Abstract
The triple isotope composition (δ17O and δ18O) of dissolved O2 in the ocean and in ice cores was recently used to assess the primary productivity over broad spatial and temporal scales. However, assessment of the productivity with the aid of this method must rely on accurate measurements of the 17O/16O versus 18O/16O relationship in each of the main oxygen-producing and -consuming reactions. Data obtained here showed that cleavage of water in photosystem II did not fractionate oxygen isotopes; the δ18O and δ17O of the O2 evolved were essentially identical to those of the substrate water. The fractionation slopes for the oxygenase reaction of Rubisco and respiration were identical (0.518 ± 0.001) and that of glycolate oxidation was 0.503 ± 0.002. There was a considerable difference in the slopes of O2 photoreduction (the Mehler reaction) in the cyanobacterium Synechocystis sp. strain PCC 6803 (0.497 ± 0.004) and that of pea (Pisum sativum) thylakoids (0.526 ± 0.001). These values provided clear and independent evidence that the mechanism of O2 photoreduction differs between higher plants and cyanobacteria. We used our method to assess the magnitude of O2 photoreduction in cyanobacterial cells maintained under conditions where photorespiration was negligible. It was found that electron flow to O2 can be as high as 40% that leaving photosystem II, whereas respiratory activity in the light is only 6%. The implications of our findings to the evaluation of specific O2-producing or -consuming reactions, in vivo, are discussed.
Variations in the 17O/16O and 18O/16O ratios in air bubbles in polar ice cores or dissolved O2 in water bodies have been used to assess the photosynthetic rates on broad spatial and temporal scales (Luz et al., 1999; Luz and Barkan, 2000; Blunier et al., 2002). To accurately assess these rates, it is essential to know, with high precision, the isotope fractionation effects due to biological producing and consuming mechanisms. To date, precise measurements of these fractionations are available only for oxygen uptake in dark respiration by the cytochrome oxidase and the alternative oxidase pathways (Angert et al., 2003; Luz and Barkan, 2005). The fractionations due to photoreduction of O2 (the Mehler reaction; Mehler, 1951) and O2 uptake by photorespiration (during oxygenation of ribulose 1,5-bisphosphate [RuBP] by Rubisco and the successive oxidation of glycolate by glycolate oxidase) are known with respect to 18O/16O (Guy et al., 1993) only. Recently, we showed that the mechanism of O2 photoreduction in higher plants differs from that in cyanobacteria (Helman et al., 2003). In higher plants, electron transfer from PSI to oxygen, either from the Fx center of PSI or ferredoxin (Foyer and Noctor, 2000), results in the formation of superoxide. The latter is disproportionated by superoxide dismutase, and the H2O2 produced is reduced to water by ascorbate peroxidase (Asada, 1999, 2000). In cyanobacteria, NADPH donates electrons to A-type flavoproteins that reduce the oxygen directly to water without the formation of reactive oxygen intermediates (Vicente et al., 2002; Helman et al., 2003). Hence, the isotopic fractionation during photoreduction might differ between plants and cyanobacteria.
In this study, we measured the triple isotopic O2 fractionation by oxygen-producing and -consuming reactions in order to assess the overall photosynthetic oxygen production and the extent of these reactions in vivo. We show that the Mehler reaction of cyanobacteria has a different triple isotopic signature of oxygen compared to other oxygen-consuming processes. Thus, besides serving as an important tool for measurements of photosynthetic productivity, the relationship between the three oxygen isotopes can be used to assess the magnitude of the Mehler reaction under natural conditions.
Determination of Triple Isotope Fractionations
Fractionations during O2 Uptake in the Absence of Photosynthesis
In cases where O2-consuming reaction occurs without O2 production, the discrimination factor ɛ against the heavy isotope is being calculated using the “Rayleigh fractionation” relationship (e.g. Guy et al., 1993, Henry et al., 1999) as:
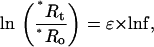 |
(1) |
where “*” can be 17 or 18; *Ro and *Rt are the isotope ratios (*O16O/16O16O) of the substrate O2 at the beginning and the time of sampling, t, respectively; and f is the remaining O2 fraction. In accordance with Mook (2000; see also http://www.iaea.org/programmes/ripc/ih/volumes/volume1.htm), we define ɛ as (α − 1), where α is a constant representing the relationship between the momentary isotope ratio of O2 incorporated into products (*Rp) and that of the substrate O2 (*Rs): *α = *Rp/*Rs. Note that ɛ, as defined here, relates to a different definition of discrimination than used by Guy et al. (1993) and Henry et al. (1999), D, ɛ = −D. Using δ notation, Equation 1 can be rewritten as:
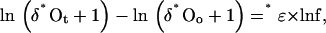 |
(2) |
where δ*O = (*R/*Rref − 1; note that, for convenience, we omit the factor 103 from the definition of δ*O and report the data in ‰). Applying Equation 2 for oxygen isotopes, 17 and 18, and rearranging, we obtain:
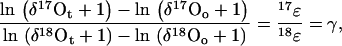 |
(3) |
where γ is the slope of the trend line representing the evolution path due to mass-dependent fractionation. The values of γ can be determined from the best fit of ln(δ17O + 1) versus ln(δ18O + 1) plots in experiments where only O2 consumption takes place. A comprehensive discussion on the three oxygen isotope relationships is provided in Luz and Barkan (2005).
