Abstract
To examine the cross talk between the abscisic acid (ABA) and ethylene signal transduction pathways, signaling events during ABA-induced stomatal closure were examined in Arabidopsis (Arabidopsis thaliana) wild-type plants, in an ethylene-overproducing mutant (eto1-1), and in two ethylene-insensitive mutants (etr1-1 and ein3-1). Using isolated epidermal peels, stomata of wild-type plants were found to close within a few minutes in response to ABA, whereas stomata of the eto1-1 mutant showed a similar but less sensitive ABA response. In addition, ABA-induced stomatal closure could be inhibited by application of ethylene or the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC). In contrast, stomata of the etr1-1 and ein3-1 mutants were able to close in response to concomitant ABA and ACC application, although to a lesser extent than in wild-type plants. Moreover, expression of the ABA-induced gene RAB18 was reduced following ACC application. These results indicate that ethylene delays stomatal closure by inhibiting the ABA signaling pathway. The same inhibitive effects of ethylene on stomatal closure were observed in ABA-irrigated plants and the plants in drought condition. Furthermore, upon drought stress, the rate of transpiration was greater in eto1-1 and wild-type plants exposed to ethylene than in untreated wild-type control plants, indicating that the inhibitive effects of ethylene on ABA-induced stomatal closure were also observed in planta.
Guard cells are highly specialized epidermal cells that are located in pairs on the aerial organs of plants. Each pair of guard cells forms a pore or “stoma” that closes and opens in response to osmotic shrinking and swelling of the guard cells, respectively. Stomata play a major role in controlling gaseous exchange, especially of photosynthetic carbon dioxide uptake, and in water release by transpiration in response to changes in the surrounding environment. The regulation of stomatal conductance is thus extremely important for the survival of plants. Abscisic acid (ABA), synthesized in response to drought stress, is known to induce stomatal closure and to reduce transpirational water loss (Schroeder et al., 2001). ABA also regulates other plant growth and developmental processes, such as embryo maturation, seed dormancy, and adaptation to environmental stresses (Leung and Giraudat, 1998). Among the various phytohormones, ABA appears to play the most important role in the control of stomatal responses (Dodd, 2003). However, as stomata respond to various external conditions that may alter the plants' phytohormone balance, stomatal movement may in fact be controlled by the interactions between multiple phytohormones. Indeed, numerous recent studies indicate a cooperative effect between ABA and other phytohormones in various physiological events, and that these effects are dependent on the species, conditions, periods, and sites in which the hormones function. Such examples include the antagonism between GAs and ABA in cereal aleurone layers (Lovegrove and Hooley, 2000); the effects of methyl jasmonate on plant transpiration (Lee et al., 1996; Wang, 1999), through the regulation of stomatal closure (Raghavendra and Reddy, 1987) in cooperation with ABA and mediated by production of reactive oxygen species (Suhita et al., 2004); as well as the interaction between ethylene and ABA.
Ethylene regulates numerous plant processes, including seed germination, root-hair initiation, leaf and flower senescence and abscission, fruit ripening, nodulation, and plant responses to a wide variety of stresses (Bleecker and Kende, 2000). Although its role in stomatal closure has been suggested (Giulivo, 1986), its effect in this process seems rather contradictory. In the early stages of Arabidopsis (Arabidopsis thaliana) seedling development, ethylene appears to act as a negative regulator of ABA action, while in Arabidopsis roots it has a positive synergistic effect on ABA action by modulating the overall carbon status (Ghassemian et al., 2000). Under water stress conditions, the increased endogenous ABA levels limit ethylene production and so maintain the growth ratio between shoots and roots (Sharp, 2002).
Despite these advances in our understanding of both ABA and ethylene, studies on the mechanisms by which ethylene interacts with ABA in guard cells are still in their infancy. Over the past decade, genetic screens that were based on ethylene's triple-response phenotype have been extensively conducted on Arabidopsis, and more than a dozen unique mutants have been identified. Ethylene is synthesized from Met via S-adenosyl-l-Met and 1-aminocyclopropane-1-carboxylic acid (ACC; Adams and Yang, 1979). The conversion from S-adenosyl-l-Met to ACC, catalyzed by ACC synthase (ACS), is generally the rate-limiting step of ethylene biosynthesis. In the ethylene over-producer1-1 (eto1-1) mutant, ethylene is overproduced due to a reduction of ETO1 activity, which promotes ACS5 degradation by a proteasome-dependent pathway (Wang et al., 2004). The ethylene perception and signal transduction pathways have also been investigated using Arabidopsis mutants. Genetically dominant mutations in ETHYLENE RESISTANT1 (ETR1) result in ethylene insensitivity (Bleecker and Kende, 2000), whereas mutations at the ETHYLENE INSENSITIVE3 (EIN3) locus cause reduced ethylene sensitivity (Roman et al., 1995).
