Abstract
The initiation and development of legume nodules induced by compatible Rhizobium species requires a complex signal exchange involving both plant and bacterial compounds. Phytohormones have been implicated in this process, although in many cases direct evidence is lacking. Here, we characterize the root and nodulation phenotypes of various mutant lines of pea (Pisum sativum) that display alterations in their phytohormone levels and/or perception. Mutants possessing root systems deficient in gibberellins (GAs) or brassinosteroids (BRs) exhibited a reduction in nodule organogenesis. The question of whether these reductions represent direct or indirect effects of the hormone deficiency is addressed. For example, the application of GA to the roots of a GA-deficient mutant completely restored its number of nodules to that of the wild type. Grafting studies revealed that a wild-type shoot or root also restored the nodule number of a GA-deficient mutant. These findings suggest that GAs are required for nodulation. In contrast, the shoot controlled the number of nodules that formed in graft combinations of a BR-deficient mutant and its wild type. The root levels of auxin and GA were similar among these latter graft combinations. These results suggest that BRs influence a shoot mechanism that controls nodulation and that the root levels of auxin and GA are not part of this process. Interestingly, a strong correlation between nodule and lateral root numbers was observed in all lines assessed, consistent with a possible overlap in the early developmental pathways of the two organs.
Nodulation is a symbiotic process whereby bacteria of the genus Rhizobium invade compatible leguminous host plants (Mylona et al., 1995; Mathesius, 2003). The invasion ultimately leads to the formation of structures called nodules, in which the bacteria fix atmospheric nitrogen to be used by the plant. As with any developmental process, nodulation is multifaceted, requiring specific signaling events regulated temporally and spatially (Ferguson and Mathesius, 2003).
Beginning in the 1980s, mutagenesis experiments using pea (Pisum sativum) produced abnormal nodulation phenotypes including nonnodulating (nod−), poorly nodulating (nod±), and hypernodulating (nod++) mutants, as well as those that fix nitrogen poorly or not at all (fix-; see refs. in Borisov et al., 2000). At present, over 200 nodulation mutants exist in pea (Borisov et al., 2000). Nodulation mutants have also been selected for in the model legume species Medicago truncatula and Lotus japonicus, which have smaller genomes than pea, making them more desirable tools for molecular studies. Mutants in these species have since been used to identify genes and gene products involved in nodule formation and functioning. This approach has been successful, and the orthologs of many nodulation genes discovered in M. truncatula or L. japonicus have subsequently been identified in important crop species such as pea (see refs. in Oldroyd and Downie, 2004).
Here, we take the reverse approach to investigate nodulation. In contrast to selecting for nodulation mutants and identifying their mutated genes, we identified the root and nodulation phenotypes of previously characterized mutants (Table I). The mutants examined here are all affected in their biosynthesis of, or responses to, the phytohormones GA or brassinosteroid (BR). Moreover, the genes and gene products of these lines have all formerly been identified (for review, see Reid et al., 2004; Table I). Unlike dwarf (le) cultivars used in many previous nodulation studies (e.g. Finale, Frisson, Rondo, Solara, Sparkle), the wild types studied here are all on a tall (LE) background. Interestingly, many pea lines used for agricultural purposes are on le backgrounds and are therefore deficient in shoot GA1 (Reid et al., 2004), as are many of the lines used for the selection of nodulation mutants. However, the effects of shoot dwarfism and reduced shoot GA1 levels on nodulation have not been described previously. This report is also the first to investigate the role(s) of endogenous BRs in nodulation. As with GA deficiencies, reductions in BR levels cause shoot dwarfism, thus allowing us to use two distinct hormone-mediated mechanisms to investigate the effects of shoot stature on nodulation and root development.
Table I.
Overview of the various pea lines investigated
| Genotype | Line Number | Gene Product | Hormone Level | Phenotype | References |
|---|---|---|---|---|---|
| Torsdag | 107 | Wild Type | |||
| lk | 212- | BR 5α-reductase | Reduced total plant BRs | Dwarf, thickened internodes | Reid (1986); Ross and Reid (1986); Nomura et al. (2004) |
| lka | 5865 | BR receptor | Increased total plant BRs | Dwarf, thickened internodes | Reid and Ross (1989); Nomura et al. (1997, 1999, 2003) |
| lkb | 5862 | BR C-24 reductase | Reduced total plant BRs | Dwarf, thickened internodes | Reid and Ross (1989); Nomura et al. (1997, 1999); Schultz et al. (2001) |
| ls-1 | 181 | Copalyl diphosphate synthase | Reduced total plant GAs | Dwarf | Ait-Ali et al. (1997); Yaxley et al. (2001) |
| lh-2 | 5843 | ent-Kaurene oxidase | Reduced total plant GAs | Dwarf | Yaxley et al. (2001); Davidson et al. (2004) |
| le-3 | 5839 | GA 3-oxidase | Reduced shoot GAs, wild-type root GAs | Dwarf | Ingram et al. (1984); Yaxley et al. (2001) |
| NA | 1766x1769 | Wild Type | |||
| na | 1766x1769 | ent-Kaurenoic acid oxidase | Reduced total plant GAs | Extreme dwarf | Yaxley et al. (2001); Davidson et al. (2003) |
| SLN | 250+ | Wild type | |||
| sln | 250- | GA 2-oxidase | Elevated seed GAs leading to elevated total plant GAs | Elongated internodes | Reid et al. (1992); Ross et al. (1993); Lester et al. (1999) |
RESULTS AND DISCUSSION
Nodulation Phenotypes of GA Mutants
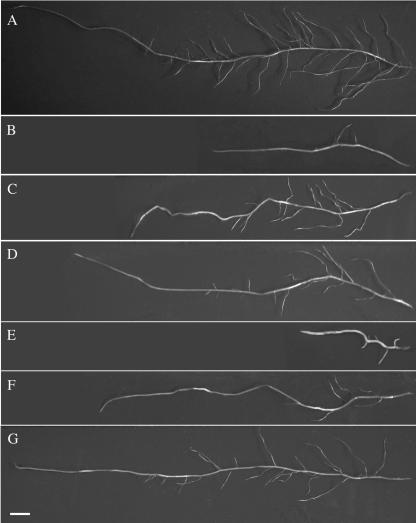
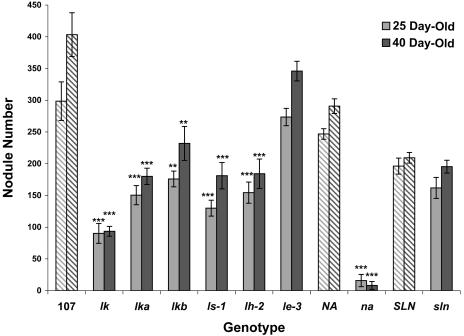
In our collection of GA-deficient mutants, na-1 causes the greatest reduction in bioactive GA1 levels in the root, followed by ls-1 and finally lh-2 (Yaxley et al., 2001). In this study, all three of these mutants developed significantly fewer nodules and significantly reduced root systems (fewer and shorter secondary and tertiary lateral roots; Fig. 1; Table II) than their wild types. The reductions in total nodule numbers were observed on a per-plant (Fig. 2) and also on a per-milligram root dry weight (DW) basis (Table III). The severity of these reductions closely paralleled the reductions in the root GA1 levels of the mutants (Yaxley et al., 2001) and strongly indicates a requirement for GAs in root and nodule initiation. Reduced root GA1 levels may affect nodule formation directly by reducing successful Rhizobium infections and nodule development. Alternatively, reductions in root GA1 levels may act indirectly by increasing the level of nodulation inhibitors, such as ethylene, and/or limiting root numbers and lengths, thereby reducing available Rhizobium infection sites. Reductions in nodule numbers were observed in both 25- and 40-d-old plants (Fig. 2), indicating that the reduced root GA1 levels are not simply delaying nodule development.
