Abstract
Transitory starch is stored during the day inside chloroplasts and broken down at night for export. Maltose is the primary form of carbon export from chloroplasts at night. We investigated the influence of daylength and circadian rhythms on starch degradation and maltose metabolism. Starch breakdown was faster in plants of Arabidopsis (Arabidopsis thaliana) ecotype Wassilewskija growing in long days. Transcript levels of genes encoding enzymes involved in starch degradation and maltose metabolism showed a strong diurnal rhythm. Under altered photoperiods, the transcript levels and the rate of starch degradation changed within one day/night cycle. However, the amount of proteins involved in starch degradation was maintained relatively constant throughout the day/night cycle. To investigate whether the diurnal cycling of the transcript levels is only a response to light or is also regulated by a circadian clock, we measured the amount of messenger RNAs in Arabidopsis leaves under continuous light and continuous darkness. The expression of genes encoding starch degradation-related enzymes was under very strong circadian control in continuous light. Under continuous light, the amount of maltose also showed a strong endogenous rhythm close to 24 h, indicating that maltose metabolism is under circadian control. Light is necessary for the cycling of transcript levels and maltose levels. Under continuous darkness, these genes were barely expressed, and no cycling of maltose levels was observed.
Starch is the most abundant carbohydrate reserve in plants. There are two types of starch: storage starch and transitory starch. Transitory starch is stored during the day inside chloroplasts and broken down at night for export. At night, starch is converted to maltodextrin by several enzymes, such as debranching enzyme, and appears to be influenced by a glucan, water dikinase (GWD) and phosphoglucan, water dikinase (PWD) (Ritte et al., 2000, 2002; Trethewey and Smith, 2000; Yu et al., 2001; Smith et al., 2003; Zeeman et al., 2004; Kotting et al., 2005). α-Amylase (AtAMY3) was thought to be involved in the conversion of starch to maltodextrin (Trethewey and Smith, 2000), but recent data indicate that AtAMY is not required for transitory starch breakdown in Arabidopsis (Arabidopsis thaliana) leaves (Yu et al., 2005). Maltodextrin is then converted to maltose and Glc by β-amylase (CT-BMY) and disproportionating enzyme (DPE1) in the chloroplast (Lao et al., 1999; Critchley et al., 2001; Scheidig et al., 2002; Schneider et al., 2002). Recent evidence indicates that maltose and Glc are the two major forms of carbon exported from chloroplasts at night (Weber et al., 2000; Servaites and Geiger, 2002; Weise et al., 2004). Maltose is exported by the maltose transporter MEX1 (Niittylä et al., 2004) and is metabolized in the cytosol by several enzymes, including disproportionating enzyme (DPE2) and possibly glucan phosphorylase (AtPHS2) (Chia et al., 2004; Lu and Sharkey, 2004; Schupp and Ziegler, 2004). Localization of DPE2 in the literature is conflicting. DPE2 protein from Arabidopsis was demonstrated to be present in the cytosol (Chia et al., 2004). The potato (Solanum tuberosum) isoform of DPE2 protein (stDPE2) was demonstrated to be present in chloroplasts (Lloyd et al., 2004). Inhibition of maltose export or maltose metabolism results in a 20- to 90-fold increase in leaf maltose content and a dwarf phenotype, showing that Glc cannot compensate for a loss of capacity for maltose metabolism (Lu and Sharkey, 2004; Niittylä et al., 2004). Antisense inhibition of cytosolic phosphorylase in potato plants has only little impact on carbohydrate metabolism (Duwenig et al., 1997). However, preliminary data from our lab using heterozygous AtPHS2 knockout plants of Arabidopsis indicate that lack of this enzyme also results in 164% increase in maltose level and 23% decrease in Suc level at night.
There are two possible roles for transitory starch. First, transitory starch may act as an overflow for newly assimilated carbon when assimilation exceeds the demand for Suc (Stitt and Quick, 1989). Second, transitory starch may provide a source of carbon for growth during the following night (Trethewey and Smith, 2000). If transitory starch is primarily a carbon overflow, then the rate of starch synthesis should be independent of daylength, while the rate of starch degradation should vary at different daylengths. If transitory starch is primarily a carbon source for nighttime growth, then the rate of starch degradation should be the same regardless of daylength. Dodd et al. (2003) reported that in Mesembryanthemum crystallinum, starch turnover regulates and limits carbon fluxes through the diel Crassulacean acid metabolism (CAM) cycle.
Accumulation and degradation of starch could be controlled by light, metabolites, daylength, or circadian rhythms. Light regulates transcript abundance of >100 genes, such as the RbcS (Rubisco small subunit) gene family and the CAB (chlorophyll a/b binding protein) gene family (Terzaghi and Cashmore, 1995). Light also acts as an entraining stimulus for circadian rhythms across a wide variety of organisms (McClung, 2001; Schultz and Kay, 2003). Light can also trigger posttranslational regulation of ADP-Glc pyrophosphorylase and Suc-P synthase in leaves (Hendriks et al., 2003; Trevanion et al., 2004). Sugars regulate carbon metabolism, gene expression, and posttranslational modification of some enzymes in starch metabolism in addition to their roles as substrates (Koch, 1996; Rolland et al., 2002; Tiessen et al., 2002; Hendriks et al., 2003; Sharkey et al., 2004). The level and turnover of starch are involved in endogenous regulation in response to carbohydrate status (Geiger et al., 2000). It has been reported that decreasing daylength can lead to stimulation of starch synthesis and that starch degradation can be affected by the length of photoperiod (Stitt et al., 1978; Chatterton and Silvius, 1979, 1980; Matt et al., 1998; Gibon et al., 2004b). In C3 plants and CAM plants, it has been suggested that diurnal starch accumulation is under circadian regulation (Li et al., 1992; Geiger et al., 1995; Borland and Taybi, 2004). Using microarray analysis, several groups have found that transcripts of some enzymes in starch degradation increase in amount before the dark period and continue to increase thereafter (Harmer et al., 2000; Schaffer et al., 2001; Smith et al., 2004). This suggests that starch mobilization could also be under circadian control. However, how these different components contribute to the regulation of starch degradation rate and maltose metabolism in response to the changes in daylength and the circadian clock is still unclear.
In this study, we investigated the influence of daylength and circadian clock on starch degradation and maltose metabolism in Arabidopsis ecotype Wassilewskija. We compared the rates of starch mobilization and carbohydrate levels in long days with those in short days. We studied how rapidly Arabidopsis can adjust the rate of starch degradation after we changed daylength. To study whether changes in carbohydrate levels and starch degradation rates are reflected in the transcript and protein levels of key enzymes, we determined the relative amount of transcripts and proteins under various conditions using real-time RT-PCR and western blots. We also analyzed whether the cycling of transcript levels, starch levels, and metabolite levels is exclusively a response to light or whether it is also regulated by a circadian clock. We determined the amount of the same transcripts, proteins, and carbohydrates under continuous light or darkness after entraining plants in a long-day regime.