To further investigate the interactions between ABA and ethylene, we have in this study compared the stomatal responses of three Arabidopsis mutants, eto1-1, etr1-1, and ein3-1, to those of wild-type plants. In addition to these phenotypic observations, the differential expression of the ABA-induced gene RAB18, which accumulates only in guard cells (Nylander et al., 2001), was investigated in these plant.
RESULTS
Ethylene Inhibits ABA-Induced Stomatal Closure
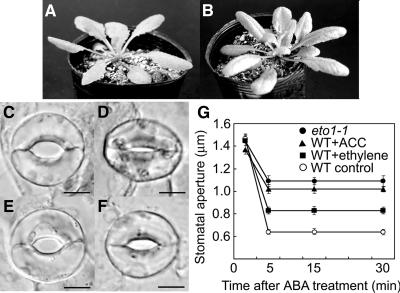
To first examine the effects of ABA on stomata, we employed an in vitro system using isolated epidermal peels in which we could measure stomatal apertures. After light illumination of wild-type plants (Fig. 1, A, C, and E), the stomatal apertures reached approximately 1.47 μm (Fig. 1G). Time-course observations demonstrated that within 5 min of start of ABA application, the stomata almost completely closed (Fig. 1D), with stomatal apertures decreasing to approximately 0.64 μm and being maintained in this condition for at least 30 min (Fig. 1G). Using the same system, we examined the effects of ethylene on ABA-induced stomatal closure. When gaseous ethylene was applied together with ABA to the isolated epidermal peels, the stomata started to close within 5 min after the treatment. However, stomatal closure was incomplete (Fig. 1, F and G), with stomatal apertures reaching about 0.83 μm and being maintained at this level until 30 min (Fig. 1, E and G). To confirm the efficacy of exogenous ethylene treatment, we applied the ethylene precursor, ACC, instead of ethylene. Following treatment with 10 μm ACC, ABA-induced stomatal closure was inhibited again, with the stomata remaining in a half-opened state. A similar pattern of changes in stomatal aperture was observed in the eto1-1 plants (Fig. 1, B and G), a mutant that overproduces ethylene without treatment with either ethylene or ACC. These observations indicate that both endogenous and exogenous ethylene have an inhibitive effect on ABA-induced stomatal closure.
Figure 1.
Ethylene treatment impairs stomatal closure induced by ABA treatment. A and B, Overall phenotype of wild-type (A) and eto1-1 (B) plants. C and E, Wild-type stomata preopened in light conditions without (C) and with (E) ethylene gas. D and F, Wild-type stomata after incubation with 10 μm ABA for 30 min without (D) and with (F) ethylene. Bars = 5 μm. G, Time-course changes in stomatal apertures (width, μm) after ABA treatment of wild-type (white circles), wild-type exposed to ethylene gas (black squares), wild type treated with ACC (black triangles), and eto1-1 plants (black circles). Bars represent means ± ses.
Ethylene Signaling Impairs ABA Regulation of Stomatal Closure
To examine the effects of ethylene on ABA-induced stomatal closure in further detail, we measured the stomatal apertures in the following three experimental systems: using ethylene biosynthesis and/or signaling mutants, treatment with ethylene reception inhibitors, and treatment with an ACS inhibitor.
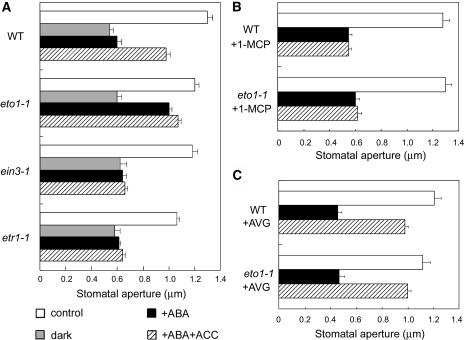
First, we compared the stomatal responses of wild-type plants with those of the three ethylene mutants, eto1-1, ein3-1, and etr1-1. The stomata in wild-type plants closed in response to either dark or ABA (Fig. 2A). In contrast, the ABA-induced stomatal closure of eto1-1 plants was suppressed, although stomatal closure in response to darkness occurred to the same extent as in wild-type plants (Fig. 2A). As with wild-type plants, stomatal apertures of the etr1-1 and ein3-1 mutants decreased to about 0.60 μm after either dark or ABA treatment (Fig. 2A). However, the ABA-induced stomatal closure could not be restored by ACC treatment in these mutants (Fig. 2A).
