Abstract
Genetically engineered tobacco (Nicotiana tabacum) with the ability to synthesis glycinebetaine was established by introducing the BADH gene for betaine aldehyde dehydrogenase from spinach (Spinacia oleracea). The genetic engineering enabled the plants to accumulate glycinebetaine mainly in chloroplasts and resulted in enhanced tolerance to high temperature stress during growth of young seedlings. Moreover, CO2 assimilation of transgenic plants was significantly more tolerant to high temperatures than that of wild-type plants. The analyses of chlorophyll fluorescence and the activation of Rubisco indicated that the enhancement of photosynthesis to high temperatures was not related to the function of photosystem II but to the Rubisco activase-mediated activation of Rubisco. Western-blotting analyses showed that high temperature stress led to the association of Rubisco activase with the thylakoid membranes from the stroma fractions. However, such an association was much more pronounced in wild-type plants than in transgenic plants. The results in this study suggest that under high temperature stress, glycinebetaine maintains the activation of Rubisco by preventing the sequestration of Rubisco activase to the thylakoid membranes from the soluble stroma fractions and thus enhances the tolerance of CO2 assimilation to high temperature stress. The results seem to suggest that engineering of the biosynthesis of glycinebetaine by transformation with the BADH gene might be an effective method for enhancing high temperature tolerance of plants.
High temperature stress is one of the environmental factors that limit plant growth (Levitt, 1980; Boyer, 1982; Frova, 1997). Photosynthesis is one of the physiological processes that are most sensitive to high temperature stress (Berry and Björkman, 1980). Inhibition of photosynthesis by high temperature stress is a common occurrence for plants in tropical and subtropical regions and the temperate zones where plants are exposed periodically to high temperatures (Larcher, 1995). It is well documented that high temperature stress causes damage to photosynthetic electron transport (Berry and Björkman, 1980). For many years, PSII has long been considered the most heat-sensitive component of the photosynthetic apparatus (Berry and Björkman, 1980). However, it appears that PSII is unaffected at temperatures that inhibit CO2 fixation (Weis, 1981a, 1981b), and that PSII is only inhibited by severe heat stress when the temperature is higher than 45°C (Havaux, 1993, 1996). Recent studies seem to support that PSII activity is not limiting at temperatures that inhibit CO2 fixation and that CO2 fixation is most sensitive to heat stress due to the inhibition of activation of Rubisco via a direct effect on Rubisco activase (Feller et al., 1998; Salvucci and Crafts-Brandner, 2004a, 2004b; Haldimann and Feller, 2004).
Glycinebetaine (GB) is one of the organic compatible solutes that can accumulate rapidly in many plants under salinity stress, drought, and low temperature (McCue and Hanson, 1990; Rhodes and Hanson, 1993; Bohnert et al., 1995). Numerous studies on the physiology, biochemistry, biophysics, and genetics of plants have suggested that GB plays an important role in plants under various types of environmental stresses (for review, see Sakamoto and Murata, 2000, 2002). GB increases the salt tolerance (Harinasut et al., 1996; Hayashi et al., 1997, 1998; Holmström et al., 2000; Park et al., 2004), cold tolerance (Hayashi et al., 1997; Alia et al., 1998a; Chen et al., 2000), and freezing tolerance of plants (Allard et al., 1998; Sakamoto et al., 2000).
GB may also enhance tolerance of plants to high temperature stress. Several in vitro studies have indicated that GB protects some enzymes and protein complexes against heat-induced inactivation (Gorham, 1995). GB is in particular effective in protecting highly complex proteins, such as the PSII complex, against heat-induced inactivation (Mamedov et al., 1993; Allakhverdiev et al., 1996). An in vivo study has further shown that transformed Arabidopsis (Arabidopsis thaliana) with accumulation of GB exhibits enhanced tolerance to high temperatures during the growth of young seedlings (Alia et al., 1998b). However, it is not clear what is the physiological basis of GB in vivo for such an enhanced tolerance of growth to high temperature stress. Since photosynthesis is among the plant functions most sensitive to high temperature stress and can be inhibited completely by high temperature before other high-temperature-induced symptoms are detected (Berry and Björkman, 1980), we propose a hypothesis that the physiological basis for enhanced tolerance of growth to high temperature stress induced by accumulation of GB in vivo may be associated with increased tolerance of photosynthesis to high temperatures. To our knowledge, no report is available on whether the accumulation of GB in vivo can enhance photosynthesis against high temperature stress.
We have established the system for the biosynthesis of GB in vivo by genetic engineering of tobacco (Nicotiana tabacum), which is unable to accumulate GB, and observed that the tolerance to salt stress is greatly increased in this transgenic tobacco (Liang et al., 1997). In this study, using transgenic tobacco, we investigated the role of GB in vivo in protecting photosynthesis from high temperature stress.
RESULTS
Expression of the BADH Gene, and Accumulation and Localization of GB in Transgenic Tobacco Plants
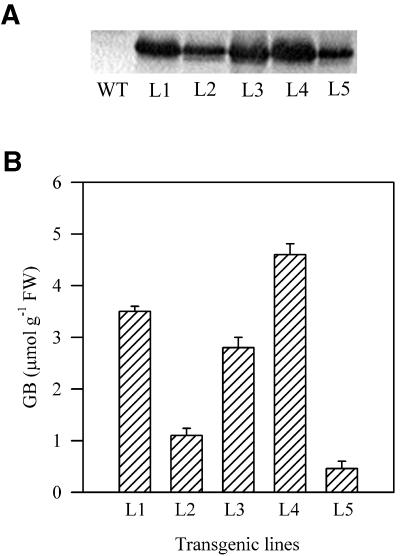
Our previous study showed that the BADH gene for betaine aldehyde dehydrogenase cloned from spinach (Spinacia oleracea) was successfully transferred to tobacco plants, which are unable to accumulate GB (Liang et al., 1997). Western-blotting analysis with the antiserum against betaine aldehyde dehydrogenase revealed the presence of a 60-kD protein corresponding to betaine aldehyde dehydrogenase in leaves of transformed plants, whereas no such protein was detected in wild-type plants (Fig. 1). The GB level in the leaves of five transgenic lines ranged from 0.46 to 4.6 μmol g−1 fresh weight, whereas no GB was detected in wild-type plants. Line 4 showed the highest accumulation of GB. We observed that there were no differences in the size or developmental stage of 2-month-old wild-type plants and transgenic plants.
Figure 1.
A, Western-blotting analysis. WT, Wild-type plants; L1 through L5, independent transgenic plants. B, GB levels in leaves of the seedlings from wild type and five independent transgenic lines. The values are mean ± se of three independent experiments. Bars indicate ses.
Although betaine aldehyde dehydrogenase is specifically targeted to the chloroplasts, we analyzed GB content in the isolated fraction of chloroplasts from leaves of transgenic plants. The percentage of GB found in the chloroplast was calculated by comparing leaf and chloroplast contents, expressed on a chlorophyll basis and corrected for the percentage of broken chloroplasts present. We estimated that 63% to 87% of total leaf GB was localized in the chloroplasts (Table I).
Table I.