RESULTS
Starch Breakdown Was Faster in Plants Growing in Long Days
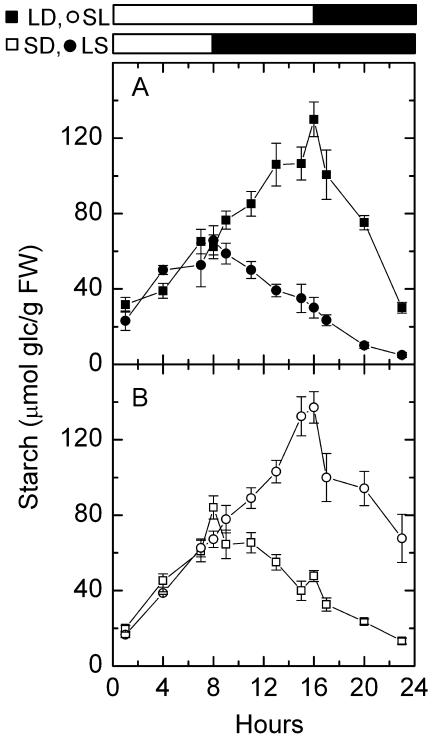
We first investigated the impact of different daylengths on the rate of starch degradation. We measured leaf starch levels of Arabidopsis plants in a 16-h-light/8-h-night regime (long days [LD]) and an 8-h-light/16-h-night regime (short days [SD]). In Arabidopsis, transitory starch was accumulated and broken down almost linearly (Fig. 1). Starch accumulation was faster in plants growing in SD than those growing in LD, but not sufficiently faster to compensate for the short photoperiod (Fig. 1; Table I). Thus, by the end of the day, plants growing in LD accumulated more starch than plants growing in SD (Fig. 1). At night, plants growing in LD/short nights had a much faster rate of starch degradation than plants growing in SD/long nights (Table I). More starch was left in the leaves at the end of night in LD (Fig. 1A) than in SD (Fig. 1B).
Figure 1.
Diurnal changes of starch in LD, LS, SD, and SL. A, LD (black squares) and LS (black circles). B, SD (white squares) and SL (white circles). White bars and black bars on the top indicate days and nights, respectively. Values are mean ± se (n = 5). FW, fresh weight.
Table I.
Rate of starch synthesis and degradation in LD, LS, SD, and SL
| Photoperiod
|
Rate of Starch Metabolism
|
|
|---|---|---|
| Synthesisa | Degradationb | |
| μmol glc g−1 FW h−1 | ||
| LD | 6.41 ± 0.47 | 13.19 ± 1.61 |
| LS | 5.34 ± 1.33 | 4.14 ± 0.19 |
| SD | 8.30 ± 1.37 | 4.39 ± 0.40 |
| SL | 8.01 ± 0.32 | 8.22 ± 2.69 |
Values are slope ± se of a linear regression of all the daytime values of starch over the duration of the day.
Values are slope ± se of a linear regression of all the nighttime values of starch over the duration of the night.
We next investigated whether the rate of starch degradation decreased after a transition from a LD to a SD regime. Arabidopsis plants were transferred from a 16-h-light/8-h-dark regime to an 8-h-light/16-h-dark regime. The amount of starch was measured before, during, and after this shift. The rate of starch degradation during the first elongated night (long day to short day [LS]), 4.14 ± 0.19 μmol Glc g−1 fresh weight (FW) h−1 (P < 0.0001), was much lower than that during a regular short night (LD), 13.19 ± 1.61 μmol glc g−1 FW h−1 (P < 0.05; Table I). Less starch was left in the leaves at the end of the first extended night than at the end of a regular short night (Fig. 1A).
We also investigated whether the rate of starch degradation increased after a transition from a SD to a LD regime. Arabidopsis plants were transferred from an 8-h-light/16-h-dark regime to a 16-h-light/8-h-dark regime. The rate of starch degradation during the first shortened night (short day to long day [SL]), 8.22 ± 2.69 μmol glc g−1 FW h−1 (P = 0.09), was much higher than that during a regular long night (SD), 4.39 ± 0.40 μmol glc g−1 FW h−1 (P < 0.0001). More starch was left in the leaves at the end of the first shortened night than at the end of a regular long night (Fig. 1B).
Transcript Levels of Starch Degradation-Related Genes Oscillated during the Day/Night Cycle
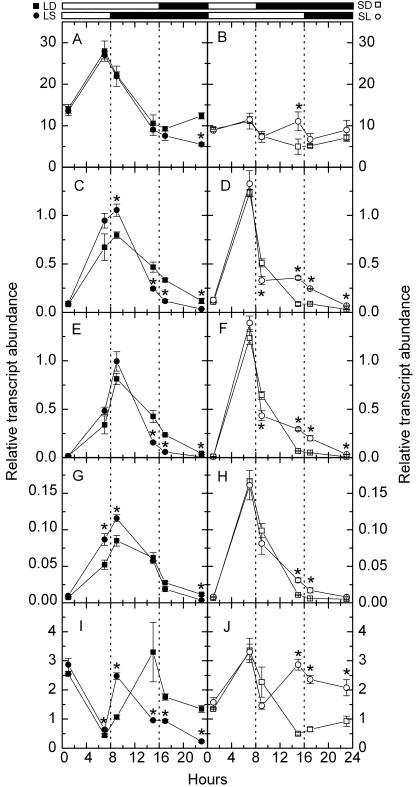
To study the expression of starch degradation-related enzymes in different daylengths, we measured relative transcript abundance of starch degradation-related enzymes, including R1 protein (SEX1), chloroplastic β-amylase (CT-BMY), cytosolic D-enzymes (DPE2), and cytoslic glucan phosphorylase (AtPHS2) by quantitative RT-PCR. Among five genes we assayed, RbcS1A had the most abundant transcript, CT-BMY had the next most abundant transcript, DPE2 and AtPHS2 had fewer transcripts than CT-BMY, and SEX1 had the least abundant transcript (Fig. 2). However, the diel changes in expression of starch degradation-related genes were much greater than the well-known alternation of RbcS1A mRNA. Typically, AtPHS2 showed up to 100-fold of change in expression in SD (Fig. 2F), while RbcS1A only showed about 3-fold of change in expression in LD (Fig. 2A). DPE2, AtPHS2, and SEX1 in LD shared the same expression pattern: an increase between 1 and 9 h of the day, followed by a decline during the rest of the day and throughout the night (Fig. 2, C, E, and G). DPE2, AtPHS2, and SEX1 in SD shared the same expression pattern: a rapid increase during the day, followed by a rapid decline at dusk and a slow decline between 1 and 7 h of the night, and a slower decline during the rest of the night (Fig. 2, D, F, and H). CT-BMY had different expression patterns: the transcript levels peaked at dawn and at dusk in LD but peaked only at dusk in SD (Fig. 2, I and J).
Figure 2.
Diurnal transcript amounts of starch degradation-related genes. A and B, RbcS1A; C and D, DPE2; E and F, AtPHS2; G and H, SEX1; I and J, CT-BMY. Left sections, LD (black squares) and LS (black circles). Right sections, SD (white squares) and SL (white circles). White bars and black bars on the top indicate days and nights, respectively. Asterisks near circles indicate that LS or SL samples (circles) are significantly different from LD or SD samples (squares) collected at the same local time (Student's t test, P = 0.05). Values are mean ± se (n = 3).
We next investigated whether the transcript levels of starch-related enzymes in plants are decreased during the first long night after a transition from a LD to a SD regime. We found that this is the case in Arabidopsis. There was a significant decrease in relative transcript abundance of DPE2, AtPHS2, and CT-BMY at 7 h of the first extended night and the rest of the first extended night (Fig. 2, C, E, and I). The effect of changes in daylength on the expression pattern of CT-BMY was dramatic. The dusk peak of CT-BMY transcript levels changed from local time 3 pm (1 h before the end of day) to local time 9 am (1 h in the night) because the day was shortened from 16 to 8 h (Fig. 2I). The effect of changes in daylength on the expression pattern of RbcS1A was not as dramatic. The only significant change was the decrease of RbcS1A transcript levels at 1 h before the end of the first extended night (Fig. 2A).