Figure 2.
ACC treatment impairs stomatal closure induced by ABA treatment only when plants can transmit the ethylene signal. A to C, Stomatal apertures of wild-type, eto1-1, ein3-1, and etr1-1 plants (A), 1-MCP-treated wild-type and eto1-1 plants (B), and AVG-treated wild-type and eto1-1 plants (C), in the preopened condition (white bars), after incubation in darkness for 2 h (gray bars), after incubation with 10 μm ABA for 2 h in the light (black bars), and after incubation with 10 μm ABA and 10 μm ACC in the light (striped bars). The data are representative of three independent experiments, showing the means of 100 stomata. Bars represent means ± ses.
We next examined the effects of a competitive inhibitor of ethylene receptor(s), 1-methylcyclopropene (1-MCP; Sisler and Serek, 1997), on stomatal responses. After an overnight treatment with 1-MCP, stomata of both wild type and eto1-1 closed after ABA treatment, and the closure was not restored even in the presence of ACC (Fig. 2B). These results suggest that ethylene signaling is necessary for the inhibition of ABA-induced stomatal closure.
We subsequently investigated the effects of ethylene in wild-type and eto1-1 plants treated with aminoethoxyvinyl Gly (AVG), an inhibitor of ACS (Yoshii and Imaseki, 1982). In wild-type plants, ABA-induced stomatal closure did not differ between AVG-treated and nontreated plants (compare Fig. 2, A and C). In contrast, AVG-treated eto1-1 plants showed an ABA-induced stomatal closure response similar to that of the wild-type plants (Fig. 2C). Such a stomatal response was not the result of the toxic side effects of AVG treatment, since AVG together with exogenous ACC inhibited ABA-induced stomatal closure in both wild-type and eto1-1 plants (Fig. 2C). These results suggest that the inhibition of ABA-induced stomatal closure observed in the eto1-1 plants resulted from the overproduction of ethylene.
Differential Expression of an ABA-Induced Gene in Guard Cells
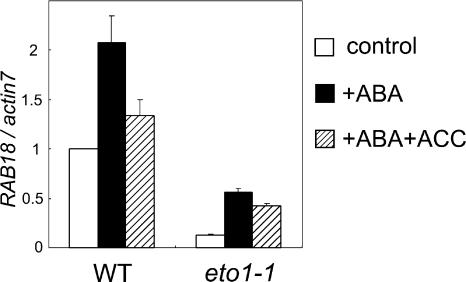
To monitor the inhibition of ABA signaling by ethylene in guard cell tissues at the molecular level, the expression of an ABA-induced gene, RAB18, was examined by quantitative real-time PCR. Following treatment with ABA, RAB18 expression was enhanced in both wild type and eto1-1, but the level of RAB18 expression in eto1-1 plants was significantly lower than that in wild-type plants (P < 0.01). Simultaneous treatment with ACC and ABA resulted in decreased RAB18 transcript levels in both wild-type (0.01 ≤ P < 0.05) and eto1-1 plants (Fig. 3). These results suggest that induction of RAB18 expression by ABA is inhibited by exogenous ACC application.
Figure 3.
ABA responses of epidermal tissues of wild-type and eto1-1 plants. Expression patterns of an ABA-induced gene, RAB18, were compared between the wild type and eto1-1. mRNA was extracted from epidermal tissues before treatment (white bars) and after treatment with 10 μm ABA (black bars) or 10 μm ABA plus 100 μm ACC (striped bars) in opening buffer without Tween 20 for 2 h. Gene expression levels were measured by real-time quantitative PCR, and the relative amounts of transcripts then calculated and normalized with actin7 mRNA. Bars represent the means ± ses (n = 6–9). The data were analyzed by ANOVA. Difference between wild type + ABA and wild type + ABA + ACC was significant (0.01 ≤ P < 0.05), and between wild type + ABA and eto1-1 + ABA was significant (P < 0.01).
Ethylene Inhibits Stomatal Closure upon ABA Treatment and Drought Stress in Planta
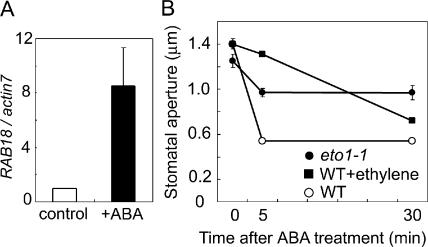
The above results indicated that ethylene repressed the ABA-induced closure of stomata in vitro but provided no evidence in planta. We therefore carried out an examination of whether ethylene affects stomatal closure even in planta. We irrigated ABA to wild type, eto1-1, and wild-type plants previously exposed to gaseous ethylene. The level of RAB18 expression was elevated in leaves of wild-type plants by ABA treatment to roots (Fig. 4A), and stomata in wild-type plants closed rapidly after ABA treatment (Fig. 4B). In contrast, stomata of eto1-1 plants and ethylene gas-exposed plants closed slowly and not completely even 30 min after ABA treatment (Fig. 4B).
