Abstract
Two maize (Zea mays) cyclin-dependent kinase (CDK) inhibitors, Zeama;KRP;1 and Zeama;KRP;2, were characterized and shown to be expressed in developing endosperm. Similar to the CDK inhibitors in Arabidopsis (Arabidopsis thaliana) and tobacco (Nicotiana tabacum), the maize proteins contain a carboxy-terminal region related to the inhibitory domain of the mammalian Cip/Kip inhibitors. Zeama;KRP;1 is present in the endosperm between 7 and 21 d after pollination, a period that encompasses the onset of endoreduplication, while the Zeama;KRP;2 protein declines during this time. Nevertheless, Zeama;KRP;1 accounts for only part of the CDK inhibitory activity that peaks coincident with the endoreduplication phase of endosperm development. In vitro assays showed that Zeama;KRP;1 and Zeama;KRP;2 are able to inhibit endosperm Cdc2-related CKD activity that associates with p13Suc1. They were also shown to specifically inhibit cyclin A1;3- and cyclin D5;1-associated CDK activities, but not cyclin B1;3/CDK. Overexpression of Zeama;KRP;1 in maize embryonic calli that ectopically expressed the wheat dwarf virus RepA protein, which counteracts retinoblastoma-related protein function, led to an additional round of DNA replication without nuclear division.
Cyclin-dependent kinase (CDK) inhibitors, or CKIs as they are known in yeast (Saccharomyces cerevisiae) and mammals, regulate cell cycle progression by binding to and inhibiting the activity of S- and M-phase CDKs (Morgan, 1997). In budding yeast, three CKIs were identified: Far1 inhibits G1 CDKs; Sic1 plays a role in the timing of S-phase by inhibiting G1/S-phase CDK complexes; and Ph081 inactivates a cyclin/CDK complex that functions in controlling gene expression under low-phosphate conditions. In fission yeast (Schizosaccharomyces pombe), a CKI called Rum1 inhibits mitotic CDK complexes. The seven CKIs described in mammals are classified in two protein families according to structural and functional similarities. The INK4 family contains an ankyrin repeat motif, and it specifically affects CDK4 and CDK6, the most divergent cell cycle-associated CDKs in mammals. The Cip/Kip family of CKIs comprises p21Cip1 (also called Waf1, Sdi1, or CAP20), p27Kip1, and p57Kip2, all of which have a conserved inhibitory domain at the N terminus. Members of the Cip/Kip family show a broader spectrum of inhibitory effects on cyclin/CDK complexes compared to the INK4 family. Importantly, both the INK4 and the Cip/Kip families inhibit CDKs involved in the G1/S transition; members of the INK4 family inhibit CDKs containing D-type cyclins, while Cip/Kip family members inhibit CDKs containing E- and A-type cyclins (for review, see Sherr and Roberts, 1995).
Recently, CKI gene sequences similar to members of the mammalian Cip/Kip family of CKIs were identified in plants. Seven genes that encode proteins with some degree of sequence homology with p27Kip1, so-called Kip-related proteins (KRPs), were isolated from Arabidopsis (Arabidopsis thaliana; Wang et al., 1998; De Veylder et al., 2001; Zhou et al., 2002a). Two of them, KRP1 and KRP2, were shown to inhibit CDK activity in vitro and in vivo (Wang et al., 1998, 2000; Lui et al., 2000; De Veylder et al., 2001). In tobacco (Nicotiana tabacum), a KRP-like inhibitor named NtKIS1a was isolated by a yeast two-hybrid screen; overexpression of this gene reduced CDK activity in planta (Jasinski et al., 2002). These studies, together with others that identified homologs of a variety of core cell cycle genes in plants, demonstrated that much of the molecular machinery controlling the cell cycle is conserved in eukaryotes.
In addition to their role in blocking cell cycle progression, CKIs also appear to help regulate endoreduplication. This type of cell cycle occurs in many eukaryotes, including plants, and it can lead to a dramatic increase in nuclear DNA content. Although the endoreduplication cell cycle is common in metabolically active tissues, the regulatory mechanisms and the components that enable endoreduplicating cells to replicate their DNA without cell division are not entirely clear (Edgar and Orr-Weaver, 2001).
Several recent studies in plants reported that KRPs inhibit endoreduplication. Jasinski et al. (2002) showed that overexpression of a tobacco KRP, NtKIS1a, in Arabidopsis blocked endoreduplication in nuclei of rosette leaves. Overexpression of Arabidopsis KRP1, KRP2, KRP6, and ICKCr, a KRP from Chenopidium rubrum (Fountain et al., 1999), was shown to inhibit endoreduplication, decreasing the ploidy level of leaves (De Veylder et al., 2001; Zhou et al., 2002a). Additionally, overexpression of KRP1 was shown to inhibit endoreduplication in Arabidopsis trichomes (Schnittger et al., 2003).
In contrast, studies in a variety of organisms demonstrated a potential positive role for CKIs in the endoreduplication process. Kikuchi et al. (1997) observed that a CKI belonging to the Cip/Kip family, p21Cip1, was up-regulated at an early stage of human UT-7 megakarocytic cell differentiation, just before nuclear polyploidization. Bates et al. (1998), Niculescu et al. (1998), and Chang et al. (2000) showed that overexpression or transient expression of p21Cip1 or p27Kip1 leads to endoreduplication in retinoblastoma (Rb)-deficient human cells. Hattori et al. (2000) reported that expression of p57Kip2, a Cip/Kip family member, is induced coincidentally with the transition to endocycles in mammalian trophoblast giant cells. Additionally, as indirect evidence of a positive role for CKIs in endoreduplication, Nakayama et al. (2000) showed that reduced synthesis in mouse hepatocytes of Skp2, a protein involved in p27Kip1 degradation, resulted in DNA polyploidization.
CDK inhibitory activity in developing maize (Zea mays) endosperm was suggested to be involved in endoreduplication (Grafi and Larkins, 1995); however, the molecular nature of the CKI is unknown. The objective of this study was to identify and characterize CKIs expressed in maize endosperm and investigate their role in endoreduplication. We describe the characterization of two maize CKI genes, Zeama;KRP;1 and Zeama;KRP;2, which are expressed in developing endosperm. Both proteins are able to inhibit plant Cdc2/CDK kinase activity associated with p13Suc1. They also specifically inhibit cyclin A1;3- and cyclin D5;1-associated CDK activities, but not cyclin B1;3/CDK. Although Zeama;KRP;1 was found to be associated with the CKI activity described by Grafi and Larkins (1995), it did not account for all of the CDK inhibitory activity present in this fraction. Overexpression of Zeama;KRP;1 in maize embryonic calli that ectopically expressed the wheat dwarf virus RepA protein, which counteracts Rb-related (RBR) protein function (Grafi et al., 1996; Gordon-Kamm et al., 2002), led to an additional round of DNA replication without nuclear division. While a clear role for Zeama;KRP;1 in endoreduplication could not be demonstrated in maize endosperm, indirect evidence suggests this protein could be involved in this process.
RESULTS
Identification of Two Maize cDNAs Encoding KRPs
Two cDNA clones with the conserved domains found in mammalian Cip/Kip CKIs were found by searching Pioneer Hi-Bred's maize expressed sequence tag database. These clones, called Zeama;KRP;1 (AY986792) and Zeama;KRP;2 (AY986793), have coding regions that are 567 and 771 bp in length, respectively. Comparison of the amino acid sequences deduced from these clones and those of mammalian CKIs showed the C terminus of the maize proteins shares 40% identity with the mammalian p27 Cip/Kip inhibitors (Fig. 1A). This region was identified as the CDK interaction/inhibition domain of the Cip/Kip inhibitors (Russo et al., 1996), where it is found, interestingly, near the N terminus. Amino acid sequence alignment of the maize KRPs and others described in Arabidopsis and tobacco (Wang et al., 1998; Jasinski et al., 2002; Zhou et al., 2002a) showed some degree (30%–50%) of conservation of amino acid sequences at the NH terminus and throughout most of the central region of the protein (Fig. 1B), with the highest degree of identity in the CDK inhibitory domain (Fig. 1C).
Figure 1.
Comparison of maize CKI protein sequences with those of the mammalian Cip/Kip family of CKIs (p21, p27, and p57), Arabidopsis (KRP1–7), and tobacco (NtKIS1a and NsKIS1a) KRPs. A, Protein sequence alignment of the Zeama;KRP;1 and Zeama;KRP;2 domains that share sequence similarity with the mammalian Cip/Kip inhibitors. B, Alignment of the amino acid sequences deduced from Zeama;KRP;1 and Zeama;KRP;2 with polypeptides deduced from the Arabidopsis and tobacco KRP cDNA sequences. The scale on the right illustrates the percentage of amino acid identity of the regions compared. C, Alignment of the conserved CDK inhibitory motifs among plant KRPs. The accession numbers of the sequences shown are as follows: KRP1(AF079587), KRP2 (AJ251851), KRP3 (AJ301554), KRP4 (AJ301555), KRP5 (AJ301556), KRP6 (AJ301557), KRP7 (AJ301558), NtKIS1a(AJ297904), and NsKIS1A(AJ297905); similarity of the amino acids among the proteins is indicated by the color code on the right.