Fractionations during O2 Uptake in the Presence of Photosynthesis
Where production and consumption of O2 occurs simultaneously, ɛ and γ cannot be calculated directly. However, their values can be determined using mass balance calculations for concentration and isotopic composition of dissolved O2. Since the experiments reported here were performed in airtight vessels, our calculations included only photosynthetic and consumption fluxes. In this case, the rate of change in O2 concentration is given as:
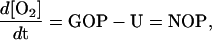 |
(4) |
where GOP and U are the rates of gross O2 production and uptake and the difference between these rates (NOP) is net O2 production. NOP is directly determined from the rate of change of O2 concentration, GOP is determined from incubations with H218O spike (e.g. Bender et al., 1987; Luz et al., 2002), and U is calculated from the difference GOP − NOP. It should be noted that GOP and NOP determined in replicate experiments may show considerable variations, for various reasons, but the ratio GOP/NOP is relatively uniform (variability approximately 10%).
Because natural O2 consists of about 99.8%, the concentration of 16O isotope, [16O2], is very close to [O2]. Thus, in experiments where H218O was not provided, the rate of change of the O2 isotope composition can be given in an analogous way to Equation 1 as:
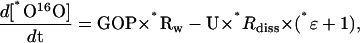 |
(5) |
where “*” denotes 17 or 18; *Rw is the *O16O/16O2 ratio in the O2 produced from water; and *Rdiss is the momentary *O16O/16O2 ratio in dissolved O2. It should be noted that an important assumption was made in writing Equation 5: that there was no fractionation during water cleavage in PSII between the substrate water and the O2 produced. This was confirmed in the experiments presented here (see also Guy et al., 1993; Luz and Barkan, 2005). In this case, GOP × *Rw can be calculated from measurements of δ18O of water, NOP in experiments without H218O spike, and the ratio GOP/NOP, which can be derived from experiments with spike. Equations 4 and 5 can be solved numerically (using the finite difference method; for details, see Luz and Barkan [2005]) to obtain 18ɛ, 17ɛ, and γ (see Eq. 3) from the best fit of [O2]final and δ*Ofinal if other parameters are known.
RESULTS AND DISCUSSION
Isotopic Discrimination during Oxygen Production in PSII
These experiments were conducted with a mutant of Synechocystis sp. strain PCC 6803 (hereafter Synechocystis) that does not exhibit photoreduction of O2 due to inactivation of flv3 (Helman et al., 2003), under conditions where photorespiration was severely inhibited (see “Materials and Methods”). Measurements of the isotopic composition of oxygen in the substrate water and in the O2 produced did not show significant discrimination against either 18O or 17O during cleavage of water in PSII (Table I). Results presented here confirmed those of Guy et al. (1993) with respect to 18O and extended this notion to 17O as well. Note that in agreement with the results of Guy et al. (1993) in experiments performed with cultures of Anacystis nidulans and Phaeodactylum tricornutum spurged with helium (He), O2 produced by PSII activity in Synechocystis was slightly enriched. The nature of this enrichment is not known; Guy et al. (1993) suggested that it emerged from some respiratory uptake that took place in the experiments with whole cells. As shown below, O2 uptake by respiratory activity in the light, in the presence of atmospheric level of O2 (considerably higher than applied here), was approximately 6% that leaving the thylakoids. Discrimination against 18O by this activity (Table II) could account for the small enrichment observed here.
Table I.
The difference in isotopic composition between oxygen evolved in photosynthesis by Synechocystis and the source water
The Δflv3 mutant unable to perform photoreduction of O2 was maintained under high CO2 level to eliminate O2 uptake by photorespiration. Note that the data provided for each difference (between the composition in evolving O2 gas and the substrate water) are based on three separate collections and isotopic measurements of O2 evolved (in the gas phase) and two determinations of O2 produced by fluorination of water.
| Experiment No. | δ18O Difference | 17Δ Difference |
|---|---|---|
| ‰ | per meg | |
| 1 | 0.59 ± 0.05 | 11 ± 8 |
| 2 | 0.54 ± 0.07 | 9 ± 8 |
| 3 | 0.27 ± 0.09 | 6 ± 6 |
Table II.