Figure 4.
Ethylene impairs stomatal closure induced by ABA treatment in planta. A, Expression patterns of an ABA-induced gene, RAB18, were compared between before and after ABA treatment. mRNA was extracted from leaves of wild-type plants before treatment (white bars) and after irrigation with 100 μm ABA (black bars) for 30 min. Gene expression levels were measured by real-time quantitative PCR, and the relative amounts of transcripts then calculated and normalized with actin7 mRNA. Bars represent the means ± ses (n = 4–8). B, Time-course changes in stomatal apertures after ABA irrigation of wild type (white circles), wild type exposed to ethylene gas (black squares), and eto1-1 plants (black circles).
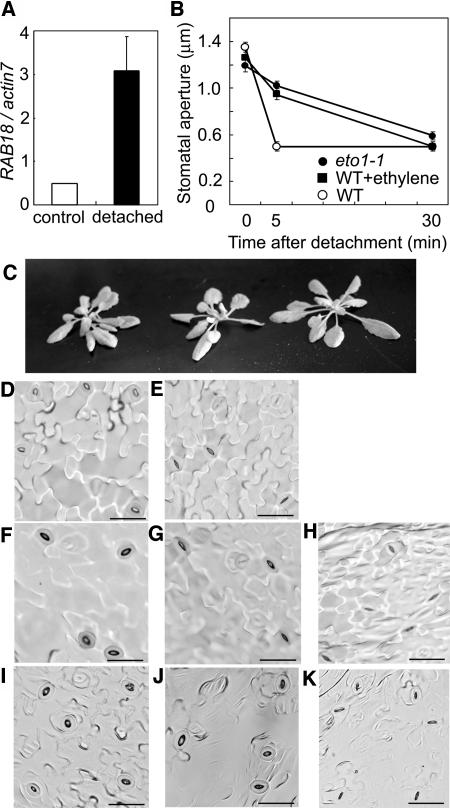
Stomata are known to close in response to drought to limit water loss by transpiration. During this process, ABA is synthesized and plays a role in closing stomata. To study the effect of ethylene on stomatal closure under drought stress, we subjected the plants, wild type, eto1-1, and wild type previously exposed to gaseous ethylene, to drought stress by detaching the aboveground portion of the seedlings from their roots. After the detachment, the level of RAB18 expression in the leaves was elevated in wild-type plants (Fig. 5A). The stomata of wild-type plants rapidly closed within 5 min, and after that the stomatal aperture was not so changed (Fig. 5, B, D, and E). Although there is no clear difference in appearance between these plants after detachment (Fig. 5C), the stomata of eto1-1 plants and wild-type plants exposed to ethylene closed slowly after detachment (Fig. 5, B, and F to K).
Figure 5.
Delay in stomatal closure of eto1-1 and wild-type plants exposed to ethylene compared to wild-type plants under drought stress. A, Expression patterns of an ABA-induced gene, RAB18, were compared between before and after detachment. mRNA was extracted from leaves of wild-type plants before detachment (white bars) and 30 min after detachment (black bars). Gene expression levels were measured by real-time quantitative PCR, and the relative amounts of transcripts then calculated and normalized with actin7 mRNA. Bars represent the means ± ses (n = 4–8). B, Time-course changes in stomatal apertures after detachment of wild type (white circles), wild type exposed to ethylene gas (black squares), and eto1-1 plants (black circles). C, Overall phenotype of wild type, eto1-1, and wild-type plant exposed to gaseous ethylene 30 min after detachment (from left to right). D to K, Stomata of wild-type plants before detachment of leaves (D), wild-type plants 5 min after leaf detachment (E), eto1-1 plants before detachment (F), eto1-1 plants 5 min (G) or 30 min after detachment (H), and wild type exposed to ethylene gas before detachment (I) and 5 min (J) or 30 min (K) after detachment, as observed by optical microscopy (×400; bars = 20 μm).
