Abstract
Substrate (futile) cycling involving carbohydrate turnover has been widely reported in plant tissues, although its extent, mechanisms, and functions are not well known. In this study, two complementary approaches, short and steady-state labeling experiments, were used to analyze glucose metabolism in maize (Zea mays) root tips. Unidirectional rates of synthesis for storage compounds (starch, Suc, and cell wall polysaccharides) were determined by short labeling experiments using [U-14C]glucose and compared with net synthesis fluxes to determine the rate of glucose production from these storage compounds. Steady-state labeling with [1-13C]glucose and [U-13C]glucose showed that the redistribution of label between carbon C-1 and C-6 in glucose is close to that in cytosolic hexose-P. These results indicate a high resynthesis flux of glucose from hexose-P that is not accounted for by glucose recycling from storage compounds, thus suggesting the occurrence of a direct glucose-P-to-glucose conversion. An enzyme assay confirmed the presence of substantial glucose-6-phosphatase activity in maize root tips. This new glucose-P-to-glucose cycle was shown to consume around 40% of the ATP generated in the cell, whereas Suc cycling consumes at most 3% to 6% of the ATP produced. The rate of glucose-P cycling differs by a factor of 3 between a maize W22 line and the hybrid maize cv Dea, and is significantly decreased by a carbohydrate starvation pretreatment.
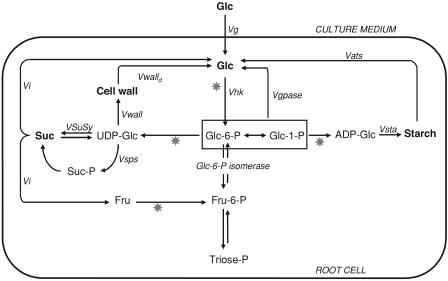
The development of nonphotosynthetic tissues is closely related to Suc import. Suc is degraded by Suc synthase (SuSy) or invertase to provide UDP-Glc or hexoses for the biosynthesis of structural or storage compounds and for ATP production (Fig. 1). In 1988, Hargreaves and ap Rees used pulse-chase experiments to show the presence of a cycle of synthesis and degradation of Suc in pea roots. This cycle, consuming ATP without any apparent physiological function, was described as a futile cycle, according to Fell's definition (Fell, 1997), and, according to more recent terminology, which refers to a possible role for these processes, it can be described as a substrate cycle (the Suc cycle). Since then, the Suc cycle has been found in many other tissues: chenopodium cells, potato (Solanum tuberosum) tubers, ripening banana (Musa cavendishii), maize (Zea mays) root tips, tomato (Lycopersicon esculentum) cells, and tomato fruit (Hatzfeld and Stitt, 1990; Hill and ap Rees, 1994; Dieuaide-Noubhani et al., 1995, 1997; N'tchobo et al., 1999; Rontein et al., 2002). One can estimate that, for the synthesis and degradation of one molecule of Suc, 1.5 to two molecules of ATP are consumed (Fig. 1). Depending on the tissue, this cycle could consume between 5% and 70% of the ATP produced by the cell (Hatzfeld and Stitt, 1990; Hill and ap Rees, 1994; Dieuaide-Noubhani et al., 1995; Fernie et al., 2002; Rontein et al., 2002).
Figure 1.
Metabolic scheme. The principal pathways considered in this study. The stars indicate the reactions consuming ATP or UTP and the flux names are indicated. Vg, Glc uptake; Vhk, hexokinase activity; Vgpase, Glc-6-phosphatase activity; Vsta, starch synthesis; Vats, starch degradation; VSuSy, Suc synthase activity; Vsps, Suc phosphate synthase activity; Vi, invertase activity; Vwall, cell wall polysaccharide synthesis; Vwalld, cell wall polysaccharide degradation.
Two other substrate cycles related to central carbohydrate metabolism have been described in plants, hexose-P ↔ triose-P cycling and the starch synthesis and degradation cycle. The occurrence of extensive cycling between starch or Suc and the triose-P has been confirmed by independent methods (Glawischnig et al., 2002), including the measurement of δ13C ratios in intact organs (Gleixner et al., 1998). However, the energy cost of these cycling phenomena has not been established. In a recent review, Portais and Delort (2002) underlined the need to distinguish between reversibility, metabolic cycles, and substrate cycles. Hexose-P ↔ triose-P cycling may be a substrate cycle or a reversibility flux, depending on the enzymes involved. It fits Fell's definition of a substrate cycle when phosphofructokinase and Fru-bisphosphate are involved. The action of inorganic pyrophosphate (PPi):Fru-6-P phosphotransferase, which catalyzes a reversible reaction without consuming ATP, generates a reversibility cycle, not a substrate cycle. The operation of these alternative pathways has the same impact on label distribution patterns (Fernie et al., 2001). Since they are not distinguishable in labeling experiments, their relative importance and thus an evaluation of the energy cost remains difficult.
In fact, substrate cycles involving sugars also exist in microorganisms (for review, see Portais and Delort, 2002) and animal cells (Landau, 1999; Jones et al., 2002), and their roles are still a matter of debate. It has been suggested that they could serve to buffer metabolite concentrations, improve sensitivity in metabolic regulation, or rapidly change the direction of net flux (Newsholme et al., 1984; Fell, 1997). In the yeast Pichia pastoris, Teusink et al. (1998) and Blomberg (2000) have suggested that the cycling of trehalose could help to regenerate inorganic phosphate (Pi) sequestered in the hexose-P pool from the first part of glycolysis, thus avoiding phosphate depletion. In plants, a possible role for substrate cycles in allowing a rapid change in the direction of net flux has been suggested by the work of Geigenberger et al. (1997). These authors have shown that fluxes through the Suc and starch cycles favoring synthesis of starch from Suc in potato could be reversed in response to water deficit, thus allowing a net accumulation of Suc. Hill and ap Rees (1995) suggested that the modification of fluxes through different substrate cycles (an increase in hexose-P ↔ triose-P cycling and a decrease in the starch and Suc cycles) could participate in keeping the ATP-to-ADP ratio constant when oxygen pressure is lowered. In tomato fruit, Suc cycling may function to increase the efficiency of sugar storage in the cytosol and vacuole (Nguyen-Quoc and Foyer, 2001). Interestingly, increasing Suc degradation activity in potato by transforming plants with a cytosolic invertase or a Suc phosphorylase leads to increases in Suc cycling and in the respiratory rate in the tuber (Trethewey et al., 1998, 1999, 2001; Fernie et al., 2002). The increase in glycolytic rate could result from an increase in the energy demand of Suc cycling, thus suggesting that glycolysis in plants is controlled by the rate of ATP utilization (Fernie et al., 2002).
A high degree of variation in flux around the Suc cycle has been reported. Two approaches for quantifying this flux have been described, both based on isotopic labeling and both having limitations that could lead to misrepresentation of the true flux. In one approach, the unidirectional flux of Suc synthesis was measured in a short time-labeling experiment from the rate of the incorporation of radioactivity into Suc (Hargeaves and ap Rees, 1988; Hatzfeld and Stitt, 1990; Hill and ap Rees, 1994, 1995). The unidirectional degradation flux was then calculated as the difference between the net flux of accumulation or degradation and the unidirectional flux of synthesis. The pitfall of this method, in the case of a substrate cycle, is that the unidirectional synthesis flux may be underestimated because of the loss of radioactivity in Suc due to degradation reactions. The second approach was based on steady-state labeling (Dieuaide-Noubhani et al., 1995; Rontein et al., 2002; Schwender et al., 2003), where fluxes were calculated from equations describing the relation between input and output fluxes of label at the level of each atom (Katz and Grunnet, 1979). In contrast to the previous method, the sequence of reactions that link two metabolites cannot be determined from steady-state labeling; various sets of reaction may give the same distribution of label, and the reaction(s) actually involved must be deduced by other methods, such as enzyme identification and compartmentation studies (Fernie et al., 2001). In maize root tips labeled with [1-13C]Glc, the observation of label redistribution from carbon C-1 to C-6 of Glc indicated that the influx of Glc from the medium was not the only source of intracellular Glc, and that a flux from hexose-P to Glc should be considered (Dieuaide-Noubhani et al., 1995). Because the turnover of cell wall polysaccharides and starch was considered to be low in growing maize root tips, and in the absence of a clear indication of Glc-6-phosphatase activity in plant cells, the whole flux from hexose-P to Glc was attributed to the Suc cycle.
The high cost in ATP of hexose-P-to-Glc cycling (Fig. 1) could make it important for the regulation of plant cell metabolism. It is thus essential to identify and quantify the pathways leading to intracellular Glc accurately so as to understand both the role of substrate cycles in plant cells and their regulation. The goal of this work was to provide experimental data on the pathways potentially responsible for the hexose-P-to-Glc conversion. Since the established pathways were not sufficient to account for the redistribution of the labeling between carbon C-1 and C-6 observed in Glc, we conclude that this redistribution results mostly from Glc-phosphatase activity. These results are discussed in relation to the measurement of Glc-6-phosphatase activity in vitro and the potential localization of this activity in the cell.
