and immature floral tissue samples from 21-d-old plants were harvested. Pollen was harvested from about 300 Columbia-0 plants by collecting flowers into 300 mL ice-cold 0.3 m mannitol, vigorously shaking for 1 min, filtering through a 75-mm nylon mesh, and concentrating by centrifugation (adapted from Honys and Twell, 2003). For analysis of sec8-m4, -m5, and -m6 expression, immature floral tissue was harvested from homozygous mutant plants. All tissues were immediately frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted using the RNeasy plant minikit (Qiagen, Valencia, CA), according to the manufacturer's instructions. The RNA yield and purity were determined spectrophotometrically. RNA derived from homozygous mutants was treated with RQ1 DNase prior to RT to eliminate any possibility of genomic contamination. RT was performed on 1.5 μg total RNA from each source using the SuperScript first-strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. The cDNA generated was used as the template for PCR amplification using appropriate primers (Supplemental Table II).
Electron Microscopy
Hand-pollinated stigmas were excised from Columbia-0 plants 1 to 4 h after pollination, allowing germination of most wild-type pollen grains, while increasing the probability that the ungerminated pollen grains from the sec8-m3 heterozygote contained the mutant allele. For the quantitative analysis (Table II), all samples were prepared from stigmas at 2 h after pollination. The excised samples were embedded in 2% low-melting-point agarose and immediately fixed and prepared for ultrathin sectioning as described by Park and Twell (2001). Briefly, samples were chemically fixed in glutaraldehyde, dehydrated, sectioned, stained with uranyl acetate and lead citrate, and viewed with a transmission electron microscope (Philips CM 12, 60 kV).
Light Microscopy and Assessment of Pollen Tube Germination and Growth Rate
Premature male gametophytes were visualized by surgically removing immature anthers from early stages of flower development and applying aniline blue (0.1% [w/v] prepared in 0.1 m phosphate buffer [pH 8.5]; adapted from Li et al., 1999) for at least 30 min prior to microscopic examination. Gentle pressure on the coverslip spread the gametophytes for optimal viewing. Mature pollen grains were stained with DAPI stain (1 μg/mL DAPI, 5% [v/v] DMSO, 1% [v/v] Triton X-100) for 1 to 2 h in the dark prior to microscopic examination. Light microscopy was performed on a Zeiss Axiovert microscope with differential interference contrast optics and a UV filter set for observing the DAPI and aniline blue fluorescent stains. Digital images were acquired using a SPOT CCD camera and software (Diagnostic Instruments, Sterling Heights, MI).
Both in vivo pollen germination studies and in vitro pollen tube growth rate assessments were performed by a researcher blind to the pollen source. In vivo pollen germination was assessed as described (Lalanne et al., 2004a). Briefly, 4 h after applying only a sparse quantity of pollen to the stigma, the stigmas were excised, placed on a microscope slide, stained with Alexander stain (Alexander, 1969), and examined. A similar procedure was followed using aniline blue (as described above) to stain pollen grains on stigmas 2 h after pollination.
In the growth rate experiments, a moderate quantity of pollen was applied to a stigma, which was then immediately excised, placed on a microscope slide that had been coated with germination media [18% (w/v) Suc, 0.01% (w/v) boric acid, 2 mm CaCl2, 1 mm Ca(NO3)2, 1 mm MgSO4, 0.5% (w/v) Noble agar; DIFCO Laboratories, Detroit], and incubated in a moist chamber. Beginning 1 h after pollination, the slides were photographed microscopically at 15- to 30-min intervals over a period of several hours. The measured lengths of individual pollen tubes as they grew onto the surface of the medium allowed the calculation of pollen tube growth rates over this series of intervals. The maximal growth rate for each pollen tube over this period was used in the comparative analysis.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material.
Supplementary Material
Acknowledgments
We thank M. Foss and Z. Vejlupkova for useful critiques of the manuscript, K. Carroll for consultations on procedures, M. Johnson for advice regarding competition experiments, Z. Yang for the LAT52 construct, and P. Schnable for communicating results prior to publication. We would like to acknowledge M. Nesson and the OSU Electron Microscopy Facility for assistance with the TEM, the OSU CGRB Central Services Lab for sequencing, and the T. Wolpert Lab for assistance with the growth and transformation of Arabidopsis. Finally, we also thank J. McElravy, J. Hines, P. Staiger, and M. Frederick for their cheerful contributions to the work of the laboratory.
This work was supported by the National Science Foundation (grant nos. IBN–0111078 and IBN–0420226 to J.E.F.), and by Grantová Agentura AV (Czech Republic grant no. A6038410 and EU–HPRN–CT–2002–002656 TIPNET to V.Z.).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.062273.