GB levels in chloroplasts isolated from leaves of transgenic plants
Values in the table are mean ± se from four independent experiments. GB content in chloroplasts was corrected for the percentage of broken chloroplasts present. The percentage of GB found in the chloroplast was calculated by comparing leaf and chloroplast contents expressed on a chlorophyll basis. Chl, Chlorophyll.
| Transgenic Lines | GB in Leaves | GB in Isolated Chloroplasts | % Intact Chloroplasts | % GB in Chloroplasts |
|---|---|---|---|---|
| μmol mg−1 Chl | μmol mg−1 Chl | |||
| L1 | 1.41 ± 0.09 | 1.06 ± 0.11 | 97.8 ± 7.0 | 76.7 ± 5.7 |
| L2 | 0.45 ± 0.04 | 0.31 ± 0.05 | 96.9 ± 8.2 | 70.6 ± 6.6 |
| L3 | 1.13 ± 0.12 | 0.85 ± 0.04 | 98.8 ± 5.1 | 76.2 ± 5.2 |
| L4 | 1.77 ± 0.08 | 1.48 ± 0.10 | 97.6 ± 5.2 | 86.6 ± 7.3 |
| L5 | 0.18 ± 0.07 | 0.11 ± 0.02 | 96.6 ± 7.1 | 62.6 ± 4.1 |
Effects of High Temperatures on Growth of Seedlings
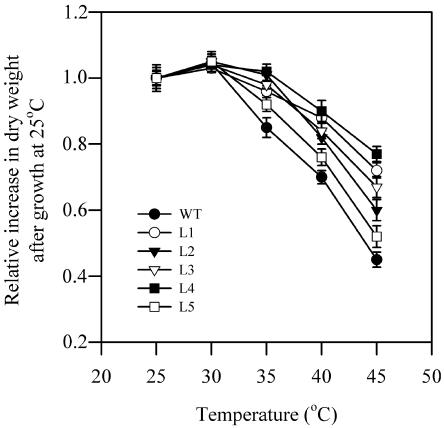
Figure 2 shows the effects of high temperature on the growth of seedlings of wild-type plants and five transgenic lines. Seedlings grown at 25°C in the greenhouse were transferred to elevated temperatures, 30°C, 35°C, 40°C, or 45°C, for 2 h in the dark. After heat treatment, the seedlings were grown again in the greenhouse for 3 d. Figure 2 shows that heat treatment significantly decreased the growth rate in wild-type and transgenic plants. The growth rate started to decrease at 35°C in wild-type plants and transgenic plants. However, the decrease in the growth rate was much greater in wild-type plants than in transgenic plants at high temperatures, in particular at 40°C and 45°C. Transgenic line 4 plants showed the highest resistance to high temperature. These results suggest that transgenic plants exhibited higher tolerance to high temperatures than wild-type plants.
Figure 2.
Effects of high temperatures on the growth of seedlings of wild-type plants and transgenic plants. Seedlings grown at 25°C in the greenhouse were transferred to different temperatures, 25°C, 30°C, 35°C, 40°C, or 45°C, in the chambers for 2 h in the dark. After heat stress, the seedlings were grown again in the greenhouse for 3 d. The growth rate of seedlings was calculated as relative increase in dry weight per seedling for 3 d. For measurements of dry weight, the seedlings were dried at 85°C for 2 d. The values are mean ± se of three independent experiments.
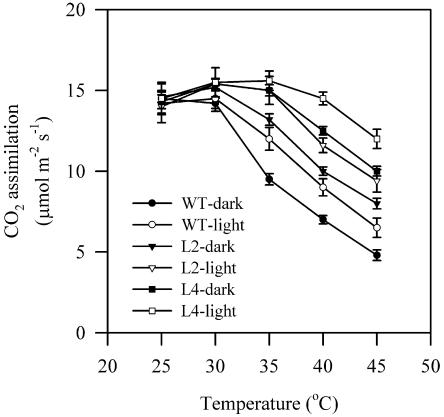
Effects of High Temperatures on CO2 Assimilation
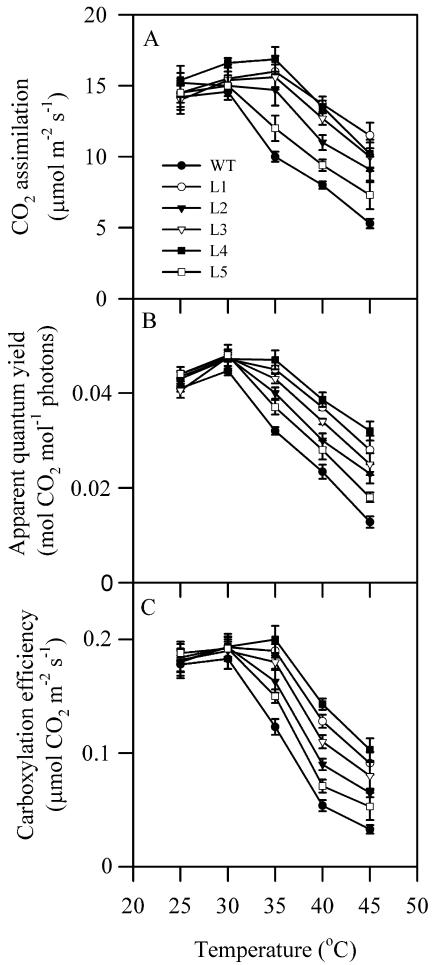
We then investigated the effects of high temperatures on CO2 assimilation in seedlings of wild-type and transgenic plants. Seedlings grown at 25°C in the greenhouse were exposed to elevated temperatures, 30°C, 35°C, 40°C, or 45°C, for 2 h in the dark and then photosynthetic gas exchange parameters were examined. Figure 3 shows that CO2 assimilation rate started to decrease significantly already at 35°C in wild-type plants. When temperatures were higher than 35°C, CO2 assimilation rate in both plants decreased significantly. However, this decrease in CO2 assimilation rate was much greater in wild-type plants than in transgenic plants. Similar results were also observed in the apparent quantum yield and the carboxylation efficiency of photosynthesis. Line 4 showed the highest resistance of CO2 assimilation rate to high temperature. These results indicate that the resistance of CO2 assimilation rate to high temperatures was significantly increased in transgenic plants.
Figure 3.
Effects of high temperatures on CO2 assimilation rate (A), the apparent quantum yield (B), and the carboxylation efficiency (C) of photosynthesis in wild-type plants and transgenic plants. Seedlings grown at 25°C in the greenhouse were exposed to different temperatures, 25°C, 30°C, 35°C, 40°C, or 45°C, in the chambers for 2 h in the dark and then the photosynthetic gas exchange parameters were determined. The values are mean ± se of three independent experiments.
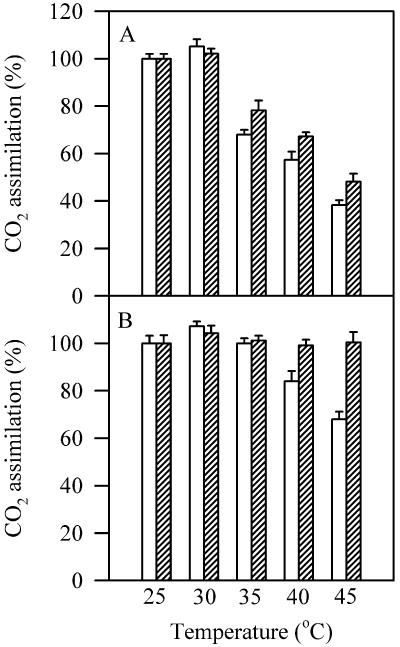
We have further compared the recovery of CO2 assimilation rate in wild-type plants and transgenic line 4 plants after 2-h recovery at 25°C following 2-h high temperature treatments. Figure 4 shows that the CO2 assimilation rate in transgenic line 4 plants showed a complete recovery, whereas wild-type plants showed only approximately 10% recovery.