We also found that the transcript levels of starch-related enzymes in Arabidopsis plants are increased during the first short night after a transition from a SD to a LD regime. There was a significant increase in relative transcript abundance of DPE2, AtPHS2, SEX1, and CT-BMY at the end of the first extended day and at the first short night (Fig. 2, D, F, H, and J). The effect of changes in daylength on the expression pattern of CT-BMY was substantial. The second peak of CT-BMY transcript levels in LD, which disappeared in SD, showed up in the first extended day (Fig. 2J). There was only one RbcS1A mRNA peak at 7 h of the day in a regular SD regime (Fig. 2B). But there were two RbcS1A mRNA peaks in the first altered day/night cycle: one at 7 h of the day and the other at 15 h of the first extended day (Fig. 2B).
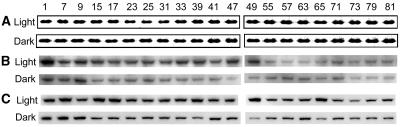
Protein Levels of Starch Degradation-Related Enzymes Did Not Oscillate during the Day/Night Cycle
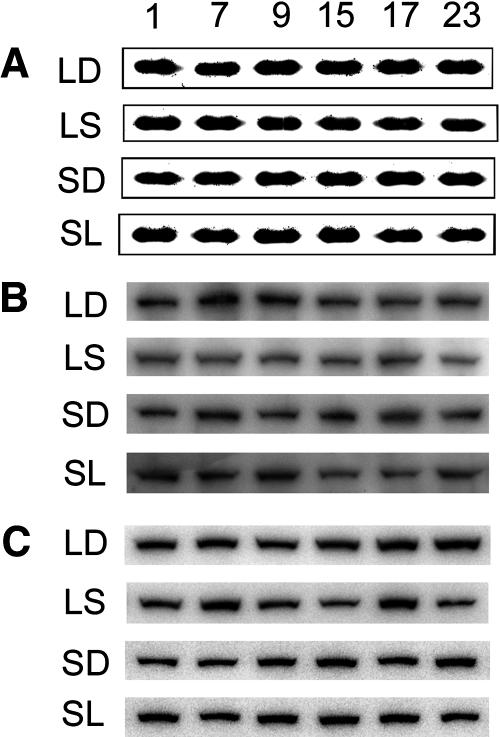
To study whether the diurnal cycling of transcript levels of starch degradation-related enzymes is reflected in the diurnal protein levels, we assayed the protein abundance of RbcS, SEX1, and DPE2 with western blots. The protein levels of RbcS (Fig. 3A), SEX1 (Fig. 3B), and DPE2 (Fig. 3C) were relatively constant throughout the day/night cycle in both LD and SD. The protein levels of these enzymes were constant during the first altered day/night cycle after a transition from a long- to a short-day regime (Fig. 3). They also were constant during the first altered day/night cycle after a transition from a short- to a long-day regime (SL) (Fig. 3).
Figure 3.
Diurnal protein abundance of RbcS, SEX1, and DPE2. A, Coomassie stains of RbcS as control; B, Western blots of SEX1; C, Western blots of DPE2 in LD, LS, SD, and SL. Numbers on the top are sample-collecting time in hours.
Metabolite Contents Had Different Profiles at Different Day/Night Regimes
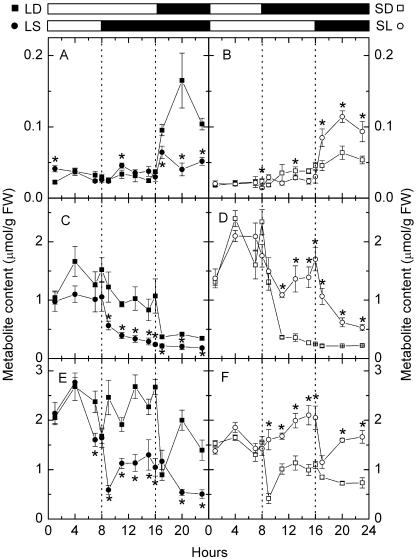
Plants growing in LD accumulated more starch and had a faster rate of starch degradation than plants growing in SD. To investigate the impact of different daylengths on the rate of starch degradation, we measured diurnal contents of G6P, Glc, α-maltose, β-maltose, and Suc in Arabidopsis leaves in LD and SD. β-Maltose is the metabolically active anomer of maltose during transitory starch degradation (Weise et al., 2005). The diurnal changes of β-maltose levels in LD were greater than those in SD (Fig. 4, A and B). The amount of β-maltose in LD was relatively constant during the day and it increased during the first 4 h of the night and decreased during the rest of the night (Fig. 4A). The amount of β-maltose in SD was again relatively constant during the day and it increased slowly during the first 12 h of the night and decreased during the rest of the night (Fig. 4B). The diurnal changes of Glc levels in LD were smaller than those in SD (Fig. 4, C and D). The average amount of daytime Glc in LD was significantly smaller than that in SD (P = 0.006; Table II), and the amount of Glc decreased rapidly during the first hour of the night in both LD and SD (Fig. 4, C and D). The average amount of daytime Suc in LD was significantly higher than that in SD (P < 0.0001; Table II). The amount of Suc decreased rapidly during the first hour of the night, then increased rapidly during the next 3 h of night, and decreased during the rest of the night in both LD and SD (Fig. 4, E and F).
Figure 4.
Diurnal changes of carbohydrate levels in LD, LS, SD, and SL. A and B, β-Maltose; C and D, Glc; E and F, Suc. Left sections, LD (black squares) and LS (black circles). Right sections, SD (white squares) and SL (white circles). White bars and black bars on the top indicate days and nights, respectively. Asterisks near circles indicate that LS or SL samples (circles) are significantly different from LD or SD samples (squares) collected at the same local time (Student's t test, P = 0.05). Values are mean ± se (n = 5).
Table II.
Comparison of daytime and nighttime carbohydrates in LD, LS, SD, and SL
Nighttime carbohydrate levels that are significantly different from daytime levels are marked with an asterisk (Student's t test, P = 0.05).
| Carbohydrate | LD | LS | SD | SL | |
|---|---|---|---|---|---|
| μmol/g FW | |||||
| G6P | Daya | 0.254 ± 0.012 | 0.271 ± 0.016 | 0.195 ± 0.011 | 0.234 ± 0.010 |
| Nightb | 0.263 ± 0.011 | 0.177 ± 0.010* | 0.167 ± 0.006* | 0.242 ± 0.019 | |
| Glc | Day | 1.251 ± 0.095 | 1.029 ± 0.095 | 1.769 ± 0.032 | 1.583 ± 0.080 |
| Night | 0.377 ± 0.015* | 0.303 ± 0.025* | 0.399 ± 0.059* | 0.738 ± 0.081* | |
| α-Maltose | Day | 0.037 ± 0.002 | 0.046 ± 0.006 | 0.029 ± 0.003 | 0.028 ± 0.001 |
| Night | 0.070 ± 0.005* | 0.032 ± 0.002* | 0.037 ± 0.003 | 0.059 ± 0.008* | |
| β-Maltose | Day | 0.030 ± 0.002 | 0.034 ± 0.003 | 0.021 ± 0.003 | 0.022 ± 0.001 |
| Night | 0.121 ± 0.015* | 0.041 ± 0.003 | 0.042 ± 0.003* | 0.097 ± 0.007* | |
| Maltose | Day | 0.067 ± 0.003 | 0.080 ± 0.007 | 0.050 ± 0.005 | 0.050 ± 0.002 |
| Night | 0.191 ± 0.015* | 0.073 ± 0.004 | 0.079 ± 0.003* | 0.156 ± 0.010* | |
| Suc | Day | 2.249 ± 0.091 | 2.169 ± 0.153 | 1.493 ± 0.062 | 1.628 ± 0.082 |
| Night | 1.430 ± 0.154* | 0.926 ± 0.074* | 0.860 ± 0.049* | 1.469 ± 0.084 | |
Daytime carbohydrate levels are mean of all the daytime samples ± se (n = 40 for LD and SL; n = 15 for SD and LS).
