Abstract
The changes in root system architecture (RSA) triggered by phosphate (P) deprivation were studied in Arabidopsis (Arabidopsis thaliana) plants grown for 14 d on 1 mm or 3 μm P. Two different temporal phases were observed in the response of RSA to low P. First, lateral root (LR) development was promoted between days 7 and 11 after germination, but, after day 11, all root growth parameters were negatively affected, leading to a general reduction of primary root (PR) and LR lengths and of LR density. Low P availability had contrasting effects on various stages of LR development, with a marked inhibition of primordia initiation but a strong stimulation of activation of the initiated primordia. The involvement of auxin signaling in these morphological changes was investigated in wild-type plants treated with indole-3-acetic acid or 2,3,5-triiodobenzoic acid and in axr4-1, aux1-7, and eir1-1 mutants. Most effects of low P on RSA were dramatically modified in the mutants or hormone-treated wild-type plants. This shows that auxin plays a major role in the P starvation-induced changes of root development. From these data, we hypothesize that several aspects of the RSA response to low P are triggered by local modifications of auxin concentration. A model is proposed that postulates that P starvation results in (1) an overaccumulation of auxin in the apex of the PR and in young LRs, (2) an overaccumulation of auxin or a change in sensitivity to auxin in the lateral primordia, and (3) a decrease in auxin concentration in the lateral primordia initiation zone of the PR and in old laterals. Measurements of local changes in auxin concentrations induced by low P, either by direct quantification or by biosensor expression pattern (DR5::β-glucuronidase reporter gene), are in line with these hypotheses. Furthermore, the observation that low P availability mimicked the action of auxin in promoting LR development in the alf3 mutant confirmed that P starvation stimulates primordia emergence through increased accumulation of auxin or change in sensitivity to auxin in the primordia. Both the strong effect of 2,3,5-triiodobenzoic acid and the phenotype of the auxin-transport mutants (aux1, eir1) suggest that low P availability modifies local auxin concentrations within the root system through changes in auxin transport rather than auxin synthesis.
Changes in mineral nutrient availability and heterogeneous distribution in the soil induce in plants various adaptive mechanisms, among which the plasticity of root development is of crucial importance (Drew, 1975; Lynch, 1995; Thaler and Pages, 1998; Farley and Fitter, 1999; Hell and Hillebrand, 2001). In several species, root proliferation in nutrient-rich regions leads to an increased ratio of root surface to explored soil volume, which facilitates the uptake of nutrients (Drew, 1975; Robinson, 1994). However, the root system architecture (RSA) does not respond the same way to all nutrients (Drew, 1975; Farley and Fitter, 1999). For instance, nitrate availability does not affect the elongation of the primary root (PR) of Arabidopsis (Arabidopsis thaliana), but determines both emergence and elongation rate of lateral roots (LRs; Zhang and Forde, 1998; Zhang et al., 1999). Iron limitation decreases the growth of LRs but increases their density (Moog et al., 1995), and induces the formation of supernumerary root hairs (Schmidt and Schikora, 2001). Phosphate (P) availability also has a strong effect on RSA, which may significantly differ from that of other ions. In Arabidopsis, P starvation has been shown to affect growth of the PR and the initiation and elongation of LRs (Williamson et al., 2001; Linkohr et al., 2002; López-Bucio et al., 2002, 2005; Al Ghazi et al., 2003), and to stimulate formation of root hairs (Bates and Lynch, 1996). In Phaseolus vulgaris, P deficiency affects the growth angle of basal roots (Bonser et al., 1996), whereas it promotes the formation of proteoid roots in Lupinus albus (Johnson et al., 1994, 1996).
In addition to the apparent complexity of the changes in root development induced by P starvation, the signaling pathways triggering these modifications remain mostly obscure. Although specific regulatory mechanisms related to the sensing of the external P availability or internal P status of the plant have to be envisaged, increasing evidence also suggests that hormones play a key role in mediating the P starvation effects on RSA. Because auxin is strongly involved in root development, a possible role of this hormone in the response of root development to P limitation has been proposed (López-Bucio et al., 2002; Al Ghazi et al., 2003). Depending on the concentration, PR elongation and LR initiation and elongation can either be stimulated or inhibited by exogenous auxin application (Evans et al., 1994; Himanen et al., 2002), whereas auxin-transport inhibitors such as 2,3,5-triiodobenzoic acid (TIBA) or napthylphthalamic acid can drastically reduce LR numbers (Blakely et al., 1982; Muday and Haworth, 1994; Fujita and Syono, 1996; Casimiro et al., 2001). Accordingly, mutants with elevated endogenous auxin concentrations, such as sur1/alf1, present an increased number of laterals (Boerjan et al., 1995; Celenza et al., 1995), while auxin-resistant (axr1, axr4) or auxin-transport mutants such as aux1 display a reverse phenotype, having fewer LRs (Pickett et al., 1990; Hobbie and Estelle, 1995; Timpte et al., 1995). The involvement of auxin signaling in the root morphological changes induced by nutrient availability has already been highlighted in the case of nitrate. Zhang et al. (1999) tested the response of several auxin-resistant mutants to a localized increase in nitrate supply and found that axr4 mutants lack the nitrate-induced stimulation of LR elongation. This was interpreted as evidence for the overlap between the nitrate and auxin response pathways. The hypothesis that auxin may be involved in the root response to P starvation recently has been proposed because auxin or auxin antagonist application as well as auxin response mutants can amplify or, on the contrary, prevent the changes in LR growth induced by P starvation (López-Bucio et al., 2002; Al Ghazi et al., 2003). However, auxin involvement in P starvation response has been challenged by Williamson et al. (2001) and Linkohr et al. (2002), who reported that root developmental responses are auxin independent. Very recently, López-Bucio et al. (2005) proposed that only some aspects of the RSA response to P availability (such as enhanced root branching) are under auxin control, while others (such as inhibition of PR growth) are independent of auxin.
To further address this question, we carried out an in-depth analysis of the possible roles of auxin in mediating the RSA response to low P. This included daily analysis of several RSA parameters from day (d) 7 to d 14 after sowing, on both wild-type plants treated or not with auxin or auxin-transport inhibitor, and three different mutants altered in auxin response pathways. This provided a large set of data that allowed us to unravel complex interactions between root architecture parameters, auxin, and P starvation response. We show here the main outcomes of this work, i.e. that many effects of P starvation on RSA (PR shortening, inhibition of LR primordia initiation, stimulation of LR primordia activation, young LR elongation) could be explained by auxin redistribution within the root system (increased accumulation in the PR apex, the initiated lateral primordia, and in young LRs, and decrease in auxin concentration in the lateral primordia initiation zone of the PR and in old laterals). A model summarizing these hypotheses of a significant redistribution of auxin in the RSA response to P limitation is presented.
RESULTS
P Starvation Only Transiently Promotes Arabidopsis LR Growth
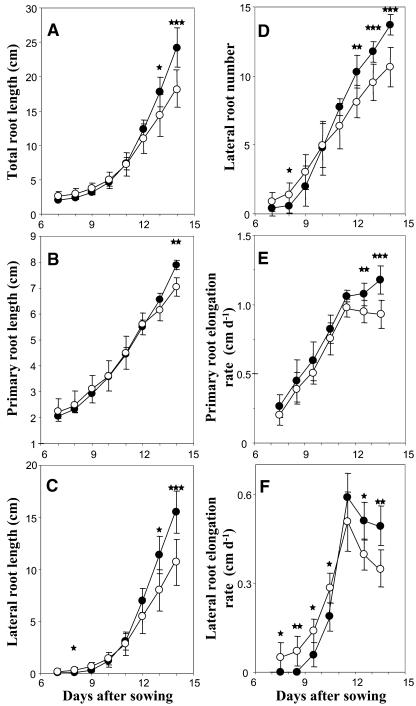
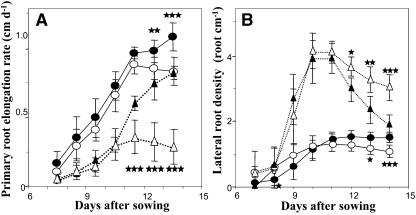
A temporal analysis of the RSA of wild-type Arabidopsis seedlings grown either on high (1 mm) or low (3 μm) P medium was carried out from d 7 to d 14 after germination. Two different phases were observed (Fig. 1). First, from d 7 to d 10, low P availability did not modify PR growth (Fig. 1, B and E) but clearly promoted LR elongation. P-starved plants produced more LR than control plants (Fig. 1D), and these roots grew faster (Fig. 1F). Thereafter, from d 11 onward, all measured RSA parameters were negatively affected by P starvation, including a fast and significant slowing down of LR appearance and elongation (Fig. 1, D and F), and a more delayed decrease in PR elongation (Fig. 1E). These responses finally led to a significant decrease in total and LR lengths (Fig. 1, A and C). In addition, when measured at d 14, P starvation also induced a significant decrease in diameter of PR and in total leaf area (data not shown).