Figure 1.
Detached secondary lateral roots of 17-d-old plants of (A) wild type (Torsdag) and (B) the BR-deficient lk, (C) the BR-receptor mutant lka, (D) the BR-deficient lkb, (E) the GA1-deficient na-1, (F) the GA1-deficient ls-1, (G) and the shoot GA1-deficient le-3. The roots were collected from the most mature region of the plants, closest to the crown. The far right-hand side of the secondary lateral root is the point at which it was detached form the primary root. Bar = 1 cm.
Table II.
Root numbers and lengths of 17-d-old GA and BR mutants and their wild types
Indicated is the number of secondary lateral roots per plant in addition to the number of tertiary lateral roots per secondary lateral root, based on the average number located on the six uppermost secondary lateral roots. Also shown are the lengths of the shoot and the longest secondary and tertiary lateral roots per plant. Results are means ± se (n = 6). Values for each mutant trait followed by an * are significantly different from that of their respective wild type at the 0.01 level.
| Genotype
|
Number
|
Length
|
|||
|---|---|---|---|---|---|
| Secondary Roots | Tertiary Roots | Shoot | Secondary Roots | Tertiary Roots | |
| cm | |||||
| Torsdag | 89 ± 3.6 | 20 ± 1.1 | 12.5 ± 0.3 | 19.7 ± 0.1 | 4.3 ± 0.2 |
| lk | 50 ± 3.6* | 4 ± 0.4* | 3.0 ± 0.2* | 10.4 ± 0.3* | 2.2 ± 0.1* |
| lka | 82 ± 2.5 | 9 ± 0.8* | 5.9 ± 0.4* | 14.8 ± 0.8* | 3.5 ± 0.2* |
| lkb | 82 ± 4.1 | 13 ± 0.9* | 5.9 ± 0.3* | 16.9 ± 0.4* | 3.7 ± 0.3 |
| ls-1 | 63 ± 4.8* | 7 ± 0.6* | 2.9 ± 0.1* | 16.7 ± 0.7* | 2.2 ± 0.3* |
| lh-2 | 63 ± 4.7* | 12 ± 1.0* | 5.7 ± 0.2* | 17.2 ± 0.7* | 2.5 ± 0.2* |
| le-3 | 100 ± 1.7 | 20 ± 1.1 | 4.2 ± 0.2* | 18.5 ± 1.2 | 3.8 ± 0.3 |
| NA | 107 ± 5.2 | 21 ± 1.1 | 21.4 ± 0.5 | 18.2 ± 0.7 | 5.9 ± 0.3 |
| na | 50 ± 2.4* | 5 ± 0.4* | 2.9 ± 0.2* | 6.1 ± 0.3* | 1.1 ± 0.1* |
| SLN | 93 ± 5.4 | 13 ± 1.0 | 28.6 ± 0.7 | 19.0 ± 1.2 | 3.2 ± 0.4 |
| sln | 98 ± 1.4 | 13 ± 0.9 | 50.0 ± 3.8* | 18.6 ± 1.2 | 2.9 ± 0.3 |
Figure 2.
Nodule numbers of 25- and 40-d-old plants inoculated with R. leguminosarum. Results are means ± se (n = 8). Dashed bars represent wild types of the mutants (black bars) situated to their right. Mutant values denoted with an *, **, or *** are significantly different from that of their wild type at the 0.05, 0.01, and 0.001 level, respectively.
Table III.
Root, shoot, and nodule DWs and nodule numbers per root and shoot DW of 25-d-old GA and BR mutants and their wild types
Plants were inoculated with R. leguminosarum 5 d following the time of sowing. Results are means ± se (n = 8). Values for each mutant trait followed by an * are significantly different from that of their respective wild type at the 0.01 level.