Nighttime carbohydrate levels are mean of all the nighttime samples ± se (n = 15 for LD and SL; n = 40 for SD and SL).
To study whether the metabolite levels in Arabidopsis leaves could change during the first altered day/night cycle after the instant changes in daylength, we measured the diurnal metabolite levels in Arabidopsis leaves after a transition from a LD to a SD regime. The amount of β-maltose did not increase during the first 8 h of extended night but increased a little more during the rest of the extended night (Fig. 4A). However, the amount of Glc dropped during the first hour of the extended night and declined slowly throughout the rest of the extended night (Fig. 4C). The amount of Suc dropped substantially during the first hour of the extended night and recovered during the next 3 h of the extended night, remained constant for another 6 h, and declined again during the rest of the night (Fig. 4E). As a response to the absence of light, the transition point between daytime and nighttime Glc and Suc levels shifted from local time 4 pm to local time 8 am (Fig. 4, C and E).
We next measured the diurnal metabolite levels in Arabidopsis leaves after a transition from a SD to a LD regime. The amount of β-maltose during the extended 8 h of light was not significantly lower than that of the same local time in a regular SD regime (P = 0.1018; Fig. 4B). The amount of β-maltose increased substantially during the first 4 h of the shortened night, to a much higher level than that of the same local time, and declined during the rest of the shortened night (Fig. 4B). The amount of Glc was high in the extended 8 h of light and declined during the shortened night (Fig. 4D). The amount of Suc was high in the extended 8 h of light and declined substantially during the first hour of the shortened night, then increased again during the rest of the shortened night (Fig. 4F). As a response to the presence of light, the transition point between daytime and nighttime Glc and Suc shifted from local time 8 am to local time 4 pm (Fig. 4, D and F).
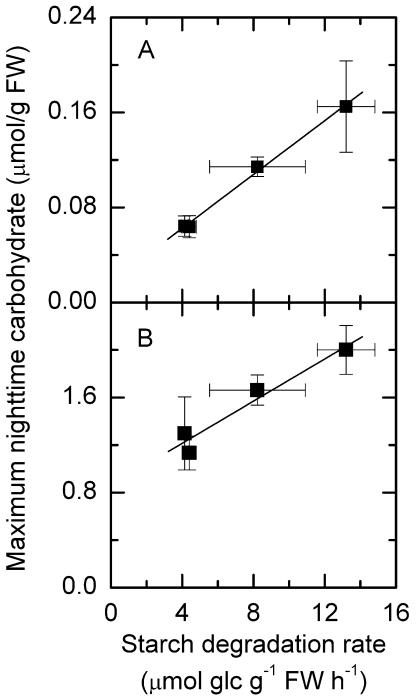
The Amounts of Maltose and Suc Produced at Night Had a Positive Linear Relationship with the Rates of Starch Degradation at Different Day/Night Regimes
We estimated average rates of starch degradation by a linear regression of all the values of starch over the duration of the night at different day/night regimes (LD, LS, SD, and SL; Table I). Then we plotted the average nighttime carbohydrate levels, maximum nighttime α-maltose, β-maltose, and Suc levels, and minimum nighttime Glc levels against average rates of starch degradation. The linear regression of maximum nighttime β-maltose and Suc levels against the rates of starch degradation is shown in Figure 5. Nighttime β-maltose levels had a positive linear correlation with the rates of starch degradation at different day/night regimes (r2 = 0.996; Fig. 5A). Nighttime Suc levels also had a positive linear correlation with the rates of starch degradation at different day/night regimes (r2 = 0.945; Fig. 5B). The amount of nighttime Glc during the altered photoperiod did not have a linear relationship with the rates of starch degradation (data not shown).
Figure 5.
Linear regression of maximum nighttime carbohydrate levels with starch degradation rates. A, β-Maltose (r2 = 0.996); B, Suc (r2 = 0.945). Average rates of starch degradation were estimated by a linear regression of all the nighttime values of starch over the duration of the night. Values on the x axis are slopes and ses of the linear regressions when estimating the rate of starch degradation. Values on y axis are mean ± se (n = 5).
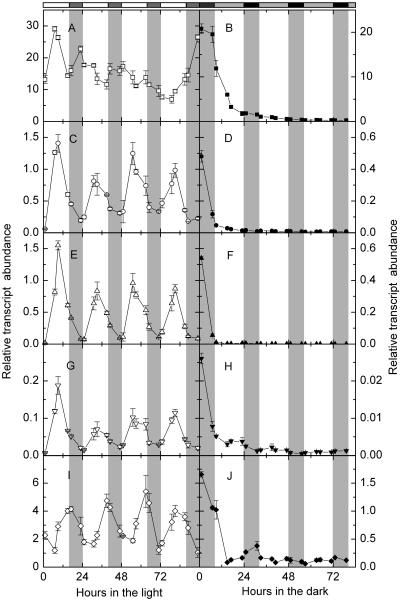
Transcript Levels of Starch Degradation-Related Genes Showed Strong Endogenous Rhythms under Continuous Light
The transcript levels of starch degradation-related enzymes oscillated during the day/night cycle (Fig. 2). To study whether the diurnal cycling of the transcript levels is a response to the presence and absence of light or is controlled by a circadian clock, we measured the relative transcript abundance of the same genes under continuous light. Under continuous light, the transcript levels of DPE2, AtPHS2, SEX1, and CT-BMY had coherent endogenous rhythms close to 24 h (Fig. 6, C, E, G, and I). They showed little or no evidence of damping throughout four cycles. DPE2, AtPHS2, and SEX1 transcript abundance cycled with a midday-specific phase (Fig. 6, C, E, and G), while CT-BMY transcript abundance cycled with a dusk-specific phase (Fig. 6I). The oscillation of RbcS1A transcript abundance damped gradually after one cycle under continuous light (Fig. 6A).
Figure 6.
Relative transcript abundance of starch degradation-related genes in continuous light or continuous darkness. A and B, RbcS1A; C and D, DPE2; E and F, AtPHS2; G and H, SEX1; I and J, CT-BMY. Left sections, Continuous light, white symbols. Right sections, Continuous darkness, black symbols. White bars and gray bars on the top are subjective days and nights. Values are mean ± se (n = 3).
Transcript Levels of Starch Degradation-Related Genes Damped Rapidly under Continuous Darkness
To study if light is necessary in keeping the cycling of transcript levels of starch degradation-related enzymes, we measured the relative transcript abundance of the same genes under continuous darkness. We found that the transcript levels of all the genes we quantified declined rapidly during the first 15 h under continuous darkness (Fig. 6, B, D, F, H, and J). The transcript levels of SEX1 and CT-BMY showed some cycling but it damped out within the first 48 h of continuous darkness (Fig. 6, H and J).
Protein Levels of Starch Degradation-Related Enzymes under Continuous Light and Continuous Darkness
To study if the protein levels of starch degradation-related enzymes remain constant under continuous light and damp under continuous darkness, we determined the protein abundance of RbcS, SEX1, and DPE2 under continuous light or darkness. The protein levels of RbcS remained relatively constant under continuous light or continuous darkness (Fig. 7A). The protein levels of SEX1 and DPE2 remained constantly high during the first 49 h of continuous light and declined to a lower level after 49 h of continuous light and remained low during the rest of continuous light (Fig. 7, B and C). The protein levels of SEX1 and DPE2 remained high during the first 9 h of continuous darkness and declined to a lower level after 15 h in continuous darkness (Fig. 7, B and C).