Figure 4.
CO2 assimilation rate (expressed as % of the values at 25°C) determined after 2-h recovery at 25°C following 2-h high temperature treatments at different temperatures (striped bars) or immediately after 2-h high temperature treatments at different temperatures (white bars) in wild-type plants (A) and transgenic line 4 plants (B). The 100% values of CO2 assimilation rate for wild type and L4 lines at 25°C were 14.2 ± 0.9 and 15.4 ± 1.2, respectively, prior to high temperature treatment, and 14.5 ± 1.1 and 15.0 ± 1.3, respectively, after 2-h recovery following high temperature treatment. The values are mean ± se of four independent experiments.
The above results clearly show that the resistance of CO2 assimilation rate to high temperatures was significantly increased in transgenic plants when heat stress was imposed in the dark. Since it is known that light is involved in the regulation of photosynthesis in response to heat stress (Weis, 1982; Havaux et al., 1991; Schrader et al., 2004), we compared the responses of CO2 assimilation rate to high temperatures in wild-type plants and transgenic plants when heat stress was imposed in the dark and in the light. Figure 5 shows that the CO2 assimilation rate was partially prevented if heat stress was imposed in the light. Moreover, the increased resistance of the CO2 assimilation rate to high temperatures in transgenic plants shown in the dark was also clearly observed when heat stress was imposed in the light.
Figure 5.
Effects of high temperatures on CO2 assimilation rate in wild-type plants and transgenic plants. Seedlings grown at 25°C in the greenhouse were exposed to different temperatures, 25°C, 30°C, 35°C, 40°C, or 45°C, in the chambers for 2 h either in the dark or in the light (300 μmol m−2 s−1, which was the equivalent of growth light intensity), and then the photosynthetic gas exchange parameters were determined. The values are mean ± se of three independent experiments.
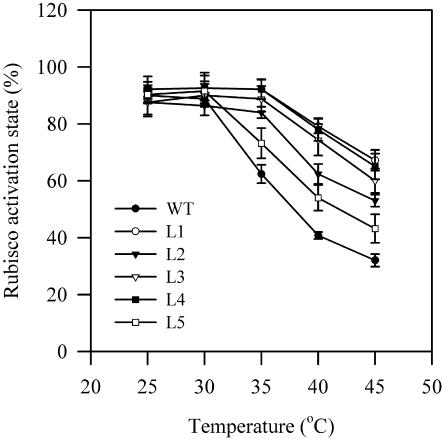
Effects of High Temperatures on Rubisco Activase-Mediated Activation of Rubisco
Our results show that there were no significant changes in the maximal efficiency of PSII photochemistry (Fv/Fm) in wild-type and transgenic plants during heat stress either in the dark or in the light (data not shown), suggesting that the decreased CO2 assimilation under high temperatures had nothing to do with the changes in the function of PSII. Thus, we further investigated whether the decreased CO2 assimilation rate under high temperatures was due to the inhibition of the dark reaction. Because it has been found recently that Rubisco activase is very sensitive to high temperature and plays an important role in limiting photosynthesis at high temperature (Feller et al., 1998; Haldimann and Feller, 2004; Salvucci and Crafts-Brandner, 2004a, 2004b), we compared the effects of high temperatures on the activity of Rubisco activase between wild-type plants and transgenic plants.
In this study, we observed that the activity of fully carbamylated Rubisco (total Rubisco activity) was not affected in the temperature range between 25°C and 45°C in wild-type and transgenic plants (data not shown). Analyses of Coomassie blue-stained proteins separated by SDS-PAGE and western blotting also show that there were no changes in the content of Rubisco in wild-type and transgenic plants during heat stress (data not shown). On the contrary, the initial Rubisco activity clearly decreased with increasing temperature. Thus, high temperature resulted in a significant decrease of the Rubisco activation state in wild-type and transgenic plants. Figure 6 shows the effects of high temperatures on the Rubisco activation state in wild-type and transgenic plants. High temperatures caused a progressive inhibition of Rubisco activation in both plants. However, the Rubisco activation state already decreased significantly at 35°C in wild-type plants. Moreover, at a given high temperature, the inhibition of the Rubisco activation state was more pronounced in wild-type plants than in transgenic plants. These results suggest that the increased tolerance of CO2 assimilation in transgenic plants is due to the increased tolerance of Rubisco activation to high temperatures.
Figure 6.
Effects of heat temperatures on Rubisco activation state calculated as the relative ratio of initial to total Rubisco activities in wild-type plants and transgenic plants. Seedlings grown at 25°C in the greenhouse were exposed to different temperatures, 25°C, 30°C, 35°C, 40°C, or 45°C, in the chambers for 2 h in the dark. The values are mean ± se of three independent experiments.
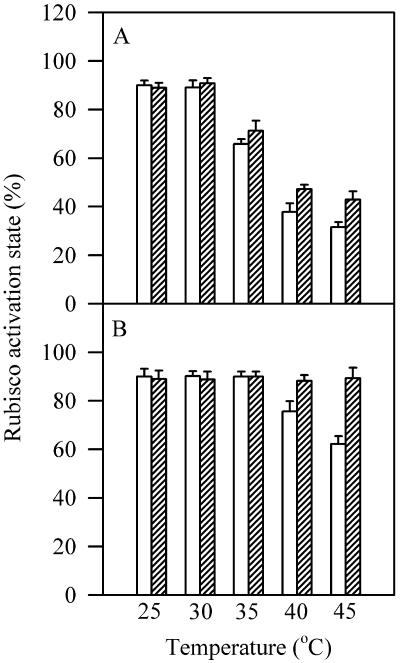
We have also compared the recovery of Rubisco activation in wild-type plants and transgenic line 4 plants after 2-h recovery at 25°C following 2-h high temperature treatments at different temperatures. Figure 7 shows that Rubisco activation in transgenic line 4 plants showed a complete recovery, whereas wild-type plants showed only a small recovery.
Figure 7.
Rubisco activation state calculated as the relative ratio of initial to total Rubisco activities determined after 2-h recovery at 25°C following 2-h high temperature treatments at different temperatures (striped bars) or immediately after 2-h high temperature treatments at different temperatures (white bars) in wild-type plants (A) and L4 transgenic plants (B). The values are mean ± se of four independent experiments.