Figure 1.
Effect of P availability on root architecture parameters. Wild-type Columbia seedlings were grown for 14 d in the presence of low (3 μm; ○) or high (1 mm; •) P concentration on vertically oriented agar plates. Average values (±sd) of eight seedlings, calculated daily from d 7 to d 14 after sowing, are given for the length of the entire root system (A), the PR (B), the cumulated length of the LRs (C), the LR number (D), the PR elongation rate (E), and the LR elongation rate (F). Significant differences (t test) are indicated by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
P Starvation Inhibits the Initiation of Lateral Primordia on the PR But Markedly Stimulates Their Activation
The contrasting effect of P starvation on the total number of visible laterals (increase before d 9 and decrease after d 11 relative to +P) was further investigated using transgenic plants expressing the β-glucuronidase (GUS) reporter gene under control of a cyclin B1-type (CycB1) promoter (Ferreira et al., 1994). As shown in Figure 2A, this allowed us to determine the total number of initiated primordia (number of primordia from the first divisions of pericycle cells to emergence, plus number of emerged laterals), and to distinguish between primordia that had aborted or had not yet been activated (no or weak staining) and those that were meristematically active (strong staining). At d 9 after germination (Fig. 2B), the total number of initiated primordia scored on the PR was markedly lower for P-starved plants than for controls, indicating a strong inhibition of initiation of LR primordia by low P availability. However, when considering the branched zone of the PR (i.e. the portion of the PR between the base and the youngest emerged lateral), it clearly appeared that the proportion of primordia that had developed into an emerged lateral was considerably higher in P-starved plants (>90%) than in controls (approximately 50%; Fig. 2B). Furthermore, most of the primordia found in the branched zone of the PR of control plants were only weakly stained (Fig. 2B), suggesting that they had aborted. Very similar results were obtained in the apical part of the PR of 14-d-old plants, which had developed after d 9 (Fig. 2C). Both results show that under P starvation, fewer primordia were initiated but a much higher proportion of these primordia actually generated LR. A modified balance between these two opposite actions of low P availability (inhibition of primordia initiation and stimulation of primordia activation) may explain why contrasting effects of P starvation were found on LR number at d 9 and d 14 (Fig. 1D).
Figure 2.
Effect of P availability on both primordia number and meristematic activity. A, Histochemical GUS staining of transgenic (CycB1::GUS) Arabidopsis plants that were cultivated on high (1 mm) or low (3 μm) P medium on vertically oriented agar plates. Meristematic activity was estimated according to staining intensity; plain boxes correspond to emerged LRs, black dots represent intensely stained primordia, and white dots correspond to weakly stained primordia. Bar = 1 cm. B and C, Schematic representation of individual entire PRs (B) of plants grown on high or low P medium for 9 d or apical part of the PR (C) of a 14-d-old plant that had developed after d 9. Bars = 1 cm.
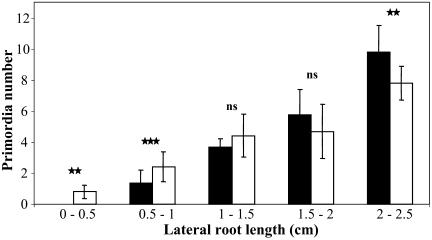
Significant changes between P-starved and control plants were also observed concerning second-order lateral primordia, located on primary laterals (Fig. 3). As on the PR, low P availability led at d 14 to a decrease in the total number of initiated primordia in old laterals (i.e. >1.5 cm). However, the opposite was found in younger laterals (i.e. <1 cm). This shows that the consequences of P starvation on lateral primordia initiation are not always associated with an inhibitory effect but also somewhat depend on the status of the root itself.
Figure 3.
Effect of P availability on the number of second-order primordia initiated on LRs. Transgenic (CycB1::GUS) Arabidopsis plants were cultured for 14 d on vertically oriented agar plates containing high (1 mm) or low (3 μm) P medium and histochemically GUS stained to easily and unambiguously score all initiated primordia. Data correspond to the average number of primordia (±sd) scored on LRs of different length of eight plants. Seedlings were cultured on low (white bars) or high (black bars) P medium. Probabilities are indicated by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, Not significant.
Auxin Alters the RSA Response to P Starvation, Even at Later Stages When the Overall Plant Growth Is Severely Affected
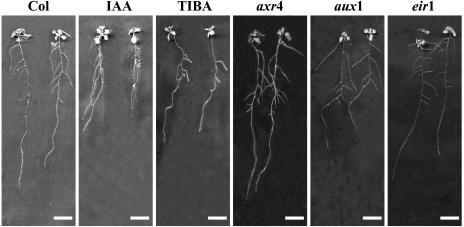
Results reported above and published data suggest that auxin may be involved in some aspects of root adaptive response to P starvation. To unravel its role, we performed an additional series of experiments, similar to that described in Figure 1, but including various auxin response mutants (axr4, aux1, and eir1) and exogenous indole-3-acetic acid (IAA) or auxin-transport inhibitor (TIBA) applications. The IAA and TIBA concentrations (0.1 μm for both) were selected on the basis of preliminary work performed on plants cultivated on high P medium and treated with a wide range of IAA levels (data not shown). Both hormone-treated wild-type and mutant plants displayed the expected changes in RSA already characterized in previous reports (Fig. 4). For instance, the IAA-treated plants presented a reduced primary root length (Evans et al., 1994) and increased LR length and density (Casimiro et al., 2001; Himanen et al., 2002), whereas the TIBA-treated plants or the axr4 mutant harbored an opposite LR phenotype (Hobbie and Estelle, 1995). The aux1 and eir1 mutants showed the well-known root agravitropic phenotype and the typical modification of LR insertion angles (Pickett et al., 1990; Roman et al., 1995).
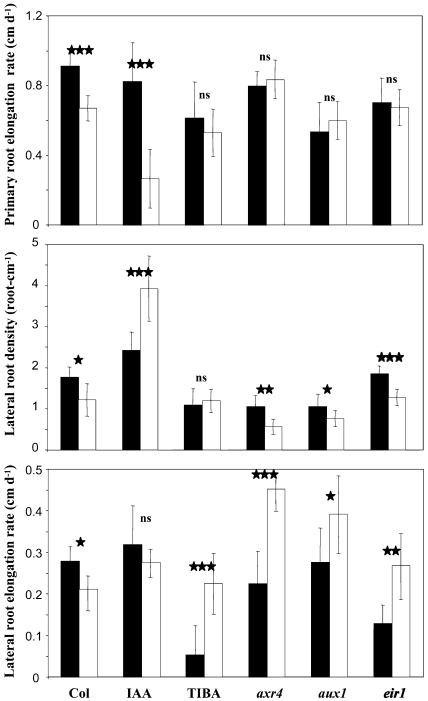
Figure 4.