Triple isotope slopes, γ, and 18O isotopic discriminations in various oxygen-consuming processes
Averages and se of estimates of the coefficients in regression analyses are provided.
| Process | n | γ | 18ɛ |
|---|---|---|---|
| Respiration (Synechocystis) | 14 | 0.5184 ± 0.0004 | −19.4 ± 0.1 |
| Respiration (Synechococcus) | 7 | 0.5174 ± 0.0003 | −19.5 ± 0.2 |
| Respiration (bacteria from Lake Kinneret, T10) | 12 | 0.5180 ± 0.0002 | −17.1 ± 0.1 |
| Rubisco oxygenase (pea) | 26 | 0.517 ± 0.001 | −21.3 ± 0.5 |
| Glycolate oxidase (spinach) | 36 | 0.501 ± 0.001 | −21.5 ± 0.2 |
| Mehler reaction (pea) | 12 | 0.526 ± 0.002 | −10.8 ± 0.2 |
| Mehler reaction (Synechocystis) | 3 | 0.497 ± 0.004 | −9.6 ± 1.2 |
Isotopic Discrimination by Oxygen-Consuming Reactions
We measured the triple isotopic fractionation during various O2-consuming processes, including (1) dark respiration by two cyanobacteria Synechocystis and a marine Synechococcus sp. strain WH 7803 (hereafter Synechococcus) and, for comparison, a bacterium isolated from Lake Kinneret (Israel); (2) photorespiration due to oxygenation of RuBP by Rubisco and glycolate oxidation; and (3) photoreduction of O2 by thylakoids isolated from pea (Pisum sativum) leaves and by intact Synechocystis cells.
Data from replicate experiments were pooled together by normalizing according to the corresponding initial values in each experiment. Data for 18ɛ and γ presented in Table II were calculated from the best fit of all data points (in the supplemental material, we provide the raw data upon which we calculated those presented in Table II). To determine the fractionation of O2 by the Mehler reaction in Synechocystis, we conducted three series of experiments, with or without the addition of H218O as explained above. The results shown in Table II are the average values.
Dark Respiration
The 18ɛ values for oxygen fractionation by Synechocystis and Synechococcus in the dark and the bacterium from Lake Kinneret (Table II) are in good agreement with published results on bacterial respiration, ranging between −19‰ (Kiddon et al., 1993) and −17.6‰ (Quay et al., 1995). The values for γ, obtained in experiments carried out with bacteria and cyanobacteria that were maintained in the dark (Table II), are similar to those observed in higher plants (0.518 ± 0.001; Angert et al., 2003; Luz and Barkan, 2005). Thus, our data provided supporting evidence to the notion (compare with Luz and Barkan, 2005) that for a wide range of organisms and conditions the slope, γ, obtained from a ln(δ17O + 1) versus ln(δ18O + 1) plot of respiration experiments shows a unified universal value of 0.5179 ± 0.0006.
Uptake of O2 by Rubisco and Glycolate Oxidase
Oxygen consumption by the photorespiratory pathway was measured only in representatives of C3 plants since this reaction is, most probably, not quantitatively important in organisms that possess efficient biochemical or biophysical CO2-concentrating mechanisms (CCMs) such as C4 plants, algae, and cyanobacteria (Chollet and Ogren, 1975; Kaplan and Reinhold, 1999). Consequent on the elevated concentration of CO2 in close proximity of Rubisco, the oxygenase activity in these organisms is most likely inhibited and the extent of oxygen consumption in photorespiration is minimized (Edwards et al., 2004). Thus, even if the fractionation of oxygen isotopes during photorespiration by these organisms differs from that measured in C3 plants, the relative effect of photorespiration on the overall isotopic composition of oxygen is probably small and thus was not examined in this study.
The extent of the discriminations, 18ɛ, by RuBP oxygenation and glycolate oxidation, examined in reactions with isolated enzymes, were very similar (−21.3‰ and −21.5‰, respectively) and in good agreement with those reported by Guy et al. (1993). In contrast, while the values of the triple oxygen isotope slopes, γ, of RuBP oxygenation were the same as for dark respiration (Table II), the γ value obtained for glycolate oxidation was much lower. The overall contribution of the photorespiratory activity to γ can be calculated from the weighted average of the two reactions. Assuming that for every two moles of O2 taken by Rubisco an additional one is consumed by glycolate oxidase (Tolbert, 1997), the expected overall slope for photorespiration should be 0.512 ± 0.002. This value is slightly higher than that reported by Angert et al. (2003; 0.506 ± 0.005), but the latter was based on a complicated isotope budget (and thus less certain) than the direct measurements performed here.