| Genotype
|
DW
|
Number of Nodules
|
||||
|---|---|---|---|---|---|---|
| Shoot | Root | Nodule Total | Nodule Average | Per Milligram Shoot Dry Weight | Per Milligram Root Dry Weight | |
| mg | mg | mg | mg | |||
| Torsdag | 208 ± 12 | 158 ± 12 | 33.4 ± 3.8 | 0.11 ± 0.012 | 1.44 ± 0.12 | 1.95 ± 0.23 |
| lk | 142 ± 15* | 115 ± 13 | 23.5 ± 3.5 | 0.29 ± 0.030* | 0.62 ± 0.07* | 0.77 ± 0.10* |
| lka | 172 ± 13 | 173 ± 15 | 30.5 ± 2.3 | 0.21 ± 0.015* | 0.87 ± 0.04* | 0.87 ± 0.04* |
| lkb | 236 ± 11 | 202 ± 16 | 42.6 ± 4.1 | 0.24 ± 0.012* | 0.74 ± 0.03* | 0.88 ± 0.04* |
| ls-1 | 111 ± 7* | 132 ± 9 | 22.4 ± 2.1 | 0.18 ± 0.016* | 1.17 ± 0.08 | 1.03 ± 0.13* |
| lh-2 | 162 ± 7* | 159 ± 8 | 29.0 ± 3.7 | 0.19 ± 0.016* | 0.94 ± 0.07* | 0.99 ± 0.12* |
| le-3 | 203 ± 19 | 170 ± 15 | 39.7 ± 4.1 | 0.15 ± 0.013 | 1.41 ± 0.13 | 1.66 ± 0.14 |
| NA | 396 ± 24 | 197 ± 10 | 62.6 ± 4.1 | 0.25 ± 0.016 | 0.64 ± 0.04 | 1.28 ± 0.08 |
| na | 184 ± 12* | 175 ± 14 | 1.3 ± 0.7* | 0.06 ± 0.025* | 0.08 ± 0.05* | 0.09 ± 0.05* |
| SLN | 357 ± 21 | 158 ± 9 | 63.6 ± 0.4 | 0.33 ± 0.017 | 0.55 ± 0.03 | 1.27 ± 0.11 |
| sln | 392 ± 28 | 142 ± 11 | 58.1 ± 5.1 | 0.37 ± 0.023 | 0.42 ± 0.04 | 1.20 ± 0.17 |
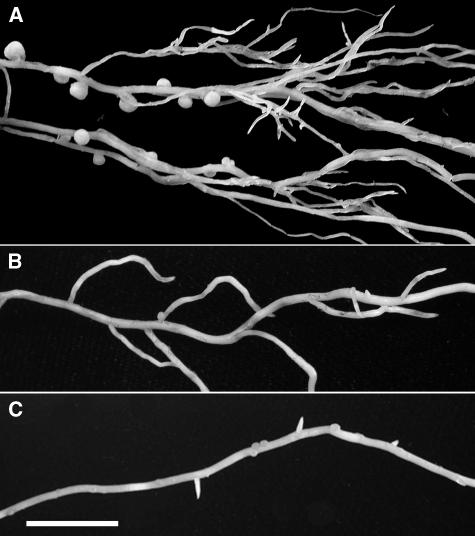
The na-1 mutant exhibited the most dramatic nodulation phenotype as few to no nodules formed (Figs. 2 and 3). Those that did form were aberrant, being small and white and resembling emerged meristems that failed to develop further (Fig. 3). Unlike the nodules observed on the other lines investigated, the few aberrant nodules of na-1 were often detected on the tertiary lateral roots of the mutant (Fig. 3B). As a consequence of their reduced size, the total DW, and average DW, of na-1 nodules were significantly reduced compared with those of its wild type (Table III). Less dramatic reductions were detected in the total nodule DWs of ls-1 and lh-2 mutant plants (Table III) compared with that of their wild type. However, although the average nodule DW was reduced in na-1, it was actually significantly elevated in ls-1 and lh-2 (Table III). Thus, it appears that GAs may also influence nodule size with slight reductions being stimulatory (ls-1 and lh-2) and large reductions inhibitory (na-1).
Figure 3.
Nodulated lateral roots of 25-d-old (A) wild-type and (B and C) na-1 plants. Wild-type nodules are large and display a white meristematic tip and a red center that represents the zone of nitrogen fixation. The few aberrant nodules that do develop on the na-1 mutant are small and white and resemble an emergent nodule meristem that failed to develop further. Bar = 1 cm.
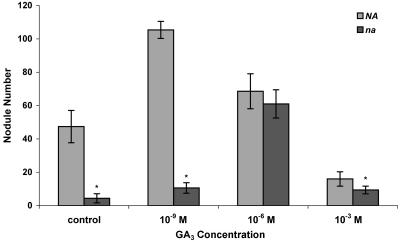
In an attempt to restore nodule numbers to that of the wild type, various concentrations of the bioactive GA3 were applied to the roots of na-1 mutants. Using this technique, concentrations of 10−6 m GA3 were found to completely restore the na-1 nodule appearance and numbers to that observed on the wild-type control (Fig. 4). This finding lends further support to our evidence that GAs are required for nodule development. Low concentrations of the hormone also stimulated nodule formation in the wild type but became inhibitory to both the wild type and the mutant as the applied concentration increased (Fig. 4). This finding is similar to that reported by Lorteau et al. (2001) for cytokinin; they found that the application of low concentrations of the phytohormone were stimulatory to pea nodule formation but became inhibitory when increased beyond a threshold level.
Figure 4.
Nodule numbers of 20-d-old wild-type and na-1 plants inoculated with R. leguminosarum and treated with various concentrations of the bioactive GA3. Results are means ± se (n = 6). Mutant values denoted with an * are significantly different from that of the wild-type control at the 0.01 level.
Grafting studies were performed using various combinations of lh-2 and its wild type (LH), Torsdag, in order to determine whether or not an LH shoot or root system could restore the reduced nodule number of the GA-deficient line (Table IV). This study revealed that either an LH root or shoot system was sufficient to restore the reduced nodule number of the mutant, both on a per-plant and a per-milligram root DW basis. This finding implies that GAs are required for nodulation. Furthermore, the root system GA level appears to play a role in nodule development, as more nodules formed on lh-2/LH grafts than on those of lh-2/lh-2 (P < 0.001), even though the shoots remained short, with a low DW (Table IV). LH/lh-2 grafts also produced more nodules than lh-2/lh-2 grafts, but it cannot be excluded that GAs were transported basipetally from the LH shoot into the mutant root system. Consistent with this suggestion is the significant promotory effect of LH shoots on the lh-2 root DW, which increased compared with that of the lh-2/lh-2 grafts (P < 0.01). Graft transmissibility of GA1 precursors (but not of GA1 itself) has been demonstrated previously (Reid et al., 1983). Interestingly, the total nodule DW was significantly reduced in grafted plants possessing an lh-2 shoot, whereas the average nodule DW was slightly increased in grafts having lh-2 roots (Table IV).
Table IV.