Morphology of hormone-treated wild-type seedlings and auxin mutants. Wild-type Columbia or mutant seedlings were grown for 14 d on high (1 mm) P medium on vertically oriented agar plates. IAA and TIBA correspond to wild-type seedlings, Col-0 ecotype, grown on 0.1 μm IAA or 0.1 μm TIBA, respectively. axr4, aux1, and eir1 indicate axr4-1, aux1-7, and eir1-1 mutant seedlings. Bars = 1 cm.
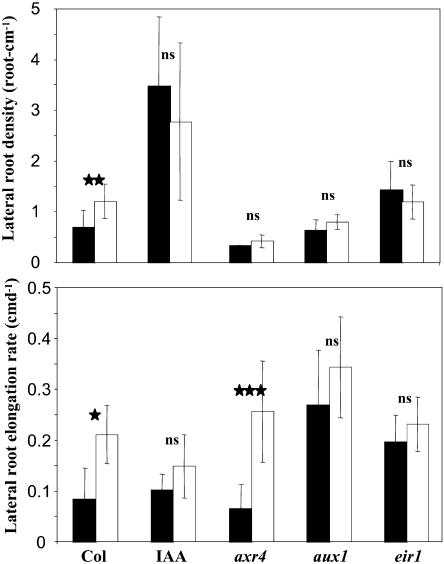
At different time points during the P starvation treatments, several RSA parameters appeared to be strongly affected by the IAA or TIBA treatments as well as by a mutant background. We focused on the RSA data obtained at d 9 and d 14 (Figs. 5 and 6, respectively) because these two time points are illustrative of the two phases of the root response to P starvation identified from the experiment shown in Figure 1. At both time points, d 9 (data not shown) and d 14 (Table I) statistical analyses were conducted. ANOVA indicated that all 17 RSA parameters were highly significantly affected by the hormonal treatments or in the mutants as compared to the wild type at d 14, whereas only 10 of them responded to P starvation (Table I). Interestingly, 12 RSA parameters were strongly affected by the hormonal treatment×P starvation interaction, showing that auxin and P availability did not act independently.
Figure 5.
Effect of P availability and hormone treatments on LR density and LR elongation rate of Arabidopsis. Seedlings were grown for 9 d on low (white bars) or high (black bars) P medium on vertically oriented agar plates. IAA corresponds to wild-type seedlings, Col-0 ecotype, grown on 0.1 μm IAA. axr4, aux1, and eir1 indicate axr4-1, aux1-7, and eir1-1 mutant seedlings. Data correspond to the average value (±sd) of eight seedlings. Significant differences (t test) are indicated by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, Not significant.
Figure 6.
Effect of P availability and hormone treatments on PR elongation rate, LR density, and LR elongation rate of Arabidopsis. Seedlings were grown for 14 d on low (white bars) or high (black bars) P medium on vertically oriented agar plates. IAA and TIBA correspond to wild-type seedlings, Col-0 ecotype, grown in presence of 0.1 μm IAA or 0.1 μm TIBA, respectively. axr4, aux1, and eir1 correspond to the axr4-1, aux1-7, and eir1-1 mutant seedlings. Data correspond to the average value (±sd) of eight seedlings. Significant differences (t test) are indicated by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, Not significant.
Table I.
Summary of the analysis of variance for 17 root architecture traits measured 14 d after germination on mutants or hormone-treated wild-type seedlings grown on high (+P) or low (−P) P medium
Treat. and P Starv. columns represent the effects of hormonal treatments (or mutants) or P starvation effect, respectively. The Treat. × P Starv. column represents the first-order interaction (hormonal treatment [or mutants] × P starvation). Probabilities are indicated by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, Not significant.
| Source of Variation at Day 14
|
|||
|---|---|---|---|
| Treat. | P Starv. | Treat. × P Starv. | |
| Primary root length (cm) | *** | *** | *** |
| Primary root elongation rate (cm d−1) | *** | ** | *** |
| Total LR length (cm) | *** | ns | ns |
| LR number | *** | ** | ** |
| LR growth arrest | *** | ns | * |
| Length of branched zone (cm) | *** | ns | ns |
| Length of unbranched zone (cm) | *** | ns | *** |
| LR elongation rate (roots longer than 0.3 cm; cm d−1) | *** | ns | *** |
| LR density (PR length) | *** | ** | * |
| LR density (length of branched zone) | *** | ** | * |
| Elongation rate of the LRs 1, 2, and 3 (cm d−1) | *** | * | *** |
| Elongation rate of the LRs 4, 5, and 6 (cm d−1) | *** | ns | ns |
| Elongation rate of the LRs 7, 8, and 9 (cm d−1) | *** | * | ns |
| Primary root diameter (mm) | *** | *** | ns |
| Primordia density | *** | *** | * |
| Primordia number | *** | *** | * |
| Length of primary root with primordia (cm) | *** | ns | *** |
At d 9, LR growth was stimulated by low P availability, and both LR density and elongation rate were higher in starved plants than in controls (Fig. 5), as observed previously in the independent experiment of Figure 1. However, IAA treatment and all investigated mutations suppressed or moderated the positive effect of P starvation on both parameters of LR growth, at the exception of the axr4 mutation. In this mutant, the increase in LR density by low P was prevented, but not the stimulation of LR elongation rate (Fig. 5). At d 14, the negative effects of P starvation on growth of primary and LR noticed in the experiment of Figure 1 were confirmed (Fig. 6). At the same time, IAA or TIBA treatments and the various mutations strongly modified most of these effects. Exogenous IAA supply amplified the decrease in PR elongation rate induced by low P, while TIBA treatment or all three mutations prevented this decrease (Fig. 6). Concerning LR density, the detrimental effect of P starvation was reverted by IAA or suppressed by TIBA treatments (Fig. 6). The increased LR density in IAA-treated plants was mainly due to a higher number of LR and not to a strongly reduced PR length (although the elongation rate of the PR begins to be drastically reduced at d 14; Fig. 6). Furthermore, TIBA or the axr4, aux1, and eir1 mutations profoundly altered the action of P starvation on the LR elongation rate since they all result in a marked increase of this parameter in P-starved plants compared with unstarved plants (Fig. 6). Some of these observations made at d 14 are particularly striking because they indicate that, depending on the auxin status of the plant, LR growth can still be either unaffected or even stimulated by P starvation. As an example, P starvation strongly stimulated LR elongation rate in the presence of TIBA, with no effect on either LR density or PR elongation rate (Fig. 6). This suggests that the slowing down of root growth after 11 d of culture on low P medium (Fig. 1) was not simply caused by nutrient shortage but represented a true adaptive response in which auxin might play a crucial role. The situation appeared to be different in the shoot, where the decrease in total leaf area resulting from growth on low P medium was unaffected by all treatments or mutations (Fig. 7), suggesting that the effect of P starvation on shoot growth might be independent of auxin signaling.
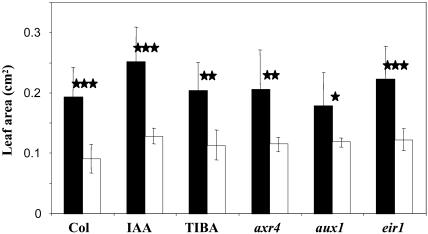
Figure 7.
Effect of P availability and hormone treatments on Arabidopsis leaf area. Wild-type Columbia or mutant seedlings were grown for 14 d on low (white bars) or high (black bars) P medium on vertically oriented agar plates. Aerial parts were excised and scanned, and projected leaf area was quantified. IAA and TIBA correspond to wild-type seedlings, Col-0 ecotype, grown in presence of 0.1 μm IAA or 0.1 μm TIBA, respectively. axr4, aux1, and eir1 correspond to the axr4-1, aux1-7, and eir1-1 mutant seedlings. Data correspond to the average value (±sd) of eight seedlings. Significant differences (t test) are indicated by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, Not significant.