Respiratory Activity in the Light
While the rate of dark respiration in various organisms including Synechocystis can be measured directly using relatively simple devices such as O2 electrodes, it is difficult to assess the extent of electron flow via cytochrome oxidase in the light, due to other O2-consuming reactions. Sharing of electron carriers between the photosynthetic and respiratory pathways in cyanobacteria (Howitt and Vermaas, 1998; Cooley et al., 2000) is an additional complicating issue. To measure the rate of O2 uptake by respiration in the light, we used a mutant of Synechocystis defective in the Mehler reaction (Helman et al., 2003). The cells were maintained under high CO2 conditions (to eliminate photorespiration) and were provided with a known amount of H218O. We accurately measured the changes in O2 concentration and its isotope abundances (see “Materials and Methods”) and could reveal the NOP/GOP ratios. An NOP/GOP ratio of 0.94 was obtained, suggesting that O2 consumption by respiratory activity in the light, simultaneously with O2 evolution, was about 6% that of O2 production. Since the amount of O2 consumed in these experiments was relatively small, we could not determine the isotope discrimination and triple isotope slope with the accuracy performed in the experiments presented in Table II. Thus, though it is most likely, we refrain from concluding that the O2 uptake observed in this mutant occurred solely by dark respiration.
Fractionation of O2 Isotopes in the Mehler Reaction
Photoreduction of O2 was measured in both Synechocystis and isolated pea thylakoids, since studies by Helman et al. (2003) showed that in cyanobacteria, unlike the case of higher plants, it does not involve formation and release of reactive oxygen species. To avoid possible complication by respiratory activity, we used a Synechocystis mutant where the genes encoding the terminal oxidases (Howitt and Vermaas, 1998) were inactivated (kindly provided by Professor W. Vermaas). Synechocystis possesses a CCM that lowers its susceptibility to O2 inhibition of photosynthesis and O2 uptake in photorespiration (Kaplan and Reinhold, 1999; Ogawa and Kaplan, 2003). To further minimize O2 consumption due to residual photorespiration, the cells were supplied with 10 mm NaHCO3 during the experiments.
Similar 18ɛ values (averaging −10.2 ‰) were obtained for O2 photoreduction in both Synechocystis and pea thylakoids (Table II). For an unknown reason, this value differs by approximately 5‰ from that reported earlier (−15.1‰; Guy et al., 1993). Contrary to the similar 18ɛ values, the γ values obtained were 0.526 in isolated pea thylakoids and 0.497 in Synechocystis (Table II). Considering that the overall error is only ±0.006 (0.004 for Synechocystis and 0.002 in pea thylakoids), the difference between the two values, 0.028, is large and significant. In addition to photoreduction of O2, only glycolate oxidase activity could account for the relatively low γ obtained (Table II). However, the fact that the overall discrimination against 18O (−9.6‰) differed markedly from that observed for glycolate oxidase (−22‰, Table II) serves as independent evidence that the low γ values did not emerge from O2 uptake by glycolate oxidase. In addition, it is most likely that the conditions of the experiments, including the high CO2 level, severely inhibited glycolate formation. Further, analysis of the genome sequence of Synechocystis (http://www.kazusa.or.jp/cyanobase) suggested that it possesses a gene annotated to encode for glycolate oxidase. Nevertheless, we are not aware of experimental evidence that, in Synechocystis, glycolate is converted to glyoxalate by glycolate oxidase rather than glycolate dehydrogenase, a reaction that does not involve direct O2 uptake. We conclude that the isotopic “fingerprint” obtained here, using a Synechocystis mutant impaired in respiration that was exposed to conditions where photorespiration is minimized, originated from photoreduction of O2. These results, independently, confirmed the previous conclusion (Helman et al., 2003) that the mechanism of O2 photoreduction in the Mehler reaction differs between plants and cyanobacteria. Thus, the γ values may be used to study the extent of various types of the Mehler reaction in different organisms.
The Extent of O2 Photoreduction during Steady-State Photosynthesis in Synechocystis
The purpose of the following set of experiments was to examine whether the triple oxygen isotope methodology can be used to assess the extent of specific O2-consuming reactions during steady-state photosynthesis in the wild type, taking the photoreduction of O2 as a test case. In the experiments described above, we determined the γ values for the Mehler reaction using a respirationless mutant maintained under high level of CO2. The 18ɛ obtained was −9.6 ± 1.2‰ and γ = 0.497 ± 0.004 (Table II). These γ values differed significantly from those associated with dark respiration (Table II).