Characterization of Maize KRP Gene Expression during Endosperm Development
To determine the expression pattern of the genes encoding Zeama;KRP;1 and Zeama;KRP;2 in maize endosperm, RNA transcripts were detected by reverse transcription (RT)-PCR and the corresponding proteins were identified by immunoblotting. Figure 2 shows the accumulation of RNAs encoded by Zeama;KRP;1 (Fig. 2A) and Zeama;KRP;2 (Fig. 2B) between 7 and 21 d after pollination (DAP). These data showed that, relative to the Actin1 control (Fig. 2C), transcripts for both genes were present at what appeared to be relatively constant amounts (note that there is a slight variation in Zeama;KRP;2 at 19 DAP) throughout this period of endosperm development.
Figure 2.
RT-PCR detection of Zeama;KRP;1 and Zeama;KRP;2 RNA transcripts during maize endosperm development. Total RNA was extracted from endosperm at 7 to 21 DAP and 50 ng were used as template for RT-PCR. Gene-specific primers were used to amplify Zeama;KRP;1 (A), Zeama;KRP;2 (B), and Actin1 (C), the latter of which was used as a loading control. The CKI cDNAs and genomic DNA were used as positive controls. Each assay was repeated at least three times, and the results illustrated are representative.
Zeama;KRP;1 and Zeama;KRP;2 protein accumulation was determined with affinity-purified polyclonal antibodies made against glutathione S-transferase (GST) fusion proteins. Zeama;KRP;1 antibodies detected a protein of approximately 21 kD in 7- to 21-DAP endosperm extracts (Fig. 3A), which is consistent with the predicted molecular mass of Zeama;KRP;1. Zeama;KRP;2 antibodies detected a protein of approximately 26 kD (Fig. 3B), which was also expected based on the molecular mass calculation. However, these antibodies also detected a protein of approximately 20 kD. The 20-kD band most likely corresponds to Zeama;KRP;1, since Zeama;KRP;2 antibodies cross-reacted with the recombinant GST-Zeama;KRP;1 protein in immunoblots (data not shown). Alternatively, this band could represent an unknown protein. The immunoblot analysis showed that, relative to actin protein, Zeama;KRP;1 was present between 7 and 21 DAP, while the Zeama;KRP;2 protein level decreased after 13 DAP (compare Fig. 3, A and B). These results show that, while the pattern of RNA transcript accumulation is similar for the two genes during this period of endosperm development, the Zeama;KRP;1 and Zeama;KRP;2 proteins are differentially expressed.
Figure 3.
Immunodetection of Zeama;KRP;1 and Zeama;KRP;2 during maize endosperm development. Protein was extracted from 7- to 21-DAP endosperms as described in “Materials and Methods,” and 50 μg were loaded per lane. A, Zeama;KRP;1 antibodies detected a single protein of approximately 21 kD (arrow). B, Zeama;KRP;2 antibodies detected a protein of approximately 26 kD (arrow), which corresponds to the molecular mass calculated for Zeama;KRP;2, and a protein of 20 kD that may represent the Zeama;KRP;1 or an unknown protein. Actin1 (C) and Ponceau S staining (data not shown) were used to standardize sample loading.
Zeama;KRP;1 and Zeama;KRP;2 Inhibit Maize Endosperm Cdc2-Related Kinases in Vitro
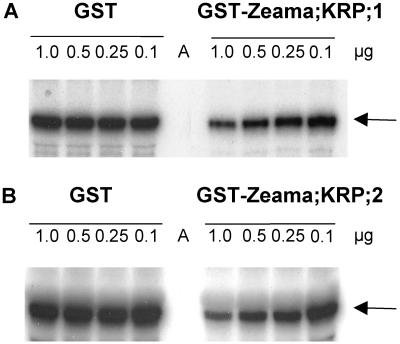
To investigate whether or not Zeama;KRP;1 and Zeama;KRP;2 have CDK inhibitory activity, their effect on CDKs obtained from maize endosperm was tested by in vitro assays. Figure 4 shows histone H1 phosphorylation by p13Suc1-associated CDK/Cdc2 kinase activities obtained from 9-DAP maize endosperm extracts. Aliquots of the CDKs were incubated with different amounts of GST or GST-tagged Zeama;KRP;1 and Zeama;KRP;2. The addition of 0.1 to 1.0 μg of GST-Zeama;KRP;1 (Fig. 4A) or GST-Zeama;KRP;2 (Fig. 4B) inhibited the p13Suc1-bound CDK activity, while increasing amounts of GST alone had no effect. The degree to which CDK activity was reduced in these assays appeared to be proportional to the CKI concentration, as would be expected for this type of CKI (Wang et al., 1998, Lui et al., 2000).
Figure 4.
Effect of Zeama;KRP;1 and Zeama;KRP;2 on p13Suc1-bound CDK activities from maize endosperm. GST-p13Suc1 agarose beads were used to pull down CDK activity from 9-DAP endosperm extracts. Subsequently, the kinase activity was incubated for 1 h with varying amounts (shown above each lane of the gel) of GST (control), GST-Zeama;KRP;1 (A), or GST-Zeama;KRP;2 (B) fusion proteins. Histone H1 (arrow) was used as substrate for the kinase assay, and the radioactive protein was analyzed by SDS-PAGE and autoradiography. Agarose beads (lane A) were used as a negative control.
Zeama;KRP;1 and Zeama;KRP;2 Specifically Inhibit Cyclin D5;1/CDK and Cyclin A1;3/CDK Activity But Not Cyclin B1;3/CDK Activity in Vitro
Although Zeama;KRP;1 and Zeama;KRP;2 inhibited p13Suc1-associated kinase activities from maize endosperm, the identity of the target CDK complexes was unknown. To determine whether the CKIs inhibited specific cell cycle-related cyclin/CDK complexes, affinity-purified antibodies that recognize cyclin A1;3 (presumably G2/M-phase), cyclin B1;3 (presumably M-phase), and cyclin D5;1 (presumably G1 to S-phase) were used to immunoprecipitate the corresponding CDKs from 9-DAP endosperm extracts. Cyclin D5;1/CDK was obtained from 9-DAP endosperms and also from developing young ears because relatively low levels of cyclin D5;1-associated kinase activity could be obtained from 9-DAP endosperm extracts.
Cyclin A1;3-containing CDKs from 9-DAP endosperm effectively phosphorylated histone H1 (Fig. 5A, GST control); in these reactions, the radioactive labeling of histone H1 was approximately 40 times over the control reaction that contained no cyclin A immunoprecipitate (Fig. 5A, lane A). The addition of 0.1 to 5.0 μg of GST-Zeama;KRP;1 to this reaction reduced the kinase activity up to 10-fold.
Figure 5.
Effect of Zeama;KRP;1 on endosperm cyclin A1;3- and cyclin B1;3-associated CDK activity. Affinity-purified cyclin A1;3 (A) and cyclin B1;3 (B) antibodies were used to immunoprecipitate CDK activity from 9-DAP endosperm extracts. The immunoprecipitated cyclin A1;3- and cyclin B1;3-containing CDKs were incubated for 1 h with the amount of GST (control) or GST-Zeama;KRP;1 fusion proteins shown above the lanes of each gel. Histone H1 (arrow) phosphorylation was used to assay kinase activity, and protein A-bound agarose beads were used as a negative control (lane A). ImageQuaNT software was used to measure radioactive histone H1; the histograms show the average and sd of the percentage of kinase activity inhibited in each assay.
Immunoprecipitates of cyclin B1;3-containing CDKs from 9-DAP endosperm contained less than one-half the level of histone H1 kinase activity as those recovered with cyclin A1;3 antibodies (compare with GST; Fig. 5, A and B). This could reflect a difference in the level of specific CDKs, cell cycle activity, or a variety of other factors. Endosperm is mitotically active at 9 DAP, although endoreduplication has begun by this stage and one might expect an increase in S-phase-associated CDKs and a reduction in M-phase CDK activity (Grafi and Larkins, 1995; Leiva-Neto et al., 2004). In contrast to the inhibition of cyclin A1;3-containing CDK activity, there was essentially no effect of GST-Zeama;KRP;1 on the cyclin B1;3-associated CDK activity (Fig. 5B). Even at the highest level of CKI used (5 μg), there was not any dramatic reduction in histone H1 phosphorylation compared to the control; the histone H1 labeling in these reactions was approximately 4 times over background, indicating that an effect on kinase activity should have been measurable in these assays.