A more detailed temporal study of the effect of auxin on both responses of PR elongation rate and LR density to low P showed that this hormone did not act in modifying the timing of the phase shifts induced by P limitation but rather in modulating the magnitude of the response to low P (Fig. 8). Indeed, the PR elongation rate in plants grown on low P started to decrease at roughly the same time point regardless of the presence or the absence of IAA in the medium, but the amplitude of this effect was markedly higher in IAA-treated plants (Fig. 8A). Similarly, the fact that IAA reverted the negative effect of low P on LR density at d 14 cannot be explained by a delayed appearance of LR in IAA-treated plants grown on high P (Fig. 8B).
Figure 8.
Effect of P availability and IAA treatment on PR elongation rate and LR density of Arabidopsis. Auxin-untreated (circles) and IAA-treated (triangles) Col-0 seedlings were grown for 14 d on low (white marks) or high (black marks) P medium on vertically oriented agar plates. Average values (±sd) of eight seedlings, calculated daily from d 7 to d 14 after sowing, are given for the PR elongation rate (A) and the LR density (B). Significant differences (t test) are indicated by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The Free IAA Levels in Different Parts of the Root Are Significantly Modified by P Starvation
The results detailed above suggest that auxin plays a central role in the response of RSA to P starvation. At least two hypotheses could be generated from the data obtained at d 14. First, low P availability induces increased transport and accumulation of auxin into the PR apex, which triggers the slowing down of PR growth in P-starved plants. This is supported by the fact that IAA supply amplified the negative effect of P starvation on PR elongation rate, while inhibition of IAA transport (TIBA, aux1, eir1) or reduced sensitivity to auxin (axr4) suppressed it (Fig. 6). Second, P starvation results in a decreased auxin accumulation in the primordia initiation zone of the PR. This would explain why low P availability reduces primordia initiation (Fig. 2, A and B) and LR density at d 14, which is reverted by IAA supply (Fig. 6).
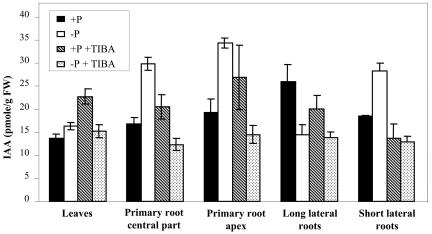
To test the hypothesis that P starvation alters RSA through local variations of auxin concentration in various portions of the root system, free IAA was quantified in different parts of seedlings grown for 14 d on high or low P medium (Fig. 9). Specific analysis of the primordia initiation zone could not be performed due to the difficulty to determine its precise location from macroscopic observations. The data obtained confirmed that, in P-starved plants compared to controls, auxin concentration was significantly increased (by 45%) in the PR apex. Increases in free IAA concentration in response to low P availability were also observed in the whole PR and in young LRs, whereas auxin concentration was decreased in old ones (Fig. 9). IAA concentration in the aerial part was not affected. All these changes were partially or totally prevented by TIBA treatment (Fig. 9), clearly demonstrating that variations in the auxin concentration in response to low P are probably due to changes in transport rather than in de novo synthesis.
Figure 9.
Free IAA levels measured in seedlings grown for 14 d on high or low P medium. Free IAA levels were measured from leaves, PR central part (from the last basal emerged LR downward but excluding the last centimeter), PR apex (1 cm), long LRs (>1.5 cm), and short LRs (<1.5 cm) of seedlings grown for 14 d. Data correspond to the average value (±se) of three independent cultures of Columbia seedlings cultured on high (black bars) or low (white bars) P medium or in the presence of 0.1 μm TIBA on high (hatched bars) or low (dotted bars) P medium.
To visualize auxin distribution and local accumulation at the microscopic level, we used the auxin-responsive reporter DR5::GUS (Ulmasov et al., 1997). A strong GUS activity was detected in the PR apex in both starved and control plants (Fig. 10, A–D). Phosphate depletion induced an increase as well as an expansion of GUS expression, leading to strong staining of the columella and the vascular tissue (Fig. 10, B and D). The LR development was followed from primordia initiation to postemergence in plants cultivated on low or high P medium (Fig. 10, E–P). For plants cultivated on high P medium, a weak GUS staining was systematically detected in stage I and II primordia (Fig. 10, E and G), whereas it was only occasionally observed at stages V and VI (Fig. 10, I and K). Later on, a strong GUS expression was observed at the tips of emerged primordia (Fig. 10M) and in young LR (Fig. 10O). In P-starved plants, a much stronger GUS activity was always observed at the different development stages (Fig. 10, F, H, J, and L), especially prior to emergence (Fig. 10L). Similarly, a stronger and wider expression was observed at the tips of emerged primordia (Fig. 10N) and in young laterals, where GUS activity was also located in the vascular tissue (Fig. 10P). These expression patterns of the DR5::GUS reporter gene thus suggest that P starvation results in an auxin overaccumulation or drastically modifies auxin sensitivity in the apex of the PR, in initiated primordia, and in young LR.
Figure 10.
Effect of P availability on auxin-responsive reporter DR5::GUS expression in PR apex and LRs. Transgenic (DR5::GUS) Arabidopsis plants were cultured for 14 d on high (A, C, E, G, I, K, M, and O) or low (B, D, F, H, J, L, N, and P) P medium followed by histochemical GUS staining. The expression pattern of the DR5::GUS was observed in the PR apical meristem (A–D) and from primordia initiation to postemergence of LRs (E–P). Primordia stages were named according to Malamy and Benfey (1997): stage I (E and F), stage II (G and H), stage V (I and J), stage VI (K and L), emergence (M and N), and LR elongation (O and P). Bars = 50 μm.
P Starvation Mimics the Effect of Auxin on the Root Phenotype of the alf3 Mutant
The hypothesis of an overaccumulation of auxin in initiated primordia in response to P starvation was further investigated using the alf3 mutant. This mutant is strongly altered in LR development, with a root system consisting of a long PR covered with many arrested LR primordia (Celenza et al., 1995). In this mutant, maturation of LR can only be rescued by addition of IAA (Celenza et al., 1995). In our culture conditions, alf3 mutant plants on high P medium presented the characteristic phenotype in the absence of IAA, i.e. very few elongating LR and many arrested primordia (Fig. 11). When grown on low P medium, however, the alf3 mutant developed many elongating LR, thus mimicking the phenotype observed in the presence of IAA. This strongly suggests that P starvation indeed induced an increase in auxin accumulation or auxin sensitivity in initiated primordia and emerging LR, which was responsible for the elongation of these roots in the alf3 mutant.
Figure 11.
Effect of P availability on root architecture of alf3 mutant. The alf3 mutant seedlings were grown for 14 d on high (+P) or low (−P) P medium on vertically oriented petri dishes. Bar = 1 cm.