Synechocystis cells maintained under high CO2 conditions (to eliminate photorespiration) were provided with a known amount of H218O (see “Materials and Methods”) and revealed the NOP/GOP ratios. When those were measured under conditions where photoreduction of O2 and respiration were essentially the sole O2-consuming process, the NOP/GOP ratio was 0.53. This suggested that during our experiments electron flow to O2 was about 47% that leaving PSII (and reflected in the gross O2 evolution). Notably, Kana (1993) also found similar high rates of O2 reduction in Trichodesmium. The 18ɛ obtained here was −9.6 ± 1.2‰ and γ = 0.497 ± 0.004, i.e. identical to those observed for photoreduction of O2 in the experiments where a mutant defective in respiration was employed (above). Since the respiratory activity in the light is 6% that of PSII (above), we conclude that, under the conditions of our experiment, photoreduction of O2 could consume as much as 40% of the O2 released in PSII. Earlier assessments of photoreduction of O2 in Synechocystis, based on measurements of 18O uptake using an on-line membrane inlet mass spectrometer, also indicated substantial O2 consumption (Helman et al., 2003). Our findings are in contrast to an earlier report that, in cyanobacteria, the magnitude of electron flow from PSI to O2 is very small. This was concluded since a mutant where katG (encoding catalase-peroxidase) was inactivated accumulated very little H2O2 (Tichy and Vermaas, 1999). This apparent contradiction is probably attributable to the fact that, in Synechocystis, photoreduction of O2 is mediated by A-type flavoproteins leading to reduction of O2 directly to water without the formation of H2O2 (Helman et al., 2003). Although measured very accurately, the magnitude of photoreduction of O2 obtained here is surprising since the rate of photosynthesis, the growth rate, and sensitivity to photoinhibition did not differ markedly between the wild type and Synechocystis mutants where genes (flv3 and flv1) encoding the A-type flavoproteins essential for the Mehler reaction were inactivated (Helman et al., 2003).
If indeed electron flow from PSI to O2 is as high as 40% that leaving PSII, as obtained here, it is not clear why inactivation of the Mehler reaction in Synechocystis did not result in a clear phenotype such as enhanced photoinhibition (Helman et al., 2003). The possibilities that PSII activity is down-regulated in the flv mutants or that the mutants utilizes other photosynthetic electron routes after PSI should await confirmation. It is well established that Synechocystis possesses multiple routes of electron transfer from PSI and those are regulated by environmental conditions such as salinity (Matthijs et al., 2002).
In conclusion, we have shown that the fractionation of three oxygen isotopes in the Mehler reaction and photorespiration differs markedly from those observed in dark respiration. In view of the large fluxes of O2 uptake associated with these mechanisms, the results presented here should be taken into account in global budgets of oxygen isotopes in order to derive better estimates of primary production. In addition, we demonstrated the application of the triple isotope and of net/gross O2 production approaches as a means to assess the extent of specific mechanisms of O2 production/uptake and conclude that the mechanism of photoreduction of O2 differs between cyanobacteria and higher plants.
MATERIALS AND METHODS
Growth Conditions
Cells of Synechocystis sp. strain PCC 6803 and mutants thereof were grown in medium BG11 (Stanier et al., 1971) supplemented with 20 mm HEPES-NaOH buffer, pH 7.8, as described by Helman et al. (2003). The cells were harvested in the logarithmic growth phase and resuspended in fresh growth media. Cells of the marine Synechococcus sp. strain WH7803 were grown on artificial seawater medium (Wyman et al., 1985) supplemented with 20 mm HEPES-NaOH, pH 8. Cultures were grown at 25°C under continuous white light of 40 to 50 μmol photons m−2 s−1, with constant agitation on an orbital shaker at 125 rpm. Bacterial cells (T-10) from Lake Kinneret were isolated by Dr. O. Hadas as described by Hadas and Berman (1998). They were grown at 37°C with mixing on nutrient broth (DIFCO Laboratories, Detroit) supplemented with 8.5 g/L NaCl. Pea plants (Pisum sativum L. cv Alaska) were grown in vermiculite for 10 d at 22°C with a 12/12-h light/dark period.
Fractionation of O2 during Water Cleavage in PSII
Cultures of mutant Δflv3 of Synechocystis (Helman et al., 2003) were grown under low level of CO2 (1:1 dilution of air and CO2-free air) to induce their CCM (Kaplan and Reinhold, 1999). The cells were harvested, resuspended to a final volume of 100 mL (cell density corresponded to 5–6 μg Chl mL−1) within a 300-mL flask containing medium BG11 supplemented with 10 mm buffer BisTris propane, pH 8.0, after removing the dissolved air by vigorously bubbling with He. The cell suspension was supplemented with NaHCO3 to a final concentration of 10 mm. The cultures were mixed by magnetic stirrer at 30°C; light intensity was 70 μmol photons m−2 s−1. The O2 produced by cleavage of water was continuously removed by zero-grade He carrier gas at a flow rate of 25 mL min−1. About 1 mL of O2 was collected in a 5A-molecular sieve trap at −196°C for 60 min and was subsequently transferred to a holding tube immersed in liquid He. The collected gas was then analyzed by mass spectrometry to determine its isotopic composition (see Barkan and Luz, 2003). The source water was sampled after each experiment and saved for isotopic analysis. The δ17O and δ18O of the water in the medium were determined by the fluorination method of Baker et al. (2002) with a precision of 0.03‰ for both δ17O and δ18O.
Photoreduction of O2 (Mehler Reaction)
Fractionation of oxygen isotopes in the Mehler reaction was determined in pea thylakoids and in cells of a Synechocystis mutant where genes encoding the terminal oxidases were inactivated.