RESULTS
Establishment of Isotopic and Metabolic Steady State upon Incubation with Glc
For steady-state labeling experiments, metabolic and isotopic steady state must be reached. Maize root tips were incubated with labeled substrates directly after excision. The time necessary to reach isotopic and metabolic steady state was determined by incubating maize roots with [U-14C]Glc and monitoring the evolution of the specific radioactivity (SR) in Glu and Asp in the last 3.5 mm of the roots. For these measurements, the roots were re-excised at 3.5 mm after incubation and labeling was monitored in these last 3.5 mm of the roots. The SR of Glu and Asp increased for 15 h and then reached a plateau, indicating that the intermediates of the glycolysis and those of the citric acid cycle had reached steady state (data not shown).
In order to further verify the time necessary to reach steady state, maize root tips were incubated with [1-13C]Glc for 25 and 30 h. The resonances assigned to Glc and Suc extracted from the last 3.5 mm of the roots showed the same enrichments after these two incubation times, thus confirming that sugars were at isotopic steady state after 25 h of incubation (data not shown). As a consequence, the time used for metabolic and isotopic steady-state labeling was set at 25 h.
Net Sugar Synthesis in Re-Excised Maize Root Tips Is Low
To determine the net fluxes of Suc, Glc, and starch synthesis, maize root tips were incubated 23 h in the nutritive solution containing Glc, re-excised to 3.5 mm, and further incubated for 2 or 4 h in the same medium. After 23-h incubation in the presence of Glc, re-excised maize root tips contained 72 ± 14 μmol of Glc/g fresh weight (FW), 34 ± 6 μmol of Suc/g FW, and 11.4 ± 2.6 μmol of starch/g FW. No significant differences for Suc, Glc, and starch contents in the last 3.5 mm were observed over a period of 4 h. These results strongly support the existence of a metabolic steady state after 25 h of incubation and show that the rates of net synthesis of Suc, Glc, and starch are negligible.
Synthesis of Cell Wall Polysaccharides and Turnover of Suc and Starch
Short time-labeling experiments were conducted after an initial incubation period of 25 h with unlabeled Glc to allow the root tips to reach metabolic steady state. The flux values obtained are presented in Table I.
Table I.
Metabolic fluxes involved in Glc/hexose-P interconvertion (nmol Glc min−1 g−1 FW) in the W22 maize line root tips
| Intermediary Metabolite | Hexose-P Consumption | Toward Glc Pool |
|---|---|---|
| Glc influx | Vg: 394 ± 73 | |
| Cell wall | Vwall: 52 ± 14 | Vwalld: ≪52 |
| Starch | Vsta: 30 ± 11 | Vats: ≤30 |
| Sucrose | Vsuc: 178 ± 36 | Vi: ≤178 |
| Hexose-P | Vres: 2,700 ± 520 | Vgpase: 2,500 ± 520 |
Glc Consumption Rate
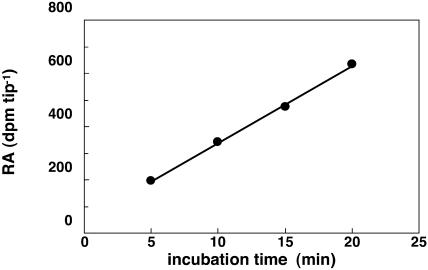
Maize root tips were incubated with [U-14C]Glc for 5, 10, 15, and 20 min. The rate of Glc consumption (Vg) was determined by dividing the slope of the curve of radioactivity incorporation by the roots (Fig. 2) by the SR of extracellular Glc. The incubation times were selected to be as short as possible to lessen the underestimation of that flux due to the catabolism of Glc, leading to losses of labeling as CO2 (by the pentose phosphate pathway, the UDP-glucuronate-decarboxylase reaction involved in synthesizing cell wall pentoses, and the citric acid cycle). Vg was found to be 394 ± 73 nmol min−1 g−1 FW.
Figure 2.
[U-14C]Glc uptake by maize root tips. Maize root tips were incubated with 200 mm Glc during 25 h and transferred into a medium containing 200 mm [U-14C]Glc (30 dpm nmol−1) during 5, 10, 15, and 20 min. The maize root tips were then grounded and radioactivity was counted. These data are from one representative experiment, repeated four times.
Rates of Starch and Suc Turnover
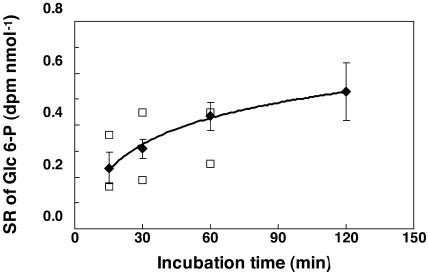
To quantify the unidirectional rates of Suc and starch synthesis, maize root tips were incubated for 15, 30, and 60 min with [U-14C]Glc. The radioactivity incorporated into the glucosyl units of Suc and starch (data not shown), and the SR of their precursors (Fig. 3) were estimated. UDP-Glc and ADP-Glc are usually rapidly exchanged with Glc-6-P through the UDP-Glc and ADP-Glc pyrophosphorylase reactions, so that Glc-6-P can be used as an indicator of the labeling of UDP-Glc and ADP-Glc. However, UDP-Glc is also produced by the SuSy reaction. Thus, in short time-labeling experiments, and if the SuSy reaction rate is high, UDP-Glc coming from Glc-6-P might be diluted by UDP-Glc coming from Suc. Using the SR of Glc-6-P instead of UDP-Glc could therefore lead to an underestimation of the rate of Suc synthesis. Figure 3 shows the increase of the SR of Glc-6-P and UDP-Glc. No significant differences were found between the SR of Glc-6-P and UDP-Glc. Therefore, Glc-6-P was considered a good indicator of the SR of UDP-Glc. This observation of labeling equilibrium agrees with previously published data (Geigenberger et al., 1997; Roscher et al. 1998) showing that many enzymes of sugar metabolism, including UDP-Glc pyrophosphorylase and hexose-P isomerase, catalyze near-equilibrium reactions.
Figure 3.
Evolution of the SR of Glc-6-P (♦) and UDP-Glc (□). Maize root tips were incubated with 200 mm Glc during 25 h and transferred into a medium containing 200 mm [U-14C]Glc (100–200 dpm nmol−1 according to the experiment) during 15, 30, 60, and 120 min. Following a perchloric extraction, the SR of Glc-6-P and UDP-Glc were obtained as described in “Materials and Methods.” They were normalized by dividing the measured values by the SR of extracellular Glc. For Glc-6-P, the values indicated after 15, 30, 60, and 120 min are the mean of four or six values (± sd), obtained in three independent experiments. For UDP-Glc, the symbols correspond to SR values obtained in two independent experiments.
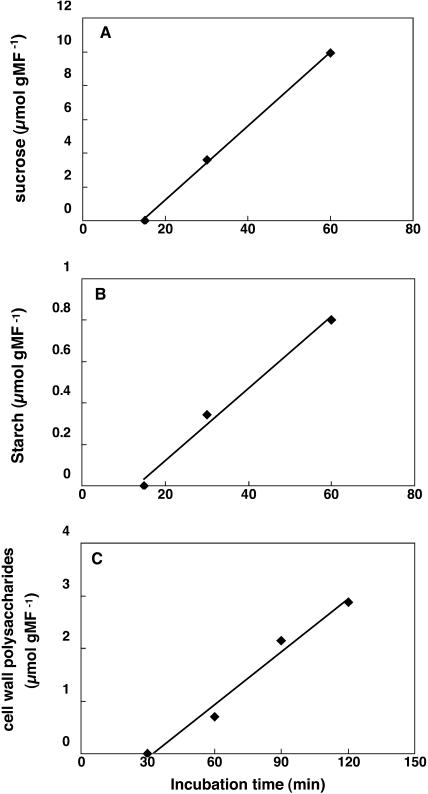
The amounts of Suc and starch produced after 15, 30, and 60 min (Fig. 4, A and B) were calculated from the radioactivity incorporated into Suc and starch glucosyls, respectively, using the following equation:
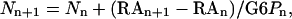 |
(1) |
where Nn is the number of moles formed, RAn is the amount of radioactivity incorporated into the compound studied (expressed in disintegrations per minute [dpm] g−1 FW), G6Pn is the SR of Glc-6-P, and n indicates the nth time point of incubation.
Figure 4.