Figure 7.
Protein levels of RbcS, SEX1, and DPE2 in continuous light or continuous darkness. A, Coomassie stains of RbcS; B, Western blots of SEX1; C, Western blots of DPE2. Numbers on the top are hours in continuous light or darkness.
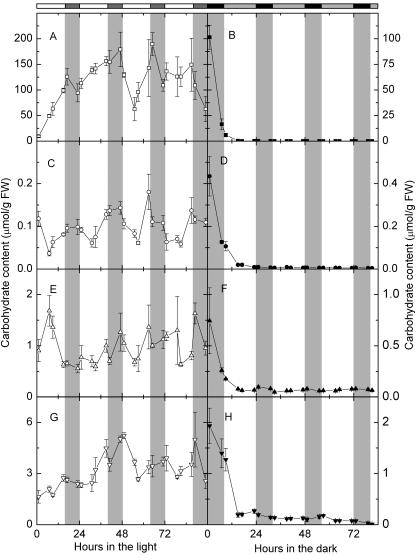
Maltose Contents Oscillated with a Strong Endogenous Rhythm under Continuous Light
To study if the metabolism of starch, maltose, and other carbohydrates is under circadian control, we measured the amount of starch, G6P, Glc, α-maltose, β-maltose, and Suc under continuous light or darkness. Under continuous light, starch increased almost linearly during the first 17 h, then decreased a little during the next 6 h (Fig. 8A). The amount of starch decreased around the subjective nights, and the intervals between the troughs of starch contents were 23, 32, 16, and 24 h (Fig. 8A). Interestingly, the biggest drop of starch levels happened between 47 and 55 h under continuous light, about the same time as the drop of protein abundance of SEX1 and DPE2 under continuous light (Fig. 7, B and C). Maltose content under continuous light showed a strong endogenous rhythm (Fig. 8B). The intervals between the troughs of maltose contents were 24, 26, and 24 h. The amount of Glc oscillated without a clear pattern except the first cycle of subjective day and night under continuous light (Fig. 8C). The amount of Suc cycled with a less solid pattern than maltose contents (Fig. 8G). The intervals between the troughs of Suc contents were 24, 32, 22, and 16 h.
Figure 8.
Carbohydrate levels in continuous light or continuous darkness. A and B, Starch; C and D, maltose; E and F, Glc; G and H, Suc. Left sections, Continuous light, white symbols. Right sections, Continuous darkness, black symbols. White bars and gray bars on the top are subjective days and nights. Values are mean ± se (n = 5).
In continuous darkness, the amount of starch, maltose, Glc, and Suc declined quickly during the first 15 h (Fig. 8, B, D, F, and H). There was a small amount of starch that was never broken down, and Glc, G6P, and Suc were never depleted throughout the 85 h in the dark. Unlike starch, Glc, G6P, or Suc, there was little maltose after the first 15 h in continuous darkness.
DISCUSSION
Plants May Adjust Their Rate of Starch Degradation According to the Changes in Daylength
We focused on how the rate of starch degradation is regulated to match the amount of starch in the leaf. Assuming starch is inert, it is hard to imagine that the level of starch could regulate the rate of degradation. One possible explanation is that a lower amount of Glc and/or higher amount of Suc at the end of the day in LD triggers the faster rate of starch degradation. Sugars can regulate carbon metabolism, gene expression, and posttranslational modification of some enzymes in starch metabolism (Koch, 1996; Rolland et al., 2002; Tiessen et al., 2002; Hendriks et al., 2003; Sharkey et al., 2004). Another possible explanation is that starch breakdown is controlled by the duration of the day directly. In Arabidopsis, two phytochromes, PhyA and PhyB, and two cryptochromes, CRY1 and CRY2, contribute to the establishment of period length (McClung, 2001). Plants may sense daylength via photoreceptors and adjust the rate of starch degradation. The rate of starch degradation during the first extended night greatly decreased. It is not likely that the rate of starch degradation is determined by the amount of Suc or Glc at the end of the first shortened day because at the end of the first shortened day, the levels of Suc and Glc were the same as those at the end of a regular LD. Our working hypothesis is that plants can adjust the rate of starch degradation according to the length of the previous day by some mechanism not involving sensing Glc or Suc levels. The endogenous rhythm of starch accumulation and breakdown does not seem to play a major role in adjusting the rate of starch degradation according to sudden changes in daylength in a short term.
Transcript Abundance of Starch Degradation-Related Genes Changes According to Changes in Daylength while Corresponding Protein Abundance Does Not
Among the four genes, DPE2, AtPHS2, and SEX1 share the same expression pattern, suggesting coordinate regulation (Smith et al., 2004). The oscillation amplitude of transcript levels for DPE2, AtPHS2, and SEX1 in SD and SL exceeds that reported by Smith et al. (2004). This is possibly because Smith et al. (2004) used a 12-h-light/12-h-dark photoperiod and we used an 8-h-light/16-h-dark or a 16-h-dark/8-h-dark photoperiod. We observed a significant decrease in transcript abundance of DPE2, AtPHS2, and CT-BMY in the first elongated night and a significant increase in transcript abundance of DPE2, AtPHS2, SEX1, and CT-BMY in the first shortened night. Therefore, we think that Arabidopsis plants can adjust transcript levels of starch degradation-related enzymes during the first altered day/night cycle.
Protein levels of two of the corresponding enzymes, SEX1 and DPE2, do not change as daylength changes. Under a 12-h-light/12-h-dark photoperiod, transcript levels of SEX1 and DPE2 peak at the end of the light period and 8 h of the light period, respectively, but the protein levels are relatively constant throughout the day/night cycle (Yu et al., 2001; Smith et al., 2004). In M. crystallinum, the protein levels of SEX1 and the activity of cytosolic starch phosphorylase are constant throughout the day/night cycle unless salt treatment induces CAM (A.M. Borland, personal communication). It appears that there is a general lack of correspondence between transcript levels and protein levels for many enzymes involved in starch degradation regardless of daylength. One exception is stDPE2, the DPE2 enzyme found in potato. The protein levels of stDPE2 increase in amount just prior to sunset and reduce in amount just before sunrise (Lloyd et al., 2004).
The lack of correspondence between transcript levels and protein levels suggests that posttranscriptional and posttranslational regulation might be important in controlling the amount of enzymes and their activities in vivo (Smith et al., 2004). It has been reported that ADP-Glc pyrophosphorylase in plants is activated by posttranslational redox modification in response to light and sugars (Sokolov et al., 1998; Tiessen et al., 2002; Hendriks et al., 2003; Geigenberger et al., 2004). It is plausible to speculate that a similar mechanism may be applied to regulate the rate of starch degradation.
Possible Circadian Regulatory Mechanism for Gene Expression of Starch Degradation-Related Enzymes
Transcript levels of starch degradation-related enzymes show robust endogenous rhythms under continuous light. This indicates that the variation of transcript levels under a regular day/night regime is not just a response to light. Circadian control plays a very important role in the oscillation of transcript levels of starch degradation-related enzymes. In Arabidopsis, novel putative β-amylase genes, BMY3 and ATβ-Amy, are also circadian regulated (Chandler et al., 2001). In M. crystallinum, the expression of genes encoding chloroplastic starch phosphorylase and β-amylase is under circadian control (Dodd et al., 2003). Light is necessary for the cycling of transcript levels of these genes. In continuous darkness, the transcript levels of genes encoding starch degradation-related enzymes decline quickly during the first 9 h, and these genes are barely expressed after 9 h in continuous darkness.