Compared with immunoprecipitates of cyclin A- and cyclin B1;3-containing CDKs, the kinase activity obtained from 9-DAP endosperm extracts with cyclin D5;1 antibodies was low. Because the level of histone H1 phosphorylation was only slightly higher than background (compare Fig. 6A with Fig. 5, A and B), it was not possible to reliably measure the effect of GST-Zeama;KRP;1 on cyclin D5;1-containing CDKs immunoprecipitated from endosperm. We therefore tested the cyclin D5;1-associated CDKs in extracts from small, unfertilized ears. We generally found high levels of activity for all three cyclin/CDK complexes in this tissue (data not shown). As shown in Figure 6B, the cyclin D5;1-associated kinase activity from young ears phosphorylated histone H1 approximately 4 times over the control reaction lacking cyclin D5;1 immunoprecipitate. The addition of 0.5 or 5.0 μg of GST-Zeama;KRP;1 to this reaction reduced histone H1 phosphorylation approximately 80%, but the relative inhibition at either concentration was not measurably different.
Figure 6.
Effect of Zeama;KRP;1 on cyclin D5;1-associated CDK activity from 9-DAP endosperm and unfertilized ears. Affinity-purified cyclin D5;1 antibodies were used to immunoprecipitate CDK activity from endosperm (A) and unfertilized ear (B) extracts. Immunoprecipitated cyclin D5;1-bound CDK was incubated for 1 h with the amount of GST (control) or GST-Zeama;KRP;1 fusion proteins indicated above the lanes of the gels. Kinase activity was assayed as described in Figure 5. The histogram shows the average and sd of the percentage of kinase activity inhibited in each assay, which was measured by using ImageQuaNT software.
The effect of GST-Zeama;KRP;2 on cyclin A1;3/CDK, cyclin D5;1/CDK, and cyclin B1;3/CDK activities was similar to that of GST-Zeama;KRP;1. Cyclin A-containing immunoprecipitates from 9-DAP endosperm extracts had a marked sensitivity to increasing concentrations of Zeama;KRP;2 in the kinase assay (Fig. 7A). On the other hand, kinase assays containing cyclin B1;3/CDKs were essentially unaffected by the addition of GST-Zeama;KRP;2 (Fig. 7B). Immunoprecipitated cyclin D5;1/CDKs from young ear extracts were equally sensitive to either 0.5 or 5 μg of GST-Zeama;KRP;2, and, at either concentration, the level of kinase activity was reduced to about one-half the GST control (Fig. 7C).
Figure 7.
Effect of Zeama;KRP;2 on cyclin A1;3- and cyclin B1;3-associated CDK activity from 9-DAP endosperm and cyclin D5;1-associated CDK activity from unfertilized ears. Affinity-purified cyclin A1;3 (A), cyclin B1;3 (B), and cyclin D5;1 (C) antibodies were used to immunoprecipitate CDK activity from 9-DAP endosperm and unfertilized ear extracts. The immunoprecipitated cyclin A1;3-, cyclin B1;3-, and cyclin D5;1-containing CDKs were incubated for 1 h with the amount of GST (control) or GST-Zeama;KRP;2 fusion proteins indicated above the lanes of each gel. Kinase activity was assayed as described in Figure 5.
Zeama;KRP;1 Does Not Account for All the CDK Inhibitory Activity in Endosperm Cells Undergoing Endoreduplication
Grafi and Larkins (1995) described a CKI that accumulated in maize endosperm coincident with the onset of endoreduplication. This inhibitor was presumed to affect the M-phase CDK, but neither the nature of the inhibitor nor the cyclin/CDK it affected was demonstrated. To better characterize this CKI, proteins from 15-DAP endosperm extracts were fractionated by ammonium sulfate (30%–80%) precipitation and separated by chromatography on G-100 Sephadex and DEAE-SP Sepharose. Activity of the partially purified inhibitor obtained after ion-exchange chromatography was assayed by incubating with human recombinant cyclin B/cdc2, using histone H1 as substrate. Figure 8 shows the inhibition of histone H1 phosphorylation in the presence of the CKI and the accumulation of this activity in endosperm between 9 and 19 DAP. The results show that the inhibitory activity peaked between 15 and 19 DAP, coincident with the maximum stage of endoreduplication (Grafi and Larkins, 1995; Leiva-Neto et al., 2004).
Figure 8.
Characterization of CKI activity in developing maize endosperm. A, Human recombinant cyclin B/cdc2 was used to assay the partially purified (see “Materials and Methods”) endosperm CKI activity in 9- to 19-DAP endosperm using histone H1 (arrow) as substrate. NETT buffer was used as the negative control in the histone H1 kinase assays (lane c). B, Histogram showing quantification of the cyclin B/cdc2 inhibitor activity in 9- to 19-DAP endosperm.
Because the nature of the CKI is unknown, we decided to test for the presence of Zeama;KRP;1 and Zeama;KRP;2 in column fractions using specific antibodies. Figure 9, A and B, shows the distribution of CDK inhibitory activity in peak fractions of the Sephadex G100 column, as assayed by human cyclin B/cdc2 kinase. For each fraction, the percent reduction in 32P-labeling of histone H1 was calculated by comparison to a control reaction that contained buffer only (Fig. 9, lane c); the relative inhibition of cyclin B/cdc2 kinase activity is illustrated by the histogram in Figure 9B. To detect Zeama;KRP;1 and Zeama;KRP;2 in these fractions, the proteins were separated by SDS-PAGE and reacted with affinity-purified antibodies by immunoblotting. Zeama;KRP;2 was not detectable (data not shown), but Zeama;KRP;1 was found in some of the fractions containing CDK inhibitory activity (Fig. 9C, lanes 1, 2, 7, and 8). Because Zeama;KRP;1 was found in fractions excluded from the Sephadex G100 matrix, it appeared to be associated with high-molecular-weight protein complexes.
Figure 9.
Association of Zeama;KRP;1 with CDK inhibitory activity in 15-DAP endosperm extract. A, CDK inhibitory activity present in fractions eluted from the G-100 column was assayed with human recombinant cyclin B/cdc2 using histone H1 (arrow) as substrate. B, Histogram showing the percent reduction of kinase activity, which was measured using ImageJ software (http://rsb.info.nih.gov/ij), compared to a noninhibited control (lane c). C, Immunodetection of Zeama;KRP;1 in the fractions shown in A. Human recombinant cyclin B/cdc2 was incubated for 10 min with fractions eluted from the Sephadex G-100 column containing 15-DAP endosperm extract. Kinase activity was assayed as described in Figure 8. NETT buffer was used as control (lane c). For the immunoblot, 25 μL of the protein present in the fractions shown in A were loaded per lane. Zeama;KRP;1 antibodies detected an approximately 21-kD protein (arrow).
Since the CDK inhibitory activity identified in this assay was purified using human recombinant cyclin B/cdc2 as the kinase, we were interested in knowing whether it also affected cyclin A1;3-, cyclin B1;3-, and cyclin D5;1-containing CDKs from maize endosperm. To reduce the contribution of Zeama;KRP;1 to the results of this assay, three column fractions that were immunoblot negative for Zeama;KRP;1 were used for this analysis. Figure 10A shows the sensitivity of endosperm cyclin A1;3-containing CDKs to the partially purified inhibitor. Compared to the level of histone H1 phosphorylation in control reactions containing either the immunoprecipitated cyclin A1;3/CDK or a column fraction lacking CDK inhibitory activity (Fig. 10A, compare lanes c and 4), addition of the three fractions containing the partially purified CKI reduced kinase activity by one-half (Fig. 10A, lanes 1–3). Similar assays with cyclin B1;3 immunoprecipitate contained a lower level of kinase activity (Fig. 10B, lanes c and 4), but there was no detectable effect of the CKI on histone H1 phosphorylation in these reactions (Fig. 10B, lanes 1–3). In contrast, the inhibitor activity in these fractions reduced kinase activity associated with cyclin D5;1 immunoprecipitates by one-half, similar to the results with cyclin A1;3-containing CDKs (compare Fig. 10, A and C). These data suggested that, in addition to Zeama;KRP;1, the CDK inhibitory activity contained other types of CKIs.
Figure 10.
Effect of CDK inhibitory activity from 15-DAP endosperm on cyclin A1;3-, cyclin B1;3-, and cyclin D5;1-containing CDKs. Affinity-purified cyclin A1;3 (A), cyclin B1;3 (B), and cyclin D5;1 (C) antibodies were used to immunoprecipitate CDKs from 9-DAP endosperm (A and B) or unfertilized ear extracts (C). The immunoprecipitated cyclin A1;3-, cyclin B1;3-, and cyclin D5;1-associated CDK activities were incubated with alternate fractions (lanes 1–3) from the Sephadex G-100 column (see “Materials and Methods”), containing CDK inhibitory activity but lacking Zeama;KRP;1. Kinase activity was assayed based on histone H1 phosphorylation (arrow). NETT buffer (lane c) and a fraction that did not contain the CDK inhibitory activity (lane 4) were used as controls. The histograms show the average and sd of the percent reduction of kinase activity, compared to a noninhibited control, and were measured using ImageQuaNT software.