DISCUSSION
The RSA Response to Low P Involves Both Stimulatory and Inhibitory Effects on Specific Root Growth Parameters and Is Dependent on Auxin Signaling
In many plant species, it has been shown that low P availability in the external medium strongly alters RSA (Drew, 1975; Johnson et al., 1994; Bonser et al., 1996; Carswell et al., 1996; Williamson et al., 2001; Linkohr et al., 2002; López-Bucio et al., 2002; Al Ghazi et al., 2003). This is often considered an adaptive response, leading toward enhancing the P uptake capacity of the plant. Despite the important implications, little is known about the physiological and molecular events responsible for sensing of P limitation, and its effect on the root system development (Ticconi and Abel, 2004). Furthermore, changes in RSA triggered by P limitation are complex, and many studies led to contrasting and sometimes conflicting conclusions. For example, Williamson et al. (2001) and Al Ghazi et al. (2003) showed that the total root length was unaffected by P starvation, whereas it was significantly reduced in the experiments reported by López-Bucio et al. (2002, 2005). Similarly, LR density appeared to be either reduced (Al Ghazi et al., 2003), unaffected (Linkohr et al., 2002), or increased (López-Bucio et al., 2002, 2005) in response to P starvation. Moreover, marked discrepancies are also obvious between the conclusions of various experiments aimed at determining the role of hormone signaling in the adaptive response of RSA to P starvation (Forde and Lorenzo, 2001). On the one hand, it has been suggested that neither auxin nor abscisic acid plays a crucial role in the modifications of total or LR length in P-starved plants (Trull et al., 1997; Williamson et al., 2001). On the other hand, a strong involvement of auxin signaling was proposed from studies indicating that P effect on PR length and LR density and elongation rate is significantly altered in both auxin mutants and auxin-treated wild-type plants (López-Bucio et al., 2002; Al-Ghazi et al., 2003). In agreement with this apparent complexity of the action of P on RSA, our results indicate that low P induced a biphasic response with, first, an increase in LR growth with no effect on PR elongation, and, later on, a general negative effect on growth of all roots. Such temporal variations might have been missed in many studies performed to date, which generally lacked time-course measurements. In previous experiments performed in very similar conditions except for lower light intensity and different wavelengths (Al Ghazi et al., 2003), most of the effects of P starvation reported above, i.e. the decrease in PR growth (Fig. 1E), the late decrease in LR number after d 10 (Fig. 1D), and the transient increase in LR elongation rate (Fig. 1F), were already observed. However, LR number was not initially stimulated in response to low P in the experiments of Al Ghazi et al. (2003). This may indicate that external conditions such as light affect the way of RSA reacts to P starvation, possibly through changes in the carbon status of the root, which has been found crucial for LR growth (Freixes et al., 2002).
Our results also delineate contrasting effects of P starvation on LR development with a strong inhibition of primordia initiation combined with a marked stimulation of their activation (Fig. 2, B and C). Such dual effects also occurred on old LR (i.e.>1.5 cm), where appearance of second-order LRs was observed after 14 d of culture on low P medium despite reduced primordia density (Fig. 3). Second-order laterals were never observed for plants grown on high P medium (data not shown). If nutritional regulation of LR elongation has been documented (see above), very little is known concerning the effect of nutrients on the primordia initiation. To date, only high carbon-to-nitrogen ratio has been reported to dramatically repress primordia initiation with little or no effect on the PR (Malamy and Ryan, 2001). On the contrary, P starvation has been reported to induce primordia initiation either in wild-type plants (López-Bucio et al., 2002) or in pdr2 mutant (Ticconi et al., 2004), but, in both cases, this phenotype was mainly the consequence of an early abortion of the root apical meristem (López-Bucio et al., 2002; Ticconi et al., 2004).
Taken together, these root adaptive responses suggest that hormones and, more likely, auxin might be involved. To study its action, we used concentrations of IAA and TIBA that were 1 to 3 orders of magnitude lower than those applied in previous studies (López-Bucio et al., 2002; Karthikeyan et al., 2002). These concentrations were sufficient to induce the expected changes in RSA (Fig. 4) without completely disturbing growth of the plant. This analysis was completed by the investigation of auxin mutants to limit potential hormonal uptake biases due to P treatment and simultaneous investigation of several levels in the response pathways. Most of the RSA responses to P starvation observed in wild-type controls were profoundly perturbed in auxin mutants or in IAA- or TIBA-treated wild-type plants (Figs. 5 and 6). Furthermore, one main outcome of our work is the conclusion that auxin signaling is involved in both the stimulatory and inhibitory effects of low P availability on RSA (Figs. 5 and 6). Data obtained at d 14 were particularly striking because they show that the general slowing down of root growth observed at this latter stage of P starvation can be to some extent prevented in mutants or treated wild-type plants. Indeed, several key parameters of RSA were actually increased rather than decreased by the 14-d P starvation period in some of these plants (Fig. 6). This suggests that the adverse effect of long P starvation on root growth cannot be solely explained by a general nutrient shortage (either P or carbon availability due to the 50% leaf area reduction). It also clearly results from specific hormone signaling and thus should be considered as part of an adaptive response. Because of its depressive action on root development, this late adaptive response is difficult to interpret as a reaction of the plant aimed at improving soil exploration and P acquisition. However, it may be part of a general hormone-dependent survival strategy, allowing slower but longer growth of the plant in adverse environment. This bears strong similarity with the hormone-mediated restriction of shoot growth in response to nitrogen starvation previously proposed by Chapin (1991).
Not all responses to P starvation seem to result from changes in auxin action. For instance, all mutants and hormone-treated wild-type plants showed a similar decrease in leaf area in response to P starvation as untreated wild-type plants (i.e. around 50% reduction at the end of the experiment; Fig. 7). Another well-documented response of the shoot to P starvation is anthocyanin accumulation (Raghothama, 1999; Martin et al., 2000; Franco-Zorrilla et al., 2002; Poirier and Bucher, 2002). In our experiments, the increase of leaf anthocyanin content in P-starved plants seemed also not affected by the various hormone treatments and mutations investigated (data not shown). Although limited to end-point measurements, these data suggest that the signals responsible for both P starvation-induced reduction of leaf expansion and leaf anthocyanin accumulation do not appear to be related to auxin signaling and remain unknown. However, these shoot responses are commonly observed after cytokinin treatments (Deikman and Hammer, 1995). This may indicate that P starvation affects leaf cytokinin homeostasis, a hypothesis in line with the known involvement of these hormones in several molecular responses to P starvation (Martin et al., 2000; Franco-Zorrilla et al., 2002; Karthikeyan et al., 2002). In addition, a role of cytokinins in the root-to-shoot signaling of nutrient sensing by the roots has already been documented in the case of nitrogen (Sakakibara, 2003).
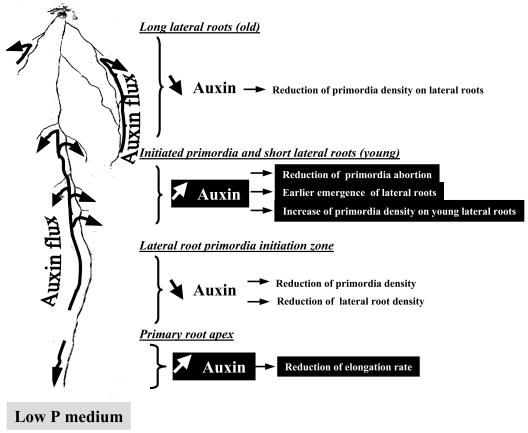
A Model Associating the RSA Response to P Starvation with a Global Redistribution of Auxin within the Root System
Our data support the idea that many effects of P starvation on RSA (Figs. 1–3) are strongly dependent on auxin signaling (Table I; Figs. 5, 6, and 8) and are associated with localized changes in auxin concentration in various parts of the root system (Figs. 9 and 10). The interpretation of these data led us to propose a model postulating that most effects of P starvation on RSA are triggered by local changes in auxin concentration (Fig. 12). According to this model, P starvation results in (1) an overaccumulation of auxin in the root apical meristem, limiting elongation of the PR (Figs. 1A and 6); (2) a lower accumulation of auxin in the LR primordia initiation zone of the PR, inducing a significant decrease in primordia initiation (Figs. 2 and 3) and, subsequently, in LR density at d 14 (Figs. 1 and 6); (3) an overaccumulation of auxin in initiated primordia and young LR, which, on the one hand, stimulates primordia activation (Fig. 2, B and C), leading to an earlier emergence of young LR (Figs. 1 and 5), and, on the other hand, increases initiation of second-order primordia in these roots (Fig. 3); and (4) a reduction of auxin concentration in old LR, where this inhibits initiation of second-order primordia (Fig. 3).
Figure 12.
Schematic model of auxin redistribution within the root system in response to P starvation.