The current model for the central oscillator in Arabidopsis is a negative feedback loop between three proteins: two myb-like DNA binding proteins, CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATION HYPOCOTYL (LHY), and the TIMING OF CAB EXPRESSION 1 (TOC1) protein (Alabadi et al., 2001; Salome and McClung, 2004). Recent evidence showed that the expression of the gene for granule-bound starch synthase I in Arabidopsis leaves is circadian regulated and is controlled by the transcription factors CCA1 and LHY (Tenorio et al., 2003). It has been demonstrated that small subunit ADP-Glc pyrophosphorylase mRNA abundance in Chlamydomonas reinhardtii is suggestive of a circadian clock control mechanism (Zabawinski et al., 2001). Results from our lab and others (Harmer et al., 2000; Schaffer et al., 2001; Dodd et al., 2003; Smith et al., 2004) indicate that the expression of genes encoding starch degradation-related enzymes is circadian regulated. It is reasonable to hypothesize that the expression of genes encoding starch degradation-related enzymes is also controlled by CCA1 and LHY. CCA1 and LHY bind to the evening element of the TOC1 promoter (EE: AAAATATCT), which is necessary for phase-specific transcription (Alabadi et al., 2001; Michael and McClung, 2002). Using programs such as PLACE (Higo et al., 1999), we searched for putative cis-acting regulatory elements. We have located the evening element, which is necessary for phase-specific transcription and GATA and I box core sequences, which are important for light-regulated transcription (Terzaghi and Cashmore, 1995) in the promoters of DPE2, AtPHS2, SEX1, and CT-BMY. Transcript levels of all four starch degradation-related genes peak at dusk in SD.
Arabidopsis plants used in this report are exposed to another time cue, temperature cycles (22°C during the day and 20°C during the night), besides light cycles. Both cues serve to synchronize the endogenous clock with local time or growth chamber time (Michael et al., 2003). Because obvious cycling of transcript levels was not observed in continuous darkness, we hypothesize that the 2°C difference between day and night temperatures is not large enough to entrain the cycling of transcript levels of starch degradation-related genes.
Glc and Suc Levels Are Light Regulated, and Maltose Levels Are Regulated by Light and a Circadian Clock
Glc and Suc levels drop quickly during the first hour of the night in both LD and SD as a response to the absence of light. The drop of Glc and Suc levels happens earlier if daylength is shortened and happens later if daylength is extended. We hypothesize that the profile of Glc and Suc contents is primarily light dependent and that the endogenous rhythm does not play a primary role in the changes seen through the day and night. Glc and Suc levels oscillate without a clear pattern in continuous light and damp out in continuous darkness. This further proves that Glc and Suc levels are primarily light regulated, and circadian clock doesn't play a major role in regulating the amount of Glc and Suc in Arabidopsis leaves.
The amounts of maltose increase quickly during the first hour of the night in LD. This massive production of maltose is not shifted earlier when darkness is imposed earlier. We hypothesize that both light and the endogenous rhythm play important roles in the cycling of maltose. Maltose levels under continuous light have a robust endogenous rhythm and damp out in continuous darkness. This further proves that maltose metabolism is under strong circadian control.
Carbohydrate, Transcript, and Protein Levels under Continuous Light and Continuous Darkness
Starch increases almost linearly during the first 48 h of continuous light with a decrease during the first subjective night. A similar pattern of starch accumulation under 48 h of continuous light was found in C3 M. crystallinum (Dodd et al., 2003). Starch decreases around subjective nights, indicating that starch degradation overrides starch synthesis around subjective nights. However, in sugar beet (Beta vulgaris), the leaf starch levels are only slightly higher after a prolonged light period (Li et al., 1992). After one subjective day/night cycle under continuous light, starch and maltose go up and down almost at the same time. Under continuous light, the amplitude of SEX1 and DPE2 transcript levels decreased after one cycle of subjective day and night. Interestingly, the protein levels of SEX1 and DPE2 did not change until 48 h later, indicating that there is a slow response of protein levels of starch degradation-related enzymes to the changes in daylength. For starch degradation-related enzymes, changes in transcript levels only have a small impact on protein levels during a regular day/night cycle but have a significant impact if daylength is altered for a few days. Recently, evidence from another lab also supports this idea (Gibon et al., 2004a).
Under continuous darkness, carbohydrate levels drop quickly during the first 15 h. Previously expressed starch-degrading enzymes may result in this rapid decrease. Interestingly, the protein abundance of SEX1 and DPE2 also drops after 15 h under continuous darkness. Because the transcript abundance declines quickly during the first 15 h, de novo translation of SEX1 and DPE2 is impossible. This also suggests that protein degradation happens under continuous darkness. Thimm et al. (2004) reported that 6 h extension of the night leads to decreased transcript levels of genes encoding proteins involved in photosynthesis, nutrient acquisition, amino acid, nucleotide, RNA, and protein synthesis and increased transcript levels of genes encoding proteins involved in amino acid and nucleotide breakdown. These results indicate that under continuous darkness, the carbohydrate levels, the transcript levels, and the protein levels are coordinated. It is very possible that the period of acute carbohydrate deficiency during the extended hours of night triggers the inhibition of biosynthesis and growth (Thimm et al., 2004). This kind of acute carbohydrate deficiency does not happen during a regular day/night cycle when the protein levels of starch degradation-related enzymes remain constant.
To sum up, plants may sense changes in daylength and adjust their rate of starch degradation the following night. The expression of genes encoding starch degradation-related enzymes is regulated by daylength and a circadian clock. The protein levels of these enzymes are constant throughout the day/night cycle, indicating that posttranscriptional regulation might be important in controlling the amount of enzymes and their activities in vivo. The amount of Glc and Suc is primarily light dependent, while the amount of maltose is regulated both by light and by the circadian clock.
MATERIALS AND METHODS
Plant Growth Conditions
Plants of Arabidopsis (Arabidopsis thaliana) ecotype Wassilewskija were grown under a 16-h-light/8-h-dark (LD) or an 8-h-light/16-h-dark photoperiod (SD), 150 μmol photon m−2 s−1. The temperature was 22°C during the day and 20°C during the night. Humidity was maintained at 60%. Plants used in the experiments were between 3 and 5 weeks old. Plants under different photoperiods were analyzed at the same leaf size. To investigate whether plants can adjust their rate of starch degradation in the first altered photoperiod, we transferred plants in LS after 8 h in the light, which shortened daylength by half. We also transferred plants in SL after 8 h in the light, which doubled daylength. To investigate whether the expression of genes encoding starch degradation-related enzymes is controlled by a circadian clock, we transferred plants entrained in 16-h-lignt/8-h-dark cycles to continuous light at the end of their regular dark period, and we transferred entrained plants to continuous darkness at the end of their regular light period. The temperature was 22°C during subjective days and 20°C during subjective nights.
Extraction and Measurements of Carbohydrates
Five rosette leaves per time point were taken and added to microfuge tubes containing 750 μL 80% (v/v) ethanol and 5% (v/v) formic acid and were frozen in liquid N2. Starch and other carbohydrates were extracted according to Lu and Sharkey (2004). Carbohydrate determination was made using NADP(H)-linked assays in a Sigma ZFP 22 dual-wavelength filter photometer (Sigma Instruments, Berlin) according to Lu and Sharkey (2004).
Preparation of RNA and Quantitative RT-PCR
Total RNA was extracted from Arabidopsis rosette leaves as described (Takaha et al., 1993), quantified with UV spectroscopy, and digested with RNase-free DNase I (Sigma-Aldrich, St. Louis). Three independent RNA extractions were preformed at each time point. Total RNA was reverse transcribed with oligo(dT) primers and Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI) according to the manufacturer's instructions.
