Abstract
PKCζ (protein kinase Cζ) is a serine/threonine protein kinase controlled by insulin, various growth factors and phosphoinositide 3-kinase. It has been implicated in controlling glucose transport in response to insulin by the translocation of GLUT4-(glucose transporter 4) containing vesicles to the plasma membrane in stimulated cells. How PKCζ modulates GLUT4 vesicle trafficking remains unknown. A yeast two-hybrid screen using full-length human PKCζ identified 80K-H protein as an interactor with PKCζ. GST (glutathione S-transferase) pull-down assays with GST-tagged 80K-H constructs confirmed the interaction and showed that the N-terminal portion of 80K-H was not required for the interaction. Immunoprecipitates of endogenous PKCζ from Cho cells, 3T3-L1 adipocytes or L6 myotubes contained endogenous 80K-H, demonstrating a physiological interaction. Insulin stimulation enhanced the association 3–5-fold. Immunoprecipitates of endogenous 80K-H contained endogenous munc18c and immunoprecipitates of endogenous munc18c contained endogenous PKCζ, with insulin markedly increasing the amount of co-immunoprecipitated protein in each case. These results show that insulin triggers interactions in vivo between PKCζ, 80K-H and munc18c. Overexpression of 80K-H constructs mimicked the action of insulin in stimulating both glucose uptake and translocation of Myc-tagged GLUT4 in Cho cells, with the level of effect proportional to the ability of the constructs to associate with munc18c. These results identify 80K-H as a new player involved in GLUT4 vesicle transport and identify a link between a kinase involved in the insulin signalling cascade, PKCζ, and a known component of the GLUT4 vesicle trafficking pathway, munc18c. The results suggest a model whereby insulin triggers the formation of a PKCζ–80K-H–munc18c complex that enhances GLUT4 translocation to the plasma membrane.
Keywords: glucose uptake, GLUT4, insulin, 80K-H, protein kinase Cζ, vesicle trafficking
Abbreviations: FL, full length; GLUT, glucose transporter; GST, glutathione S-transferase; HA, haemagglutinin; LDM, low-density microsome; PKC, protein kinase C; VAMP-2, vesicle-associated membrane protein 2
INTRODUCTION
PKCζ (protein kinase Cζ) is a 67 kDa serine/threonine kinase belonging to the atypical PKC subfamily. PKCζ has been implicated in the control of various signalling pathways [1]. PKCζ can be activated by insulin and various mitogens through the activation of phosphoinositide 3-kinase. Various studies have shown a clear role for PKCζ and its close relative PKCλ in insulin-stimulated glucose transport [2–4] and glucose transport through the pyk2 receptor [5]. Insulin stimulates glucose transport into the cell by translocation of a membrane vesicle containing GLUT4 (glucose transporter 4) to the plasma membrane. This regulated vesicle trafficking shares similar properties to synaptic vesicle trafficking [6–8]. Despite the discovery of several proteins that co-ordinate docking and fusion of the GLUT4 vesicle to the plasma membrane, there has been no direct link established with the signalling cascade initiated by insulin.
Atypical PKCs are known to mediate signalling responses either by phosphorylating targets or by binding other proteins. The atypical PKC V1 sequence in the regulatory domain and other domains of PKCζ are clearly important in binding proteins that regulate apoptosis and cell polarity such as p62, Par-4, Par-6 and ASIP [32]. The failure to discover relevant phosphorylated substrates for the atypical PKCs increases the prospect that enhancement of glucose transport involves scaffolding links.
In the present study, we identified 80K-H protein as an interactor of PKCζ. 80K-H is related to VASAP-60, which is involved in vesicle trafficking events [9]. 80K-H is widely expressed, and present at significant levels in the major insulin-sensitive tissues including fat and muscle cells ([23] and results herein). Therefore we investigated whether 80K-H had any role in GLUT4 vesicle transport. Using a model system, we were able to identify that overexpression of 80K-H fragments enhanced GLUT4 vesicle trafficking to the plasma membrane. Moreover, 80K-H interacts with both PKCζ and munc18c in an insulin-controlled fashion. The results indicate that insulin triggers the formation of a PKCζ–80K-H–munc18c complex that may free syntaxin-4 and VAMP-2 (vesicle-associated membrane protein 2) to interact.