Root, shoot, and nodule DWs, and nodule numbers per plant and root and shoot DW of 30-d-old graft combinations of LH and lh-2 mutants
Plants were grafted 6 d after sowing and inoculated with R. leguminosarum at 10 d. Results are means ± se (n = 8). Values for each trait followed by an * are significantly different from the LH/LH graft combination at the 0.01 level.
| Graft Type
|
DW
|
Number of Nodules
|
|||||
|---|---|---|---|---|---|---|---|
| Shoot | Root | Nodule Total | Nodule Average | Per Plant | Per Milligram Shoot DW | Per Milligram Root DW | |
| mg | mg | mg | mg | ||||
| LH/LH | 259 ± 12 | 81 ± 5 | 34.4 ± 2.8 | 0.42 ± 0.053 | 87 ± 9 | 0.34 ± 0.03 | 1.07 ± 0.07 |
| LH/lh-2 | 249 ± 12 | 93 ± 6 | 31.3 ± 1.9 | 0.46 ± 0.066 | 78 ± 11 | 0.32 ± 0.05 | 0.87 ± 0.14 |
| lh-2/LH | 165 ± 17* | 81 ± 8 | 23.7 ± 3.3* | 0.32 ± 0.047 | 75 ± 6 | 0.49 ± 0.07 | 0.99 ± 0.12 |
| lh-2/lh-2 | 151 ± 12* | 62 ± 7 | 23.1 ± 2.6* | 0.53 ± 0.062 | 44 ± 4* | 0.30 ± 0.02 | 0.76 ± 0.07* |
The le-3 mutant, which has decreased shoot GA1 levels but wild-type root GA1 levels (Yaxley et al., 2001), and the sln mutant, which has elevated root and shoot GA1 levels early in development (Reid et al., 1992; Yaxley et al., 2001), both had a similar number and size of lateral roots and nodules as their wild types (Figs. 1 and 2; Tables II and III). Importantly, the normal root and nodule phenotypes of the le-3 mutant indicate that the effects of GA1 deficiency on these characteristics, as observed in na-1, ls-1, and lh-2, are not mediated by dwarfism of the shoot. Furthermore, the results with le-3 are consistent with those of the grafting experiment with lh-2 (Table IV), as neither dwarfism nor a reduced shoot GA1 level impaired the root system DW nor the nodule number of a root system having a normal level of GA1. Moreover, the wild-type level of GA1 in the le-3 root system is insufficient to rescue the shoot dwarfism of the mutant. This finding is consistent with that observed using the lh-2 grafts (Table IV).
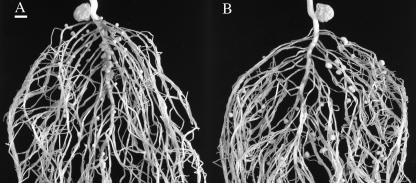
The elevated GA1 levels of sln do not appear to influence the root system or the overall number of nodules that form per plant (Figs. 1 and 2; Tables II and III). Despite these findings, high GA1 levels may actually be inhibitory to nodule organogenesis. The source of the elevated GAs of sln is the seed (Ross et al., 1993). As the sln seedling develops, this excess GA is mobilized throughout the plant until it is eventually metabolized and maintained at near SLN levels (Ross et al., 1993). By this time, the primary roots of both SLN and sln are well established and appear similar. However, although numerous nodules formed on the primary roots of SLN plants, no nodules developed on the primary roots of sln mutants (Fig. 5). This may suggest that the elevated GA levels of the mutant prevented nodules from establishing, which is consistent with the finding that treatment with high concentrations of GA3 reduced the number of nodules that formed on wild-type plants (Fig. 4). This inhibition in sln is temporary, as nodulation was not prevented on lateral roots, of which many formed following the metabolism of the majority of the excess GA1. Elevated GA1 levels might act directly to inhibit the infection process or nodule development, or indirectly, by affecting assimilate distribution.
Figure 5.
Nodulated root systems of the 25-d-old (A) wild type, SLN, and (B) the GA1-overproducing, sln. SLN, like the other wild-type lines investigated here, formed many nodules on both primary and secondary roots, whereas sln only developed nodules on secondary roots. Bar = 1 cm.
Nodulation Phenotypes of BR Mutants
In our collection of BR mutants, lk has the most severe reduction in bioactive BRs in the shoot (Nomura et al., 2004), followed by lkb (Nomura et al., 1997). A reduction in BR levels in the roots has also been confirmed for lkb (Symons and Reid, 2004). Here, we demonstrate that, in addition to shoot dwarfism, the BR synthesis mutants lk and lkb and the BR response mutant lka also have fewer and shorter lateral roots (Fig. 1; Table II). These findings support recent reports that BRs have a role in lateral root development (Bao et al., 2004). Interestingly, despite all three BR mutants producing fewer and shorter lateral roots (Fig. 1; Table II), only the lk root system DW was significantly reduced compared with that of Torsdag (Table III).
Nodule numbers were reduced in all three BR mutants compared with that of Torsdag. These reductions occurred in both 25- and 40-d-old plants, indicating that nodule development was not delayed, but rather diminished, as was observed with the GA1-deficient mutants (Fig. 2). The nodule numbers were also reduced on a per-milligram root DW basis (Table III), indicating that the reductions were not simply correlated with the size of the root systems. Instead, these diminished nodule numbers might be caused by reduced BR levels, or perception, directly or indirectly effecting nodule development, as is discussed above for mutants having reduced root GA1 levels.
The average nodule DW was significantly increased for all of the BR mutants, compared with that of Torsdag (Table III). Thus, in the case of lk, although the root system DW decreased, the average nodule DW increased. This finding illustrates that nodule size is not simply a reflection of root system DW. Interestingly, with the exception of the severely reduced na-1, reductions in root GA1 levels also resulted in increased nodule DWs. Producing large nodules may be a compensatory mechanism to increase nitrogen fixation in response to reduced nodule numbers.
Recently, BRs were shown to be relatively immobile within pea (Symons and Reid, 2004). For this reason, BR application studies similar to that performed using GA3 and na-1 were not considered to be the best method to investigate nodulation here. In addition, a BR mutant similar to that of le-3 having normal BR levels in the root, but decreased levels in the shoot, is not available. As a result, grafting studies involving lkb and its wild type (LKB), Torsdag, were the only method available to examine the effects of decreased root and shoot BR levels on nodulation. Results from these studies illustrate that the shoot controlled the number of nodules that formed in these graft combinations (Table V). This finding contrasted with that observed with the lh-2 graft combinations (Table IV). Grafted plants having an lkb shoot developed fewer nodules than those having an LKB shoot on a per-plant, as well as per-milligram root DW basis (Table V). In addition, the root and shoot DWs of grafted plants with an lkb shoot were not significantly reduced from those with an LKB shoot (Table V). This indicates that the reduced nodule numbers on grafted plants having an lkb shoot were not simply the result of a smaller root or shoot system. Instead, our findings suggest that BRs may be influencing a nodulation mechanism of the shoot that is involved in regulating the nodule numbers of the root. One such mechanism known to exist in the shoot involves the receptor kinase HAR-1/SYM29/NARK (e.g. Wopereis et al., 2000; for review, see Oldroyd and Downie, 2004). To date, it is unknown what effects, if any, BRs have on this receptor; however, the mutants examined in this report appear to be excellent candidates for investigating this potential relationship.