Gene-specific primers were designed to span two exons and were synthesized at Integrated DNA Technologies (Coralville, IA): 5′-TTC CTG ACC TTA CCG ATT CC-3′ and 5′-GCA TTG GGG TAC TCC TTC TT-3′ for RbcS1A; 5′-TAC GTC AAC TGG AGC ACC TC-3′ and 5′-TCA TAG CAT GAG CTG GAA GC-3′ for DPE2; 5′-CGC CAA GTA CAG TCC ACA TT-3′ and 5′-CAA GCT CAT AAC CCA GCG TA-3′ for AtPHS2; 5′-TGG GAA CGT AAG GGT AAA CA-3′ and 5′-GCT CTG GTT GCT TGG AAA CT-3′ for SEX1; 5′-AAA GCA CGG TCT CAA ACT CC-3′ and 5′-CAC AGA ATC ACA TCC CAA GG-3′ for CT-BMY; 5′-CAT CCA AGC TGT TCT CTC CT-3′ and 5′-CTT ACA ATT TCC CGC TCT GC-3′ for Actin 2 (ACT2).
Quantitative PCR was performed on a Stratagene Mx3000P QPCR system with Brilliant SYBR Green master mix according to the manufacturer's instructions (Stratagene, La Jolla, CA). All samples were assayed in triplicate. The following standard thermal profile was used for all PCR reactions: 95°C for 10 min; 40 cycles of 95°C for 30 s, 55°C for 1 min, and 72°C for 1 min. Absence of genomic DNA contamination was confirmed by the dissociation curve following 40 PCR cycles according to the manufacturer's instructions. Ct values for all analyzed genes were normalized to the threshold cycle (Ct) values of ACT2. PCR efficiency was estimated from the standard curve for each gene with purified PCR products as the template. Validation of the RT-PCR data was confirmed by standard curves of individual RT-PCR products.
Protein Extraction and Western Blots
Total soluble protein was extracted from Arabidopsis rosette leaves as described by Heck et al. (1995). The protein concentration was determined using the Bradford technique as modified by Bio-Rad Laboratories (Hercules, CA). The protein concentration of the samples was determined from the standard curve prepared from bovine serum albumin samples at the same time. A total of 12 μg of soluble protein per lane was separated on a NuPAGE NOVEX 4% to 12% Bis-Tris gel and transferred to a polyvinylidene difluoride (PVDF) membrane using the NOVEX XCell II Mini-Cell system according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). The membranes (Amersham Biosciences, Piscataway, NJ) were incubated with immune serum that was diluted 1:5000. The immunoreactive protein was visualized using the ECL western blotting system according to the manufacturer's instructions (Amersham Biosciences). The secondary donkey anti-rabbit antibody linked horseradish peroxidase (Amersham Biosciences) was diluted 1:2000. The blots were exposed to Kodak X-OMAT AR x-ray films (Eastman Kodak, Rochester, NY) for 10 min. The PVDF membranes were stained with Coomassie Brilliant Blue to visualize all the proteins. Location of RbcS protein on the blots was determined by western blots. Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under the following accession numbers: At1g10760 (GWD1, SEX1), At1g67090 (RbcS1A), At2g40840 (DPE2), At3g18780 (ACT2), At3g46970 (AtPHS2), At4g15210 (Atβ-Amy), At4g17090 (CT-BMY), and AF402598 (BMY3).
Acknowledgments
We thank Gerhard Ritte for providing antiserum to potato SEX1 protein and Alison M. Smith for providing peptide-specific antiserum to DPE2. We also thank Anne M. Borland, C. Robertson McClung, Gerhard Ritte, and Andreas P.M. Weber for critical reading of the manuscript.
This work was supported by the Chemical Sciences, Geosciences, and Biosciences Division, U.S. Department of Energy.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.061903.
References
- Alabadi D, Oyama T, Yanovsky MJ, Harmon FG, Mas P, Kay SA (2001) Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293: 880–883 [DOI] [PubMed] [Google Scholar]
- Borland AM, Taybi T (2004) Synchronization of metabolic processes in plants with Crassulacean acid metabolism. J Exp Bot 55: 1255–1265 [DOI] [PubMed] [Google Scholar]
- Chandler JW, Apel K, Melzer S (2001) A novel putative β-amylase gene and ATβ-Amy from Arabidopsis thaliana are circadian regulated. Plant Sci 161: 1019–1024 [Google Scholar]
- Chatterton NJ, Silvius JE (1979) Photosynthate partitioning into starch in soybean leaves. 1. Effects of photoperiod versus photosynthetic period duration. Plant Physiol 64: 749–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton NJ, Silvius JE (1980) Photosynthate partitioning into leaf starch as affected by daily photosynthetic period duration in 6 species. Physiol Plant 49: 141–144 [Google Scholar]
- Chia T, Thorneycroft D, Chapple A, Messerli G, Chen J, Zeeman SC, Smith SM, Smith AM (2004) A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J 37: 853–863 [DOI] [PubMed] [Google Scholar]
- Critchley JH, Zeeman SC, Takaha T, Smith AM, Smith SM (2001) A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J 26: 89–100 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Griffiths H, Taybi T, Cushman JC, Borland AM (2003) Integrating diel starch metabolism with the circadian and environmental regulation of Crassulacean acid metabolism in Mesembryanthemum crystallinum. Planta 216: 789–797 [DOI] [PubMed] [Google Scholar]
- Duwenig E, Steup M, Willmitzer L, Kossmann J (1997) Antisense inhibition of cytosolic phosphorylase in potato plants (Solanum tuberosum L.) affects tuber sprouting and flower formation with only little impact on carbohydrate metabolism. Plant J 12: 323–333 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M, Fernie AR (2004) Metabolic control analysis and regulation of the conversion of sucrose to starch in growing potato tubers. Plant Cell Environ 27: 655–673 [Google Scholar]
- Geiger DR, Servaites JC, Fuchs MA (2000) Role of starch in carbon translocation and partitioning at the plant level. Aust J Plant Physiol 27: 571–582 [Google Scholar]
- Geiger DR, Shieh WJ, Yu XM (1995) Photosynthetic carbon metabolism and translocation in wild-type and starch-deficient mutant Nicotiana sylvestris L. Plant Physiol 107: 507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Blaesing OE, Hannemann J, Carillo P, Hohne M, Hendriks JHM, Palacios N, Cross J, Selbig J, Stitt M (2004. a) A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16: 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Blasing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Hohne M, Gunther M, Stitt M (2004. b) Adjustment of diurnal starch turnover to short days: Depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39: 847–862 [DOI] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang H-S, Han B, Zhu T, Wang X, Kreps JA, Kay SA (2000) Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290: 2110–2113 [DOI] [PubMed] [Google Scholar]
- Heck GR, Perry SE, Nichols KW, Fernandez DE (1995) AGL15, a MADS domain protein expressed in developing embryos. Plant Cell 7: 1271–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks JHM, Kolbe A, Gibon Y, Stitt M, Geigenberger P (2003) ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol 133: 838–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 509–540 [DOI] [PubMed] [Google Scholar]
- Kotting O, Pusch K, Tiessen A, Geigenberger P, Steup M, Ritte G (2005) Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, water dikinase. Plant Physiol 137: 242–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao NT, Schoneveld O, Mould RM, Hibberd JM, Gray JC, Kavanagh TA (1999) An Arabidopsis gene encoding a chloroplast-targeted β-amylase. Plant J 20: 519–527 [DOI] [PubMed] [Google Scholar]