Determination of the unidirectional rates of Suc synthesis, starch, and cell wall polysaccharides. Maize root tips were incubated with 200 mm Glc during 25 h and transferred into a medium containing 200 mm [U-14C]Glc (30 to 380 dpm nmol−1 according to the experiment) during 15, 30, and 60 min. A time of 90 min was added in experiments performed for cell wall polysaccharide studies. Because Suc was partially degraded by invertase activities in the grinding buffer used for the separation of cell wall polysaccharides, separate experiments were carried out for Suc and cell wall polysaccharide analysis (see “Materials and Methods”). Starch was purified as Glc, after ethanolic extraction as well as from samples allowing cell wall polysaccharide separation. Radioactivity incorporated into glucosyl moieties of Suc and starch and into polysaccharides was counted and normalized by dividing by the SR of external Glc. The quantities of radioactivity incorporated during a time interval were then converted to the amount of sugar produced, expressed in mol/g FW, using the equation Nn+1 = Nn + (RAn+1 − RAn)/G6Pn. Nn, RAn, and G6Pn correspond, respectively, to the number of moles, the radioactivity incorporated into the compound studied (expressed in dpm/g FW), and the SR of Glc-6-P; n indicates the nth time point of incubation. The results presented here correspond to one typical experiment, repeated five, five, and three times for Suc, starch, and cell wall polysaccharides, respectively. These data were used to quantify the unidirectional rate of Suc, starch, and cell wall polysaccharide syntheses.
We have emphasized in “Materials and Methods” that it is difficult to measure the flux of synthesis of a compound involved in a cycle of synthesis and degradation because of the loss of radioactivity by the degradation reaction. After 60 min, the SR of the glucosyl moiety of Suc reached 0.09 dpm nmol−1, which is 21% of that of the precursor Glc-6-P (0.43 dpm nmol−1). This indicates that the pathway is still far from isotopic steady state, which makes it reasonable to assume that losses of radioactivity are negligible. The same applies to starch, where the SR of glucosyl units was about 0.05 dpm nmol−1. The linearity of the curves of Suc and starch synthesis (Fig. 4) confirms that losses of radioactivity due to turnover were weak enough to avoid underestimation of the rate of incorporation of the tracer into its product. Therefore, we can estimate the unidirectional rate of Suc synthesis, Vsuc, which corresponds to the sum of the fluxes through the Suc-P synthase and the SuSy reactions, was estimated at 178 ± 36 nmol min−1 g−1 FW. The unidirectional rate of starch synthesis, Vsta, was 30 ± 11 nmol min−1 g−1 FW (Table I).
These unidirectional rates of Suc and starch synthesis would, in the absence of degradation, lead to an increase over 4 h of approximately 43 and 7 μmol of Suc and starch, respectively, which are significant amounts compared to root tip contents of 34 ± 6 μmol of Suc g−1 FW and 11.4 ± 2.6 μmol of starch g−1 FW. However, the content in Suc or starch did not change over 4 h (data not shown). These results indicate that the net flux to Suc was very low compared to the unidirectional flux of Suc synthesis, i.e. that the synthesis and degradation of Suc and starch occurred at the same rates.
Rate of Cell Wall Polysaccharide Synthesis
Maize root tips were incubated for 30, 60, and 120 min with [U-14C]Glc. Cell wall polysaccharides were separated from other compounds (see “Materials and Methods”). Using Equation 1, the unidirectional rate of synthesis of cell wall polysaccharides (Fig. 4C) was found to be 52 ± 14 nmol min−1 g−1 FW.
Total Glc-P-to-Glc Recycling
Total Glc-P-to-Glc recycling was quantified after labeling to isotopic steady state and modeling, as described in “Materials and Methods.”
Analysis of Sugar Enrichments
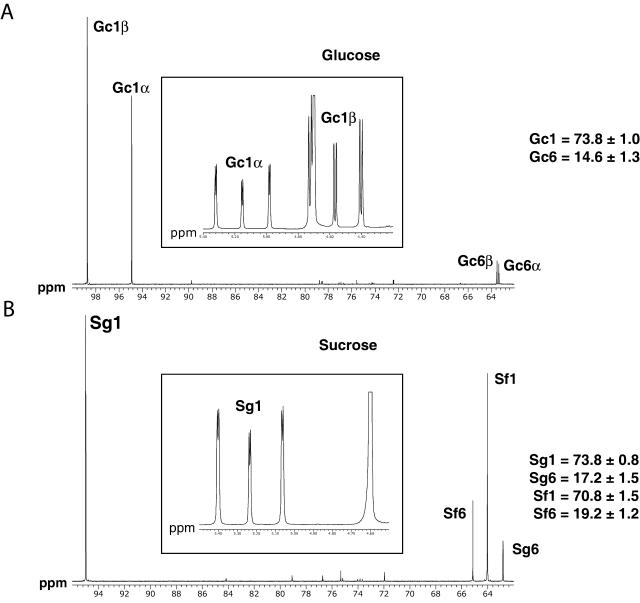
Figure 5 shows typical NMR spectra of Glc and Suc. The 13C enrichments, measured and then corrected by the dilution factors (see “Materials and Methods”), are shown in Table II. At isotopic steady state, the labeling of the glucosyl and fructosyl moieties of Suc reflects the labeling of cytosolic Glc-6-P and Fru-6-P, respectively (Fig. 1). Compared to the carbon enrichment of external Glc (98% above natural abundance), we observed a dilution of the total enrichment of the hexose moieties of Suc (Sg1 + Sg6 = 92.2% above natural abundance), and a redistribution of label from the carbon C-1 position to C-6. Previous results in root tips of the maize cv Dea hybrid (Dieuaide-Noubhani et al., 1995) indicated that these effects result from a combination of the pentose phosphate pathway in the plastid, the reversibility of glycolysis between triose-P and hexose-P, and a cytosolic transaldolase activity. These data on the W22 line are consistent with the same metabolic scheme. However, it is notable that there was less redistribution from C-1 to C-6 in the glucosyl (C-6/C-1 = 0.23 ± 0.02) than in the fructosyl moiety (C-6/C-1 = 0.27 ± 0.02) of Suc (P < 0.05 in t test), suggesting that the hexose-P isomerase reaction is not at equilibrium. On the other hand, the C-1 to C-6 redistribution observed in Glc was close to that observed for the Suc glucosyl, which indicates that the Glc-P-to-Glc cycling is high compared to the influx of Glc.
Figure 5.
13C and 1H (insert) spectra of cellular Glc (A) and Suc (B). Maize root tips were incubated for 25 h with d-[1-13C]Glc 200 mm. Glc and Suc were purified and analyzed by 1H- and 13C-NMR as described in “Materials and Methods.” Gciα, Gciβ, Sgi, and Sfi correspond to the resonance of the carbon i of Glc-α and Glc-β, the glucosyl and fructosyl moieties of Suc, respectively. For the studied carbon atoms, the enrichment values were obtained as described in “Materials and Methods” and reported on the right of the spectra. The spectra are representative of one labeling experiment repeated four times.
Table II.
Specific 13C enrichments of the carbon atoms of soluble sugars
Maize root tips were incubated for 25 h with 200 mm d-[1-13C]Glc. The Glc and Suc were purified by HPLC and analyzed by 1H- and 13C-NMR. The enrichment values of the C-1 carbons were calculated from 1H-NMR spectra, those of the C-2 to C-6 carbons, from 13C-NMR spectra. The results reported as measured enrichments are the average ±sd of five independent experiments. For Glc and Suc, as the enrichments of carbons C-2 to C-5 are close to natural abundance, they are not reported in the table. The measured enrichments obtained after a steady-state labeling with [1-13C]Glc were corrected by the diluting factor according to the following formula: Ecorrected = (Emeasured − 1.1). DF + 1.1, where DF is the diluting factor (see “Materials and Methods”), 1.066 and 1.038 for Glc and Suc, respectively. These values are reported as corrected enrichments.
| Glucose
|
Sucrose
|
||
|---|---|---|---|
| Glucosyl | Fructosyl | ||
| Measured enrichments (%) | |||
| C-1 | 73.8 ± 1.0 | 73.8 ± 0.8 | 70.8 ± 1.5 |
| C-6 | 14.6 ± 1.3 | 17.2 ± 1.5 | 19.2 ± 1.2 |
| Corrected enrichments (%) | |||
| C-1 | 78.6 ± 2.7 | 76.6 ± 1.1 | 73.4 ± 1.8 |
| C-6 | 15.5 ± 1.7 | 17.8 ± 1.6 | 19.9 ± 1.3 |
The ratio of enrichment C-1 to C-6 of Fru was quantified in two independent experiments according to 13C-NMR spectra (data not shown). Its value, 3.9 ± 0.2, was close to that measured for the fructosyl moiety of Suc (3.7 ± 0.3; see Table IV), thus indicating that Fru was produced mainly from the Suc turnover via invertase or through the reversibility of the SuSy reaction, or from Fru 6-P through a hexose-phosphatase reaction.
Table IV.