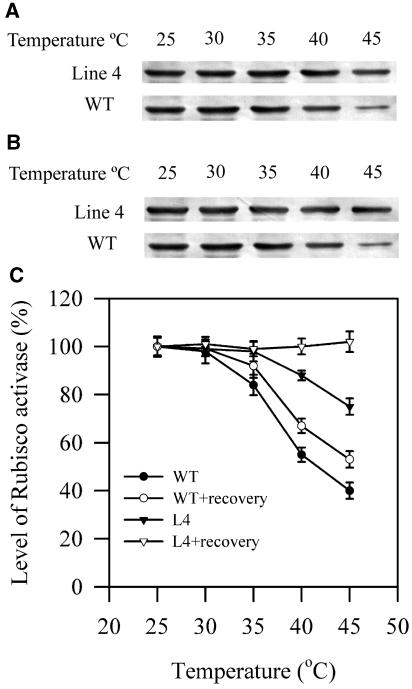
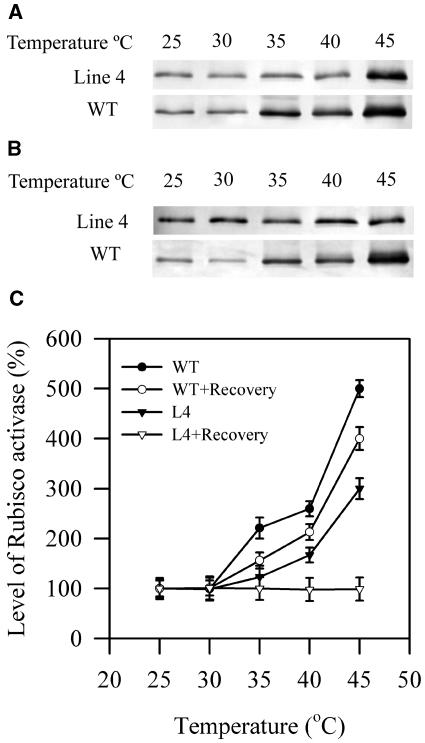
We further investigated the causes of this increased tolerance of Rubisco activation to high temperatures in transgenic plants. We examined whether the inhibition of Rubisco activation was associated with the decrease in the content of Rubisco activase. Western-blotting analysis was used to examine the effects of high temperatures on the content of Rubisco activase in the soluble fractions and the thylakoid fractions in wild-type and transgenic plants. Figure 8 shows that the content of Rubisco activase in soluble fractions decreased at high temperatures, and this decrease was more pronounced in wild-type plants than in transgenic line 4 plants. The changes in the content of Rubisco activase in soluble fractions in transgenic line 4 plants were largely reversible, whereas Rubisco activase in soluble fractions in wild-type plants showed only a small recovery after a recovery at 25°C for 2 h following high temperature treatments.
Figure 8.
A, Content of Rubisco activase in the soluble fractions of extracts of leaves in wild-type plants and transgenic line 4 plants after exposure to different high temperatures for 2 h. B, Content of Rubisco activase in the soluble fractions of extracts of leaves in wild-type plants and transgenic line 4 plants after 2-h recovery at 25°C following 2-h high temperature treatments at different temperatures. C, Quantitation of results in A and B. The values are mean ± se of three independent experiments.
On the other hand, the content of Rubisco activase in thylakoid fractions increased at high temperatures, and this increase was more pronounced in wild-type plants than in transgenic line 4 plants (Fig. 9). In addition, the changes in the content of Rubisco activase in thylakoid fractions in transgenic line 4 plants were largely reversible, whereas Rubisco activase in thylakoid fractions in wild-type plants showed only a small recovery after a recovery at 25°C for 2 h following high temperature treatments (Fig. 9).
Figure 9.
A, Content of Rubisco activase in the thylakoid fractions of extracts of leaves in wild-type plants and transgenic line 4 plants after exposed to different high temperatures for 2 h. B, Content of Rubisco activase in the thylakoid fractions of extracts of leaves in wild-type plants and transgenic line 4 plants after 2-h recovery at 25°C following 2-h high temperature treatments at different temperatures. C, Quantitation of results in A and B. The values are mean ± se of three independent experiments.
These results indicate that high temperatures caused association of Rubisco activase with thylakoids from the stroma, and such association was much more pronounced in wild-type plants than in transgenic plants. Thus, the results suggest that the enhanced tolerance of the activation of Rubisco to high temperatures in transgenic plants was due to high maintained content of Rubisco activase in the stroma fraction, which was associated with the lesser association of Rubisco activase with thylakoid membranes from the stroma fractions under high temperatures.
Effects of in Vitro GB on Temperature-Dependent Association of Rubisco Activase with the Thylakoid Membrane
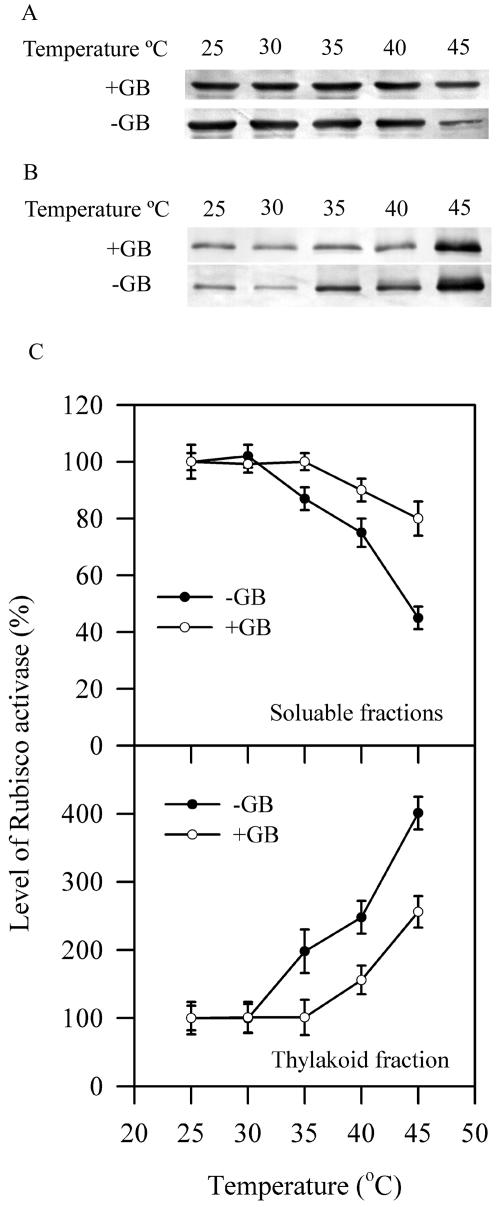
To further investigate the role of GB in the association of Rubisco activase with the thylakoid membranes during high temperature, we compared the effects of in vitro GB at the concentration comparable to that in the transgenic plants (line 4) on the association of Rubisco activase with the thylakoid membranes. The broken chloroplasts were obtained after an osmotic shock from intact chloroplasts of wild-type plants. The soluble fractions and thylakoid fractions were isolated from the broken chloroplasts that were incubated with exogenous GB (equivalent to 1.5 μmol mg−1 chlorophyll) at different temperatures for 10 min. Figure 10 clearly shows that exogenous GB decreased significantly the association of Rubisco activase with thylakoid membranes from the stroma fractions under high temperatures.
Figure 10.
Effects of in vitro GB on temperature-dependent association of Rubisco activase with the thylakoid membranes. A, Soluble fractions. B, Thylakoid fractions. Soluble fractions and thylakoid fractions were isolated from the broken chloroplasts, which were incubated without or with exogenous GB (equivalent to 1.5 μmol mg−1 chlorophyll) at different temperatures for 10 min. The broken chloroplasts were obtained after an osmotic shock from intact chloroplasts of wild-type plants. C, Quantitation of results in A and B. The values are mean ± se of three independent experiments.