Pea Thylakoids
All steps of thylakoid preparations were carried out at 4°C. Pea leaves were ground three times, 5 s each, in a Wareing blender containing 10 mm Tris-HCl, pH 8, 0.4 m Suc, and 10 mm NaCl. The homogenate was filtered through two layers of cheesecloth and two layers of Miracloth before centrifugation at 12,000g for 10 min. The pellet was resuspended in a buffer containing 5 mm Tricine, pH 7.5, 5 mm MgCl2, and 10 mm NaCl. To eliminate O2 production by PSII, the thylakoids were resuspended in 1 m Tris buffer, pH 8, for 20 min in the dark with occasional mixing. The Tris-washed thylakoid membranes were then centrifuged at 12,000g for 10 min and washed twice with a reaction buffer containing 5 mm HEPES-NaOH, pH 7.0, 200 mm Suc, and 2 mm MgCl2. Prior to each experiment, the thylakoid membranes were placed in a Clark-type oxygen electrode to confirm that oxygen evolution was completely inhibited. Diphenylcarbazide was used as an electron donor for the Mehler reaction; stock solution of 100 mm in ethanol was added to the reaction buffer to a final concentration of 4 mm. The addition of the ethanol (4%, v/v) forced some oxygen out of the reaction buffer. The thylakoid membranes were added to the medium only after the removal of the bubbles. The reaction was performed in the presence of superoxide dismutase 15 μg/mL, catalase 15 μg/mL, and 10 mm NH4Cl. Irradiance was 400 μmol photons m−2 s−1. Chlorophyll concentration was 25 μg/mL.
Wild-Type and Mutant of Synechocystis
To measure the isotopic fractionation of oxygen consumption by the water-water cycle in cyanobacteria, we used mutant ΔctaDIEI/ctaDIIEII/cydAB where the genes encoding terminal oxidases were inactivated (Howitt and Vermaas, 1998). To minimize oxygen uptake by photorespiration, the experiments were performed in the presence of 10 mm NaHCO3, irradiance was 100 μmol photons m−2 s−1, and chlorophyll concentration was 5 μg/mL. Unlike the experiments with pea thylakoids, oxygen evolution by PSII by the Synechocystis mutant was not inhibited, and it was necessary to accurately measure the amount of oxygen production. This was performed using parallel experiments where a known amount of H218O was provided followed by isotopic mass balance calculations (see “Determination of Triple Isotope Fractionations” above). The experiments were thus performed in two reaction vessels maintained under identical conditions. A known amount of H218O was added to one of the vessels (typically 0.1 mL of 95% enriched H218O in a total volume of 300 mL), and the extent of 18O produced served to assess gross production. Similar experiments were performed with wild type and with Synechocystis mutant cells where sll0550 (flv3) was inactivated leading to complete inhibition of photoreduction of O2 (Helman et al., 2003).
Oxygenase Activity of Rubisco
Isolation of Rubisco
The isolations were carried out at 4°C. Pea leaves were ground in a Wareing blender containing 50 mm Bicine, pH 8, 1 mm EDTA, 1 mm dithiothreitol (DTT), and 2% polyvinylpyrrolidone. The homogenate was filtered through two layers of cheesecloth and two layers of Miracloth before centrifugation at 12,000g for 40 min. The supernatant was filtered again through Miracloth and centrifuged at 100,000g for 60 min. A two-step ammonium sulfate precipitation was then performed, at 30% and at 60% w/v, for Rubisco isolation from the supernatant. Prior to each experiment the pellet was resuspended in 1 mL dialysis buffer containing 5 mm HEPES, pH 7.2, protease inhibitors, and 1 mm DTT, at 4°C.
Oxygenase Activity Measurements
After dialysis, Rubisco was activated by preincubation in 50 mm Bicine, pH 8, 10 mm MgCl2, 1 mm DTT, and 10 mm NaHCO3 for 30 min on ice. The reaction buffer contained 50 mm Bicine, pH 8, 5 mm MgCl2, and 1 mm NaHCO3; the reaction was initiated by the addition of RuBP (Sigma R-0878, Sigma-Aldrich, St. Louis) to a final concentration of 0.4 mm.
Glycolate Oxidase
Oxygen consumption by glycolate oxidase was measured using an enzyme isolated from spinach leaves (Spinacia oleracea; Sigma G8260). The reaction medium contained 50 mm Tris buffer, pH 8, 70 μM flavine mononucleotide, and 4 mm glycolate. The reaction was carried out in the dark to prevent photooxidation of the flavine mononucleotide.
Dark Respiration
Consumption of O2 during dark respiration and its isotopic fractionations were measured in cells of Synechococcus sp. strain WH7803, Synechocystis sp. strain PCC 6803, and bacteria T10 isolated from Lake Kinneret. The cells were maintained in their respective growth media during the incubation.