Comparison between maize Dea and W22 incubated with Glc with or without starvation pretreatment
Maize root tips were incubated with [U-14C]Glc and [1-13C]Glc (see “Materials and Methods”) after starvation pretreatment (maize root tips were incubated 4 h without Glc and then 25 h plus labeled Glc) or not. For Dea, with starvation pretreatment, the ratio C-1 to C-6 and the fluxes were calculated from data published by Dieuaide-Noubhani et al. (1995). All fluxes, Vres, Vgpase, and Vi were determined as for W22 (see “Results”). The values of relative fluxes were calculated for a rate of Glc consumption (Vg) equal to 1. The values of absolute fluxes were then calculated for Vg equal to 350 and 394 nmol min−1 g−1 FW for Dea and W22, respectively. Results are given as mean ± sd (n = 5 for W22 without starvation pretreatment, and n = 2 for W22 with starvation pretreatment). For Dea without starvation pretreatment, results are from one experiment.
| Without Starvation Pretreatment
|
With Starvation Pretreatment
|
|||
|---|---|---|---|---|
| Dea | W22 | Dea | W22 | |
| Enrichment ratio C-1 to C-6 | ||||
| Sucrose glucosyl | 4.6 | 4.3 ± 0.4 | 5.7 | 5.9 ± 0.4 |
| Sucrose fructosyl | 3.8 | 3.7 ± 0.3 | 5.4 | 5.0 ± 0.2 |
| Glucose | 6.5 | 5.1 ± 0.5 | 8.6 | 8.1 ± 0.4 |
| Relative fluxes | ||||
| Vres | 2.9 | 6.86 ± 1.33 | 2.1 | 3.0 ± 0.2 |
| Absolute fluxes (μmol min−1 g−1 FW) | ||||
| Vres | 1.0 | 2.70 ± 0.52 | ||
| Vi | 0.2 | 0.15 ± 0.03 | ||
| Vgpase | 0.8 | 2.55 ± 0.52 | ||
Calculation of the Glc-P-to-Glc Flux
Of the three pathways that may produce Glc from Glc-P, we considered here only the two routes involving invertase and Glc-phosphatase activities. This approximation could be made because the turnover of starch is low (see above) and because the turnover of cell wall polysaccharides is probably negligible in growing tissues (Haigler et al., 2001). Two equations can be written to describe the metabolic and isotopic steady-state labeling of the C-1 and C-6 carbons of intracellular Glc; these involve the fluxes Vg (rate of Glc uptake) and Vres (rate of Glc resynthesis) resulting from two enzymatic paths, invertase or Glc-phosphatase.
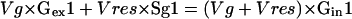 |
(2) |
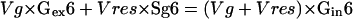 |
(3) |
Gex1, Sg1, and Gin1 represent the enrichment of the C-1 of extracellular Glc, the glucosyl moiety of Suc, and intracellular Glc, respectively, and Gex6, Sg6, and Gin6 represent the enrichments of C-6 in the same molecules. To solve these two equations, the enrichments of C-1 and C-6 of Suc and Glc must be determined. However, steady-state labeling of maize root tips with [U-14C]Glc reveals the presence of unlabeled pools of sugars, probably located in the vacuole (see “Materials and Methods”). For each soluble sugar, a diluting factor was determined, allowing calculation of the enrichments of metabolically active pools (corrected enrichment; Table II) from the measured enrichments.
A new equation (Eq. 4) can be deduced from these two equations involving the enrichment ratios C-1 to C-6 for all sugars considered and the absolute enrichment of C-6 of Suc glucosyl. Using a ratio of enrichments in flux calculation eliminates the error introduced by the Glc diluting factors.
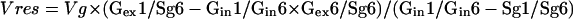 |
(4) |
Using the enrichments presented in Table II to solve Equation 4, Vres was found to be 2.7 ± 0.5 μmol min−1 g−1 FW. This flux must then be compared to the flux through Suc cycling, estimated at 178 nmol min−1 g−1 FW from 14C pulse labeling (Table I). The large difference between these two values implies that the Suc cycle cannot account for the observed labeling of Glc. Therefore, a flux through a Glc-phosphatase must be considered. By subtraction of the flux through the Suc cycle, the flux catalyzed by the Glc-phosphatase was estimated at 2.5 ± 0.5 μmol min−1 g−1 FW.
Phosphatase Activities in Maize Root Tips
The existence of this new cycle requires substantial phosphatase activity. Phosphatase activity was therefore measured in extracts obtained from maize Dea and W22 root tips, using Glc-6-P as substrate (Table III). Because of the uncertainty concerning the subcellular localization of Glc-6-phosphatase in plant cells, we measured the activity at pH 5.5 and pH 7.5, corresponding to the vacuolar and cytosolic pH in maize root tips (R.M. Brouquisse, D. Rolin, and C. Roby, unpublished data). At both pHs, the activity measured in W22 root tips was higher than in Dea root tips. It is interesting to note that the activities measured at pH 7.5 were low compared to the flux measured in vivo. On the other hand, the activities measured at the vacuolar pH were of the same order as the fluxes measured in vivo.
Table III.
Glc-6-phosphatase activities
Glc-6-phosphatase activities were measured in maize Dea and W22 root tips at pH 5.5 (vacuolar pH) and pH 7.5 (cytosolic pH) as described in “Materials and Methods.” The values represent the mean (±sd) of two (activity measured at pH 5.5) and four (activity measured at pH 7.5) separate experiments.
| pH 5.5 | pH 7.5 | |
|---|---|---|
| Glc-6-phosphatase activity (μmol min−1 g−1 FW) | ||
| Dea | 1.5 ± 0.3 | 0.55 ± 0.1 |
| W22 | 1.5 ± 0.2 | 0.84 ± 0.1 |
Fluxes through the Substrate Cycle Depend on the Maize Variety and Are Sensitive to Starvation Pretreatment
In order to compare these data on W22 with those obtained on the Dea hybrid (Dieuaide-Noubhani et al., 1995), steady-state labeling with [1-13C]Glc was performed (1) with W22 according to the protocol previously used for Dea, i.e. after starvation pretreatment (4 h without Glc in medium before feeding with [1-13C]Glc) and (2) with Dea according to this protocol (see “Materials and Methods”), i.e. without starvation pretreatment. The ratios of enrichments C-1 to C-6 were determined for the intracellular Glc and the glucosyl and fructosyl moieties of Suc, and the relative Glc-P-to-Glc conversion fluxes (Vres) calculated from these data and Eq. (4). Results are shown in Table IV.
Effects of Starvation Pretreatment
For both lines, starvation pretreatment induced a substantial decrease of the randomization of labeling from C-1 to C-6 in Glc and in Suc. Moreover, it appears that starvation led to a significant reduction of the recycling of Glc-P to Glc in the W22 variety, but had little or no effect in Dea.
Comparison of W22 and Dea Metabolism
The flux of Glc-P-to-Glc conversion was higher in W22 than in Dea, regardless of Glc starvation pretreatment. In order to estimate the Glc-phosphatase flux (Vgpase) in Dea maize, we determined the flux of Suc synthesis (Vsuc) and the Glc influx (Vg) after 25 h of incubation with Glc (Table IV). In Dea, Vg (350 nmol min−1 g−1 FW) was similar to that observed in W22 (Table I). In the absence of Suc, accumulation in Dea (Dieuaide-Noubhani et al., 1995), Vi, the unidirectional flux of Suc degradation, is equal to Vsuc, thus indicating that the maximal flux through invertase was Vi = 0.2 μmol min−1 g−1 FW, whereas Vgpase was around 0.8 μmol min−1 g−1 FW. These data clearly show that the difference measured in Vres was a consequence of the increase of Glc-phosphatase activity in W22 compared to Dea, the Suc hydrolysis rate being comparatively low in both genotypes.
DISCUSSION
Carbohydrate Starvation Reduces Substrate Cycling and Perturbs Metabolism
Internal pools of sugars can limit metabolic studies in plants because they delay the time necessary for reaching steady-state labeling. In a previous study, Dieuaide-Noubhani et al. (1995) starved maize root tips for 4 h before labeling. This treatment reduced the intracellular pools of sugars and the time required to reach isotopic steady state at 12 h, but it could potentially alter metabolic rates.
The prestarvation treatment significantly affects carbon enrichments (data not shown) and increases the C-1 to C-6 ratios (Table IV) in the hexose units of Suc and starch and in free Glc. The increase of the C-1 to C-6 ratio in free Glc indicates that the prestarvation treatment lowers hexose-P cycling by a factor of 2 in the W22 line (see Table IV). The C1-C6 randomization in Suc and starch, i.e. in hexose-P, has been observed in most plant materials (Hatzfeld and Stitt, 1990, Viola et al., 1991, Dieuaide-Noubhani et al., 1995, Rontein et al., 2002). It has been attributed to triose-P recycling through the PPi-dependent Fru-6-P phosphotransferase reaction (Hatzfeld and Stitt, 1990, Krook et al. 1998). In addition, it is established that the enrichment of C-1 is decreased by flux of hexose-P through the oxidative pentose phosphate pathway, and a careful examination of the enrichments of the C-1 and C-6 Glc carbons showed that part of this randomization was due to transaldolase activity (Dieuaide-Noubhani et al., 1995). The lower C-1 to C-6 ratio observed in control, compared to starvation pretreated root tips (Table IV), indicates that one or more of these fluxes of central metabolism are decreased by the starvation pretreatment relative to Glc uptake rate. In the absence of a complete analysis of fluxes, it is difficult to determine whether the variation of the C-1 to C-6 ratio is due to an increase in Vg, a decrease in the other fluxes, or both. However, it has been established that a number of treatments leading to an increased demand for carbohydrates induce the expression of Glc transporters at the plasma membrane (Sakr et al., 1997; Truernit et al., 1996). According to our data, Dea maize roots incubated immediately after excision absorbed Glc at a rate estimated at 1.1 nmol min−1 per root, which is 3 times lower than the value measured by Dieuaide-Noubhani et al. (1995). Thus, the higher C-1 to C-6 ratio observed after prestarvation treatment is likely to result, at least in part, from an increased Glc uptake.