DISCUSSION
This study showed that the accumulation of GB in vivo by introducing the BADH gene for betaine aldehyde dehydrogenase into tobacco resulted in the enhanced tolerance of growth and photosynthesis to high temperatures in transgenic plants. Although there is a study showing that accumulated GB in Arabidopsis by genetic engineering exhibits enhanced tolerance to high temperatures during the growth of young seedlings (Alia et al., 1998b), no report is available for the physiological basis of GB in vivo for such an enhanced tolerance of growth to high temperature stress. The results in this study support our hypothesis that the physiological basis for enhanced tolerance of growth to high temperature stress induced by accumulation of GB in vivo may be associated with increased tolerance of photosynthesis to high temperatures. The results suggest a new physiological function of the accumulation of GB in vivo, i.e. protecting photosynthesis against high temperature stress.
The mechanisms for increased tolerance by GB accumulation in vivo to various stresses have been proposed. Many studies have shown that GB accumulation in vivo results in increased tolerance to cold and salt stress. The mechanism for tolerance of cold and salt stress can be explained by the fact that GB stabilizes the PSII complex by stimulating its repair when plants are exposed to cold and salt stress (Papageorgiou et al., 1991; Hayashi et al., 1997; Alia et al., 1999; Holmström et al., 2000; Sakamoto and Murata, 2000; Park et al., 2004). On the other hand, several in vitro studies have shown that GB can protect the PSII complex against heat-induced inactivation (Mamedov et al., 1993; Allakhverdiev et al., 1996). Although PSII has long been considered the most temperature-sensitive component of photosynthesis (Berry and Björkman, 1980), recent studies have demonstrated that PSII is not affected at moderately high temperature (35°C–45°C; Havaux, 1993; Feller et al., 1998; Haldimann and Feller, 2004). Indeed, we observed that PSII was not affected by heat stress in this study, suggesting that enhanced tolerance of photosynthesis induced by GB to high temperature stress was not related to the function of PSII.
To further understand why accumulation of GB in vivo can enhance CO2 assimilation against high temperatures, we investigated whether such an enhancement of CO2 assimilation was associated with Rubisco activase-mediated activation of Rubisco. Rubisco per se is a relatively thermostable enzyme, and its enzyme activity is still stable at temperatures above 50°C, whereas Rubisco activase has been reported to be particularly sensitive to inactivation by elevated temperatures (Crafts-Brandner et al., 1997; Eckhardt and Portis, 1997). Rokka et al. (2001) found that most of the Rubisco activase was sequestered to the thylakoid membrane from the soluble stroma fraction at high temperature (42°C) ,and, at lower temperatures (38°C–40°C), the association of Rubisco activase with the thylakoid membrane occurred more slowly. Under physiological conditions, Rubisco activase functions in chloroplast stroma in removing inhibitory sugar phosphates from the active site of Rubisco in an ATP-dependent manner, and the decrease in the content of Rubisco activase in chloroplast stroma would result in a decrease in the activation of Rubisco and, thus, a decrease in CO2 assimilation rate (Portis, 1990, 1992). Our results show that the activity of Rubisco activase decreased significantly at high temperatures, and such a decrease was much pronounced in wild-type plants than in transgenic plants (Fig. 6). The decrease in the activity of Rubisco activase was not due to the decrease in the content of Rubisco since there was no change in the content of Rubisco in both plants during heat stress. In this study, we observed that the content of Rubisco activase in the soluble fractions decreased while the association of Rubisco activase with the thylakoid membranes increased with increasing temperature in wild-type and transgenic plants. However, the decrease in the content of Rubisco activase in the soluble fractions in transgenic plants was much less than in wild-type plants, whereas the increase in the content of Rubisco activase in the thylakoid membrane fractions in transgenic plants was much less than in wild-type plants (Figs. 8 and 9). Thus, our results suggest that compared to wild-type plants, the enhanced tolerance of CO2 assimilation to high temperature stress in transgenic plants was associated with increased thermotolerance of Rubisco activase, which resulted in the lesser association of Rubisco activase with the thylakoid membranes from stroma fraction. The results also suggest that the accumulation GB in vivo can maintain the activation of Rubisco by preventing the sequestration of Rubisco activase to the thylakoid membrane from the soluble stroma fraction under high temperature stress. Thus, our results seem to indicate that the accumulation of GB in vivo leads to increased thermotolerance of Rubisco activase. This function of GB is of significance for increasing tolerance of plants to high temperatures since Rubisco activase has been shown to be particularly sensitive to inactivation by high temperatures (Crafts-Brandner et al., 1997; Eckhardt and Portis, 1997).
The results presented here strongly suggest a role for GB against heat-induced inactivation of Rubisco by preventing the association of Rubisco activase with the thylakoids from the chloroplast stroma. Although the mechanism of this thermoprotection in vivo is not clear, it is tempting to propose a possible role for GB. It has been shown that the temperature-dependent association of Rubisco activase with the thylakoid membrane was due to a conformational change in the Rubisco activase itself, which leads to a specific association of Rubisco activase with thylakoid-bound polysomes but not to heat-induced alterations in the thylakoid membrane (Rokka et al., 2001). The molecular features of GB allow it to interact with both hydrophilic and hydrophobic domains of macromolecules, such as enzymes and protein complexes. It has been documented that, in vitro, GB stabilizes the structures and activities of enzymes against the damaging effects of excessive salt, cold, and heat (Gorham, 1995). For example, it has been shown that GB protects the Rubisco enzyme from inactivation during salt stress by the stabilization of its conformation (Nomura et al., 1998) and that GB acts as a molecular chaperone in Escherichia coli, assisting in enzymatic refolding (Bourot et al., 2000). Thus, we tentatively propose that the accumulation of GB in vivo may prevent the high-temperature-induced conformational change in the Rubisco activase itself by acting as a molecular chaperone that inhibits a specific association of Rubisco activase with thylakoid-bound polysomes and may maintain the high activation of Rubisco even at high temperature stress.
The results in this study showed that high temperatures induced no damage to PSII, which has been reported by many studies showing that PSII is not affected at moderately high temperature (35°C–45°C; Havaux, 1993; Feller et al., 1998; Haldimann and Feller, 2004). Does no damage to PSII mean that high temperature has no effect on other processes related to photosynthetic electron transport? It has been reported that photosynthetic electron transport is the functional limitation of photosynthesis in field-grown Pima cotton (Gossypium barbadense) plants at high temperature (Wise et al., 2004). Moderate heat stress induces a reversible thylakoid membrane leakiness, and such a membrane leakiness may alter the ATP and NAD (P)H levels and may play a role in the decrease of photosynthetic rate under heat stress (Pastenes and Horton, 1996; Bukhov et al., 1999; Schrader et al., 2004). Heat stress also induces a cyclic electron transport around PSI that can balance the loss of protons due to membrane leakiness so that a sizeable pH can be maintained despite membrane leakiness (Bukhov et al., 1999; Schrader et al., 2004). In addition, several studies have shown that moderate heat stress results in an increase in PSI activity at the expense of the redox status of the stroma (Boucher et al., 1989; Havaux, 1996; Bukhov et al., 1998, 2000; Schrader et al., 2004) as well as a substantial transition from state 1 to state 2 (Pastenes and Horton, 1996; Schrader et al., 2004). It has been proposed that the deactivation of Rubisco can be an adaptive mechanism in response to the decreased capacity for electron transport under heat stress to maintain the thylakoid energy and reduce accumulation of high levels of photorespiratory intermediates (Schrader et al., 2004). Thus, the binding of Rubisco activase to thylakoid membranes observed in this study can be explained by an adaptive mechanism in response to the decreased photosynthetic electron transport, which may result from membrane leakiness and increased cyclic electron transport.