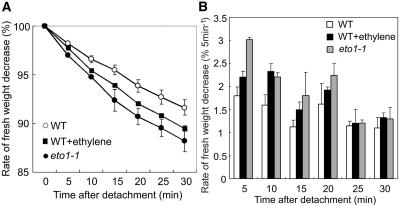
To gain further insight into the observed delay of stomatal closure, the changes in fresh weight under drought stress were measured in the eto1-1 plants and wild-type plants exposed to ethylene. Results demonstrated that eto1-1 plants and wild-type plants exposed to ethylene showed an accelerated decrease of the fresh weight in comparison to nontreated wild-type plants (Fig. 6A). The rate of the fresh weight decrease in eto1-1 and ethylene-treated plants was higher than in nontreated plants 20 min after detachment (Fig. 6B). This result will reflect the fact that the eto1-1 plants and wild-type plants exposed to ethylene fail to limit their transpirational water loss by the delay of stomatal closure in an early stage of drought condition.
Figure 6.
Accelerated decrease of the fresh weight in eto1-1 and wild-type plants exposed to ethylene gas. A, Changes in fresh weight decrease ratio of aerial parts of wild type (white circles), wild-type plants exposed to ethylene gas (black squares), and eto1-1 plants (black circles) after detachment from roots. B, The rate of the fresh weight decrease calculated every 5 min after detachment from roots. Wild type (white bars), wild-type plants exposed to ethylene gas (black bars), and eto1-1 plants (gray bars) are shown. Fresh weights of the aerial parts after detachment were measured, and the ratios of reduced weights and original fresh weights were then calculated. The data are representative of three independent experiments, and the means of 20 individual plants are shown. Error bars represent ses.
DISCUSSION
The prime objective of this study was to elucidate the cross talk between ABA and ethylene signal transduction on stomatal closure using Arabidopsis leaf epidermal peels as a model. Toward this goal, we evaluated the inhibition of ABA-induced stomatal closure by ethylene using genetic, molecular, biochemical, and physiological approaches.
Ethylene Inhibits ABA-Induced Stomatal Closure
Gaseous ethylene or ACC treatment of the epidermal peels inhibited ABA-induced stomatal closure (Fig. 1). In contrast, inhibition of ABA-induced stomatal closure by ACC treatment was not observed if the ethylene reception or signaling pathway was inhibited (Fig. 2, A and B). Besides, ACC treatment did not induce the dark-closed stomata into the open state (data not shown). Taken together, these results suggest that ethylene physiologically inhibited ABA-induced stomatal closure through the ethylene signaling pathway.
In our experimental system, we found that in response to ABA, stomata of Arabidopsis plants closed much faster than other plants. For example, in Vicia faba, stomata started to close 8 min after ABA treatment and reached maximal closure within 20 min (Roelfsema et al., 2004). A rapid response to ABA might be necessary for the survival strategy of plants like Arabidopsis, in which whole plant size as well as stomatal size are much smaller than plants like V. faba. Still, the same inhibitive effect of ethylene against ABA was observed also in stomata of V. faba (data not shown).
Effect of Ethylene on Stomatal Closure in Planta
Since the expression of RAB18 was elevated in leaves in in planta analysis by ABA irrigation, we assumed that the amount of ABA was also elevated by this culture condition. As the same inhibitive effects of ethylene on stomatal closure were observed, it is suggested that the inhibitive effect of ethylene against ABA coincided with functions also in planta.
However, in our three time-course experiments, there were some differences in patterns of stomatal closure upon ethylene gas treatment or in eto1-1 plants (Figs. 1G, 4B, and 5B). In response to ABA irrigation, the stomata of wild-type plants exposed to ethylene gas gradually closed by 30 min (Fig. 4B), while those of peels floated on buffer kept half-open (Fig. 1G). This difference will come from the amount of ethylene exposure on plants. Ethylene gas exposed to the plant tissue in advance of ABA irrigation might diminish gradually (Fig. 4B), while ethylene gas once dissolved in the buffer might remain at least through the experimental period (Fig. 1G). This effusion of ethylene gas exposed to the plant tissue will explain the difference between the eto1-1 and wild-type plants exposed to ethylene gas since in eto1-1 plants ethylene should be overproduced continuously. In addition, the gradual closure of the stomata in wild-type plants exposed to ethylene gas may suppose an idea that the effect of ethylene against ABA is reversible.
On the other hand, in contrast to the ABA-irrigated plants and peels floated on buffer, the stomata of detached leaves of both wild-type plants treated with gaseous ethylene and eto1-1 plants finally closed at the same level as control wild-type plants (Fig. 5B), though they exhibited a retardation of stomatal closure compared to the control (Fig. 5, B, and D to K). This complete closure in the detached plants by 30 min might be caused by the loss of water in guard cell itself, since the stomata are known to be closed by water loss from the guard cells upon dehydrated condition (Asai et al., 1999). Although we did not measure ABA amount in our experiments, an increase of the ABA concentration in detached leaflets within 15 min in V. faba (Harris and Outlaw, 1991) will support the idea that the stomatal closure after detachment will be caused by ABA response, and ethylene may inhibit the ABA signaling.