Table V.
Root, shoot, and nodule DWs, nodule numbers per plant, and root and shoot DW and root levels of IAA and GA1 of 30-d-old graft combinations of LKB and lkb mutants.
Plants were grafted 6 d after sowing and inoculated with R. leguminosarum at 10 d. Results are means ± se (n = 8) for physiological traits and means ± se of two replicates, each consisting of six root systems, for hormone analysis. Values for each trait followed by an * are significantly different from the LKB/LKB graft combination at the 0.01 level.
| Graft Type
|
DW
|
Number of Nodules
|
Hormone Level
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Nodule Total | Nodule Average | Per Plant | Per Milligram Shoot DW | Per Milligram Root DW | IAA | GA1 | |
| mg | mg | mg | mg | ng g−1fresh weight | ng g−1fresh weight | ||||
| LKB/LKB | 410 ± 35 | 130 ± 15 | 65 ± 9.5 | 0.57 ± 0.085 | 136 ± 26 | 0.35 ± 0.03 | 1.09 ± 0.20 | 3.93 ± 0.69 | 0.022 ± 0.0005 |
| LKB/lkb | 420 ± 25 | 180 ± 23 | 75 ± 11.5 | 0.83 ± 0.252 | 129 ± 26 | 0.34 ± 0.09 | 0.94 ± 0.36 | 3.23 ± 0.32 | 0.020 ± 0.0020 |
| lkb/LKB | 310 ± 39 | 120 ± 12 | 48 ± 9.5 | 0.98 ± 0.239 | 56 ± 5* | 0.20 ± 0.03 | 0.48 ± 0.06* | 3.08 ± 0.06 | 0.020 ± 0.0025 |
| lkb/lkb | 430 ± 43 | 200 ± 15* | 69 ± 10.6 | 1.58 ± 0.236* | 46 ± 7* | 0.11 ± 0.01* | 0.23 ± 0.03* | 3.16 ± 0.19 | 0.024 ± 0.0005 |
Recently, Symons and Reid (2004) demonstrated that BRs are not graft-transmissible. Thus, the level of BRs in an lkb root system would be reduced compared with that of LKB, even if grafted to an LKB shoot. Therefore, the increased number of nodules observed on lkb roots grafted to an LKB shoot cannot be explained by an increase in root BRs. In addition, despite having normal levels of BRs, LKB root systems grafted to an lkb shoot produced fewer nodules compared with those grafted to an LKB shoot. Together, these findings indicate that the root level of BRs does not have a direct effect on nodule numbers. Based on these results, we investigated whether or not shoot BRs regulate root and nodule development by altering the levels of other hormones in the roots. For example, our findings with the GA mutants indicate a role for GA in the development of roots and nodules. In addition, the phytohormone auxin is known to have a prominent role in both root and nodule development (Ferguson and Mathesius, 2003) and is produced at high levels in the shoot, followed by a reported acropetal transport to the root system. Thus, we measured the levels of GA1 and the auxin, indole acetic acid (IAA), in the root systems of the various Torsdag and lkb graft combinations. This revealed that the levels of both GA1 and IAA were similar among all of the graft combinations (Table V), demonstrating that the reduced BR levels of lkb do not alter the root GA1 or IAA levels. Therefore, the reductions in root and nodule numbers of the BR mutants do not appear to be attributed to changes in the root levels of GA1 or IAA.
Correlation between Root and Nodule Formation
A correlation between the number of nodules and the number of lateral roots was detected across all of the mutant and wild-type lines examined (Fig. 6). Correlations between nodule and lateral root numbers were first described by Nutman (1948) who noted that the more lateral roots a line of red clover developed the more nodules it formed. These findings indicate that a strong correlation between nodule and root formation exists and may suggest that roots utilize an autoregulatory mechanism similar to that identified in nodulation (e.g. Caetano-Anollés and Gresshoff, 1991). Consistent with this suggestion is the observation that the hypernodulating mutant of L. japonicus, har-1, exhibits stimulated root initiation when grown in the absence of Mesorhizobium loti (Wopereis et al., 2000).
Figure 6.
The correlation between the average number of tertiary lateral roots observed on the oldest six secondary lateral roots of 17-d-old plants and the number of nodules of 25-d-old plants inoculated with R. leguminosarum. Results are means ± se for the nodule number (n = 6–8).
It has been postulated that nodulation evolved from preexisting mechanisms of early lateral root development (Hirsch and LaRue, 1997; Mathesius, 2003). This theory is supported by root-nodule hybrids that have been observed on roots of Medicago sativa (Dudley et al., 1987) and Trifolium repens (McIver et al., 1997) following inoculation with specific Rhizobium strains. Roots also emerge from apical meristems of actinorhizal nodules of Casuarina cunninghamiana (Torrey, 1976) and Myrica gale (Torrey and Callaham, 1978). The nodule apex can also be converted into a root apex by adjusting growing temperatures from low to high (see refs. in Dart, 1977). Moreover, mycorrhizal nodules develop on Podocarpaceae species, even in sterile soil free of the fungus (Russell et al., 2002). These structures are not simply lateral roots modified by the endosymbiont, but rather novel outgrowths that have diverged from the root developmental pathway prior to their emergence.