It should be pointed out that the reduced Rubisco activation state should have no effect on the apparent quantum of photosynthesis. However, the results in this study showed that high temperatures reduced the apparent quantum yield of photosynthesis (Fig. 3). As discussed above, heat stress may induce membrane leakiness and increased cyclic electron transport, which lead to the decrease in photosynthetic electron transport although heat stress induced no damage to PSII. Thus, the reduced apparent quantum yield may be explained by the decreased photosynthetic electron transport due to membrane leakiness and increased cyclic electron transport under high temperatures. In addition, it has been shown that heat stress induced a reduction in the quantum yield of PSII electron transport, which mainly originated from a reversible down-regulation of PSII activity in response to an inhibition of photosynthetic carbon metabolism by heat stress since heat stress had no significant effect on the maximal quantum yield of PSII photochemistry (Haldimann and Feller, 2004). The decrease in the quantum yield of PSII electron transport would result in the decrease in the apparent quantum yield of photosynthesis. Therefore, the decrease in the apparent quantum yield observed in this study could also explained by the down-regulation of PSII activity in response to an inhibition of photosynthetic carbon metabolism.
Although the genetic engineering of the synthesis of GB to tolerate abiotic stress appears promising (Sakamoto and Murata, 2000, 2002; Chen and Murata, 2002; Yuwansiri et al., 2002), there are still no reports on the enhanced tolerance of photosynthesis to high temperature stress. Our study demonstrates the importance of transformation with the BADH gene for enhancing tolerance of growth and photosynthesis to high temperature stress because photosynthesis is among the plant functions most sensitive to high temperature damage. Therefore, our results may benefit efforts to improve crop yields in tropical and subtropical regions as well as in temperate zones where plants are often periodically exposed to heat temperature stress.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The expression cassette contained (1) the BADH gene that had been isolated from spinach (Spinacia oleracea) under the control of the promoter 35S ribosomal RNA from cauliflower mosaic virus, (2) the transit peptide of the small subunit of Rubisco of tobacco (Nicotiana tabacum), and (3) the terminator of the gene for nopaline synthase. This cassette was inserted into binary vector pBin19 at the SacI and SalI sites. The resulting plasmid, pBinBAD-S, was introduced into Agrobacterium tumefaciens LBA4404 (An et al., 1988). Tobacco (wild-type K326) was transformed with the resultant plasmid by the standard Agrobacterium-mediated method as described previously (Liang et al., 1997). T1 plants, obtained by self-pollination of T0 plants, were backcrossed to wild type, and the resulting T2 plants were allowed to self-fertilize. T3 plants obtained by self-pollination of T2 plants were used in this study. Transgenic plants were selected for kanamycin resistance and verified by PCR or western blotting using antisera raised against BADH.
Five independent lines of transgenic tobacco plants (L1, L2, L3, L4, and L5) were selected for this study. The seeds of these transgenic plants were allowed to germinate on agar in the presence of 50 μg L−1 kanamycin. The seeds of wild-type plants were allowed to germinate on agar in the absence of kanamycin. After growth for 2 weeks, plants were transferred to vermiculite for 2 weeks and then were transplanted to soil. The plants were grown in a greenhouse at 25°C ± 1°C with photosynthetic photon flux density of 300 μmol m−2 s−1, a relative humidity of 75% to 80%, and a photoperiod of 14/10-h light/dark. The seedlings after growth for 2 months were subjected to various experiments. To study the effects of high temperatures on growth and photosynthetic physiology and biochemistry parameters, the whole plants were exposed to various temperatures (25°C–45°C) for 2 h in the dark. In addition, to examine the role of light in the responses of photosynthetic rate to heat stress, heat treatments on the whole plants were also imposed in the light (300 μmol m−2 s−1, which was equivalent of growth light intensity). All the measurements on physiological and biochemical parameters were carried out on the youngest fully expanded leaves.
Analysis of Gas Exchange
Measurements of net photosynthetic gas exchange were made on a fully expanded attached leaf of tobacco seedlings using an open system (Ciras-1, PP Systems, Norfolk, UK). After exposure to elevated temperatures for 2 h, the whole plants were returned to 25°C and gas exchange was then analyzed. The light-saturating photosynthetic rate was made at a CO2 concentration of 360 μL L−1 and at temperature 25°C with relative humidity 80% and saturating light (800 μmol m−2 s−1). The apparent quantum yield and carboxylation efficiency of photosynthesis were determined as the slope of photosynthesis-light and CO2 response curves, respectively. The measurements on these photosynthetic parameters lasted approximately 10 min, during which no significant recovery was observed on these parameters.
For recovery experiments, the whole plants were placed at 25°C for 2 h following 2-h heat stress treatments, and photosynthetic parameters were then determined under the conditions as mentioned above.
To clarify whether the measurements of photosynthesis made at 25°C following 2-h heat treatments were a measure of the aftereffects of high temperature or not, light-saturated photosynthetic rate was determined at respective high temperature by irradiating the whole plants with 800 μmol m−2 s−1 during the last 10 min of 2-h different high temperature treatments. We observed that there were no significant differences in photosynthetic rate determined either at high temperature or at 25°C, suggesting that the measurements of photosynthesis at 25°C following 2-h high temperature treatments were a direct result of high temperature.
Measurements of Chlorophyll Fluorescence
Chlorophyll fluorescence was measured with a PAM-2000 chlorophyll fluorescence system under atmospheric conditions (Heinz Walz, Effeltrich, Germany). After a dark adaptation period of 10 min, minimum fluorescence (Fo) was determined by a weak red light. Maximum fluorescence of dark-adapted state (Fm) was measured during a subsequent saturating light pulse (8,000 μmol m−2 s−1 for 0.8 s). The measurements were performed on the attached leaves of tobacco seedlings. The maximal efficiency of PSII photochemistry was determined as the ratio of variable to maximal chlorophyll fluorescence (Fv/Fm; Krause and Weis, 1991).
GB Extraction and Quantification
The method developed by Rhodes et al. (1989) was essentially followed. Leaf samples were ground in methanol:chloroform:water (12:5:1). The aqueous phase was fractioned by Dowex-1-OH− and Dowex-50-H+ ion-exchange chromatograph. The GB fraction was eluted with 6 m NH4OH, dried under a stream of N2 at 45°C, and dissolved in 1 mL of distilled water. HPLC (Waters 600) and Millenium Chromatography Manager System Control software were used to determine the content of GB and to obtain spectral data, respectively.
Determination of Light-Dependent Activation of Rubisco
To determine light-dependent activation of Rubisco, the whole plants were irradiated with saturating light 800 μmol m−2 s−1 for 10 min at 25°C to promote full activation of Rubisco following 2-h high temperature treatments in the dark or after a defined period of recovery following heat treatments at different high temperatures in the dark. After illumination, leaf tissues were harvested immediately for the determination of the initial and total Rubisco activities.