To examine the contribution of Zeama;KRP;1 to the total CKI activity, we assayed kinase activity before and after immunodepletion of Zeama;KRP;1 from the column fractions. Affinity-purified antibodies recognizing Zeama;KRP;1 were added to aliquots of the samples used in Figure 9, and the protein was removed by reaction with protein A-bound agarose beads. Figure 11 shows the relative inhibition of human cyclin B/cdc2 kinase activity after adding aliquots of the Sephadex G100 column fractions before (Fig. 11A) and after (Fig. 11B) immunoprecipitation of Zeama;KRP;1. To ensure that Zeama;KRP;1 was completely removed, aliquots of immunodepleted fractions were immunoblotted with Zeama;KRP;1 antibodies and shown not to contain detectable levels of this protein (data not shown). Figure 11C shows a comparison of the level of histone H1 phosphorylation by human cyclin B/cdc2 kinase before (gray bar) and after (white bar) removal of Zeama;KRP;1. The fractions illustrated in Figure 11C correspond to the kinase assays shown in Figure 11, A and B. Although there was evidence of increased histone H1 phosphorylation in several samples (e.g. Fig. 11C, fractions 1–5), kinase activity was relatively unaffected in others (Fig. 11C, fractions 6–10). Notably, the immunodepletion of Zeama;KRP;1 had relatively little effect on the CKI activity.
Figure 11.
Effect of Zeama;KRP;1 immunodepletion on the partially purified CDK inhibitory activity. A, CDK inhibitory activity from aliquots of the fractions shown in Figure 10 was assayed with human recombinant cyclin B/cdc2 after incubation with protein A-bound agarose beads; the Zeama;KRP;1 (arrow) in these fractions was subsequently detected by immunoblotting. B, CDK inhibitory activity from aliquots of the fractions shown in A was assayed after immunodepletion of Zeama;KRP;1. Kinase activity was assayed as described in Figure 11A, followed by immunodetection of Zeama;KRP;1. C, Histogram showing the percent reduction of kinase activity, measured by ImageJ software, before (gray bar) and after (white bar) immunoprecipitation of Zeama;KRP;1. Affinity-purified Zeama;KRP;1 antibodies were used to immunodeplete Zeama;KRP;1 protein from fractions as described in “Materials and Methods.” Protein A bound to agarose beads was used as a negative control.
Ectopic Expression of Zeama;KRP;1 Can Induce Endoreduplication in Maize Calli
Overexpression of the p21 and p27 Cip/Kip CKIs leads to endoreduplication in human Rb-negative cells (Bates et al., 1998; Niculescu et al., 1998; Chang et al., 2000). To determine whether overexpression of Zeama;KRP;1 and Zeama;KRP;2 can cause endoreduplication in cells in which the activity of the maize RBR is reduced or eliminated, embryonic calli were transformed with gene constructs expressing the wheat dwarf virus RepA protein in the presence and absence of the maize KRPs. RepA was previously shown to interact with the maize RBR protein and stimulate the cell cycle (Grafi et al., 1996; Gordon-Kamm et al., 2002). Maize embryos were transformed by particle bombardment as described by Gordon-Kamm et al. (2002), and, after culturing them on selective media, the levels of Zeama;KRPs and RepA transcripts were measured by RT-PCR and the nuclear ploidy of cells was determined by flow cytometric analysis.
Figure 12 shows the analysis of nuclear ploidy from two transgenic events expressing RepA only (Fig. 12, A and B), and four events expressing both RepA and Zeama;KRP;1 (Fig. 12, C–F). Calli overexpressing only Zeama;KRP;1 grew very slowly, and, for reasons that are unclear, no transgenic calli expressing Zeama;KRP;2 were obtained. Consequently, we were unable to obtain nuclei from calli overexpressing only the KRPs to determine the effect on nuclear ploidy. Based on RT-PCR measurements, there were variable levels of Zeama;KRP;1 and RepA transcripts in the calli (Fig. 12G). In general, the level of Zeama;KRP;1 RNA detected exceeded that of RepA RNA, which could have been a consequence of the additional endogenous expression of the Zeama;KRP;1 gene. Calli transformed with only the RepA gene generally contained lower levels of Zeama;KRP;1 RNA than those ectopically expressing Zeama;KRP;1. Only 2C and 4C nuclei were detected in calli transformed with RepA only (Fig. 12, A and B), but some cells in each of the calli overexpressing Zeama;KRP;1 and ectopically expressing RepA underwent an additional cycle of DNA replication without nuclear division, resulting in 8C nuclei. The callus with the highest level of Zeama;KRP;1 expression had the lowest percentage of endoreduplicated nuclei (Fig. 12D). Calli with the highest percentage of endoreduplicated nuclei appeared to contain somewhat higher levels of Zeama;KRP;1 and RepA RNAs compared to calli transformed with RepA only, but there was no clear relationship between the percentage of endoreduplicated nuclei and the level of RepA and Zeama;KRP;1 RNAs. However, the amount of RNA transcripts detected in these assays might not reflect the level of functional proteins in the cells.
Figure 12.
Effect of Zeama;KRP;1 overexpression on nuclear ploidy of maize embryonic calli expressing the wheat dwarf virus RepA protein. Calli were cobombarded with plasmids expressing the RepA and Zeama;KRP;1 genes under control of the ubiquitin promoter and nuclei were analyzed with a PARTEC flow cytometer. Flow cytometric analysis of nuclei from transgenic calli expressing RepA only (A and B) and RepA plus Zeama;KRP;1 (C–F). The percentage of endoreduplicated nuclei shown in each image was calculated by dividing the total number of nuclei with a ploidy equal to or greater than 8C by total number of nuclei and multiplying by 100. The histogram (G) shows the level of Zeama;KRP;1 RNA (white bar) and RepA RNA (gray bar) in these calli, relative to Actin1 RNA.
DISCUSSION
Differential Expression of Zeama;KRP;1 and Zeama;KRP;2 in Developing Maize Endosperm
We report the identification and characterization of two maize CKIs, Zeama;KRP;1 and Zeama;KRP;2, which are expressed in developing endosperm tissue. These proteins have a high degree of homology with Arabidopsis and tobacco KRPs (Wang et al., 1998; De Veylder et al., 2001; Jasinski et al., 2002; Zhou et al., 2002a) and, like them, share a sequence of approximately 20 amino acids in common with animal Cip/Kip CKIs (Fig. 1A); this region was identified as the CDK interaction/inhibition domain in the mammalian CKIs (Russo et al., 1996). Zeama;KRP;1 RNA and protein are present throughout the early and middle stages of endosperm development (7–21 DAP), which encompasses the period when endoreduplication begins (Figs. 2 and 3). In contrast, while Zeama;KRP;2 RNA was detected in endosperm between 7 and 21 DAP, the protein decreases in concentration after 13 DAP (Fig. 3). Thus, Zeama;KRP;2 could play a role in the onset of endoreduplication, but it does not appear to play a role in later stages of the polyploidization process. We cannot explain the functional significance for the apparent stability of Zeama;KRP;2 RNA but not the protein it encodes; however, this relationship underscores the importance of measuring both protein and RNA concentrations before drawing conclusions regarding gene expression.
Zeama;KRPs Are CKIs That Can Affect Cyclin A/CDK and Cyclin D5;1/CDK Activities
Crystal structure analysis of the inhibitory domain of the mammalian p27Kip1 bound to cyclin A/CDK2 revealed a mechanism of inhibition where strong interactions between the inhibitor, the cyclin, and the CDK allow deformation of and interference with the CDK active site (Russo et al., 1996). Because of the sequence similarity between the plant and mammalian CKIs of the Cip/Kip family, it has been inferred based on indirect evidence that the plant KRPs inhibit CDK activity through interaction with cyclin/CDK complexes (Wang et al., 1998; De Veylder et al., 2001; Jasinski et al., 2002; Zhou et al., 2002a, 2002b; Schnittger et al., 2003).
The maize CKIs, Zeama;KRP;1 and Zeama;KRP;2, inhibited p13Suc1-bound CDKs from developing maize endosperm (Fig. 4). However, it is now well documented that p13Suc1 has affinity for CDKA;1 (De Veylder et al., 1997), which has high activity at both the S- and M-phase of the cell cycle (for review, see Mironov et al., 1999). Consequently, to more precisely identify the nature of the cyclin/CDK complexes affected by Zeama;KRP;1 and Zeama;KRP;2, we tested their inhibitory effects on what are presumably S-phase (cyclin A1;3 and cyclin D5;1) and M-phase (cyclin B1;3) CDKs. Immunoprecipitates of cyclin A1;3- and cyclin B1;3-containing CDK complexes from 9-DAP endosperm produced histone H1 kinase activities that were significantly above background levels; however, the kinase activity obtained from cyclin D5;1 immunoprecipitates of 9-DAP endosperm was very low. Therefore, cyclin D5;1-containing CDKs from small, unfertilized ears were used instead. While different concentrations of GST alone did not affect histone H1 phosphorylation by cyclin D5;1/CDKs or cyclin A1;3/CDKs, different concentrations of the recombinant GST-Zeama;KRPs did (Figs. 6B and 7C). D-type cyclins have been proposed to mediate the G1/S transition in plants and animals (Dewitte and Murray, 2003); consequently, these results are consistent with the hypothesis that the Zeama;KRPs could function as G1/S transition inhibitors.