References
- Alexander M (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44: 117–122 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur KM, Vejlupkova Z, Meeley RB, Fowler JE (2003) Maize ROP2 GTPase provides a competitive advantage to the male gametophyte. Genetics 165: 2137–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek SY, Falbel TG (2002) Membrane trafficking during plant cytokinesis. Traffic 3: 621–629 [DOI] [PubMed] [Google Scholar]
- Camacho L, Malhó R (2003) Endo/exocytosis in the pollen tube apex is differentially regulated by Ca2+ and GTPases. J Exp Bot 54: 83–92 [DOI] [PubMed] [Google Scholar]
- Chen YC, McCormick S (1996) sidecar pollen, an Arabidopsis thaliana male gametophytic mutant with aberrant cell divisions during pollen development. Development 122: 3243–3253 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Edlund AF, Swanson R, Preuss D (2004) Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell (Suppl) 16: S84–S97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias M, Drdova E, Ziak D, Bavlnka B, Hala M, Cvrckova F, Soukupova H, Zarsky V (2003) The exocyst complex in plants. Cell Biol Int 27: 199–201 [DOI] [PubMed] [Google Scholar]
- Feijó JA, Costa SS, Prado AM, Becker JD, Certal AC (2004) Signalling by tips. Curr Opin Plant Biol 7: 589–598 [DOI] [PubMed] [Google Scholar]
- Fowler JE, Quatrano RS (1997) Plant cell morphogenesis: plasma membrane interactions with the cytoskeleton and cell wall. Annu Rev Cell Dev Biol 13: 697–743 [DOI] [PubMed] [Google Scholar]
- Fu Y, Yang Z (2001) Rop GTPase: a master switch of cell polarity development in plants. Trends Plant Sci 6: 545–547 [DOI] [PubMed] [Google Scholar]
- Golovkin M, Reddy AS (2003) A calmodulin-binding protein from Arabidopsis has an essential role in pollen germination. Proc Natl Acad Sci USA 100: 10558–10563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubet F, Misrahi A, Park SK, Zhang Z, Twell D, Dupree P (2003) AtCSLA7, a cellulose synthase-like putative glycosyltransferase, is important for pollen tube growth and embryogenesis in Arabidopsis. Plant Physiol 131: 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ (1998) Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 93: 731–740 [DOI] [PubMed] [Google Scholar]
- Grini PE, Schnittger A, Schwarz H, Zimmermann I, Schwab B, Jurgens G, Hulskamp M (1999) Isolation of ethyl methanesulfonate-induced gametophytic mutants in Arabidopsis thaliana by a segregation distortion assay using the multimarker chromosome 1. Genetics 151: 849–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Wang Z, Yang Z (2004) ROP/RAC GTPase: an old new master regulator for plant signaling. Curr Opin Plant Biol 7: 527–536 [DOI] [PubMed] [Google Scholar]
- Guo W, Grant A, Novick P (1999) Exo84p is an exocyst protein essential for secretion. J Biol Chem 274: 23558–23564 [DOI] [PubMed] [Google Scholar]
- Hazuka CD, Foletti DL, Hsu SC, Kee Y, Hopf FW, Scheller RH (1999) The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J Neurosci 19: 1324–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17: 159–187 [DOI] [PubMed] [Google Scholar]
- Hicks GR, Rojo E, Hong S, Carter DG, Raikhel NV (2004) Geminating pollen has tubular vacuoles, displays highly dynamic vacuole biogenesis, and requires VACUOLESS1 for proper function. Plant Physiol 134: 1227–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D (2003) Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol 132: 640–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 5: R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, TerBush D, Abraham M, Guo W (2004) The exocyst complex in polarized exocytosis. Int Rev Cytol 233: 243–265 [DOI] [PubMed] [Google Scholar]
- Inoue M, Chang L, Hwang J, Chiang SH, Saltiel AR (2003) The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature 422: 629–633 [DOI] [PubMed] [Google Scholar]
- Iwano M, Shiba H, Miwa T, Che FS, Takayama S, Nagai T, Miyawaki A, Isogai A (2004) Ca2+ dynamics in a pollen grain and papilla cell during pollination of Arabidopsis. Plant Physiol 136: 3562–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, von Besser K, Zhou Q, Smith E, Aux G, Patton D, Levin JZ, Preuss D (2004) Arabidopsis hapless mutations define essential gametophytic functions. Genetics 168: 971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Brousseau SA, McCormick S (2004) A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically expressed genes. Plant J 39: 761–775 [DOI] [PubMed] [Google Scholar]
- Jurgens G, Geldner N (2002) Protein secretion in plants: from the trans-Golgi network to the outer space. Traffic 3: 605–613 [DOI] [PubMed] [Google Scholar]
- Kandasamy MK, Nasrallah JB, Nasrallah ME (1994) Pollen-pistil interactions and developmental regulation of pollen tube growth in Arabidopsis. Development 120: 3405–3418 [Google Scholar]