Sample Preparation and Mass-Spectrometric Measurements
All the experiments were carried out in airtight stirred Erlenmeyer flasks (300 mL) at room temperature. Duplicate water samples were taken from each flask for mass-spectrometric measurements carried out according to Luz et al. (2002) and Barkan and Luz (2003). Briefly, 150 mL of medium were transferred into preevacuated gas extraction vessels (300-mL flasks with Louwers Hapert O-ring stopcocks containing 1 mL of saturated HgCl2 solution to stop the biological activity). The water and headspace in the flasks were equilibrated for 24 h at room temperature, and the water was sucked out leaving only headspace gases. The flasks were then connected to a preparation line for the purification of O2-argon (Ar) mixture.
The δ18O and δ17O of O2 in the purified oxygen-Ar mixture were determined by dual inlet mass spectrometry on a multicollector instrument (Finnigan MAT Delta-Plus, Bremen, Germany) that allows simultaneous measurement of m/z 32, 33, and 34. Each mass spectrometric measurement consists of three separate runs, during which the ratio of sample to reference is determined 30 times. The pressures of the sample and reference gases were balanced before each of the three runs. The reported δ-values are the average of three runs. The analytical error (se of the mean, n = 90, multiplied by Student's t factor for 95% confidence limits) in δ18O and δ17O was 0.003 and 0.006‰, respectively.
The ratio O2/Ar is reported in the standard δ-notation as:
 |
(6) |
where 32 and 40 represent ion beam intensities for m/z 32 and 40, respectively. They were obtained by sequential measurements in the same collector by peak jumping. Based on two consecutive runs, the precision (absolute difference from the average) of δO2/Ar is 0.2‰. The isotopic and O2/Ar ratios are reported with respect to atmospheric O2.
NOP (fraction with respect to the initial amount) was calculated from δO2/Ar values as in Luz et al. (2002):
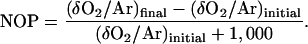 |
(7) |
The isotope discrimination 18ɛ was calculated from the best fit of a plot ln(f) versus ln[(δ18Ofinal + 1)/ [(δ18Oinitial + 1)], where f was determined from δO2/Ar measurements as:
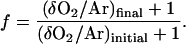 |
(8) |
Supplementary Material
Acknowledgments
We thank Professor W. Vermaas, who provided us with mutant ΔctaDIEI/ctaDIIEII/cydAB of Synechocystis sp. strain PCC 6803 where genes encoding respiratory oxidases were inactivated; and Professor T. Ogawa, who raised the Synechocystis sp. strain PCC 6803 mutants where genes encoding the A-type flavoproteins were inactivated.
This work was supported by grants from the Israel Science Foundation, the U.S.-Israel Binational Science Foundation, The Ring Foundation, and the German Ministerium for Bildung, Wissenshaft, Forschung, und Technologie.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.063768.
References
- Angert A, Rachmilevitch S, Barkan E, Luz B (2003) Effects of photorespiration, the cytochrome pathway, and the alternative pathway on the triple isotopic composition of atmospheric O2. Global Biogeochem Cycles 17: 1089, doi/10.1029/2003GB002056 [Google Scholar]
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Asada K (2000) The water-water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond Ser B Biol Sci 355: 1419–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L, Franchi IA, Maynard J, Wright IP, Pillinger CT (2002) A technique for the determination of 18O/16O and 17O/16O isotopic ratios in water from small liquid and solid samples. Anal Chem 74: 1665–1673 [DOI] [PubMed] [Google Scholar]
- Barkan E, Luz B (2003) High-precision measurements of 17O/16O and 18O /16O of O2 and O2/Ar ratio in air. Rapid Commun Mass Spectrom 17: 2809–2814 [DOI] [PubMed] [Google Scholar]
- Bender ML, Grande K, Johnson K, Marra J, Williams P, Sieburth J, Pilson M, Langdon C, Hitchcock J, Orchardo J, et al (1987) A comparison of four methods for the determination of planktonic community metabolism. Limnol Oceanogr 32: 1085–1098 [Google Scholar]
- Blunier T, Barnett B, Bender ML, Hendricks MB (2002) Biological oxygen productivity during the last 60,000 years from triple oxygen isotope measurements. Global Biogeochem Cycles 16: doi/10.1029/2001GB001460
- Chollet R, Ogren WL (1975) Regulation of photorespiration in C3 and C4 plants. Bot Rev 41: 137–179 [Google Scholar]