Glc-P-to-Glc Conversion Cannot Be Explained by the Suc Cycle Alone and Suggests Glc-Phosphatase Activity
In steady-state labeling experiments, C-1 to C-6 scrambling of the intracellular Glc pool indicates that it is formed not only from exogenous Glc, but also from molecules derived from hexose-P. Using steady-state modeling, the total flux from hexose-P to Glc (Vres) was found to be 3 and 6 times the Glc influx in the Dea hybrid and the W22 line, respectively. This confirms the importance of hexose-P recycling in maize root tips (Dieuaide-Noubhani et al., 1995).
The hexose-P could have been directly degraded to Glc by a Glc-phosphatase or incorporated into Suc, starch, and cell wall polysaccharides before being returned to the Glc pool (see Fig. 1). In previous studies (Dieuaide-Noubhani et al., 1995; Rontein et al., 2002), the simplifying hypothesis was that the Glc-P-to-Glc conversion occurred only via Suc cycling. In this work, the possible contribution of turnover of different cellular sugar fractions to Glc resynthesis was evaluated by measuring their rate of synthesis using pulse-labeling experiments and calculating the reverse flux (Glc production) from the net accumulation. According to our data, Suc turnover accounts for only 7% of the total flux of Glc-P-to-Glc conversion. This low value is probably an overestimate because Suc is not degraded by invertase only, but also by SuSy, whose activity is included in the total Suc turnover measurement but does not contribute to Vres.
Starch and cell wall polysaccharide synthesis and turnover rates are low, and their minor contribution to Glc resynthesis was neglected. To confirm whether these turnovers contribute significantly to Vres, and to evaluate the possible error in Vgpase flux caused by ignoring them, we considered the unidirectional rates of starch and cell wall polysaccharides syntheses as the maximum rate of their Glc-P-to-Glc conversion. From this, it may be seen that starch and cell wall polysaccharide turnover contribute little to Vres, reducing the flux Vgpase by at most 6% and 2%, respectively. Furthermore, the amylolitic pathway leading to Glc may contribute to Vres, but the phosphorolytic pathway leading to Glc-1-P cannot; the relative contribution of these two pathways to starch degradation in maize root tips is not known.
Another possible source of Glc with C-1 to C-6 scrambling might be degradation of Suc to UDP-Glc and Fru by SuSy, followed by the direct conversion of Fru to Glc by a Glc-Fru isomerase. In microorganisms, a Xyl isomerase able to catalyze the isomerization reaction between Glc and Fru has been described (Bhosale et al., 1996). In Dea maize, a consensus sequence (TC 260043; www.tigr.org) showing 89% identity with a cDNA (EMBL accession no. X95257) coding for a Xyl isomerase of barley was described. However, according to Kristo et al. (1996), the barley enzyme does not catalyze Glc isomerization. The absence of a Glc-Fru isomerase activity in maize root tips is supported by our data. (1) In an attempt to test whether Glc and Fru might be directly interconverted, desalted protein extracts from maize root tips were incubated with 10 mm [U-14C]Fru or 10 mm [U-14C]Glc as substrates. No radioactivity transfer between Glc and Fru pools was detected (data not shown). The sensitivity of this assay was sufficient to detect a Glc/Fru isomerase activity of less than 1% of the calculated Vres flux. (2) In the hypothesis of rapid exchange between Glc and Fru through an isomerase reaction, the labeling of Fru would be intermediate between those of Glc and Suc fructosyl, or even identical to that of Glc. The similarity of the C-1 to C-6 ratios in Fru and Suc indicates that Fru is mainly formed from Suc, thus excluding the role of a Glc-Fru isomerase in Glc labeling in maize root tips.
Is There Molecular Evidence for Glc-Phosphatase in Plant Cells?
No Glc-phosphatase has yet been characterized in plants. However, several reports described phosphatase activity with Glc-6-P or Glc-1-P as substrates. In a recent study of maize root tips, Cortès et al. (2003) established the operation of such a phosphatase by measuring the dephosphorylation of the Glc-6-P analog, 3-O-methylglucose-6-P, using in vivo 31P-NMR spectroscopy. A Suc-6(F)-P phosphohydrolase (EC 3.1.3.24) was purified from rice leaves. The activity is dependent on Mg2+ and is highly specific for Suc 6(F)-P (Lunn et al., 2000). Of particular interest is the acidic phosphoenolpyruvate (PEP) phosphatase purified by Duff et al. (1989). It was designated as a PEP phosphatase owing to the high Vmax to Km ratio for this substrate, compared to 14 other natural substrates. However, its activity and affinity toward Glc-1-P and Glc-6-P are significant when the concentration of these metabolites in plant tissues is taken into account. This enzyme was found to be localized in the vacuole (Duff et al., 1991), and was associated with the bypass of the pyruvate kinase step of glycolysis that may be useful during phosphate starvation. Using a nonaqueous extraction method in cells of potato tubers, Farré et al. (2001) have shown that most PEP and Glc-6-P are cytosolic, but a small proportion of PEP, and as much as 34% of Glc-1-P, is located in the vacuole of potato tubers, making it plausible that the PEP phosphatase could hydrolyze Glc-1-P under physiological conditions.
Energy Cost and Possible Role of the Glc-P Cycle in Plant Cells
The cycling of each Glc via Glc-6-phosphatase costs one ATP, meaning that 2.5 ± 0.5 μmol of ATP are consumed/min g−1 FW. In W22, the respiratory rate was 1.2 ± 0.2 μmol O2 min−1 g−1 FW. Assuming that 30 molecules of ATP are produced from the degradation of each Glc molecule (Geigenberger and Stitt, 1991), the rate of ATP synthesis can be estimated as 6 ± 1 μmol min−1 g−1 FW. Thus, 41% of the ATP generated in the cell seems to be wasted by the Glc-P cycle. As a comparison, the Suc cycle requires one to three molecules of ATP per molecule of Suc, depending on the enzymes involved in Suc synthesis, and the equivalence of PPi with ATP as phosphate donor (Dieuaide-Noubhani et al., 1995; Trethewey et al., 1999). The attribution of Glc-P-to-Glc recycling to a phosphatase reaction rather than to the Suc cycle decreases the ATP cost about 2-fold. According to these data, the Suc cycle only consumes 3% to 6% of the ATP produced by respiration, which is in agreement with previously measured values (Trethewey et al., 1999).
In animal cells, Glc-6-phosphatases are involved in extracellular transport. By analogy, Moore and McClelen (1985) have suggested that Glc-6-phosphatase could be involved in mucilage secretion by root cap cells. However, this function is probably limited to a specific root domain and is probably achieved by an extracellular phosphatase. The turnover of Glc-P described in this work is certainly not associated with this process. Indeed, after correction by the diluting factors, Glc and Suc were found to be identically labeled in the mature domain and in the root tip (data not shown), thus indicating that the Glc-P turnover occurs in all root tissues, not only in the cap cells. It has been shown that phosphatase activities increase in response to Pi starvation and are thus implied in the remobilization of Pi (Plaxton, 1998; Raghotama, 1999). However, because Pi starvation inhibits growth (Plaxton 1998), it is unlikely to occur in maize root tips that are continuously growing during the 25 h of incubation. On the other hand, we cannot exclude the possibility that synthesis and degradation of hexose-P occur successively and not simultaneously: Active synthesis could decrease the Pi content in cytosol and would be followed by degradation of hexose-P in order to regenerate Pi. Recently, Urbanczyk-Wochniak et al. (2003) have suggested that glycolytic flux in plant cells is controlled by the rate of ATP utilization and that Suc turnover could increase the glycolytic rate by increasing the energy demand. A similar role could be ascribed to the hexose-P cycle.
CONCLUSION
Of the three established pathways that may introduce glucosyl units from Glc-P into the intracellular Glc pool, namely, turnover of cell wall, starch, or Suc, the major one appears to be Suc hydrolysis by invertase. However, the quantitative evaluation of these fluxes shows that they only account for a small fraction of total Glc-P turnover. This leads us to hypothesize the occurrence of a new substrate cycle between hexose-P and Glc in maize root tips, the simplest hypothesis being a Glc-phosphatase catalyzed reaction. The high activity measured in vivo (Table I) suggests an important role for this pathway of carbohydrate metabolism both in terms of carbon flux and energy cost.