Determination of light-dependent activation of Rubisco was followed essentially according to Feller et al. (1998). Leaf tissues taken after 10 min of illumination were immediately extracted using a glass homogenizer in the extraction buffer containing 100 mm Tricine, pH 8.0, 5 mm MgCl2, 0.1 mm EDTA, 5 mm dithiothreitol, 1% (w/v) polyvinylpyrrolidone, 1% (w/v) casein, 0.05% (v/v) Triton X-100, 1 mm phenylmethylsulfonyl fluoride, and 20 μmol leupeptin. The resuspended extract was assayed at 30°C either immediately to determine the initial Rubisco activity or after incubation for 10 min at 30°C in an assay mixture containing 10 mm NaHCO3 and Mg2+ but lacking ribulose-1,5-bisphosphate to determine the activity of fully carbamylated Rubisco (total Rubisco activity). The activation state of Rubisco (Perchorowicz et al., 1981) was calculated as the relative ratio of initial to total Rubisco activities.
To clarify whether the measurements of light-dependent activation of Rubisco made at 25°C following 2-h heat treatments in the dark were a measure of the aftereffects of high temperature or not, we also determined the initial and total activities of Rubisco immediately after irradiating the whole plants with saturating light 800 μmol m−2 s−1 during the last 10 min of 2-h high temperature treatments. We observed that there were no significant differences in the initial and total activities of Rubisco between the leaf tissues taken immediately after 10 min of illumination at 25°C following 2-h high temperature treatments in the dark and the leaf tissues taken immediately after the last 10 min of illumination of 2-h high temperature treatment. This suggests that the measurements of the initial and total activities of Rubisco at 25°C after 10 min of illumination following 2-h high temperature treatments in the dark were a direct result of high temperature. In addition, it should be pointed out that no significant recovery was observed on the initial and total activities of Rubisco at 25°C during 10 min of illumination following 2-h high temperature treatments.
Isolation of Thylakoids and Chloroplast
Thylakoids were isolated from the leaves of wild-type and transgenic tobacco plants. Fresh leaves were homogenized in a medium containing 0.4 m Suc, 50 mm Tricine, pH 7.6, and the homogenate was filtrated through four and then 16 layers of gauze. The filtrate was centrifuged at 500g for 2 min to remove large debris. The supernatant was centrifuged at 3,000g for 10 min. The pellet was washed twice by the buffer (50 mm Tricine, 10 mm NaCl, 5 mm MgCl2, pH 7.6) at 10,000g for 10 min. The resulting washed pellet was thylakoid membranes. All procedures were carried out at 0°C to 4°C.
Intact chloroplasts were isolated by centrifugation on a Percoll density gradient according to the protocol of Mullet and Chua (1983). The percentage of intact chloroplasts was determined by measuring ferricyanide photoreduction before and after osmotic shock. Chlorophyll content was determined in 80% (v/v) acetone according to Porra et al. (1989).
SDS-PAGE and Immunological Analyses
Samples were solubilized in the presence of 6 m urea and separated by SDS-PAGE (Laemmli, 1970) using 15% (w/v) acrylamide gels with 6 m urea. For immunoblotting, polypeptides were electrophoretically transferred to a nitrocellulose membranes (Millipore, Saint-Quentin, France) and proteins were detected with antibodies raised against betaine aldehyde dehydrogenase (Z. Liang, Institute of Botany, Chinese Academy of Sciences), Rubisco activase (G. Chen, Shanghai Institute of Plant Physiology and Ecology), and Rubisco large subunit (L. Zhang, Institute of Botany, Chinese Academy of Sciences). Protein content was determined by the dye-binding assay according to Bradford (1976).
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number gi|170099.
Acknowledgments
We thank Professor Lixin Zhang, Institute of Botany, Chinese Academy of Sciences, for constructive discussion and for providing the antibody of the large subunit of Rubisco, and Professor Genyun Chen, Shanghai Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, for providing the antibody of Rubisco activase.
This work was supported by the Frontier Project of the Knowledge Innovation Engineering of the Chinese Academy of Sciences (grant no. KSCXZ–SW–326), by the Program of 100 Distinguished Young Scientists of the Chinese Academy of Sciences (to C.L.), and by the Natural Science Foundation of China (grant no. 30370849 to X.Y.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.063164.
References
- Alia, Hayashi H, Chen THH, Murata N (1998. a) Transformation with a gene for choline oxidase enhances the cold tolerance of Arabidopsis during germination and early growth. Plant Cell Environ 21: 232–239 [Google Scholar]
- Alia, Hayashi H, Sakamoto A, Murata N (1998. b) Enhancement of the tolerance of Arabidopsis to high temperatures by genetic engineering of the synthesis of glycinebetaine. Plant J 16: 155–161 [DOI] [PubMed] [Google Scholar]
- Alia, Sakamoto A, Nonaka H, Hayashi H, Saradhi PP, Chen THH, Murata N (1999) Enhanced tolerance to light stress of transgenic Arabidopsis plants that express the codA gene for a bacterial choline oxidase. Plant Mol Biol 40: 279–288 [DOI] [PubMed] [Google Scholar]
- Allakhverdiev SI, Feyziev YM, Ahmed A, Hayashi H, Aliev JA, Klimov VV, Murata N, Carpentier R (1996) Stabilization of oxygen evolution and primary electron transport reactions in photosystem II against heat stress with glycinebetaine and sucrose. J Photochem Photobiol B Biol 34: 149–157 [DOI] [PubMed] [Google Scholar]
- Allard F, Houde M, Krol M, Ivanov A, Huner NPA, Sarhan F (1998) Betaine improves freezing tolerance in wheat. Plant Cell Physiol 39: 1194–1202 [Google Scholar]
- An G, Ebert PR, Mitra A, Ha SB (1988) Binary vectors. In SB Gelvin, RA Schilperoort, eds, Plant Molecular Biology Manual A3. Kluwer Academic, Dordrecht, The Netherlands, pp 1–9
- Berry JA, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31: 491–543 [Google Scholar]
- Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7: 1099–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher N, Harnois J, Carpentier R (1989) Heat-stress stimulation of electron flow in a photosystem I submembrane fraction. Biochem Cell Biol 68: 999–1004 [DOI] [PubMed] [Google Scholar]
- Bourot S, Sire O, Trautwetter A, Touze T, Wu LF, Blanco C, Bernard T (2000) Glycinebetaine-assisted protein folding in a lysA mutant of Escherichia coli. J Biol Chem 275: 1050–1056 [DOI] [PubMed] [Google Scholar]
- Boyer JS (1982) Plant productivity and environment. Science 218: 443–448 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein using the principal of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Bukhov NG, Boucher N, Carpentier R (1998) Loss of the precise control of photosynthesis and increased yield of non-radiative dissipation of excitation energy after mild heat stress treatment of barley leaves. Physiol Plant 104: 563–570 [Google Scholar]
- Bukhov NG, Samson G, Carpentier R (2000) Nonphotosynthetic reduction of the intersystem electron transport chain of chloroplasts following heat stress. Steady-state rate. Photochem Photobiol 72: 351–357 [PubMed] [Google Scholar]
- Bukhov NG, Wiese C, Neimanis S, Heber U (1999) Heat sensitivity of chloroplasts and leaves: leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth Res 59: 81–93 [Google Scholar]
- Chen THH, Murata N (2002) Enhancement of tolerance to abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5: 250–257 [DOI] [PubMed] [Google Scholar]