- Cooley JW, Howitt CA, Vermaas WFJ (2000) Succinate: quinol oxidoreductases in the cyanobacterium Synechocystis sp strain PCC 6803: presence and function in metabolism and electron transport. J Bacteriol 182: 714–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GE, Franceschi VR, Voznesenskaya EV (2004) Single-cell C-4 photosynthesis versus the dual-cell (Kranz) paradigm. Annu Rev Plant Biol 55: 173–196 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signalling. New Phytol 146: 359–388 [Google Scholar]
- Guy RD, Fogel ML, Berry JA (1993) Photosynthetic fractionation of the stable isotopes of oxygen and carbon. Plant Physiol 101: 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadas O, Berman T (1998) Seasonal abundance and vertical distribution of Protozoa (flagellates, ciliates) and bacteria in Lake Kinneret, Israel. Aquat Microb Ecol 14: 161–170 [Google Scholar]
- Helman Y, Tchernov D, Reinhold L, Shibata M, Ogawa T, Schwarz R, Ohad I, Kaplan A (2003) Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr Biol 13: 230–235 [DOI] [PubMed] [Google Scholar]
- Henry BK, Atkin OK, Day DA, Millar AH, Mentz RI, Farquhar GD (1999) Calculation of the oxygen isotope discrimination factor for studying plant respiration. Aust J Plant Physiol 26: 773–780 [Google Scholar]
- Howitt CA, Vermaas WFJ (1998) Quinol and cytochrome oxidases in the cyanobacterium Synechocystis sp. PCC 6803. Biochemistry 37: 17944–17951 [DOI] [PubMed] [Google Scholar]
- Kana TM (1993) Rapid oxygen cycling in Trichodesmium thiebautii. Limnol Oceanogr 38: 18–24 [Google Scholar]
- Kaplan A, Reinhold L (1999) The CO2 concentrating mechanisms in photosynthetic microorganisms. Annu Rev Plant Physiol Plant Mol Biol 50: 539–570 [DOI] [PubMed] [Google Scholar]
- Kiddon J, Bender ML, Orchardo J, Caron DA, Goldman JC, Dennett M (1993) Isotopic respiration of oxygen by respiring marine organisms. Global Biogeochem Cycles 7: 679–694 [Google Scholar]
- Luz B, Barkan E (2000) Assessment of oceanic productivity with the triple-isotope composition of dissolved O2. Science 288: 2028–2031 [DOI] [PubMed] [Google Scholar]
- Luz B, Barkan E (2005) The isotopic ratios 17O/16O and 18O/16O in molecular oxygen and their significance in bio-geochemistry. Geochim Cosmochim Acta 69: 1099–1110 [Google Scholar]
- Luz B, Barkan E, Bender ML, Thiemens MH, Boering KA (1999) Triple-isotope composition of atmospheric oxygen as a tracer of biosphere productivity. Nature 400: 547–550 [Google Scholar]
- Luz B, Barkan E, Sagi Y, Yacobi YZ (2002) Evaluation of community respiratory mechanisms with oxygen isotopes: a case study in Lake Kinneret. Limnol Oceanogr 47: 33–42 [Google Scholar]
- Matthijs HCP, Jeanjean R, Yeremenko N, Huisman J, Joset F, Hellingwerf KJ (2002) Hypothesis: versatile function of ferredoxin-NADP(+) reductase in cyanobacteria provides regulation for transient photosystem I-driven cyclic electron flow. Funct Plant Biol 29: 201–210 [DOI] [PubMed] [Google Scholar]
- Mehler AH (1951) Studies on reactivities of illuminated chloroplasts: mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys 33: 65–77 [DOI] [PubMed] [Google Scholar]
- Mook WG, editor (2000) Enviromental Isotopes in the Hydrological Cycle, Vol 1: Introduction. United Nations Educational, Scientific and Cultural Organization. International Atomic Energy Agency, Paris
- Ogawa T, Kaplan A (2003) Inorganic carbon acquisition systems in cyanobacteria. Photosynth Res 77: 105–115 [DOI] [PubMed] [Google Scholar]
- Quay PD, Wilbur DO, Richey JE, Devol AH, Benner R, Forssberg BR (1995) The 18O:16O of dissolved oxygen in rivers and lakes in the Amazon Basin: determining the ratio of respiration to photosynthesis rates in freshwaters. Limnol Oceanogr 40: 718–729 [Google Scholar]
- Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35: 171–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichy M, Vermaas W (1999) In vivo role of catalase-peroxidase in Synechocystis sp. strain PCC 6803. J Bacteriol 181: 1875–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert NE (1997) The C-2 oxidative photosynthetic carbon cycle. Annu Rev Plant Physiol Plant Mol Biol 48: 1–23 [DOI] [PubMed] [Google Scholar]
- Vicente JB, Gomes CM, Wasserfallen A, Teixeira M (2002) Module fusion in an A-type flavoprotein from the cyanobacterium Synechocystis condenses a multiple-component pathway in a single polypeptide chain. Biochem Biophys Res Commun 294: 82–87 [DOI] [PubMed] [Google Scholar]
- Wyman M, Gregory RPF, Carr NG (1985) Novel role for phycoerythrin in a marine cyanobacterium, Synechococcus strain DC2. Science 230: 818–820 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