Still, in those plants in which the delay in stomatal closure was observed, an acceleration of water loss rate appeared to result in their fresh weights decreasing faster than wild-type plants (Fig. 6, A and B). A rapid decrease in fresh weight was also observed in the wild-type plants treated with ACC, but not in the etr1-1 and ein3-1 plants (data not shown). Therefore, the delay in stomatal closure appears to result from the inhibition by ethylene, and the result of our in vitro experiments could be extrapolated to the in planta observations.
In this article, we have shown that ethylene inhibits ABA-induced stomatal responses. What is the physiological role of ethylene inhibition of ABA-mediated stomatal closure? One possibility may be that ethylene ensures the minimum supply of carbon dioxide for photosynthesis by keeping the stomata half-opened. It is well known that both ethylene and ABA are affected by drought stress of plants (Leung and Giraudat, 1998), and the rate of photosynthesis is almost dependent on the stomatal aperture. Indeed, even though the antagonistic relationship between ABA and ethylene under drought has been reported (Spollen et al., 2000), ethylene synthesis is often promoted in response to drought (Xu and Zou, 1993). With regard to the relationship between ethylene and photosynthesis, it has been reported that the rates of ethylene release, photosynthesis, and transpiration increased simultaneously in rice (Oryza sativa) and were especially promoted during the light period (Saito et al., 1996). Furthermore, in unstressed wild-type Arabidopsis plants, ethylene production levels were found to be controlled by light and by the circadian clock (Thain et al., 2004). Therefore, ethylene may play a role in keeping a minimum level of photosynthesis upon drought stress for a long period.
Another possible role of ethylene on the inhibition of ABA-mediated stomatal closure may be related to the involvement of ethylene in regulating leaf senescence, since defects in ethylene synthesis or perception were shown to delay leaf senescence (Davies and Grierson, 1989; Picton et al., 1993; Grbic and Bleecker, 1995; John et al., 1995). Drought can also induce senescence and is known to promote increased ethylene production in plants (McMichael et al., 1972; Apelbaum and Yang, 1981). A recent report demonstrated that the loss of ACS expression in transgenic maize (Zea mays) plants resulted in a delay in drought-induced leaf senescence that was associated with increased stomatal conductance (Young et al., 2004), indicating that ethylene promotes drought-induced leaf senescence.
In this study, we showed a physiological effect of ethylene on ABA-induced stomatal closure. Among various ABA signal transduction mechanisms, rapid ABA-induced Ca2+ influx and S-type anion channel activation are required for RAB18 expression (Schroeder et al., 2001). Although we did not measure anion channel activities in this study, the repression of the ABA-induced RAB18 expression by ACC treatment (Fig. 3) suggests that ethylene signaling might impair the Ca2+ influx or the S-type anion channel activation. At least, ethylene seems not to interfere with the early ABA-signaling pathway since the stomata started to close by ABA application even in the presence of ethylene, but some later stage of ABA-signaling since the stomata were kept half-opened by ethylene treatment. It would be excellent to find out the cross point of interaction between ABA and ethylene signaling pathways. However, to clarify this point needs further study.
MATERIALS AND METHODS
Plant Materials and Culture Conditions
Arabidopsis (Arabidopsis thaliana) seeds of Col-0, eto1-1 (stock no. CS3072), etr1-1 (CS237), and ein3-1 (CS8052) were obtained from the Arabidopsis Biological Resource Center (Columbus, OH). All mutants used were of the Col-0 background. Seeds were germinated and grown on vermiculite that was irrigated daily with mineral nutrients, as described by Naito et al. (1994), in growth chambers at 23.5°C, a relative humidity of 60%, and under a photosynthetic photon flux density of 50 μmol photons m−2 s−1 in 12-h-light/12-h-dark cycles.
Measurement of Stomatal Aperture
The abaxial epidermis was peeled from rosette leaves of 5- to 6-week-old plants 3 h after the beginning of the light period. Epidermal peels were floated, peeled-side down, on opening buffer (10 mm KCl, 10 mm CaCl2 and 10 mm MES, 0.01% Tween 20, pH 6.5) and incubated under light conditions for 2 h to open the stomata. Subsequently, the epidermal peels with preopened stomata were transferred to the same buffer supplemented with 10 μm ABA (Sigma-Aldrich, St. Louis), either with or without bubbling 100 μL L−1 ethylene gas (GL Sciences, Tokyo) or alternatively with the addition of 10 μm ACC. For dark conditions, preopened epidermal peels were incubated in the dark for 2 h. Stomatal apertures were measured from the pore widths that were observed by light microscopy (Olympus BX51; Tokyo), using a fitted camera (Olympus DP70 digital camera unit), and measured with a digital ruler in Adobe Photoshop 6 (Adobe Systems, Mountain View, CA).