The action of auxin in triggering the inhibition by low P of the elongation of the PR (point 1 of the model) is suggested first by the observation that exogenous auxin supply amplified this effect (Fig. 6) and, second, by the fact that the axr4 mutant with reduced sensitivity to auxin had similar PR elongation rate at both levels of P supply (Figs. 6 and 8A). Our interpretation is that P starvation stimulates auxin accumulation in the PR apex, which resulted in a reduced elongation rate of the root. This is in agreement with the well-known negative action of auxin on PR elongation (Evans et al., 1994; Hobbie and Estelle, 1995; Timpte et al., 1995), and is further supported by our data showing that low P indeed resulted in a higher auxin concentration and an increased activity of the auxin-inducible DR5 promoter in the PR apex (Figs. 9 and 10).
The postulated decrease by P starvation of the auxin concentration in the LR initiation zone of the PR (point 2 of the model) could not be confirmed by direct auxin measurements or visualization of DR5 activity, mostly because of the difficulty in precisely locating this zone. However, low P availability strongly decreased the total number of primordia initiated along the primary axis (Fig. 2, B and C), an effect that certainly explains the lower LR number and density found in P-starved plants at d 14 (Figs. 1 and 6). Because cell cycle reactivation in the xylem pericycle, which leads to the initiation of primordia, is a step of LR development critically dependent on auxin (Casimiro et al., 2001, 2003; Himanen et al., 2002), this suggests that initiation of primordia in the PR of P-starved plants is limited by a local decrease in auxin concentration. Accordingly, the reduction of LR density at d 14 by low P supply was prevented by auxin supply (Figs. 6 and 8B). Furthermore, direct evidence that P starvation alters primordia initiation through local variations of auxin concentration is provided by the analysis of second-order primordia located on first-order laterals (Fig. 3). Clearly, the changes induced by low P of the density of these primordia correlated with those of local auxin concentrations (Figs. 3 and 9). Both primordia density and auxin concentration increased in young LRs (point 3 of the model) but decreased in older ones (point 4 of the model).
The hypothesis that P starvation promotes LR development through increased auxin accumulation in initiated primordia or newly emerged young LRs (point 3 of the model) is strongly supported by several lines of evidence. First, low P dramatically stimulated activation of initiated primordia (Fig. 2, B and C), a process relying on the auxin-mediated establishment and activity of a new meristem (Celenza et al., 1995; Malamy and Benfey, 1997; Himanen et al., 2002). Second, the increase in auxin concentration in both primordia and young LRs of P-starved plants was clearly confirmed by both auxin assays and localization of DR5 activity (Figs. 9 and 10). Third, P starvation was able to mimic the effect of auxin supply in overcoming the inhibition of LR emergence and growth in the alf3 mutant (Fig. 11). Fourth, as stated above, low P stimulates initiation of second-order LR primordia in young LRs (Fig. 3).
Although our data are consistent with local changes in auxin concentration in response to low P, we cannot exclude that in the portions of the root system that were only investigated using DR5::GUS expression (primordia initiation zone of the PR and initiated primordia), the effect of low P could be associated with a modified sensitivity to auxin and not to external changes in auxin concentration.
In a recent study, López-Bucio et al. (2005) also investigated the involvement of auxin in the response of various aspects of root development to P starvation. In agreement with our model, their data show that low P stimulates activation of LR primordia since a much higher proportion of initiated primordia evolved from stage A (up to three cell layers) to stage D (LR longer than 0.5 mm) in P-starved plants as compared to controls. As we do, they attribute this effect to increased concentration of (or responsiveness to) auxin in LR primordia. On the other hand, their conclusions concerning the inhibition of PR growth by low P markedly differ from ours. In their experiments, P starvation resulted in an almost arrest of PR growth at a very early stage (i.e. 6 d after planting), which was associated with a decrease in DR5 promoter activity in the PR apex, suggesting lower auxin concentration (i.e. the reverse of what is seen in our plants). The reasons for this discrepancy are unclear, but it should be noted that, in our conditions, the negative effect of low P on PR growth is much more delayed (observed at d 13 versus d 6 after planting), and significantly lower (PR length decreased by 10% versus 80%), than in López-Bucio et al. (2005).
Because TIBA strongly altered the effects of P starvation on both RSA (Figs. 5 and 6) and local auxin concentrations (Fig. 9), our model also hypothesizes that the morphological responses of the root system to low P are due to modifications of auxin transport. The clearest example of this may be the earlier appearance (at d 7–9) of LRs in P-starved plants compared with controls (Fig. 1; Fig. 5, LR density). This is postulated to result from an increase in auxin concentration occurring in the initiated primordia and young LRs (Fig. 12). Accordingly, both exogenous IAA supply and axr4 mutation suppressed the promoting effect of low P on LR density at d 9 (Fig. 5). Interestingly, earlier appearance of LR at low P availability is also abolished at d 9 in the aux1 mutant, in which the basipetal auxin transport (from cotyledons or young leaves to the roots) is impaired due to decreased phloem unloading (Swarup et al., 2001). In such young plants (<10 d), auxin in the roots mostly originates from this AUX1-mediated basipetal transport (Ljung et al., 2001; Bhalerao et al., 2002). This demonstrates that the earlier emergence of LRs in P-starved plants requires downward auxin flux from the shoots and is thus due to altered distribution of the hormone in the plant.
The interpretation of the effects of TIBA or of mutations of auxin transporters on the RSA response to low P in 14-d-old plants might be more challenging because, at this later stage, all parts of the seedlings can potentially synthesize IAA de novo (Ljung et al., 2001). In particular, both Ljung et al. (2001) and Bhalerao et al. (2002) proposed that roots could be the major source of auxin in plants older than 10 d. Such multiplicity in auxin sources suggests complex fluxes within the root system of 14-d-old plants.
Despite this complexity, several aspects of the P starvation-induced RSA response could be explained by changes in auxin transport. For instance, the decrease in auxin concentration in the primordia initiation zone of the PR and its increase in young laterals could result from enhanced auxin flux from the former to the latter. Phosphorus depletion may act in two different ways, either by increasing basipetal and lateral transport to the initiated primordia or more likely by enhancing the auxin sink activity of newly formed primordia according to the “fountain” model proposed by Benkova et al., (2003). This would lead to a local auxin impoverishment around initiated primordia that in turn would limit new primordia initiation in the neighborhood and, ultimately, LR density at d 14 (Fig. 6). In agreement with this, TIBA treatment did revert the detrimental effect of low P on LR density at d 14 (Fig. 6). At the opposite, both aux1 and eir1 mutations did not alter this detrimental effect (Fig. 6). This may be explained by the localization of the AUX1 and EIR1 transporters not related to the LR primordia initiation zone, i.e. protophloem cells and LR cap for AUX1 (Swarup et al., 2001, 2004) and LR cap for EIR1 (Luschnig et al., 1998; Müller et al., 1998; Friml, 2003). With the same rationale, auxin accumulation in PR tip in response to P starvation may result from a slowing down of auxin basipetal transport since TIBA treatment as well as AUX1 and EIR1 mutations suppress the negative effect of P starvation on the PR elongation rate by reducing PR growth in unstarved plants to the level of wild-type P-starved plants. Several reports agree with this interpretation since TIBA treatment and eir1 mutation have been found to induce an overaccumulation of auxin in the root tip (Sabatini et al., 1999; Ottenschlager et al., 2003).