In contrast to animals, plants contain a complex set of A-type cyclins. In Arabidopsis, there are two A1 (CYCA1;1 and CYCA1;2) and four A2 and A3 (CYCA2;1 to CYCA2;4 and CYCA3;1 to CYCA3;4) genes (Chaubet-Gigot, 2000; Vandepoele et al., 2002), and maize A-type cyclins are likely to show similar complexity. Different Arabidopsis A-type cyclins were reported to be expressed sequentially at different time points from late G1/early S-phase until mid-M-phase (Reichheld et al., 1996). Consequently, it is possible the maize A1;3-type cyclin/CDK functions during S-phase and/or the G2/M transition, as was suggested for the Medicago sativa A2-type cyclin-associated CDK (Roudier et al., 2000). Although a function at a specific cell cycle phase cannot be presently attributed to maize cyclin A1;3, its associated kinase activity is most abundant at early stages of endosperm development, and declines sharply at the onset of endoreduplication. This suggests a role for cyclin A1;3 in G2/M- rather than S-phase (R.A. Dante, P.A. Sabelli, H. Nguyen, J.T. Leiva-Neto, Y. Tao, K.S. Lowe, G. Hoerster, W.J. Gordon-Kamm, R. Jung, and B.A. Larkins, unpublished data).
Neither of the maize KRPs appeared to inhibit cyclin B1;3/CDK activity from maize endosperm (Figs. 5B and 7B). Even at the highest concentration of KRP (5 μg/reaction), there was no apparent reduction in kinase activity in these reactions (compare Fig. 5, A and B). Based on histone H1 phosphorylation, there was less cyclin B1;3-associated CDK activity immunoprecipitated from 9-DAP endosperm than that obtained with cyclin A1;3 antibodies. This may have some developmental significance, but it is also possible that histone H1 is not as good a substrate for cyclin B1;3- compared to cyclin A1;3-containing CDKs. Zeama;CycB1;3 was proposed to be a mitotic cyclin based on its activity in Xenopus oocytes (Sun et al., 1997) and developmental expression (Sun et al., 1999). Our results are in accordance with those reported by Zhou et al. (2002a), which showed that, based on the yeast two-hybrid assay, ICK1 does not interact with CycB1- and CycB2-containing CDKs.
Zeama;KRPs Account for Only Part of the CDK Inhibitory Activity That Peaks Coincident with Endoreduplication
Grafi and Larkins (1995) identified a CDK inhibitory activity in 15-DAP maize endosperm that affected the p13Suc1-bound kinase activity obtained during the period of endoreduplication. Based on earlier studies that showed that maturation (mitosis)-promoting factor binds p13Suc1 and phosphorylates histone H1, they proposed this kinase activity was related to maturation (mitosis)-promoting factor and that the inhibitor specifically affected an M-phase CDK. However, De Veylder et al. (1997) subsequently showed that the Arabidopsis, fission yeast, and human Suc1/Cks1 protein can bind CDKA;1, and the associated kinase activity is high at S-, G2-, and M-phase (for review, see Mironov et al., 1999). This implies that the endosperm CKI identified by Grafi and Larkins (1995) could act at S- and/or M-phase.
We attempted to determine the nature of the endosperm CKI by partially purifying it and testing its effect on cyclin-associated CDK activities that function at different phases of the cell cycle. Although a significant degree of enrichment was made possible by chromatographic purification (Fig. 8), inhibitor fractions eluted from the DEAE-SP Sepharose column were found to contain multiple polypeptides, and it was not possible to identify a CKI. The partially purified CKI activity did not inhibit the ZeamaCycB1:3/CDK from 9-DAP endosperm, but it did inhibit cyclin D5;1/CDK and cyclin A1;3/CDK activity (Fig. 10), similar to Zeama;KRP;1 and Zeama;KRP;2. However, based on immunodepletion and immunodetection assays, it was shown that Zeama;KRP;2 is not present and Zeama;KRP;1 only accounts for a portion of this CDK inhibitory activity (Fig. 11). It appears, therefore, that the CDK inhibitory activity described by Grafi and Larkins (1995) contains both M- and S-phase CKIs, including Zeama;KRP;1. Since we do not know the precise role of cyclin A1;3, cyclin B1;3, or cyclin D5;1 in cell cycle regulation during endosperm development, it is not possible to conclude whether or not Zeama;KRP;1 and Zeama;KRP;2 are functionally involved in endoreduplication. However, the observation (see below) that overexpression of Zeama;KRP;1 can induce an additional cycle of DNA synthesis in RepA-expressing, RBR-inhibited maize cells implies that it might function in the polyploidization process.
Zeama;KRP;1 Overexpression Can Lead to Endoreduplication in RepA-Expressing Embryonic Calli
Although endoreduplication is a widespread process, its molecular mechanisms are still not fully understood. Based on studies in plants and animals, it appears that a common regulatory mechanism that creates an endoreduplication cell cycle involves the loss of mitotic regulators and oscillation of S-phase CDKs. Recently, several studies have demonstrated a potential role for CKIs in the endoreduplication process of various organisms (Kikuchi et al., 1997; Bates et al., 1998; Niculescu et al., 1998; Chang et al., 2000; Hattori et al., 2000; Nakayama et al., 2000).
To determine whether Zeama;KRP;1 can affect endoreduplication, we expressed the gene encoding it in maize embryonic calli in the presence and absence of the wheat dwarf virus RepA protein. RepA expression in these cells, as well as in tobacco cell cultures, was shown to stimulate cell cycle activity, apparently by inhibiting RBR function (Gordon-Kamm et al., 2002). Rb/RBR inhibits cell cycle progression by coupling with transcription factors (E2F/Dp) that promote expression of genes required for S-phase (Weinberg, 1995; Gutierrez et al., 2002). Rb/RBR inhibitory activity can be released either as a consequence of phosphorylation or binding of specific viral proteins, such as RepA (reviewed by Gutierrez, 2000). Since Grafi et al. (1996) suggested that ZmRb phosphorylation increases during the period of endoreduplication, it was hypothesized that inactivation of this protein is associated with the polyploidization process.
Overexpression of Zeama;KRP;1 in maize embryonic calli ectopically expressing RepA allowed an additional round of DNA replication, resulting in 8C nuclei (Fig. 12). This result is consistent with those from similar types of studies in mammalian cell cultures (Bates et al., 1998; Niculescu et al., 1998, Chang et al., 2000). The number of cells undergoing endoreduplication in the maize calli ranged from 6% to 21% based on flow cytometric analysis. In general, calli with endoreduplicated nuclei had a higher level of Zeama;KRP;1 RNA than RepA RNA, but there was no obvious relationship in the size of this ratio. However, the callus with the highest expression of Zeama;KRP;1 had the lowest percentage of endoreduplicated nuclei and there was no evidence of endoreduplication in calli expressing RepA only. Overexpression of the Zeama;KRPs by themselves severely reduced growth of embryonic maize calli, as was observed in several previous studies in which CKIs were constitutively expressed (Wang et al., 2000, Jasinski et al., 2002, Zhou et al., 2002a, 2002b).
Overexpression of CKIs in Arabidopsis and tobacco plants reduced and/or blocked endoreduplication. Therefore, it is possible that a constitutive high level of CKI is not sufficient to induce endoreduplication. Rather, a preexisting block of the Rb pathway at the transition from the mitotic to the endoreduplication cell cycle could be required to initiate this process. As reported by Hattori et al. (2000), the CKI, p57Kip2, accumulates at the onset of endoreduplication, but its level oscillates during polyploidization. A mutation that stabilized p57Kip2 blocked S-phase entry, ultimately inhibiting endoreduplication progression. It is possible that high levels of CKI in the presence of a blocked Rb pathway promotes only one additional cycle of DNA replication in maize embryonic calli, while in tissues like endosperm, where endoreduplication is associated with differentiation, a different mechanism is in operation.
MATERIALS AND METHODS
Isolation of Maize CKI cDNA Clones
By searching Pioneer Hi-Bred's maize (Zea mays) expressed sequence tag database, two putative maize cDNA clones were identified based on conserved domains of the mammalian Cip/Kip family of CKIs. Comparison of the deduced amino acid sequences of the maize clones with those of the mammalian Cip/Kip family of CKIs, and those of Arabidopsis (Arabidopsis thaliana; ICK1/KRP1-7) and tobacco (Nicotiana tabacum; NtKIS1a and NsKIS1), were performed using MACAW software. One of the cDNA clones was arbitrarily identified as Zeama;KRP;1 and the other as Zeama;KRP;2.