MATERIALS AND METHODS
Materials and Incubations
Maize seeds (Zea mays L. cv Dea, Pioneer France Maïs, or W22) were germinated for 3 d in darkness at 25°C as described by Brouquisse et al. (1991). The 3.5-mm tips of primary roots were excised.
Short-Term Labeling Experiments
Maize root tips (10 roots/mL) were incubated for 25 h in medium A, as described by Brouquisse et al. (1991), supplemented with 200 mm Glc, and bubbled with a N2/O2 mixture (50:50, v/v). After incubation, the root tips were washed with abundant water to eliminate exogenous Glc, and then further incubated with 200 mm d-[U-14C]Glc for 5 to 60 min. Roots were harvested by filtration, washed with ice-cold water, and re-excised at 3.5 mm before being frozen in liquid N2.
Steady-State Labeling Experiments
In 13C-labeling experiments, 150 to 200 excised root tips were incubated for 25 h with 200 mm d-[U-13C]Glc (99% enrichment) or d-[1-13C]Glc (99% enrichment) in medium A, washed with abundant ice-cold water, re-excised at 3.5 mm, and frozen in liquid N2. When indicated, a starvation pretreatment (4 h in medium A) was performed before incubating maize roots with labeled Glc.
Preparation of Extracts
Acid Extracts of Glc-6-P and UDP-Glc
Water-soluble metabolites, including Glc-6-P and UDP-Glc, were extracted using perchloric acid as described by Brouquisse et al. (2001). After extraction, the extracts were dried.
Ethanolic Extraction and Fractionation of Ethanol-Soluble Compounds
The extraction of soluble components was performed using boiling aqueous solutions of ethanol (successively 80% and 50%), as described by Salon et al. (1988). The extract was resuspended in 1 mL water after evaporation. A neutral fraction containing soluble sugars was then prepared by passing the sample through cationic (AG 50W-X8 Superfine, H+ form; Bio-Rad Laboratories, Hercules, CA) and anionic (AG 1-X8 Superfine, formate form; Bio-Rad) columns, according to the manufacturer's instructions. Amino acids were eluted from the cationic column with 1.4 m NH4OH.
Starch Extraction
The residue remaining after ethanolic extraction was used for the analysis of starch. It was washed with 20% ethanol (v/v) and with water (1 mL/10 roots), and starch was converted to Glc enzymatically as described by Moing et al. (1994).
Cell Wall Polysaccharide Extraction
We used a protocol described by Gibeaut and Carpita (1991), with some modifications. Maize root tips were homogenized in 50 mm TES buffer, pH 5.5, containing 50 mm NaCl and 30 mm ascorbate. The extract was centrifuged for 10 min at 4,000 rpm. The pellet was washed successively with 0.5 m NaCl (3×), water (2×), methanol:chloroform (1:1, v/v; 2× for 30 min at 45°C), methanol (1×), and water (1×). Proteins were degraded by incubating the pellet with pronase, as described by Dieuaide-Noubhani et al. (1997). Starch was removed as described previously. The remaining pellet contained the cell wall polysaccharides.
Analysis of Metabolites
Normalization of the Results
According to the [1-14C]Glc labeling experiment, the SR of the external Glc varied between 20 and 150 dpm/nmol. To allow comparison of data from different experiments, all values are given after normalization of the SR of external Glc to 1, i.e. after dividing the radioactivity measured by the SR of the external Glc.
Purification of Sugar by HPLC
The neutral fraction was separated using a PL Hi-Plex calcium column (Polymer Laboratories, Amherst, MA), as described by Moing et al. (1994). Sugar detection was performed with an ERMA ERC-7515 refractometer. Sugar quantification was achieved with Millenium software (Waters, Milford, MA), after external calibration. Each peak was collected. In the case of 14C-labeling experiments, radioactivity was counted to determine the SR of each sugar. For the quantification of radioactivity incorporated in Suc glucosyl, the repartition of the labeling between the glucosyl and fructosyl moieties was determined in the first experiments. The purified Suc was hydrolyzed by yeast invertase in 0.2 m acetate buffer, pH 4.8. The sample was passed through anionic and cationic columns as described previously and the SR of the glucosyl and fructosyl moieties of Suc were determined after HPLC separation. The results showed that the glucosyl moiety accounted for 53% of the radioactivity measured in Suc independently of the incubation time (data not shown). The radioactivity incorporated into glucosyl was then estimated from the radioactivity incorporated into Suc, multiplied by 0.53. In the case of 13C-labeling experiments, fractions were dried before NMR analysis.
Purification of Amino Acids by HPLC
Amino acids from ethanolic extract were analyzed by reverse-phase HPLC (Thermo Separation Products, Riviera Beach, FL) on a Nova-Pack C18 column after o-phthaldialdehyde derivatization (Salon et al., 1988). Detection was performed with a Waters 486 spectrophotometer by following the absorption at 350 nm. Only Asp and Glu peaks were collected and counted. SR of Asp and Glu was determined as in a previous study (Salon et al., 1988).
Quantification and Determination of the SR of Glc-6-P
The SR of Glc-6-P was determined from the perchloric extracts, resuspended in water. First, the quantity of Glc-6-P present in the perchloric extract was determined by using an enzymatic assay described by Michal (1984), after some modifications. The sample was incubated in 100 mm KH2PO4 buffer at pH 7.5, containing 3 mm NADP. The reaction, carried out at 25°C, was started by adding 3 units of Glc-6-P dehydrogenase and the production of NADH was monitored at 340 nm. In a second step, the radioactivity incorporated into Glc-6-P was determined by following a procedure similar to that described by Dieuaide-Noubhani et al. (1995) for Glc analysis. The reaction was performed in a Warburg flask containing 100 mm KH2PO4 buffer at pH 7.5, containing 3 mm NADP, 3 units of Glc-6-P dehydrogenase, and 2 units of 6-phosphogluconate dehydrogenase. Whatman paper soaked with KOH 2% (w/v) was placed in the central well and 0.1 m HCl in the sidearm of the flask. The reaction was started by adding the extract and the flask was hermetically closed for 100 min. At the end of the incubation time, HCl was mixed with the reaction medium in order to stop the reaction and to liberate CO2. After 30 min, the paper filter was recovered, the central well was rinsed twice, and the radioactivity contained in CO2 (corresponding to carbon C-1 of Glc-6-P) was counted. Glc-6-P radioactivity was calculated by multiplying CO2 radioactivity by 6. Quantitative degradation and recovery of CO2 were confirmed with defined solutions of [U-14C]Glc-6-P.
Quantification and Determination of the SR of UDP-Glc
The analysis of the UDP-Glc was performed from perchloric extracts, according to the protocol of Räbinä et al. (2001). After analysis of Glc-6-P, the extracts were dried and resuspended in 1 mL of 10 mm NH4HCO3. The sugar nucleotides were partially purified on Supelclean Envi-Carb SPE columns (Supelco, Bellefonte, PA), previously conditioned with 3 mL of 80% (v/v) acetonitrile containing 0.1% (v/v) trifluoroacetic acid, and washed with 2 mL water. After loading the sample, the column was successively washed with 2 mL each of water, 25% (v/v) acetonitrile-water, and 50 mm aqueous triethylammonium acetate (TEAA) buffer at pH 7.0. The sugar nucleotides were then eluted with 3 mL of 25% (v/v) acetonitrile-water containing 50 mm TEAA at pH 7.0. The sugar nucleotides were then separated by HPLC on a Satisfaction RP18-AB 5-μm column, 250 × 4.6 mm (CIL, Cluzeau, France). The mobile phase was a gradient system with acetonitrile (A) and 20 mm TEAA buffer, pH 6.0 (B). The flow rate was 1 mL min−1. The separation was performed for 51 min, with 100% solvent B for 15 min, from 100% to 98% solvent B in 20 min, 98% to 96% solvent B in 5 min, 96% solvent B for 10 min, 96% to 100% solvent B in 1 min. A re-equilibration period of 9 min was used between individual runs. The detection wavelength was set at 254 nm. Identification and quantification of UDP-Glc were performed after external calibration. UDP-Glc was collected and the radioactivity was counted.
Determination of Glc and Suc Enrichments
NMR analyses were performed at 24°C with a Bruker Avance 500 spectrometer equipped with a 5-mm cryoprobe optimized for detecting 13C. 1H-NMR spectra were obtained at 500.16 MHz with a pulse of 10 μs (corresponding to an angle of 90°) using a recycling time greater than 6T1. 13C-NMR spectra were obtained at 125.77 MHz with a pulse of 6.7 μs (corresponding to an angle of 90°) using a recycling time greater than 6T1. For Suc and Glc, T1 was measured with an inversion-recovery sequence and found to be, respectively, 1.7 and 2.7 s for 1H carried by the C-1, 0.6 and 1.3 s for 13C-1, and 0.3 and 0.63 for 13C-6. Peak assignment was performed according to previous studies (Dieuaide-Noubhani et al., 1995; Rontein et al., 2002) and from spectra of pure compounds. The scan number was 128 and 512 for 1H and 13C spectra, respectively.