In conclusion, our data support the hypothesis that many processes of the adaptive response of the RSA to P starvation are triggered by auxin redistribution within the root system. Nevertheless, the proposed model does not explain all the root responses to P starvation that were observed in our experiments. For instance, the increase in auxin concentration found in young LR in response to P starvation might account for the higher elongation rate of laterals in 9-d-old plants cultured on low P medium as compared with controls (Fig. 5). However, this hypothesis is challenged by several observations (Figs. 5 and 6). First, exogenous auxin supply did not stimulate LR elongation rate in unstarved plants. Second, LR elongation was markedly increased by low P in the auxin-resistant mutant axr4. Third, LR elongation rate was drastically modified in both auxin-transport mutants. Taken together, these results suggest that auxin signaling is only partially involved in the response of LR elongation to low P. Other signaling pathways, including those involving other phytohormones, have to be considered. Trull et al. (1997) clearly demonstrated that abscisic acid is involved neither in molecular nor in the root architecture P starvation response. Martin et al. (2000), Franco-Zorrilla et al. (2002), and Karthikeyan et al. (2002) reported that cytokinins modulate the molecular response to P starvation, whereas Ma et al. (2003) recently reported that the LR elongation, in response to P starvation, was affected by ethylene. Taken together, these results suggest that the control of LR elongation by P availability relies on a complex cross talk between auxin and other phytohormones that remains to be elucidated.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 (Col-0) and the different mutant strains axr4-1 (CS8018; Hobbie and Estelle, 1995), aux1-7 (CS3074; Pickett et al., 1990), and eir1-1 (CS8058; Roman et al., 1995) were from the Nottingham Arabidopsis Stock Centre (http://nasc.nott.ac.uk/). The alf3 mutant (Celenza et al., 1995), CycBl::GUS transgenic line (Ferreira et al., 1994), and DR5::GUS (Ulmasov et al., 1997) were kindly provided by Drs. Grisafi (Whitehead Institute for Biomedical Research, Cambridge, MA), Beeckman (Vlaams Interuniversitair Instituut voor Biotechnologie, Ghent, Belgium), and Bellini (Institut National de la Recherche Agronomique, Versailles, France), respectively.
Seeds were surface-sterilized in 4% (w/v) BAYROCHLOR (Bayrol, Mundolsheim, France), 50% (v/v) ethanol during 10 min, and then washed three times with 100% ethanol and three times in sterile water. Sterile seeds were sown on 12- × 12-cm petri dishes (Greiner Bio-one, Frickenhausen, Germany) containing 40 mL of sterile culture medium, sealed with Parafilm. The high P culture medium contained 0.5 mm CaSO4, 2 mm KNO3, 0.5 mm MgCl2, 0.05 mm NaFe-EDTA, 1 mm KH2PO4, 2.5 mm MES, 50 μm H3BO3, 12 μm MnCl2, 1 μm CuCl2, 1 μm ZnCl2, 30 nm NH4Mo, adjusted to pH 5.7 with 1 n KOH and solidified with 0.8% (w/v) Bactoagar (DIFCO, BD Bioscience, Sparks, MD). All chemicals were purchased from Sigma Chemicals (St. Quentin, France). In low P medium, although KH2PO4 was replaced by 1 mm KCl, the P content was estimated at 3 μm due to the slight P content of Bactoagar.
After sowing on either high or low P medium, petri dishes were cold treated at 4°C for 24 h in darkness to promote and synchronize germination and, subsequently, were transferred in a near vertical position to a growth chamber under a temperature of 21.5°C and a photoperiod of 16 h of light (150 μmol m−2 s−1) using fluorescent and metallic halogens lamps. At d 7, a subset of 15 seedlings was transplanted into new petri dishes containing fresh medium (either high or low P medium; five seedlings per plate).
Hormone Treatments
Low and high P culture media were supplemented with either 0.1 μm IAA or 0.1 μm TIBA. These compounds were filter-sterilized and added to medium at 60°C. IAA and TIBA were purchased from Sigma Chemicals.
Morphological Analysis
The root systems of plants grown in vertical agar plates were scanned daily at 300 dpi (EPSON perfection 2450 Photo; Seiko Epson, Nagano, Japan). Root growth parameters were determined after analysis of scanned images using the Optimas image analysis software (MediaCybernetics, Silver Spring, MD) as described by Freixes et al. (2002). Only LRs longer than 1 mm were considered. Several variables were estimated at each time point: PR length, PR elongation rate, LR length, number of elongating LRs longer than 1 mm, number of LRs stopping elongation, length of branched zone measured from the root/hypocotyl junction to the most apical visible LR and length of unbranched zone measured from the root tip to the most apical visible LR, elongation rate of LR (only roots longer than 3 mm were taken into account to measure rapid elongation), elongation rate of the three uppermost LRs, elongation rate of the LRs 4, 5, and 6, elongation rate of the LRs 7, 8, and 9, and LR density calculated as LR number divided by the length of the PR or by the length of the branched zone. The elongation rates of LRs represent the mean values of elongation rates of individual LRs. On d 14, an additional variable was obtained: the total rosette leaf area was measured on scanned rosettes using the Image Analysis software. The last variables were measured using a microscope (Leitz DMBR; Leica, Wetzlar, Germany). These were the number of primordia in the unbranched zone of the PR, the length of the region spanning from the first visible primordium to the first visible emerged root (PR region with primordia), the density of primordia (as the number of primordia divided by the length of the unbranched zone), and the diameter of PR (measured on the same plants as those used for growth analysis). Data were exported to an Excel worksheet (Microsoft, Redmond, WA) for final processing, and statistical comparisons of means between treatments or genotypes were performed using the Student's t test of the Statistica software (Statsoft, Tulsa, OK). Data are the means of eight replicates ± se. For analysis of variance, the two-factor ANOVA with a lsd post hoc test from the Statistica software (Statsoft) was used for assessing differences (P = 0.05) at d 9 and d 14.
Histochemical analysis of the GUS reporter enzyme activity was adapted from Craig (1992). Samples tissues were fixed in 4% (w/v) paraformaldehyde, 0.5% (v/v) glutaraldehyde in 0.1 m phosphate buffer for 1 min, rinsed twice, and incubated for 12 h in reaction buffer containing 1 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid as the substrate. Plant pigments were cleared with ethanol, and the GUS staining patterns were analyzed on an Olympus BX61 microscope (Tokyo) and a digital camera (Colorview 2) driven by Analysis software (Soft Imaging System USA, Lakewood, CO).
Auxin Quantification
The frozen samples were ground in liquid nitrogen and extracted overnight at −20°C in 80% (v/v) methanol. For recovery calculations, 138 pmol of 13C6-IAA (Cambridge Isotope Laboratories, Andover, MA) was added to the samples. After centrifugation (20,000g, 15 min, 4°C), the supernatant was collected and passed through a C18 cartridge (Varian, Harbor City, CA). Afterward, IAA was purified by means of a DEAE-Sephadex A25 cartridge (formic acid conditions; Amersham Pharmacia, Uppsala) coupled to a C18 cartridge. Prior to analyses, samples were methylated by methyl ester (Prinsen et al., 2000) and quantified by microLC-(ES+) MS/MS in SRM mode (Prinsen et al., 1998). Chromatograms were analyzed using the Masslynx software (Micromass, Vilvoorde, Belgium), and the IAA concentration was calculated according to the principle of isotope dilution.
Acknowledgments
We thank Drs. Catherine Bellini, Paola Grisafi, and Tom Beeckman for kindly providing us seeds of DR5::GUS, alf3, and CycBl::GUS, respectively. We gratefully acknowledge Alain Gojon for stimulating discussion and critical reading of our manuscript. Hugues Baudot and Gaëlle Viennois are thanked for their valuable technical assistance.
This work was supported in part by the Ecogene program of the Institut National de la Recherche Agronomique.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.060061.