- Chen WP, Li PH, Chen THH (2000) Glycinebetaine increases chilling tolerance and reduces chilling-induced lipid peroxidation in Zea mays L. Plant Cell Environ 23: 609–618 [Google Scholar]
- Crafts-Brandner SJ, van de Loo FJ, Salvucci ME (1997) The two forms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiol 114: 439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt NA, Portis AR Jr (1997) Heat denaturation profiles of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and Rubisco activase and the inability of Rubisco activase to restore activity of heat-denaturated Rubisco. Plant Physiol 113: 243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller U, Crafts-Brandner SJ, Salvucci E (1998) Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase activase-mediated activation of Rubisco. Plant Physiol 116: 539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frova C (1997) Genetics dissection of thermotolerance in maize. In S Grillo, A Leone, eds, Physical Stress in Plants. Springer-Verlag, New York, pp 31–38
- Gorham J (1995) Betaines in higher plants—biosynthesis and role in stress metabolism. In RM Wallsgrove, ed, Amino Acids and Their Derivatives in Higher Plants. Cambridge University Press, Cambridge, UK, pp 171–203
- Haldimann P, Feller U (2004) Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ 27: 1169–1183 [Google Scholar]
- Harinasut P, Tsutsui K, Takabe T, Nomura M, Takabe T, Kishitani S (1996) Exogenous glycinebetaine accumulation and increased salt-tolerance in rice seedlings. Biosci Biotechnol Biochem 60: 366–368 [DOI] [PubMed] [Google Scholar]
- Havaux M (1993) Characterization of thermal damage to the photosynthetic electron transport system in potato leaves. Plant Sci 94: 19–33 [Google Scholar]
- Havaux M (1996) Short-term responses to photosystem I to heat stress. Photosynth Res 47: 85–97 [DOI] [PubMed] [Google Scholar]
- Havaux M, Greppin H, Strasser RJ (1991) Functioning of photosystems I and II in pea leaves exposed to heat stress in the presence or absence of light. Planta 186: 88–98 [DOI] [PubMed] [Google Scholar]
- Hayashi H, Alia, Sakamoto A, Nonaka H, Chen THH, Murata N (1998) Enhanced germination under high-salt conditions of seeds of transgenic Arabidopsis with a bacterial gene (codA) for choline oxidase. J Plant Res 111: 357–362 [Google Scholar]
- Hayashi H, Alia Mustardy L, Deshnium P, Ida M, Murata N (1997) Transformation of Arabidopsis thaliana with the codA gene for choline oxidase: accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J 12: 133–142 [DOI] [PubMed] [Google Scholar]
- Holmström K-O, Somersalo S, Mandal A, Palva ET, Welin B (2000) Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J Exp Bot 51: 177–185 [DOI] [PubMed] [Google Scholar]
- Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42: 313–349 [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Larcher W (1995) Ecophysiology and stress physiology of functional groups. In W Larcher, ed, Physiological Plant Ecology. Springer-Verlag, Berlin, pp 340–353
- Levitt L (1980) Responses of plants to environmental stresses. In TT Kozlowski, ed, Physiological Ecology. Academic Press, New York, pp 347–448
- Liang Z, Ma D, Tang L, Hong Y, Luo A, Dai X (1997) Expression of the spinach aldehyde dehydrogenase (BADH) gene in transgenic tobacco plants. Chin J Biotechnol 13: 236–240 [PubMed] [Google Scholar]
- Mamedov M, Hayashi H, Murata N (1993) Effects of glycinebetaine and unsaturation of membrane lipids on heat stability of photosynthetic electron-transport and phosphorylation reactions in Synechocystis PCC6803. Biochim Biophys Acta 1142: 1–5 [Google Scholar]
- McCue KF, Hanson AD (1990) Drought and salt tolerance: towards understanding and application. Trends Biotechnol 8: 358–362 [Google Scholar]
- Mullet JE, Chua NH (1983) In vitro reconstitution of synthesis, uptake, and assembly of cytoplasmically synthesized chloroplast proteins. Methods Enzymol 97: 502–509 [Google Scholar]
- Nomura M, Hibino T, Takabe T, Sugyama T, Yokota A, Miyake H, Takabe T (1998) Transgenically produced glycinebetaine protects ribulose 1,5-bisphosphate carboxylase/oxygenase from inactivation in Synechococcus sp. PCC7942 under salt stress. Plant Cell Physiol 39: 425–432 [Google Scholar]
- Papageorgiou GC, Fugimura Y, Murata N (1991) Protection of the oxygen-evolving PS II complex by glycinebetaine. Biochim Biophys Acta 1057: 361–366 [Google Scholar]
- Park E-J, Jeknic Z, Sakamoto A, DeNoma J, Yuwansiri R, Murata N, Chen THH (2004) Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J 40: 474–487 [DOI] [PubMed] [Google Scholar]
- Pastenes C, Horton P (1996) Effect of high temperature on photosynthesis in beans. I. Oxygen evolution and chlorophyll fluorescence. Plant Physiol 112: 1245–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz JT, Raynes DA, Jensen RG (1981) Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci USA 78: 2985–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Portis AR Jr (1990) Rubisco activase. Biochim Biophys Acta 1051: 15–28 [DOI] [PubMed] [Google Scholar]
- Portis AR Jr (1992) Regulation of ribulose-1,5-bisphosphate carboxylase/oxygenase activity. Annu Rev Plant Physiol Plant Mol Biol 43: 415–437 [Google Scholar]
- Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol 44: 357–384 [Google Scholar]
- Rhodes D, Rich PJ, Brunk DG, Rhodes JC, Pauly MH, Hansen LA (1989) Development of two isogenic sweet corn hybrids differing for glycinebetaine content. Plant Physiol 91: 1112–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokka A, Zhang L, Aro E-M (2001) Rubisco activase: an enzyme with a temperature-dependent dual function? Plant J 25: 463–471 [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Murata N (2000) Genetic engineering of glycinebetaine synthesis in plants: current status and implications for enhancement of stress tolerance. J Exp Bot 51: 81–88 [PubMed] [Google Scholar]
- Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25: 163–171 [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Valverde R, Alia, Chen THH, Murata N (2000) Transformation of Arabidopsis with the codA gene for choline oxidase enhances freezing tolerance of plants. Plant J 22: 449–453 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004. a) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120: 179–186 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004. b) Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol 134: 1460–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader SM, Wise RR, Wacholtz WF, Ort DR, Sharkey TD (2004) Thylakoid membrane responses to moderately high leaf temperature in Pima cotton. Plant Cell Environ 27: 725–735 [Google Scholar]
- Weis E (1981. a) Reversible heat-inactivation of the Calvin cycle: a possible mechanism of the temperature regulation of photosynthesis. Planta 151: 33–39 [DOI] [PubMed] [Google Scholar]
- Weis E (1981. b) The temperature sensitivity of dark-inactivation and light-activation of the ribulose-1,5-bisphosphate carboxylase in spinach chloroplasts. FEBS Lett 129: 197–200 [Google Scholar]
- Weis E (1982) Influence of light on the heat sensitivity of the photosynthetic apparatus in isolated spinach chloroplasts. Plant Physiol 70: 1530–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RR, Olson AJ, Schrader SM, Sharkey TD (2004) Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ 27: 717–724 [Google Scholar]
- Yuwansiri R, Park EJ, Jeknic Z, Chen THH (2002) Enhancing cold tolerance in plants by genetic engineering of glycinebetaine synthesis. In PH Li, T Palva, eds, Plant Cold Hardiness: Gene Regulation and Genetic Engineering. Kluwer Academic/Plenum Publishers, Dordrecht, The Netherlands, pp 259–275