Lateral roots and nodules share many aspects of their development. For example, they are both derived via postembryonic mechanisms involving dedifferentiating and dividing cells adjacent to xylem poles (Mathesius, 2003). One proposed difference in their development is the site of initial cellular divisions; the pericycle for roots and the cortex for nodules. However, peanut nodules originate predominately from the pericycle (Allen and Allen, 1940), and pericycle divisions do occur during nodule development of pea (Bond, 1948) and T. repens (McIver et al., 1997). In addition, nonleguminous Actinorhizal nodules, myconodules, and Parasponia nodules are all derived from the pericycle (see refs. in Hirsch and LaRue, 1997). Moreover, ENOD40, a signal thought to be involved in cell division, is expressed in the pericycle of M. sativa prior to nodule primordium initiation (Compaan et al., 2001). Furthermore, Kawaguchi et al. (1996) demonstrated that bioactive GAs induce pericycle divisions leading to nodule-like structures in L. japonicus. These structures were free of central vascular cells and were therefore not simply deformed lateral roots. Collectively, these findings point to a role for the pericycle in nodulation, possibly including cell divisions as are known to occur in lateral root development (e.g. Dubrovsky et al., 2000). The involvement of the pericycle may be mediated by hormones, which may explain why parallel declines in nodule and root numbers were observed in our mutants that have hormone-deficient root systems. Transcript profiling of early lateral root initiation in Arabidopsis (Arabidopsis thaliana) has detected numerous genes expressed in the pericycle (Himanen et al., 2004). Perhaps a similar investigation into the pericycle using a legume species, with and without Rhizobium inoculation, would help discriminate between gene products shared by, and unique to, root and nodule initiation.
Correlations between nodulation and the remaining characteristics measured were not observed. For example, there was no correlation between shoot stature and nodulation, as sln was taller than its wild type and le-3 was shorter, but they both produced wild-type numbers of nodules (Fig. 2). Also, there is no correlation between the rate of leaf expansion and nodulation because, when compared with their wild types, GA deficient mutants had fewer leaves, whereas BR mutants had more (data not shown), yet both formed fewer nodules (Fig. 2). Shoot and root DW also did not form a correlation with nodulation. The DW of lh-2 shoots was similar to that of le-3 (Table III), but lh-2 formed significantly fewer nodules than le-3 (Fig. 2). In addition, the BR mutants all formed significantly fewer nodules than Torsdag (Fig. 2), despite of no consistent differences in their root system DWs compared with Torsdag (Table III). Furthermore, the length of secondary lateral roots does not appear to be the limiting factor of the development of tertiary lateral roots and nodules. For example, lkb and ls-1 secondary lateral roots are similar in length (Fig. 1; Table II), but ls-1 developed fewer tertiary lateral roots (Fig. 1; Table II) and nodules (Fig. 2) than lkb.
CONCLUSIONS
The results presented here illustrate that reduced root levels of GAs significantly decrease the number of nodules in pea (Fig. 2). These decreases in nodule numbers were observed at both 25 and 40 d, indicating that they were not simply the result of a delay in nodule formation. The application of GA3 restored the nodule number of na-1, suggesting a direct role for GAs in nodule development. In addition, grafting experiments illustrated that normal GA1 levels in the root are sufficient to elicit the formation of a normal number of nodules. In contrast, BRs do not have a direct effect on nodule numbers, but act to influence a shoot mechanism involved in regulating nodule numbers. Interestingly, with the exception of the severely inhibited na-1, significant increases in the average nodule DW were found on all GA and BR mutants having reduced nodule numbers (Table III). This might suggest the existence of a mechanism that compensates for changes in nodule numbers by regulating the size of individual nodules. Taken together, our findings support the theory proposed by Libbenga et al. (1973) that a delicate balance in hormone levels is required to achieve optimum nodule development. This theory is further supported by our finding that GAs, in addition to cytokinins (Lorteau et al., 2001), are stimulatory to pea nodule formation at low concentrations but inhibitory when increased beyond a threshold level.
Reductions in root GA and BR levels also diminished lateral root numbers and lengths (Yaxley et al., 2001; Table II). Interestingly, this appears to be opposite to the effects of cytokinins, which reportedly inhibit nodulation but stimulate lateral root development (Lohar et al., 2004). It is likely that hormones have multiple roles in root and nodule development (Ferguson and Mathesius, 2003) and are required to different degrees at various stages of development. Overall, mutants have proven to be valuable tools for understanding the processes of root and nodule development and for isolating genes relating to these processes. In pea, an extensive collection of nodulation mutants has been assembled (Borisov et al., 2000), but there remains a need for additional root mutants, which would aid in determining the developmental aspects that are shared in, and are unique to, the root-nodule relationship.
MATERIALS AND METHODS
Plant Growing Conditions
An overview of the various plant lines used in this report, including any mutated genes and their resulting effects on the plant, is provided in Table I. For nodulation studies, plants were sown one per pot in 100-mm Space Saver pots (Reko, Australia) and for root analysis experiments, seeds were sown seven per pot in 200-mm Plastamatic pots (Melbourne, Australia). All pots contained a 1:1 mixture of grade 3 vermiculite (Australian Vermiculite and Perlite, Fairfield, Australia) and 10 mm dolerite aggregate (HBMI, Kingston, Australia). This mixture was topped with approximately 2 cm of a pasteurized peat/sand potting mix composed of a 1:1 mixture of peat moss (Te - Em, New Brunswick, Canada) and coarse river sand (Island Resources, Scottsdale, Australia). Pasteurization was achieved using a steam/air mix at 70°C for 45 min. The pH was adjusted to 7.0 with dolomite lime and limestone.
Plants were grown in a controlled environment glasshouse with temperatures maintained at 20°C day (18 h) and 15°C night (6 h) ± 1°C. Relative humidity was maintained at a minimum of 40%. The photoperiod of 18 h consisted of natural daylight supplemented and extended morning and evening by 4 GE (Hungary) Lucagrow LU400/HO High Pressure Sodium 400 W globes and 2 incandescent globes (60 W Pearl, Thorn, Australia) delivering an additional approximately 150 μmol photons m−2 s−1 at the pot surface.
Plants were placed on capillary mats (Bottom Up Irrigation, Fertool Distributors, Hallam, Australia) and watered using an automated overhead sprinkling system (70 lines per hour at 150 kPa) for 2 min each morning and evening. For nodule count studies, each pot was provided with 25 mL of Rhizobium leguminosarum bv viciae 128C53K (Nitragin Inoculants, Liphatech, Milwaukee, WI) grown in yeast-mannitol broth and diluted with water to approximately OD600 0.01, which represents 5 × 106 cells mL−1. Based on a previous experiment, inoculation was delayed in these studies until 5 d after planting to maximize nodulation. For root characterization experiments, at the time of sowing, 150 mL of the bacterial solution was applied. Plants grown in excess of 25 d were also provided with a modified Hoagland solution containing only 1 mm 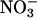 to prevent the inhibition of nodulation.
to prevent the inhibition of nodulation.