- Li B, Geiger DR, Shieh WJ (1992) Evidence for circadian regulation of starch and sucrose synthesis in sugar-beet leaves. Plant Physiol 99: 1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JR, Blennow A, Burhenne K, Kossmann J (2004) Repression of a novel isoform of disproportionating enzyme (stDPE2) in potato leads to inhibition of starch degradation in leaves but not tubers stored at low temperature. Plant Physiol 134: 1347–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Sharkey TD (2004) The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta 218: 466–473 [DOI] [PubMed] [Google Scholar]
- Matt P, Schurr U, Klein D, Krapp A, Stitt M (1998) Growth of tobacco in short-day conditions leads to high starch, low sugars, altered diurnal changes in the Nia transcript and low nitrate reductase activity, and inhibition of amino acid synthesis. Planta 207: 27–41 [DOI] [PubMed] [Google Scholar]
- McClung CR (2001) Circadian rhythms in plants. Annu Rev Plant Physiol Plant Mol Biol 52: 139–162 [DOI] [PubMed] [Google Scholar]
- Michael TP, McClung CR (2002) Phase-specific circadian clock regulatory elements in Arabidopsis. Plant Physiol 130: 627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael TP, Salome PA, McClung CR (2003) Two Arabidopsis circadian oscillators can be distinguished by differential temperature sensitivity. Proc Natl Acad Sci USA 100: 6878–6883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303: 87–89 [DOI] [PubMed] [Google Scholar]
- Ritte G, Lloyd JR, Eckermann N, Rottmann A, Kossmann J, Steup M (2002) The starch-related R1 protein is an alpha-glucan, water dikinase. Proc Natl Acad Sci USA 99: 7166–7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritte G, Lorberth R, Steup M (2000) Reversible binding of the starch-related R1 protein to the surface of transitory starch granules. Plant J 21: 387–391 [DOI] [PubMed] [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell Suppl 14: S185–S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome PA, McClung CR (2004) The Arabidopsis thaliana clock. J Biol Rhythms 19: 425–435 [DOI] [PubMed] [Google Scholar]
- Schaffer R, Landgraf J, Accerbi M, Simon V, Larson M, Wisman E (2001) Microarray analysis of diurnal and circadian-regulated genes in Arabidopsis. Plant Cell 13: 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidig A, Frohlich A, Schulze S, Lloyd JR, Kossmann J (2002) Downregulation of a chloroplast-targeted beta-amylase leads to a starch-excess phenotype in leaves. Plant J 30: 581–591 [DOI] [PubMed] [Google Scholar]
- Schneider A, Hausler RE, Kolukisaoglu U, Kunze R, van der Graaff E, Schwacke R, Catoni E, Desimone M, Flugge UI (2002) An Arabidopsis thaliana knock-out mutant of the chloroplast triose phosphate/phosphate translocator is severely compromised only when starch synthesis, but not starch mobilisation is abolished. Plant J 32: 685–699 [DOI] [PubMed] [Google Scholar]
- Schultz TF, Kay SA (2003) Circadian clocks in daily and seasonal control of development. Science 301: 326–328 [DOI] [PubMed] [Google Scholar]
- Schupp N, Ziegler P (2004) The relation of starch phosphorylases to starch metabolism in wheat. Plant Cell Physiol 45: 1471–1484 [DOI] [PubMed] [Google Scholar]
- Servaites JC, Geiger DR (2002) Kinetic characteristics of chloroplast glucose transport. J Exp Bot 53: 1581–1591 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Laporte M, Lu Y, Weise S, Weber APM (2004) Engineering plants for elevated CO2: a relationship between starch degradation and sugar sensing. Plant Biol 6: 280–288 [DOI] [PubMed] [Google Scholar]
- Smith AM, Zeeman SC, Thorneycroft D, Smith SM (2003) Starch mobilization in leaves. J Exp Bot 54: 577–583 [DOI] [PubMed] [Google Scholar]
- Smith SM, Fulton DC, Chia T, Thorneycroft D, Chapple A, Dunstan H, Hylton C, Zeeman SC, Smith AM (2004) Diurnal changes in the transcriptome encoding enzymes of starch metabolism provide evidence for both transcriptional and posttranscriptional regulation of starch metabolism in Arabidopsis leaves. Plant Physiol 136: 2687–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov LN, Dejardin A, Kleczkowski LA (1998) Sugars and light/dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana (thale cress). Biochem J 336: 681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Bulpin PV, Rees TA (1978) Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim Biophys Acta 544: 200–214 [DOI] [PubMed] [Google Scholar]
- Stitt M, Quick P (1989) Photosynthetic carbon partitioning: its regulation and possibilities for manipulation. Physiol Plant 77: 633–641 [Google Scholar]
- Takaha T, Yanase M, Okada S, Smith SM (1993) Disproportionating enzyme (4-alpha-glucanotransferase; EC 2.4.1.25) of potato. Purification, molecular cloning, and potential role in starch metabolism. J Biol Chem 268: 1391–1396 [PubMed] [Google Scholar]
- Tenorio G, Orea A, Romero JM, Merida A (2003) Oscillation of mRNA level and activity of granule-bound starch synthase I in Arabidopsis leaves during the day/night cycle. Plant Mol Biol 51: 949–958 [DOI] [PubMed] [Google Scholar]
- Terzaghi WB, Cashmore AR (1995) Light-regulated transcription. Annu Rev Plant Physiol Plant Mol Biol 46: 445–474 [Google Scholar]
- Thimm O, Blasing O, Gibon Y, Nagel A, Meyer S, Kruger P, Selbig J, Muller LA, Rhee SY, Stitt M (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37: 914–939 [DOI] [PubMed] [Google Scholar]
- Tiessen A, Hendriks JHM, Stitt M, Branscheid A, Gibon Y, Farre EM, Geigenberger P (2002) Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell 14: 2191–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trethewey RN, Smith AM (2000) Starch metabolism in leaves. In RC Leegood, TD Sharkey, S von Caemmerer, eds, Photosynthesis: Physiology and Metabolism. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 205–231
- Trevanion SJ, Castleden CK, Foyer CH, Furbank RT, Quick WP, Lund JE (2004) Regulation of sucrose-phosphate synthase in wheat (Triticum aestivum) leaves. Funct Plant Biol 31: 685–695 [DOI] [PubMed] [Google Scholar]
- Weber A, Servaites JC, Geiger DR, Kofler H, Hille D, Groner F, Hebbeker U, Flugge U-I (2000) Identification, purification, and molecular cloning of a putative plastidic glucose translocator. Plant Cell 12: 787–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise SE, Kim KS, Stewart RP, Sharkey TD (2005) β-Maltose is the metabolically active anomer of maltose during transitory starch degradation. Plant Physiol 137: 756–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise SE, Weber APM, Sharkey TD (2004) Maltose is the major form of carbon exported from the chloroplast at night. Planta 218: 474–482 [DOI] [PubMed] [Google Scholar]
- Yu T-S, Kofler H, Hausler RE, Hille D, Flugge U-I, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, et al (2001) The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell 13: 1907–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T-S, Zeeman SC, Thorneycroft D, Fulton DC, Dunstan H, Lue W-L, Hegemann B, Tung S-Y, Umemoto T, Chapple A, et al (2005) α-Amylase is not required for breakdown of transitory starch in Arabidopsis leaves. J Biol Chem 280: 9773–9779 [DOI] [PubMed] [Google Scholar]
- Zabawinski C, Van den Koornhuyse N, D'Hulst C, Schlichting R, Giersch C, Delrue B, Lacroix JM, Preiss J, Ball S (2001) Starchless mutants of Chlamydomonas reinhardtii lack the small subunit of a heterotetrameric ADP-glucose pyrophosphorylase. J Bacteriol 183: 1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Smith SM, Smith AM (2004) The breakdown of starch in leaves. New Phytol 163: 247–261 [DOI] [PubMed] [Google Scholar]