The absolute 13C enrichments of Glc-α and Glc-β C-1, Suc glucosyl C-1, and starch glucosyl C-1 were determined from 1H-NMR spectra, as the ratio of the area of the satellites (1H-13C J-coupling) area to the total area of the multiplet. The relative enrichments of Glc, Suc, and starch glucosyl carbons C-2 to C-6 were determined by 13C-NMR in comparison with the absolute enrichment of Glc, Suc glucosyl, and starch glucosyl C-1 measured by 1H-NMR.
Preliminary experiments of labeling with [1-13C]Glc showed that the enrichment of the Glc C-1 carbon was lower than that of the Suc glucosyl C-1 carbon. However, Suc glucosyl C-1 comes from internal Glc C-1, so it should be labeled as more than or the same as internal Glc. This discrepancy between the theoretical and the experimental values is a good argument in favor of the existence of an unlabeled pool of Glc. In the same way, unlabeled pools of Suc and starch could also exist. To estimate the quantitative importance of these unlabeled pools of sugars, maize root tips were incubated with [U-13C]Glc. The enrichments of the C-1 of purified Glc, Suc, and starch glucosyl units were determined by 1H-NMR. A diluting factor was determined (see Table I) and applied to the enrichment determined in [1-13C]Glc labeling experiments to obtain the corrected enrichments.
Quantification of Glc-6-Phosphatase Activity
The enzymatic activity was measured in extracts obtained from 30 to 50 maize root tips re-excised to 3.5 mm after 25-h incubation. Thus, the roots were ground at 4°C in 50 mm Tris buffer (pH 7.5) containing 2.6 mm dithiothreitol, 10 mm EDTA, 10 μm chymostatine, 1% (w/v) Triton X-100, and 1% (w/v) bovine serum albumin. The homogenate was centrifuged for 5 min at 15,000 rpm. The supernatant was then desalted through a G25 column equilibrated with 50 mm Tris buffer, pH 7.5, containing 2.6 mm dithiothreitol and 10 mm EDTA. After desalting, chymostatine (10 μm final concentration) was added to the extract.
Neutral and acidic activities were measured by incubating the extract in a 50 mm acetate, 50 mm MES, 50 mm Tris buffer, pH 7.5 or pH 5.5. The reaction, measured at 25°C, was initiated with 9 mm [U-14C]Glc-6-P (100 dpm/nmol). Aliquots of 200 μL were sampled after 20 and 40 min, and the reaction was stopped by adding 500 μL of boiling water and incubating 5 min at 95°C. The samples were then applied to an anionic column (AG 1-X8 Superfine, formate form; Bio-Rad), and the resin was washed with 5 volumes of water. The effluent, containing the [U-14C]Glc produced by Glc-6-phosphatase activity, was counted. The phosphatase activity was deduced from the production of labeled Glc between 20 and 40 min. In these experimental conditions, the activity was linear over a time period of 60 min.
Flux Measurements
Short Time-Labeling Method
The unidirectional rate of synthesis of a cellular compound can be measured after short time labeling by dividing the rate of radioactivity incorporated by the SR of the precursor. Several precautions have to be taken to measure accurate fluxes with this approach: (1) The labeled substrate must enter the tissue rapidly and uniformly; (2) the SR of the precursor, UDP-Glc, and the ADP-Glc for Suc and starch, respectively, have to be determined; and (3) the incubation time has to be as short as possible to ensure that the SR of the product is low, compared to that of its precursor, in order to avoid underestimating the flux. This last point is crucial in the case of starch and Suc, both involved in substrate cycles, because radioactivity could be lost by the degradation reaction (invertase and SuSy activities).
Steady-State Labeling Method
When isotopic labeling is long enough to reach steady state, both the concentration and labeling of metabolic intermediates are constant. For each compound X located at a metabolic crossroads, equations can be written describing metabolic steady state (ΣVs = ΣVd, where Vs and Vd represent rates of synthesis and degradation, respectively, expressed per mass of tissue) and isotopic steady state [Σ(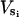 * SLi)= SLX * ΣVd, where
* SLi)= SLX * ΣVd, where 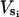 corresponds to the rate of synthesis of the compound X from a precursor i, SLi and SLX are the specific labeling of compounds i and X, respectively). Such equations can be written for each carbon of a metabolite. SL corresponds to SR or to 13C enrichment in the case of radioactive or 13C-enriched compounds, respectively. Determining the specific labeling of the compound involved allows quantification of relative fluxes. Absolute fluxes can be deduced after the quantification of one flux rate using an independent method. In this work, fluxes were determined relative to the rate of Glc uptake (Vg), which was quantified by 14C-labeling experiments. All fluxes were expressed as hexose nmol min−1 g−1 FW, except for Suc synthesis and degradation expressed as Suc nmol min−1 g−1 FW.
corresponds to the rate of synthesis of the compound X from a precursor i, SLi and SLX are the specific labeling of compounds i and X, respectively). Such equations can be written for each carbon of a metabolite. SL corresponds to SR or to 13C enrichment in the case of radioactive or 13C-enriched compounds, respectively. Determining the specific labeling of the compound involved allows quantification of relative fluxes. Absolute fluxes can be deduced after the quantification of one flux rate using an independent method. In this work, fluxes were determined relative to the rate of Glc uptake (Vg), which was quantified by 14C-labeling experiments. All fluxes were expressed as hexose nmol min−1 g−1 FW, except for Suc synthesis and degradation expressed as Suc nmol min−1 g−1 FW.
Acknowledgments
We thank Yair Shachar-Hill (Michigan State University, East Lansing, MI) for critical reading of the manuscript, and Michael Maucourt and Catherine Deborde for their technical assistance with NMR spectrometry.
This work was supported by a grant from the Institut National de la Recherche Agronomique (INRA), Université Victor Segalen Bordeaux 2 and Université Bordeaux 1 and the Conseil Regional d'Aquitaine, and by a doctoral fellowship from INRA and the Conseil Regional d'Aquitaine (to A.P.A.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.062083.
References
- Bhosale SH, Rao MB, Deshpande VV (1996) Molecular and industrial aspects of glucose isomerase. Microbiol Rev 60: 280–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg A (2000) Metabolic surprises in Saccharomyces cerevisiae during adaptation to saline conditions: questions, some answers and a model. FEMS Microbiol Lett 182: 1–8 [DOI] [PubMed] [Google Scholar]
- Brouquisse RM, Evrard A, Rolin D, Raymond P, Roby C (2001) Regulation of protein degradation and protease expression by mannose in maize root tips. Pi sequestration by mannose may hinder the study of its signaling properties. Plant Physiol 125: 1485–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouquisse RM, James F, Raymond P, Pradet A (1991) Study of glucose starvation in excised maize root tips. Plant Physiol 96: 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortès S, Gromova M, Evrard A, Roby C, Heyraud A, Rolin DB, Raymond P, Brouquisse RM (2003) In plants, 3-O-methylglucose is phosphorylated by hexokinase but not perceived as a sugar. Plant Physiol 131: 824–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieuaide-Noubhani M, Canioni P, Raymond P (1997) Sugar-starvation-induced changes of carbon metabolism in excised maize root tips. Plant Physiol 115: 1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieuaide-Noubhani M, Raffard G, Canioni P, Pradet A, Raymond P (1995) Quantification of compartmented metabolic fluxes in maize root tips using isotope distribution from 13C- or 14C-labeled glucose. J Biol Chem 270: 13147–13159 [DOI] [PubMed] [Google Scholar]
- Duff SMG, Lefebvre DD, Plaxton WC (1989) Purification and characterization of a phosphoenolpyruvate phosphatase from Brassica nigra suspension cells. Plant Physiol 90: 734–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff SMG, Lefebvre DD, Plaxton WC (1991) Purification, characterization and subcellular localization of an acid phosphatase from Brassica nigra suspension cells. Arch Biochem Biophys 286: 226–232 [DOI] [PubMed] [Google Scholar]
- Farré EM, Tiessen A, Roessner U, Geigenberger P, Trethewey RN, Willmitzer L (2001) Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiol 127: 685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell D (1997) Understanding the Control of Metabolism. Portland Press, London, pp 213–225