Nodule Count Studies
Investigation of Mutant and Wild-Type Lines
Plants were harvested 25 d after planting. This timing allowed nodules to develop to a stage where they could be clearly distinguished and their appearance accurately assessed. For each plant, the number of nodes was recorded, counting the cotyledon as node zero. The roots and shoots were separated at the cotyledon, which was excised and discarded. The root system was gently rinsed clean of potting substrates and placed in a tray of water. Nodules were counted, removed with forceps and, together with the roots and shoots, placed in a 60°C oven for a minimum of 3 d to obtain their DWs.
Additional plants were allowed to persist until 40 d after planting, coinciding with the flowering time of many of the lines, including wild types. The same traits examined using 25-d-old plants were then assessed. By 40 d, the formation of new nodule structures should be minimal due to the plants' autoregulation of nodulation (Caetano-Anollés and Gresshoff, 1991). Thus, assessing the number of nodules at this age confirms that the numbers determined at 25 d have remained relatively stable and are not increasing indefinitely with age. This approach helps verify that autoregulation of nodulation is functional and provides confirmation of a reduction, as opposed to a delay, in nodule development.
GA Treatments
The effect of GA on nodule formation was examined using the GA-deficient na-1 and its wild type (Table I). Seeds of the two lines were sown according to the methods used for the root characterization experiments (described above). The roots of the seedlings were treated with 150 mL of either water (control) or various concentrations (10−9, 10−6, or 10−3 M) of the bioactive GA3. These treatments commenced 3 d after planting and continuing twice per week until harvest. The plants were harvested 20 d after planting, rinsed clean of soil substrates and their nodules counted.
Grafting Experiments
For grafting experiments, seeds of Torsdag and lkb, or lh-2 (Table I) were sown as detailed above for the nodule count investigation. At 6 d after planting, the seedlings were grafted using the methods of Reid et al. (1983). These mutants were chosen because of their common background (i.e. Torsdag; Table I) and their relative similarity in terms of both shoot stature and nodule numbers (Table III). At 10 d after planting, the plants were inoculated with 25 mL of the bacteria, thus allowing the grafts to establish prior to inoculation. The graft combinations were then scored 30 d after planting.
Analysis of Root Characteristics
Plants were harvested 17 d after planting, allowing for the development of secondary and tertiary lateral roots. The plants were uprooted, gently cleaned in water, and placed in a tray of water. The length of the shoot and the longest secondary and tertiary lateral root was measured. The total number of nodes and secondary lateral roots were recorded. In addition, the number of tertiary lateral roots located on each of the upper (i.e. closest to the crown) six secondary lateral roots was counted.
Hormone Analysis
The roots of 30-d-old grafted plants were cleaned of soil, separated from their shoots and cotyledons, and weighed. IAA and GA1 were then extracted from these root systems, and their levels quantified, using the methods outlined in Ross (1998). Two replicates, consisting of six root systems per replicate, were analyzed.
Acknowledgments
We thank Ian Cummings, Tracy Jackson, Nathan Hoeme, Tiernan O'Rourke, and Noel Davies for technical assistance.
This work was supported by the Australian Research Council, by the Centre of Excellence for Integrative Legume Research (to B.J.F.), by the Thomas Crawford Memorial Scholarship, and by the Tasmanian International Research Scholarship.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.062414.
References
- Ait-Ali T, Swain SM, Reid JB, Sun T-P, Kamiya Y (1997) The Ls locus of pea encodes the gibberellin biosynthesis enzyme ent-kaurene synthase A. Plant J 11: 443–454 [DOI] [PubMed] [Google Scholar]
- Allen ON, Allen EK (1940) Response of the peanut plant to inoculation with rhizobia, with special reference to morphological development of the nodules. Bot Gaz 102: 121–142 [Google Scholar]
- Bao F, Shen J, Brady SR, Muday GK, Asami T, Yang Z (2004) Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol 134: 1624–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond L (1948) Origin and developmental morphology of root nodules of Pisum sativum. Bot Gaz 109: 411–434 [Google Scholar]
- Borisov AY, Barmicheva EM, Jacobi LM, Tsyganov VE, Voroshilova VA, Tikhonovich IA (2000) Pea (Pisum sativum L.) Mendelian genetics controlling development of nitrogen-fixing nodules and arbuscular mycorrhiza. Czech J Gen Plant Breed 36: 106–110 [Google Scholar]
- Caetano-Anollés G, Gresshoff PM (1991) Plant genetic control of nodulation. Annu Rev Microbiol 45: 345–382 [DOI] [PubMed] [Google Scholar]
- Compaan B, Yang WC, Bisseling T, Franssen H (2001) ENOD40 expression in the pericycle precedes cortical cell division in Rhizobium-legume interaction and the highly conserved internal region of the gene does not encode a peptide. Plant Soil 230: 1–8 [Google Scholar]
- Dart PJ (1977) Infection and development of leguminous nodules. In RWF Hardy, ed, A Treatise on Dinitrogen Fixation. Wiley, New York, pp 367–472
- Davidson SE, Elliott RC, Helliwell CA, Poole AT, Reid JB (2003) The pea gene NA encodes ent-kaurenoic acid oxidase. Plant Physiol 131: 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson SE, Smith JJ, Helliwell CA, Poole AT, Reid JB (2004) The pea gene LH encodes ent-kaurene oxidase. Plant Physiol 134: 1123–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Doerner PW, Coln-Carmona A, Rost TL (2000) Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiol 124: 1648–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Jacobs TW, Long SL (1987) Microscopic studies of cell divisions induced in alfalfa roots by Rhizobium meliloti. Planta 171: 289–301 [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Mathesius U (2003) Signaling interactions during nodule development. J Plant Growth Regul 22: 47–72 [Google Scholar]