RT-PCR Analysis of RNA Transcripts
Total RNA was isolated from maize tissue using an Absolutely RNA miniprep kit, as recommended by the manufacturer (Stratagene, San Diego). RT-PCR reactions were performed with 50 (B73 endosperm) or 8 ng (maize embryonic calli) of total RNA using the Titan One Tube RT-PCR system (Roche, Basel), as recommended by the manufacturer. RT-PCR reactions with RNA from maize embryonic calli contained [α-32P]dCTP (10 mCi/mL; NEN, Boston). Zeama;KRP;1, Zeama;KRP;2, Actin1, and RepA transcripts were reverse transcribed and PCR amplified by a total of 40 (endosperm) or 25 cycles (maize embryonic calli) with the following gene-specific primer combinations: Zeama;KRP;1 forward, 5′-ATGGGCAAGTACATGCGCAAGGCC-3′, Zeama;KRP;1 reverse, 5′-GCTTCACCCACTCAAACCTGCCTGGG-3′; Zeama;KRP;2 forward, 5′-GTTACGCAGGTCGGTCGGCGTCCGG-3′, Zeama;KRP;2 reverse, 5′-CCTCTGCAGCGTCGTAGGAGCGG-3′; Actin1 forward, 5′-ATTCAGGTGATGGTGTGAGCCACAC-3′, Actin1 reverse, 5′-GCCACCGATCCAGACACTGTACTTCC-3′; RepA forward, 5′-TCTGCACCCAGGTTCCGTGTCTATTCC-3′, RepA reverse, 5′-CTCGAGGGACAGAGAGTAGAGATGTTC-3′. The annealing temperatures were as follows: Zeama;KRPs and Actin1, 68°C, RepA 65°C. The nonradioactive RT-PCR products were separated by electrophoresis in agarose gel and visualized by UV excitation of ethidium bromide-stained DNA. The radioactive RT-PCR products were separated by electrophoresis in 4% (w/v) nondenaturing acrylamide gel containing 1× Tris-borate EDTA (TBE; 90 mm Tris-borate, 2 mm EDTA), after which the gel was dried and exposed to x-ray film and a PhosphorImager screen. Radioactive signals were measured with a Storm 860 PhosphorImager (Molecular Dynamics, Sunnyvale, CA) and analyzed with ImageQuaNT software (Amersham, Piscataway, NJ). All RT-PCR assays were performed at least three times for each RNA sample.
Production of Recombinant GST-Zeama;KRP;1 and GST-Zeama;KRP;2 Proteins
The Zeama;KRPs were PCR amplified from their respective cDNAs using gene-specific primers (GSTKRP1 forward, 5′-CGGGATCCATGGGCAAGTACATGCGCAAGGCC-3′, GSTKRP1 reverse, 5′-CGGAATTCTCAGTCTAGCTTCACCCACTCAAACC-3′; GSTKRP2 forward, 5′-CGGGATCCATGGGGAAGTACATGCGCAAGTGC-3′, GSTKRP2 reverse, 5′-GGAATTCTCAGATGCTGACCACCGGCGCCC-3′), subcloned in frame into the pGEx4T-3 expression vector (Amersham) and the resulting construct used to transform BL21 codon plus (DE3)-RIL cells (Stratagene). Recombinant GST-Zeama;KRP;1 and GST-Zeama;KRP;2 protein production was induced with 1 mm isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 37°C. The GST-tagged proteins were purified using glutathione agarose resin (Sigma, St. Louis) as follows: After 15-min centrifugation at 4,225g in a Sorvall GS-3 rotor (DuPont, Wilmington, DE), the bacterial pellet was resuspended with lysis buffer (50 mm Tris-HCl, pH 8.0, 100 mm NaCl, 1 mm EDTA, pH 8.0) containing freshly added 1 mm dithiothreitol (DTT), 1 mm phenylmethylsulfonyl fluoride (PMSF), and 1× Complete EDTA-free protease inhibitor cocktail (Roche, Hamburg, Germany), and incubated for 30 min on ice with 1 mg/mL lysozyme. Sarcosyl was added to 1%, the lysate sonicated three times for 30 s, and then Triton X-100 added to 1% and the extract centrifuged for 15 min at 15,000g. The supernatant was filtered through two to four layers of cheesecloth and applied to glutathione agarose beads. Two washes were performed with a 10× bed column volume of lysis buffer that contained 0.5% and 0.1% Triton X-100, respectively. The recombinant proteins were eluted with a 10× bed column volume of lysis buffer containing 20 mm reduced l-glutathione (Sigma) and no PMSF or Triton X-100. The proteins were dialyzed at 4°C for 3 h against 1,000 volumes of kinase assay buffer (50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 1 mm β-glycerol phosphate, and the protease inhibitor cocktail) containing 2 mm EGTA and 0.4 mm DTT, as described by Wang et al. (1998). After dialysis, the proteins were stored at −80°C until used.
Immunodetection of Zeama;KRP;1 and Zeama;KRP;2 Proteins
Polyclonal rabbit antisera were raised against the complete Zeama;KRP;1 protein and against amino acids 1 to 120 of the Zeama;KRP;2. Both recombinant proteins were expressed from cDNA clones with a GST tag in Escherichia coli, as described above, separated by 15% SDS-PAGE, and the corresponding bands (according to their calculated molecular weight) were excised and sent to Strategic Biosolutions (Widham, ME) for production of polyclonal antisera.
For affinity purification of Zeama;KRP;1 and Zeama;KRP;2 antibodies, the crude antisera were centrifuged at 5,000g for 30 min at 4°C in a Sorvall SS-34 rotor; proteins were precipitated with 50% ammonium sulfate by stirring at 4°C overnight and collected by centrifugation at 3,000g for 30 min at 4°C. The protein pellet was resuspended in 50 mm Tris-HCl, pH 7.5, to the original volume, incubated by rocking for 6 h with GST covalently bound to 1,1′-carbonyldimidazole-activated, 0% cross-linked, beaded agarose (reactive gel 6×; Pierce, Rockford, IL). After 6 h, the supernatant was collected and incubated by rocking at 4°C overnight with GST-Zeama;KRP;1 or GST-Zeama;KRP;2 covalently bound to beaded agarose (reactive gel 6×; Pierce). The agarose beads were washed with 50 mm Tris-HCl, pH 7.5 (50× bed column volume), and the antibodies were eluted with 100 mm Gly, pH 2.4, and collected in microcentrifuge tubes containing 1 m Tris-HCl, pH 8.0, which was needed to adjust the pH to approximately 7.0. Fractions that contained antibodies were pooled and concentrated with Amicon Ultra-15, according to the manufacturer's instructions (Millipore, Bedford, MA).
For immunodetection of Zeama;KRP;1 and Zeama;KRP;2, 9- to 21-DAP dissected B73 endosperms were ground in 3 volumes of NETT buffer (20 mm Tris-HCl, pH 8.0, 100 mm NaCl, 20 mm EDTA, pH 8.0, 0.5% Triton X-100, 5 mm NaF, 1 mm Na3VO4), with freshly added 1 mm PMSF, 1 mm DTT, and 1× protease inhibitor cocktail, and centrifuged at 12,000g at 4°C for 10 min in an Eppendorf microfuge (Eppendorf, Hamburg, Germany). The protein concentration of the supernatant was determined by Bradford assay (Bio-Rad, Hercules, CA). Fifty micrograms of protein from each sample was separated by 12.5% SDS-PAGE and blotted onto nitrocellulose using a wet transfer apparatus (mini trans-blot cell; Bio-Rad) at 200 Vh. The membrane was blocked with Tween plus Tris-buffered saline (TTBS; 20 mm Tris-HCL, pH 7.5, 150 mm NaCl, and 0.005% Tween 20) plus 5% nonfat dry milk for 1 h, followed by overnight incubation on a rotating plate at 4°C with a 1:1,000 dilution of polyclonal rabbit anti-Zeama;KRP;1 and a 1:100 dilution of anti-Zeama;KRP;2 antibody. After three washes with TTBS for 15 min each, the membranes were incubated for 1 h with a 1:25,000 dilution of anti-rabbit IgG conjugated with horseradish peroxidase (Sigma). The membranes were washed with TTBS three times for 15 min each and incubated with chemiluminescent substrate (Super Signal West Pico; Pierce) for 5 min. Then the membranes were exposed to x-ray films, which were subsequently developed (QX 134 plus; Konica, Tokyo). All immunoblot analyses were performed at least two times.
Effect of Zeama;KRP;1 and Zeama;KRP;2 on p13 Suc1-, Cyclin A1;3-, Cyclin D5;1-, and Cyclin B1;3-Associated CDK Activity
Three to five grams of dissected endosperm from 9-DAP kernels were ground in 10 to 15 mL of NETT buffer. The homogenate was centrifuged at 12,000g for 10 min at 4°C. The supernatant was preincubated with a 30-μL slurry of glutathione agarose beads (Sigma; Grafi and Larkins, 1995) by rocking at 4°C for 1 to 2 h. After removal of the agarose beads with a quick spin in the microfuge, Cdc2-like protein kinases were purified from the extract by incubating with 30 μL of GST-p13Suc1-conjugated agarose beads for 2 to 3 h at 4°C. Subsequently, the p13Suc1-conjugated agarose beads were washed two times with NETT buffer and once with kinase buffer (50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 20 mm EGTA, 2 mm DTT, 1 mm β-glycerol phosphate, and the protease inhibitor cocktail).