- Fernie AR, Roscher A, Ratcliffe RG, Kruger NJ (2001) Fructose 2,6-bisphosphate activates pyrophosphate: fructose-6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta 212: 250–263 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Tiessen A, Stitt M, Willmitzer L, Geigenberger P (2002) Altered metabolic fluxes result from shifts in metabolite levels in sucrose pyrophosphorylase-expressing potato tubers. Plant Cell Environ 25: 1219–1232 [Google Scholar]
- Geigenberger P, Reimholz R, Geiger M, Merlo L, Canale L, Stitt M (1997) Regulation of sucrose to starch metabolism in potato tubers in response to short-term water deficit. Planta 201: 502–518 [Google Scholar]
- Geigenberger P, Stitt M (1991) Regulation of carbon partitioning between sucrose and nitrogen assimilation in cotyledons of germinating Ricinus communis L. seedlings. Planta 185: 563–568 [DOI] [PubMed] [Google Scholar]
- Gibeaut DN, Carpita NC (1991) Tracing cell wall biogenesis in intact cells and plants. Plant Physiol 97: 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glawischnig E, Gierl A, Tomas A, Bacher A, Eisenreich W (2002) Starch biosynthesis and intermediary metabolism in maize kernels. Quantitative analysis of metabolite flux by nuclear magnetic resonance. Plant Physiol 130: 1717–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleixner G, Scrimgeour C, Schmidt HL, Viola R (1998) Stable isotope distribution in the major metabolites of source and sink organs of Solanum tuberosum L.: a powerful tool in the study of metabolic partitioning in intact plants. Planta 207: 241–245 [Google Scholar]
- Haigler CH, Ivanova-Datcheva M, Hogan PS, Salnikov VV, Hwang S, Martin K, Delmer DP (2001) Carbon partitioning to cellulose synthesis. Plant Mol Biol 47: 29–51 [PubMed] [Google Scholar]
- Hargreaves JA, ap Rees T (1988) Turnover of starch and sucrose in roots of Pisum sativum. Phytochemistry 27: 1627–1629 [Google Scholar]
- Hatzfeld WD, Stitt M (1990) A study of the rate of recycling of trioses phosphates in heterotrophic Chenopodium rubrum cells, potato tubers, and maize endosperm. Planta 180: 198–204 [DOI] [PubMed] [Google Scholar]
- Hill SA, ap Rees T (1994) Fluxes of carbohydrate metabolism in ripening bananas. Planta 192: 52–60 [Google Scholar]
- Hill SA, ap Rees T (1995) The effect of hypoxia on the control of carbohydrate metabolism in ripening bananas. Planta 197: 313–323 [Google Scholar]
- Jones ME, Berry MN, Phillips JW (2002) Futile cycles revisited: a Markov chain model of simultaneous glycolysis and gluconeogenesis. J Theor Biol 217: 509–523 [DOI] [PubMed] [Google Scholar]
- Katz K, Grunnet N (1979) Estimation of metabolic pathways in steady state in vitro. Rates of tricarboxylic acid and pentose cycle. In HL Kornberg, JC Metcalfe, DH Northcote, CI Pogson, KF Tipton, eds, Techniques in the Life Sciences, Vol B2/1. Elsevier/North-Holland Scientific Publishers, Limerick, Ireland, pp 1–18
- Kristo P, Saarelainen R, Fagerström R, Aho S, Korhola M (1996) Protein purification, and cloning and characterization of the cDNA and gene for xylose isomerase of barley. Eur J Biochem 237: 240–246 [DOI] [PubMed] [Google Scholar]
- Krook J, Vreugdenhil D, Dijkema C, Van der Plas L (1998) Sucrose and starch metabolism in carrot (Daucus carota L.) cell suspensions analysed by 13C-labelling: indications for a cytosol and a plastid-localized oxidative pentose phosphate pathway. J Exp Bot 49: 1917–1924 [Google Scholar]
- Landau BR (1999) Quantifying the contribution of gluconeogenesis to glucose production in fasted human subjects using stable isotopes. Proc Nutr Soc 58: 963–972 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Ashton AR, Hatch MD, Heldt HW (2000) Purification, molecular cloning, and sequence analysis of sucrose-6(F)-phosphate phosphohydrolase from plants. Proc Natl Acad Sci USA 97: 12914–12919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michal G (1984) D-glucose 6-phosphate and D-fructose 6-phosphate. In HU Bergmeyer, J Bergmeyer, M Graβl, eds, Methods of Enzymatic Analysis, Ed 3, Vol VI. Verlag Chemie GmbH, Weinheim, Germany, pp 191–198
- Moing A, Escobar-Gutierrez A, Gaudillère JP (1994) Modeling carbon export out of mature peach leaves. Plant Physiol 106: 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R, McClelen CE (1985) The involvement of glucose-6-phosphatase in mucilage secretion by root cap cells of Zea mays. Ann Bot (Lond) 56: 139–142 [DOI] [PubMed] [Google Scholar]
- Newsholme EA, Challis RAJ, Crabtree B (1984) Substrate cycles: their role in improving sensitivity in metabolic control. Trends Biochem Sci 9: 277–280 [Google Scholar]
- Nguyen-Quoc B, Foyer CH (2001) A role for ‘futile cycles’ involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. J Exp Bot 52: 881–889 [DOI] [PubMed] [Google Scholar]
- N'tchobo H, Dali N, Nguyen-Quoc B, Foyer CH, Yelle S (1999) Starch synthesis in tomato remains constant throughout fruit development and is independent on sucrose supply and sucrose synthase activity. J Exp Bot 50: 1457–1463 [Google Scholar]
- Plaxton W (1998) Metabolic aspects of phosphate starvation in plants. In JP Lynch, J Deikman, eds, Phosphorus in Plant Biology: Regulatory Roles in Molecular, Cellular, Organismic and Ecosystem Processes. American Society of Plant Physiologists, Rockville, MD, pp 229–241
- Portais JC, Delort AM (2002) Carbohydrate cycling in micro-organisms: what can (13)C-NMR tell us? FEMS Microbiol Rev 26: 375–402 [DOI] [PubMed] [Google Scholar]
- Räbinä J, Maki M, Savilahti EM, Jarvinen N, Penttila L, Renkonen R (2001) Analysis of nucleotide sugars from cell lysates by ion-pair solid-phase extraction and reversed-phase high-performance liquid chromatography. Glycoconj J 18: 799–805 [DOI] [PubMed] [Google Scholar]
- Raghotama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Rontein D, Dieuaide-Noubhani M, Dufourc EJ, Raymond P, Rolin D (2002) The metabolic architecture of plant cells. Stability of central metabolism and flexibility of anabolic pathway during the growth cycle of tomato cells. J Biol Chem 277: 43948–43960 [DOI] [PubMed] [Google Scholar]
- Roscher A, Emsley L, Raymond P, Roby C (1998) Unidirectional steady state rates of central metabolism enzymes measured simultaneously in a living plant tissues. J Biol Chem 273: 25053–25061 [DOI] [PubMed] [Google Scholar]
- Sakr S, Noubahni M, Bourbouloux A, Riesmeier J, Frommer WB, Sauer N, Delrot S (1997) Cutting, ageing and expression of plant membrane transporters. Biochim Biophys Acta 1330: 207–216 [DOI] [PubMed] [Google Scholar]
- Salon C, Raymond P, Pradet A (1988) Quantification of carbon fluxes through the tricarboxylic acid cycle in early germinating lettuce embryos. J Biol Chem 263: 12278–12287 [PubMed] [Google Scholar]
- Schwender J, Ohlrogge JB, Shachar-Hill Y (2003) A flux model of glycolysis and the oxidative pentose phosphate pathway in developing Brassica napus embryos. J Biol Chem 278: 29442–29453 [DOI] [PubMed] [Google Scholar]
- Teusink B, Walsh MC, van Dam K, Westerhoff HV (1998) The danger of metabolic pathways with turbo design. Trends Biochem Sci 23: 162–169 [DOI] [PubMed] [Google Scholar]
- Trethewey RN, Fernie AR, Bachmann A, Fleischer-Notter H, Geigenberger P, Willmitzer L (2001) Expression of a bacterial sucrose phosphorylase in potato tubers results in a glucose-independent induction of glycolysis. Plant Cell Environ 24: 357–365 [Google Scholar]
- Trethewey RN, Geigenberger P, Riedel K, Hajirezaei MR, Sonnewald U, Stitt M, Riesmeier JW, Willmitzer L (1998) Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J 15: 109–118 [DOI] [PubMed] [Google Scholar]
- Trethewey RN, Riesmeier JW, Willmitzer L, Stitt M, Geigenberger P (1999) Tuber-specific expression of a yeast invertase and a bacterial glucokinase in potato leads to an activation of sucrose phosphate synthase and the creation of a sucrose futile cycle. Planta 208: 227–238 [DOI] [PubMed] [Google Scholar]
- Truernit E, Schmid J, Epple P, Illig J, Sauer N (1996) The sink-specific and stress-regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 8: 2169–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanczyk-Wochniak E, Leisse A, Roessner-Tunali U, Lytovchenko A, Reismeier J, Millmitzer L, Fernie AR (2003) Expression of a bacterial xylose isomerase in potato tubers results in an altered hexose composition and a consequent induction of metabolism. Plant Cell Physiol 44: 1359–1367 [DOI] [PubMed] [Google Scholar]
- Viola R, Davies HV, Chudeck AR (1991) Pathways of starch and sucrose biosynthesis in developing tubers of potato (Solanum tuberosum L.) and seeds of fava bean (Vicia fava L.). Planta 183: 202–208 [DOI] [PubMed] [Google Scholar]