Treatment 1-MCP and AVG
Treatment of samples with 1-MCP was conducted by evaporating 5.6 mg of 1-MCP, dissolved in 85 μL of distilled water, in a closed chamber (5.8 L) for 12 h. The final concentration of 1-MCP in the gas phase was expected to be about 500 pL L−1 (Tamaoki et al., 2003). For treatment with AVG, 100 μm AVG was added to the opening buffer during the experiment.
Expression Analyses
For analyzing ABA responses of epidermal tissues, rosette leaves from 5- to 6-week old-plants were homogenized in opening buffer for 2 min and the epidermal fragments then collected on a 20-μm (pore size) nylon mesh as described by Allen et al. (1999). These fragments were then incubated in the opening buffer without Tween 20 for 2 h, and then treated with 10 μm ABA or with 10 μm ABA and 100 μm ACC for another 2 h. The epidermal fragments were again collected on a 20-μm nylon mesh and immediately frozen in liquid nitrogen. Total RNA was extracted from 100 mg of frozen tissue using the Qiagen Plant RNeasy kit (Qiagen, Valencia, CA), according to the manufacturer's specifications. For reverse transcription-PCR analysis, 5 μg of total RNA was reverse transcribed with M-MLV Reverse Transcriptase (Promega, Madison, WI) and the resulting cDNAs then used for the subsequent PCR steps.
Real-time quantitative PCR was conducted in a Smart Cycler II system (Cepheid, Sunnyvale, CA) and using SYBR Premix Taq (Takara Bio, Shiga, Japan) according to the manufacturer's specifications. As an internal standard for cDNA amounts, a 143-bp fragment of actin-7 cDNA was amplified with PCR primers 5′-GGAAATTGTCCGTGACATAAAGGAG-3′ (upstream primer) and 5′-CTCTCAGCTCCGATGGTTATGACTT-3′ (downstream primer). A 226-bp fragment of the RAB18 cDNA was amplified with PCR primers 5′-ACGAGTACGGAAATCCGATG-3′ (upstream primer) and 5′-ACCACCACTTTCCTTGTGGA-3′ (downstream primer).
Treatment with ABA and Drought Stress in Planta
Treatment with ABA in planta was carried out by irrigation of 100 μm ABA dissolved in distilled water for 30 min. For drought stress, the aerial parts of 5-week plants were detached from the roots. Total RNA was extracted from rosette leaves of treated plants, before or 30 min after the treatment, and real-time quantitative PCR was conducted as described above.
Stomatal Imaging of in Vivo Leaves
Stomatal shapes were observed by Suzuki's Universal Micro-Printing (SUMP) method using SUMP liquid and SUMP plate (SUMP Laboratory, Tokyo). Here, the abaxial sides of the leaves were pressed onto 10 μL of SUMP liquid placed on a cover glass until the liquid became solid. The copied SUMP resin images were then observed by light microscopy (Olympus BX51).
Ethylene Gas Treatment and Water Loss Measurements
Five-week-old wild-type plants were exposed to a dose of 100 μL L−1 ethylene gas (GL Sciences) for 3 h in a transparent plastic, air-tight container. An appropriate amount of 100% ethylene was injected into the chamber to bring the gas composition to 100 μL L−1, and the presence of ethylene gas was verified by observing the triple response of etiolated Arabidopsis seedlings (Guzmán and Ecker, 1990). Conditions within the container were maintained at 23.5°C, with a photosynthetic photon flux density of 50 μmol photons m−2 s−1 under continuous light. Control plants were kept in the same conditions described above but without ethylene gas. After the treatments, the aerial parts of the plants were detached from the roots and their fresh weights measured.
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas (grant no. 17051008) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to S.H.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.063503.