- Lalanne E, Honys D, Johnson A, Borner GH, Lilley KS, Dupree P, Grossniklaus U, Twell D (2004. a) SETH1 and SETH2, two components of the glycosylphosphatidylinositol anchor biosynthetic pathway, are required for pollen germination and tube growth in Arabidopsis. Plant Cell 16: 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne E, Michaelidis C, Moore JM, Gagliano W, Johnson A, Patel R, Howden R, Vielle-Calzada JP, Grossniklaus U, Twell D (2004. b) Analysis of transposon insertion mutants highlights the diversity of mechanisms underlying male progamic development in Arabidopsis. Genetics 167: 1975–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lin Y, Heath RM, Zhu MX, Yang Z (1999) Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11: 1731–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S (2004) Control of male gametophyte development. Plant Cell (Suppl) 16: S142–S153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M, Garza D, Scheller RH, Schwarz TL (2003) Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron 37: 433–447 [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Matsui M (2003) Selection of hygromycin-resistant Arabidopsis seedlings. Biotechniques 34: 28–30 [DOI] [PubMed] [Google Scholar]
- Novick P, Guo W (2002) Ras family therapy: Rab, Rho and Ral talk to the exocyst. Trends Cell Biol 12: 247–249 [DOI] [PubMed] [Google Scholar]
- Otegui MS, Staehelin LA (2004) Electron tomographic analysis of post-meiotic cytokinesis during pollen development in Arabidopsis thaliana. Planta 218: 501–515 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Preuss D (2000) Pollen tube targeting and axon guidance: parallels in tip growth mechanisms. Trends Cell Biol 10: 517–524 [DOI] [PubMed] [Google Scholar]
- Park SK, Howden R, Twell D (1998) The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development 125: 3789–3799 [DOI] [PubMed] [Google Scholar]
- Park SK, Twell D (2001) Novel patterns of ectopic cell plate growth and lipid body distribution in the Arabidopsis gemini pollen1 mutant. Plant Physiol 126: 899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR (1999) Transport-vesicle targeting: tethers before SNAREs. Nat Cell Biol 1: E17–E22 [DOI] [PubMed] [Google Scholar]
- Procissi A, de Laissardiere S, Ferault M, Vezon D, Pelletier G, Bonhomme S (2001) Five gametophytic mutations affecting pollen development and pollen tube growth in Arabidopsis thaliana. Genetics 158: 1773–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefler GM, Balasingam G, Lucas KG, Wang S, Hsu SC, Firestein BL (2003) Exocyst complex subunit sec8 binds to postsynaptic density protein-95 (PSD-95): a novel interaction regulated by cypin (cytosolic PSD-95 interactor). Biochem J 373: 49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Prybylowski K, Petralia RS, Chang K, Wang YX, Racca C, Vicini S, Wenthold RJ (2003) NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat Cell Biol 5: 520–530 [DOI] [PubMed] [Google Scholar]
- Schekman R, Novick P (2004) 23 genes, 23 years later. Cell 116: S13–S15 [DOI] [PubMed] [Google Scholar]
- Segui-Simarro JM, Austin JR II, White EA, Staehelin LA (2004) Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell 16: 836–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinebrunner I, Wu J, Sun Y, Corbett A, Roux SJ (2003) Disruption of apyrases inhibits pollen germination in Arabidopsis. Plant Physiol 131: 1638–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara K, Asano S, Tanaka K, Iwamatsu A, Okawa K, Ohta Y (2002) The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat Cell Biol 4: 73–78 [DOI] [PubMed] [Google Scholar]
- Tang W, Ezcurra I, Muschietti J, McCormick S (2002) A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell 14: 2277–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P (1996) The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J 15: 6483–6494 [PMC free article] [PubMed] [Google Scholar]
- Twell D, Yamaguchi J, Wing RA, Ushiba J, McCormick S (1991) Promoter analysis of genes that are coordinately expressed during pollen development reveals pollen-specific enhancer sequences and shared regulatory elements. Genes Dev 5: 496–507 [DOI] [PubMed] [Google Scholar]
- Vega IE, Hsu SC (2001) The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J Neurosci 21: 3839–3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen T-J, Hochholdinger F, Sauer M, Bruce W, Schnable PS (2005) The roothairless1 gene of maize encodes a homolog of sec3, which is involved in polar exocytosis. Plant Physiol 138: 1637–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengier D, Valsecchi I, Cabanas ML, Tang WH, McCormick S, Muschietti J (2003) The receptor kinases LePRK1 and LePRK2 associate in pollen and when expressed in yeast, but dissociate in the presence of style extract. Proc Natl Acad Sci USA 100: 6860–6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte JR, Munro S (2002) Vesicle tethering complexes in membrane traffic. J Cell Sci 115: 2627–2637 [DOI] [PubMed] [Google Scholar]
- Yamada K, Lim J, Dale JM, Chen H, Shinn P, Palm CJ, Southwick AM, Wu HC, Kim C, Nguyen M, et al (2003) Empirical analysis of transcriptional activity in the Arabidopsis genome. Science 302: 842–846 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nishimura M, Hara-Nishimura I, Noguchi T (2003) Behavior of vacuoles during microspore and pollen development in Arabidopsis thaliana. Plant Cell Physiol 44: 1192–1201 [DOI] [PubMed] [Google Scholar]
- Yang Z (2002) Small GTPases: versatile signaling switches in plants. Plant Cell 14: S375–S388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ (2004) Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J Cell Sci 117: 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.