Rabbit polyclonal antibodies that cross-react with maize cyclin A1;3, cyclin B1;3, and cyclin D5;1 were prepared as described by R.A. Dante, P.A. Sabelli, H. Nguyen, J.T. Leiva-Neto, Y. Tao, K.S. Lowe, G. Hoerster, W.J. Gordon-Kamm, R. Jung, and B.A. Larkins (unpublished data). Monospecific antibodies were purified by affinity chromatography with GST fusions of the cyclin proteins. Immunoprecipitation of cyclin A1;3-, cyclin D5;1-, and cyclin B1;3-associated CDK activity was performed similarly to the p13Suc1 pull downs, except that endosperm extracts or extracts from 10- to 15-cm-long immature ears were incubated with approximately 5 μg of affinity-purified cyclin D5;1 antibodies, 0.5 μg of affinity-purified cyclin A1;3 antibodies, or 1.0 μg of affinity-purified cyclin B1;3 antibodies for 2 h on a rocker at 4°C. Subsequently, protein A agarose beads (Sigma) were added and incubated for 2 h by rocking at 4°C. The protein A agarose beads were centrifuged and washed as previously described. As a negative control, endosperm or immature ear extract was incubated with protein A agarose beads only.
For the CDK inhibition assay, the concentration of the recombinant proteins, GST-Zeama;KRP;1 and GST-Zeama;KRP;2, and GST alone was estimated by side-by-side comparison with known concentrations of bovine serum albumin (fraction V; Pierce) after 12.5% SDS-PAGE, scanning the gel, and measuring the bands using Image J software. The kinase activity associated with anti-cyclin A1;3, anti-cyclin D5;1, and anti-cyclin B1;3 immunoprecipitates, or p13Suc1 pulls downs, was incubated with different amounts of GST or recombinant Zeama;KRP proteins plus kinase buffer, 100 μL total volume, by rocking for 1.5 h at 4°C. The p13Suc1-conjugated agarose beads or protein A agarose beads were collected by centrifugation, washed with kinase buffer one time, and incubated with 7 μL of kinase buffer, 1 μL of 2.5 μg/μL histone H1 (Sigma), 1 μL of 4 mm ATP, and 1 μL of [γ-32P]ATP, 10 μCi/μL (Amersham). After 25-min incubation at room temperature, the reactions were stopped by adding 5 μL of 5× Laemmli buffer (Laemmli, 1970), heated in boiling water for 5 min, and the proteins resolved by 12.5% SDS-PAGE. The gels were vacuum dried and exposed to x-ray film and PhosphorImager screen. Radioactive signals were measured with a Storm 860 PhosphorImager (Molecular Dynamics) and analyzed with ImageQuaNT software (Amersham). The effect of Zeama;KRP proteins on histone H1 phosphorylation was calculated as a percentage of the radioactive labeling present in a particular sample (with a certain amount of GST-Zeama;KRP protein) relative to that measured in the control reaction with the corresponding amount of GST protein. All inhibition assays were performed at least two times with four different concentrations of the recombinant proteins.
Partial Purification and Analysis of a CKI from Maize Endosperm
One hundred grams of 15-DAP maize kernels were homogenized in 200 mL of NETT buffer and centrifuged at 12,000 rpm for 20 min at 4°C in a Sorvall GS-A rotor. The supernatant was filtered through one layer of Miracloth, and the proteins recovered by precipitation with 30% to 80% ammonium sulfate. The pellet was resuspended in 20 mL NETT buffer, applied to a 2.5- × 100-cm Sephadex G100 column, and 8-mL samples collected. Fractions with CDK inhibitory activity were pooled and applied to linked DEAE-SP Sepharose columns (each 2 × 15 cm). The columns were washed with 1 m NaCl and then equilibrated with 7× bed column volume of NETT buffer. The SP Sepharose column was eluted with a linear gradient of 0 to 1.0 m NaCl and fractions containing CDK inhibitory activity were analyzed.
For the CKI assay, 1 μL of 5 units/μL human recombinant cyclin B/cdc2 (Promega, Madison, WI), or the kinase activity associated with the previously described maize cyclin immunoprecipitates, and 2 μL of alternate fractions collected from the Sephadex G100 column were incubated with 20 μL of kinase buffer at room temperature for 10 min. When the assay was performed with the purified CDK inhibitory fraction from developing endosperm, 2 μL of fractions collected from the SP Sepharose column were used. Fractions from the Sephadex G-100 column were used in most assays because too little activity was recovered after affinity chromatography on DEAE-SP Sepharose. The kinase reaction was initiated by adding 1 μL of 2.5 μg/μL histone H1 (Sigma), 0.5 μL of 25 mm ATP, and 0.5 μL of [γ-32P]ATP (10 μCi/μL; Amersham) and stopped after 20 min by adding 5 μL of 5× Laemmli buffer (Laemmli, 1970) and heating in boiling water for 5 min; histone H1 was resolved by 12.5% SDS-PAGE. The gel was vacuum dried and exposed to an x-ray film and PhosphorImager screen. Radioactive signals were measured as described above. All the inhibition assays were performed at least three times.
Immunodepletion of Zeama;KRP;1 protein from Sephadex G100 fractions was performed by incubating alternate 1-mL fractions with approximately 5 μg of polyclonal Zeama;KRP;1 antibodies for 2 h on a rocker at 4°C. Subsequently, protein A agarose beads (Sigma) were added and incubated for 2 h by rocking at 4°C. The protein A agarose beads were centrifuged, the supernatant transferred to a clean microfuge tube, and an aliquot was immediately used for CKI assays. As a control, 1 mL of the same fraction was incubated with only protein A agarose beads.
Expression of Zeama;KRP;1 and Zeama;KRP;2 in Transgenic Maize Calli
Embryogenic maize calli were cobombarded with genes expressing the wheat dwarf virus RepA protein and/or Zeama;KRP;1 and Zeama;KRP;2, as described by Gordon-Kamm et al. (2002). The Zeama;KRP;1 and Zeama;KRP;2 recombinant DNA constructs were made by PCR amplification of the full-length cDNAs using the following primer combinations: KRP1ubi forward, 5′-CGGGATCCATGGGCAAGTACATGCGCAAGGCC-3′, KRP1ubi reverse, 5′-CGGATCCTCAGTCTAGCTTCACCCACTCAAACC-3′; KRP2ubi forward, 5′-GGATCCATGGGGAAGTACATGCGCAAGTG-3′, KRP2ubi reverse, 5′-CCATGGTCAGATGCTGACCACCGGCGC-3′. The PCR products were subcloned into PHP17720, an expression vector that contains a maize ubiquitin (UBI1) promoter and the 3′ downstream region of the proteinase inhibitor, pinII (An et al., 1989). Expression of the RepA gene was also regulated by a maize ubiquitin promoter and pinII terminator (Gordon-Kamm et al., 2002).
Maize calli were obtained from high type II embryos, as described by Gordon-Kamm et al. (2002). Particle-mediated transformation was performed as described by Songstad et al. (1996). After incubating the embryos on 560P medium in the dark at 28°C for 4 or 5 d, they were transferred onto 560Y medium and subcultured scutellum side up for 3 h before transformation. The scutellar surface was targeted with a PDS-100 Helium Gun (Bio-Rad) at one shot per sample using 650PSI rupture discs. Approximately 67 ng of DNA were delivered per shot. After bombardment, the embryos were maintained on 560L medium. Transformants were transferred 2 to 7 d after bombardment onto 560R for selection. Plates were maintained at 28°C in the dark and transferred to fresh medium every 2 weeks until being analyzed.
Flow Cytometric Analysis of Nuclei
Maize embryonic calli transformed with Zeama;KRP;1, GUS, RepA, or RepA + Zeama;KRP;1 were chopped with a single-edged razor blade in the presence of 0.8 mL of filtered ice cold PARTEC (Munster, Germany) buffer (200 mm Tris-HCl, pH 7.5, 4 mm MgCl2 and 0.1% Triton X-100; Dilkes et al., 2002). The homogenate was aspirated through two layers of cheesecloth with a plastic pipette, passed through a 100-μm nylon mesh, and then combined with an additional 0.8 mL of PARTEC buffer. Nuclei were stained with 40 μL of a 100 mg/mL solution of DAPI and analyzed with a PARTEC CCAII flow analyzer (PARTEC). For each sample, at least 10,000 nuclei were collected and analyzed using a logarithmic scale display. Each flow cytometric histogram was saved using PARTEC CA3 software and analyzed with WinMDI 2.8 software (available at http://facs.scripps.edu/software.html). The ratio of G1 to G2 nuclei was calculated by dividing the total number of G1 nuclei by the total number of G2 nuclei. The percentage of endoreduplicated nuclei was calculated by dividing the total number of nuclei with a ploidy equal to or greater than 8C by total number of nuclei and multiplying it by 100.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AY986792 and AY986793.