References
- Adams DO, Yang SF (1979) Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA 76: 170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI (1999) Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell 11: 1785–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A, Yang SF (1981) Biosynthesis of stress ethylene induced by water deficit. Plant Physiol 68: 594–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai N, Nakajima N, Kondo N, Kamada H (1999) The effect of osmotic stress on the solutes in guard cells of Vicia faba L. Plant Cell Physiol 40: 843–849 [Google Scholar]
- Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Davies KM, Grierson D (1989) Identification of cDNA clones for tomato (Lycopersicon esculentum Mill.) mRNAs that accumulate during fruit ripening and leaf senescence in response to ethylene. Planta 179: 73–80 [DOI] [PubMed] [Google Scholar]
- Dodd IC (2003) Hormonal interactions and stomatal responses. J Plant Growth Regul 22: 32–46 [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, Mc P, Court P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12: 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulivo C (1986) Hormonal control of water transport in soil-plant-atmosphere continuum. Acta Hortic 179: 385–393 [Google Scholar]
- Grbic V, Bleecker AB (1995) Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J 8: 595–602 [Google Scholar]
- Guzmán P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MJ, Outlaw WH (1991) Rapid adjustment of guard-cell abscisic-acid levels to current leaf-water status. Plant Physiol 95: 171–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John I, Drake R, Farrell A, Cooper W, Lee P, Horton P, Grierson D (1995) Delayed leaf senescence in ethylene-deficient ACC-oxidase antisense tomato plants: molecular and physiological analysis. Plant J 7: 483–490 [Google Scholar]
- Lee TM, Lur HS, Lin YH, Chu C (1996) Physiological and biochemical changes related to methyl jasmonate-induced chilling tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ 19: 65–74 [Google Scholar]
- Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49: 199–222 [DOI] [PubMed] [Google Scholar]
- Lovegrove A, Hooley R (2000) Gibberellin and abscisic acid signalling in aleurone. Trends Plant Sci 5: 102–110 [DOI] [PubMed] [Google Scholar]
- McMichael BL, Jordan WR, Powell RD (1972) Effect of water stress on ethylene production by intact cotton petioles. Plant Physiol 49: 658–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito S, Yokota MY, Chino M, Komeda Y (1994) Expression of a soybean (Glycine max [L.] Merr.) seed storage protein gene in transgenic Arabidopsis thaliana and its response to nutritional stress and to abscisic acid mutations. Plant Physiol 104: 497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander M, Svensson J, Palva ET, Welin BV (2001) Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana. Plant Mol Biol 45: 263–279 [DOI] [PubMed] [Google Scholar]
- Picton S, Barton SL, Bouzayen M, Hamilton AJ, Grierson D (1993) Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene-forming enzyme transgene. Plant J 3: 469–481 [Google Scholar]
- Raghavendra AS, Reddy KB (1987) Action of proline on stomata differs from that of abscisic acid, G-substances, or methyl jasmonate. Plant Physiol 83: 732–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema MR, Levchenko V, Hedrich R (2004) ABA depolarizes guard cells in intact plants, through a transient activation of R- and S-type anion channels. Plant J 37: 578–588 [DOI] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139: 1393–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Tani A, Kiyota M, Ohe M (1996) Rates of ethylene release, photosynthesis and transpiration of rice measured in closed-type chamber. Acta Hortic 440: 55–59 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Sharp RE (2002) Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ 25: 211–222 [DOI] [PubMed] [Google Scholar]
- Sisler EC, Serek M (1997) Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiol Plant 100: 577–582 [Google Scholar]
- Spollen WG, LeNoble ME, Samuels TD, Bernstein N, Sharp RE (2000) Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol 122: 967–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol 134: 1536–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki M, Matsuyama T, Kanna M, Nakajima N, Kubo A, Aono M, Saji H (2003) Differential ozone sensitivity among Arabidopsis accessions and its relevance to ethylene synthesis. Planta 216: 552–560 [DOI] [PubMed] [Google Scholar]
- Thain SC, Vandenbussche F, Laarhoven LJJ, Dowson-Day MJ, Wang ZY, Tobin EM, Harren FJM, Millar AJ, Straeten DVD (2004) Circadian rhythms of ethylene emission in Arabidopsis. Plant Physiol 136: 3751–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KLC, Yoshida H, Lurin C, Ecker JR (2004) Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature 428: 945–950 [DOI] [PubMed] [Google Scholar]
- Wang SY (1999) Methyl jasmonate reduces water stress in strawberry. J Plant Growth Regul 18: 127–134 [DOI] [PubMed] [Google Scholar]
- Xu CC, Zou Q (1993) Effect of drought on lipoxygenase activity, ethylene and ethane production in leaves of soybean plants. Acta Bot Sin 35: 31–37 [Google Scholar]
- Yoshii H, Imaseki H (1982) Regulation of auxin-induced ethylene biosynthesis. Repression of inductive formation of 1-aminocyclopropane-1-carboxylate synthase by ethylene. Plant Cell Physiol 23: 639–649 [Google Scholar]
- Young TE, Meeley RB, Gallie DR (2004) ACC synthase expression regulates leaf performance and drought tolerance in maize. Plant J 40: 813–825 [DOI] [PubMed] [Google Scholar]