- Himanen K, Vuylsteke M, Vanneste S, Vercruysse S, Boucheron E, Alard P, Chriqui D, Montagu MV, Inzé D, Beeckman T (2004) Transcript profiling of early lateral root initiation. Proc Natl Acad Sci USA 101: 5146–5151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AM, LaRue TA (1997) Is the legume nodule a modified root or stem or an organ sui generis? Crit Rev Plant Sci 16: 361–392 [Google Scholar]
- Ingram TJ, Reid JB, Murfet IC, Gaskin P, Willis CL, MacMillan J (1984) Internode length in Pisum: the Le gene controls the 3β-hydroxylation of gibberellin A20 to gibberellin A1. Planta 83: 1048–1053 [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Imaizumi-Anraku H, Fukai S, Syono K (1996) Unusual branching in the seedlings of Lotus japonicus: gibberellins reveal the nitrogen-sensitive cell divisions within the pericycle on roots. Plant Cell Physiol 37: 461–470 [Google Scholar]
- Lester DR, Ross JJ, Smith JJ, Elliott RC, Reid JB (1999) Gibberellin 2-oxidation and the SLN gene of Pisum sativum. Plant J 19: 65–73 [DOI] [PubMed] [Google Scholar]
- Libbenga KR, van Iren F, Bogers RJ, Schraag-Lamers MF (1973) The role of hormones and gradients in the initiation of cortex proliferation and nodule formation in Pisum sativum L. Planta 114: 29–39 [DOI] [PubMed] [Google Scholar]
- Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DM (2004) Cytokinins play opposite roles in lateral root formation, and nematode and Rhizobial symbiosis. Plant J 38: 203–214 [DOI] [PubMed] [Google Scholar]
- Lorteau M-A, Ferguson BJ, Guinel FC (2001) Effects of cytokinin on ethylene production and nodulation in pea (Pisum sativum) cv. Sparkle. Physiol Plant 112: 421–428 [DOI] [PubMed] [Google Scholar]
- Mathesius U (2003) Conservation and divergence of signalling pathways between roots and soil microbes: the Rhizobium-legume symbiosis compared to the development of lateral roots, mycorrhizal interactions and nematode-induced galls. Plant Soil 255: 105–119 [Google Scholar]
- McIver J, Djordjevic MA, Weinman JJ, Rolfe BG (1997) Influence of Rhizobium leguminosarum biovar trifolii host specific nodulation genes on the ontogeny of clover nodulation. Protoplasma 172: 166–179 [Google Scholar]
- Mylona P, Pawlowski K, Bisseling T (1995) Symbiotic nitrogen fixation. Plant Cell 7: 869–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Bishop GJ, Kaneta T, Reid JB, Chory J, Yokota T (2003) The LKA gene is a BRASSINOSTEROID INSENSITIVE 1 homolog of pea. Plant J 36: 291–300 [DOI] [PubMed] [Google Scholar]
- Nomura T, Jager CE, Kitasaka Y, Takeuchi K, Fukami M, Yoneyama K, Matsushita Y, Nyunoya H, Takatsuto S, Fujioka S, Smith JJ, et al (2004) Brassinosteroid deficiency due to truncated steroid 5α-reductase causes dwarfism in the lk mutant of pea. Plant Physiol 135: 2220–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Kitasaka Y, Takatsuto S, Reid JB, Fukami M, Yokota T (1999) Brassinosteroid/sterol synthesis and plant growth as affected by lka and lkb mutations of pea. Plant Physiol 119: 1517–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T (1997) Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiol 113: 31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutman PS (1948) Physiological studies on nodule formation. I. The relationship between nodulation and lateral root formation in red clover. Ann Bot (Lond) 12: 81–96 [Google Scholar]
- Oldroyd GE, Downie JA (2004) Calcium, kinases and nodulation signalling in legumes. Nat Rev Mol Cell Biol 5: 566–576 [DOI] [PubMed] [Google Scholar]
- Reid JB (1986) Internode length in Pisum. Three further loci, lh, ls and lk. Ann Bot (Lond) 57: 577–592 [Google Scholar]
- Reid JB, Murfet IC, Potts WC (1983) Internode length in Pisum. II. Additional information on the relationship and action of loci Le, La, Cry, Na and Lm. J Exp Bot 34: 349–364 [Google Scholar]
- Reid JB, Ross JJ (1989) Internode length in Pisum. Two further gibberellin insensitivity genes lka and lkb. Physiol Plant 75: 81–88 [Google Scholar]
- Reid JB, Ross JJ, Swain SM (1992) Internode length in Pisum: a new, slender mutant with elevated levels of C19 gibberellins. Planta 188: 462–467 [DOI] [PubMed] [Google Scholar]
- Reid JB, Symons GM, Ross JJ (2004) Regulation of gibberellin and brassinosteroid biosynthesis by genetic, environmental and hormonal factors. In PJ Davis, ed, Plant Hormones: Biosynthesis, Signal Transduction, Action! Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 179–203
- Ross JJ (1998) Effects of auxin transport inhibitors on gibberellins in pea. J Plant Growth Regul 17: 141–146 [Google Scholar]
- Ross JJ, Reid JB (1986) Internode length in Pisum: the involvement of ethylene with the gibberellin-insensitive erectoides phenotype. Physiol Plant 67: 673–679 [Google Scholar]
- Ross JJ, Reid JB, Swain SM (1993) Control of stem elongation by gibberellin A1: evidence from genetic studies including the slender mutant, sln. Aust J Plant Physiol 20: 585–599 [Google Scholar]
- Russell AJ, Bidartondo MI, Butterfield BG (2002) The root nodules of the Podocarpaceae harbour arbuscular mycorrhizal fungi. New Phytol 156: 283–295 [DOI] [PubMed] [Google Scholar]
- Schultz L, Kerckhoffs LHJ, Klahre U, Yokota T, Reid JB (2001) Molecular characterization of the brassinosteroid-deficient lkb mutant in pea. Plant Mol Biol 47: 491–498 [DOI] [PubMed] [Google Scholar]
- Symons GM, Reid JB (2004) Brassinosteroids do not undergo long-distance transport in pea. Implications for the regulation of endogenous brassinosteroid levels. Plant Physiol 135: 2196–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey JG (1976) Initiation and development of root nodules of Casuarina (Casuarinaceae). Am J Bot 63: 335–344 [Google Scholar]
- Torrey JG, Callaham D (1978) Determinate development of nodule roots in actinomycete-induced root nodules of Myrica gale L. Can J Bot 56: 1357–1364 [Google Scholar]
- Wopereis J, Pajuelo E, Dazzo FB, Jiang Q, Gresshoff PM, de Bruijn FJ, Stougaard J, Szczyglowski K (2000) Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J 23: 97–114 [DOI] [PubMed] [Google Scholar]
- Yaxley JR, Ross JJ, Sherriff LJ, Reid JB (2001) Gibberellin biosynthesis mutations and root development in pea. Plant Physiol 125: 627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]