This work was supported by grants from the Department of Energy (DE–96ER20242) and Pioneer Hi-Bred International (to B.A.L.) and by scholarships from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, Brazil (to C.M.C. and R.A.D).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.063917.
References
- An G, Mitra A, Choi HK, Costa MA, An K, Thornburg RW, Ryan CA (1989) Functional analysis of the 3′ control region of the potato wound-inducible proteinase inhibitor II gene. Plant Cell 1: 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S, Ryan KM, Phillips AC, Vousden KH (1998) Cell cycle arrest and DNA endoreduplication following p21waf1/cip1 expression. Oncogene 17: 1691–1703 [DOI] [PubMed] [Google Scholar]
- Chang B, Broude EV, Fang J, Kalinichenko TV, Abdryashitov R, Poole JC, Roninson IB (2000) p21Waf1/Cip1/Sdi1-induced growth arrest is associated with depletion of mitosis-control proteins and leads to abnormal mitosis and endoreduplication in recovering cells. Oncogene 19: 2165–2170 [DOI] [PubMed] [Google Scholar]
- Chaubet-Gigot N (2000) Plant A-type cyclins. Plant Mol Biol 43: 659–675 [DOI] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GT, Krols L, Terras F, Landrieu I, van der Schueren E, Maes S, Naudts M, Inze D (2001) Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13: 1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Sergers G, Glad N, Casteels P, Van Montagu M, Inzé D (1997) The Arabidopsis Ckst1At protein binds the cyclin-dependent kinases cdc2aAt and cdc2bAt. FEBS Lett 412: 446–452 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Murray JA (2003) The plant cell cycle. Annu Rev Plant Biol 54: 235–264 [DOI] [PubMed] [Google Scholar]
- Dilkes BP, Dante RA, Coelho C, Larkins BA (2002) Genetic analyses of endoreduplication in Zea mays endosperm: evidence of sporophytic and zygotic maternal control. Genetics 160: 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar B, Orr-Weaver TL (2001) Endoreduplication cell cycles: more for less. Cell 105: 297–306 [DOI] [PubMed] [Google Scholar]
- Fountain MD, Renz A, Beck E (1999) Isolation of a cDNA encoding a G1-cyclin-dependent kinase inhibitor (ICDK) from suspension cultured photoautotrophic Chenopidium rubrum L. cells. Plant Physiol 120: 33910409053 [Google Scholar]
- Gordon-Kamm W, Dilkes BP, Lowe K, Hoerster G, Sun X, Ross M, Church L, Bunde C, Farrell J, Hill P, et al (2002) Stimulation of the cell cycle and maize transformation by disruption of the plant retinoblastoma pathway. Proc Natl Acad Sci USA 99: 11975–11980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafi G, Burnett RJ, Helentjaris T, Larkins BA, DeCaprio JA, Sellers WR, Kaelin WG (1996) A maize cDNA encoding a member of the retinoblastoma protein family: involvement in endoreduplication. Proc Natl Acad Sci USA 93: 8962–8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafi G, Larkins BA (1995) Endoreduplication in maize endosperm- involvement of M-phase promoting factor inhibition and induction of S-phase-related kinases. Science 269: 1262–1264 [DOI] [PubMed] [Google Scholar]
- Gutierrez C (2000) DNA replication and cell cycle in plants: learning from geminiviruses. EMBO J 19: 792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C, Ramirez-Parra E, Castellano MM, Del Pozo JC (2002) G1 to S transition: more than a cell cycle engine switch. Curr Opin Plant Biol 5: 480–486 [DOI] [PubMed] [Google Scholar]
- Hattori N, Davies TC, Anson-Cartwright L, Cross JC (2000) Periodic expression of the cyclin-dependent kinase inhibitor p57kip2 in trophoblast giant cells defines a G2-like gap phase of the endocycle. Mol Biol Cell 11: 1037–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski S, Riou-Khamlichi C, Roche O, Perennes C, Bergounioux C, Glab N (2002) The CDK inhibitor NtKIS1a is involved in plant development, endoreduplication and restores normal development of cyclin D3;1-overexpressing plants. J Cell Sci 115: 973–982 [DOI] [PubMed] [Google Scholar]
- Kikuchi J, Furukawa Y, Iwase S, Terui Y, Nakamura M, Kitagawa S, Kitagawa M, Komatsu N, Miura Y (1997) Polyploidization and functional maturation are two distinct processes during megakaryocytic differentiation: involvement of cyclin-dependent kinase inhibitor p21 in polyploidization. Blood 89: 3980–3990 [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Leiva-Neto JT, Grafi G, Sabelli PA, Dante RA, Woo YM, Maddock S, Gordon-Kamm W, Larkins BA (2004) A dominant negative mutant of cyclin-dependent kinase A reduces endoreduplication but not cell size or gene expression in maize endosperm. Plant Cell 16: 1854–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui H, Wang H, DeLong C, Fowke LC, Crosby WL, Fobert PR (2000) The Arabidopsis Cdc2a-interacting protein ICK2 is structurally related to ICK1 and is a potent inhibitor of cyclin dependent kinase activity in vitro. Plant J 21: 379–385 [DOI] [PubMed] [Google Scholar]
- Mironov V, De Veylder L, Van Montagu M, Inzé D (1999) Cyclin-dependent kinases and cell division in plants—the nexus. Plant Cell 11: 509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO (1997) Cyclin-dependent kinases: engines, clocks and microprocessors. Annu Rev Cell Dev Biol 13: 261–291 [DOI] [PubMed] [Google Scholar]
- Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, et al (2000) Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploid and centrosome overduplication. EMBO J 19: 2069–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu AB, Chen X, Smeets M, Hengs L, Prives C, Reed SI (1998) Effects of p21 (Cip/Waf) at both G1/S and G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol 18: 629–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichheld JP, Chaubet N, Shen WH, Renaudin JP, Gigot C (1996) Multiple A-type cyclins express sequentially during the cell cycle in Nicotiana tabacum BY2 cells. Proc Natl Acad Sci USA 93: 13819–13824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudier F, Fedorova E, Györgyey J, Feher A, Brown S, Kondorosi A, Kondorosi E (2000) Cell cycle function of a Medicago sativa A2-type cyclin interacting with a PSTAIRE-type cyclin-dependent kinase and a retinoblastoma protein. Plant J 23: 73–83 [DOI] [PubMed] [Google Scholar]
- Russo AA, Jeffrey PD, Patten AK, Massangué J, Pavletich NP (1996) Crystal structure of the p27Kip1 cyclin-dependent kinase inhibitor bound to the cyclinA-Cdk2 complex. Nature 382: 325–331 [DOI] [PubMed] [Google Scholar]
- Schnittger A, Weinl C, Bouyer D, Schobinger U, Hulskamp M (2003) Misexpression of the cyclin-dependent kinase inhibitor ICK1/KRP1 in single-celled Arabidopsis trichomes reduces endoreduplication and cell size and induces cell death. Plant Cell 15: 303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM (1995) Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 10: 1149–1163 [DOI] [PubMed] [Google Scholar]
- Songstad DD, Armstrongn CL, Petersen WL, Hairston B, Honchee MAW (1996) Production of transgenic maize plants and progeny by bombardment of Hi II immature embryos. In Vitro Cell Dev Biol Plant 32: 170–183 [Google Scholar]
- Sun Y, Flannigan BA, Madison JT, Setter TL (1997) Alternative splicing of cyclin transcripts in maize endosperm. Gene 195: 167–175 [DOI] [PubMed] [Google Scholar]
- Sun Y, Flannigan BA, Setter TL (1999) Regulation of endoreduplication in maize (Zea mays L.) endosperm. Isolation of a novel B1-type cyclin and its quantitative analysis. Plant Mol Biol 41: 245–258 [DOI] [PubMed] [Google Scholar]
- Vandepoele K, Raes J, De Veylder L, Rouzé P, Rombautus S, Inzé D (2002) Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell 14: 903–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Qi Q, Schorr P, Cutler AJ, Crosby WL, Fowke L (1998) ICK1, a cyclin-dependent kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. Plant J 15: 501–510 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou Y, Gilmer S, Whitwill S, Fowke LC (2000) Expression of the plant cyclin-dependent kinase inhibitor ICK1 affects cell division, plant growth and morphology. Plant J 24: 613–623 [DOI] [PubMed] [Google Scholar]
- Weinberg RA (1995) The retinoblastoma protein and the cell cycle control. Cell 81: 323–330 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fowke LC, Wang H (2002. a) Plant CDK inhibitors: studies of interactions with cell cycle regulators in the yeast two-hybrid system and functional comparisons in transgenic Arabidopsis plants. Plant Cell Rep 20: 967–975 [Google Scholar]
- Zhou Y, Wang H, Gilmer S, Whitwill S, Keller W, Fowke LC (2002. b) Control of petal and pollen development by the plant cyclin-dependent kinase inhibitor ICK1 in transgenic Brassica plants. Planta 215: 248–257 [DOI] [PubMed] [Google Scholar]