References
- Al-Ghazi Y, Muller B, Pinloche S, Tranbarger TJ, Nacry P, Rossignol M, Tardieu F, Doumas P (2003) Temporal response of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signalling. Plant Cell Environ 26: 1053–1066 [Google Scholar]
- Bates TR, Lynch JP (1996) Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ 19: 529–538 [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Bhalerao RP, Eklof J, Ljung K, Marchant A, Bennett M, Sandberg G (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29: 325–332 [DOI] [PubMed] [Google Scholar]
- Blakely LM, Durham M, Evans TA, Blakely RM (1982) Experimental studies on lateral root formation in radish seedlings roots. 1. General methods, developmental stages and spontaneous formation of laterals. Bot Gaz 143: 341–352 [Google Scholar]
- Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Vanonckelen H, Vanmontagu M, Inze D (1995) Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7: 1405–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonser AM, Lynch J, Snapp S (1996) Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytol 132: 281–288 [DOI] [PubMed] [Google Scholar]
- Carswell C, Grant BR, Theodorou ME, Harris J, Niere JO, Plaxton WC (1996) The fungicide phosphonate disrupts the phosphate-starvation response in Brassica nigra seedlings. Plant Physiol 110: 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inze D, Sandberg G, Casero PJ, Bennett M (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL Jr, Grisafi PL, Fink GR (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9: 2131–2142 [DOI] [PubMed] [Google Scholar]
- Chapin FS (1991) Integrated responses of plants to stress. Bioscience 41: 29–36 [Google Scholar]
- Craig S (1992) The GUS reporter gene. Application to light and transmission electron microscopy. In SR Gallagher, ed, GUS Protocols: Using the GUS Gene as a Reporter of Gene Expression. Academic Press, San Diego, pp 115–124
- Deikman J, Hammer PE (1995) Induction of anthocyanin accumulation by cytokinins in Arabidopsis thaliana. Plant Physiol 108: 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC (1975) Comparison of the effect of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol 75: 479–490 [Google Scholar]
- Evans ML, Ishikawa H, Estalle MA (1994) Responses of Arabidopsis roots to auxin studied with high temporal resolution: comparison of wild type and auxin-response mutants. Planta 194: 215–222 [Google Scholar]
- Farley RA, Fitter AH (1999) Temporal and spatial variation in soil resources in a deciduous woodland. J Ecol 87: 688–696 [Google Scholar]
- Ferreira PC, Hemerly AS, Engler JD, van Montagu M, Engler G, Inze D (1994) Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell 6: 1763–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B, Lorenzo H (2001) The nutritional control of root development. Plant Soil 232: 51–68 [Google Scholar]
- Franco-Zorrilla JM, Martin AC, Solano R, Rubio V, Leyva A, Paz-Ares J (2002) Mutations at CRE1 impair cytokinin-induced repression of phosphate starvation responses in Arabidopsis. Plant J 32: 353–360 [DOI] [PubMed] [Google Scholar]
- Freixes S, Thibaud MC, Tardieu F, Muller B (2002) Root elongation and branching is related to local hexose concentration in Arabidopsis thaliana seedlings. Plant Cell Environ 25: 1357–1366 [Google Scholar]
- Friml J (2003) Auxin transport: shaping the plant. Curr Opin Plant Biol 6: 7–12 [DOI] [PubMed] [Google Scholar]
- Fujita H, Syono K (1996) Genetic analysis of the effects of polar auxin transport inhibitors on root growth in Arabidopsis thaliana. Plant Cell Physiol 37: 1094–1101 [DOI] [PubMed] [Google Scholar]
- Hell R, Hillebrand H (2001) Plant concepts for mineral acquisition and allocation. Curr Opin Biotechnol 12: 161–168 [DOI] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inze D, Beeckman T (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14: 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, Estelle M (1995) The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J 7: 211–220 [DOI] [PubMed] [Google Scholar]
- Johnson JF, Allan DL, Vance CP (1994) Phosphorus stress-induced proteoid roots show altered metabolism in Lupinus albus. Plant Physiol 104: 657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JF, Vance CP, Allan DL (1996) Phosphorus deficiency in Lupinus albus. Altered lateral root development and enhanced expression of phosphoenolpyruvate carboxylase. Plant Physiol 112: 31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Mukatira UT, D'Urzo MP, Damsz B, Raghothama KG (2002) Regulated expression of Arabidopsis phosphate transporters. Plant Physiol 130: 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser HM (2002) Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J 29: 751–760 [DOI] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28: 465–474 [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Hernandez-Abreu E, Sanchez-Calderon L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129: 244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Hernandez-Abreu E, Sanchez-Calderon L, Perez-Torres A, Rampey RA, Bartel B, Herrera-Estrella L (2005) An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis. Identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiol 137: 681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J (1995) Root architecture and plant productivity. Plant Physiol 109: 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Baskin TI, Brown KM, Lynch JP (2003) Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol 131: 1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Malamy JE, Ryan KS (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 127: 899–909 [PMC free article] [PubMed] [Google Scholar]
- Martin AC, del Pozo JC, Iglesias J, Rubio V, Solano R, de La Pena A, Leyva A, Paz-Ares J (2000) Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis. Plant J 24: 559–567 [DOI] [PubMed] [Google Scholar]
- Moog PR, van der Kooij TA, Bruggemann W, Schiefelbein JW, Kuiper PJ (1995) Responses to iron deficiency in Arabidopsis thaliana: The Turbo iron reductase does not depend on the formation of root hairs and transfer cells. Planta 195: 505–513 [DOI] [PubMed] [Google Scholar]
- Muday GK, Haworth P (1994) Tomato root growth, gravitropism, and lateral development: correlation with auxin transport. Plant Physiol Biochem 32: 193–203 [PubMed] [Google Scholar]
- Müller A, Guan C, Galweiler L, Tanzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17: 6903–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenschlager I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100: 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett FB, Wilson AK, Estelle M (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94: 1462–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y, Bucher M (2002) Phosphate transport and homeostasis in Arabidopsis. In C Somerville, E Meyerowitz, eds, The Arabidopsis Book. The American Society of Plant Biologists, Rockville, MD, http://www.aspb.org/publications/arabidopsis/ [DOI] [PMC free article] [PubMed]
- Prinsen E, Van Dongen W, Esmans EL, Van Onckelen H (1998) Micro and capillary liquid chromatography tandem mass spectrometry: a new dimension in phytohormone research. J Chromatogr 826: 25–37 [Google Scholar]
- Prinsen E, Van Laer S, Sevgi Ö, Van Onckelen H (2000) Auxin analysis. In GA Tucker, JA Roberts, eds, Methods in Molecular Biology: Plant Hormone Protocols, Vol 141. Humana Press, Totowa, NJ, pp 49–65 [DOI] [PubMed]
- Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Robinson D (1994) The response of plants to non uniform supplies of nutrients. New Phytol 127: 637–674 [DOI] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139: 1393–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Sakakibara H (2003) Nitrate-specific and cytokinin-mediated nitrogen signaling pathways in plants. J Plant Res 116: 253–257 [DOI] [PubMed] [Google Scholar]
- Schmidt W, Schikora A (2001) Different pathways are involved in phosphate and iron stress-induced alterations of root epidermal cell development. Plant Physiol 125: 2078–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev 15: 2648–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Kargul J, Marchant A, Zadik D, Rahman A, Mills R, Yemm A, May S, Williams L, Millner P, et al (2004) Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 16: 3069–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler P, Pages L (1998) Modelling the influence of assimilate availability on growth and architecture. Plant Soil 201: 307–320 [Google Scholar]
- Ticconi CA, Abel S (2004) Short on phosphate: plant surveillance and countermeasures. Trends Plant Sci 9: 548–555 [DOI] [PubMed] [Google Scholar]
- Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S (2004) Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J 37: 801–814 [DOI] [PubMed] [Google Scholar]
- Timpte C, Lincoln C, Pickett FB, Turner J, Estelle M (1995) The AXR1 and AUX1 genes of Arabidopsis function in separate auxin-response pathways. Plant J 8: 561–569 [DOI] [PubMed] [Google Scholar]
- Trull MC, Guiltinan MJ, Lynch JP, Deikman J (1997) The responses of wild-type and ABA mutant Arabidopsis thaliana plants to phosphorus starvation. Plant Cell Environ 20: 85–92 [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LC, Ribrioux SP, Fitter AH, Leyser HM (2001) Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol 126: 875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HJ, Barlow PW, Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA 96: 6529–6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HJ, Forde BG (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279: 407–409 [DOI] [PubMed] [Google Scholar]