EXPERIMENTAL
Yeast two-hybrid system
The LexA yeast two-hybrid system was purchased from ClonTech Laboratories (Basingstoke, U.K.). The human matchmaker brain cDNA library was also from ClonTech Laboratories. Full-length human PKCζ was subcloned from the pcDNA3 vector, as described by Hodgkinson et al. [10]. The PKCζ was ligated into EcoRI–XhoI-digested pLexA to generate an in-frame fusion with the LexA-binding domain, confirmed by sequencing and expression of the full-length protein in yeast. The EGY48[p8opLacZ] yeast strain was first transformed with pLexA-PKCζ, tested for expression of the hybrid protein by Western blotting using the LexA antibody (ClonTech Laboratories). Subsequently, the EGY48[p8opLacZ, pLexA–PKCζ] was transformed into the pB42AD–brain cDNA library (100 μg) and plated on to SD/dex/kan/-his/-trp/-ura. After three days of growth, the transformants were collected and 5×106 cells were plated on to SD/gal/raf/kan/-his/-trp/-ura/-leu selection medium plates supplemented with X-gal (80 μg/ml). Leu+LacZ+ colonies were collected over a period of three days. Library plasmids were rescued by transformation of KC8 bacteria grown on M9TrpDOAmp plates. Putative interacting library plasmids were reintroduced into EGY48[p8opLacZ, pLexA-PKCζ] as the positive control and [pLexA–laminin, pLexA–p53] as negative controls with the selection on SD/gal/raf/kan/-his/-trp/-ura/-leu/Xgal plates.
Plasmid constructs
A full-length 80K-H clone, expressing the full 400 amino acids of the protein, was isolated by yeast two-hybrid screen. This was subcloned from pB42AD in pGex-5X-1. Deletions of the 80K-H protein were first expressed as GST (glutathione S-transferase) fusions. Using the pGex-5X-1 full-length 80K-H construct as template, the deletions were amplified using Pfu DNA polymerase. The forward primer was sequence-specific and contained an EcoRI site, the reverse primer was to the pGex plasmid and was 3′ of the XhoI site (5′-CCGTCATCATCTAGACGCGCGA-3′). EcoRI–XhoI digestion of the PCR fragments facilitated in-frame cloning into pGex-5X-1. Forward primers were: 80K-H 172 5′-TGACGGAATTCAAGGAGGAGCAGCCGCCA-3′; 80K-H 248 5′-CGCAACGAATTCGAGGAGGCCGAGCGGTC-3′; 80K-H 331 5′-CCACGACGAATTCAGTGCCATGAAGTATGA-3′; 80K-H 360 5′-GCGGGAAAGAATTCATGGTGACCAGCACC-3′; 80K-H 390 5′-CCGCCTGAATTCCCCACCGAAGACCAT-3′. The EcoRI site is underlined, the number after the 80K-H refers to the first amino acid of the 80K-H protein following the EcoRI site, thus 80K-H 172 represents an 80K-H truncated protein encompassing amino acid residues 172–400. The full-length 80K-H, 80K-H 172 and 80K-H 248 were subsequently subcloned into pcDNA4HisMax-C (Invitrogen), inframe with the 6×His and Xpress epitopes. GLUT4 was amplified from a mouse clone supplied by Invitrogen (Clone ID 4207674, accession number BC014282), which contained the full-length GLUT4 sequence. The full-length GLUT4 was amplified from the plasmid by Pfu DNA polymerase using the primers 5′-ATGCGGTCGGGTTTCCAG-3′ and 5′-TCAGTCATTCACATCTGGC-3′. The PCR product was further amplified by Pfu DNA polymerase using the primers 5′-GCATGGAATTCATGCCGTCGGGTTTCCAG-3′ and 5′-GCTAGCTCGAGTCAGTCATTCACATCTGGC-3′. Digestion of this PCR product with EcoRI and XhoI allowed ligation into pcDNAmyc vector [10,11], generating a GLUT4–Myc fusion. HA (haemagglutinin)-tagged PKCζ has been described previously [10,11].
Transient cell transfection in cos-1 cells of HA-tagged PKCζ constructs
Exponentially growing cos-1 cells [10,11] (2×106) were transiently transfected with plasmid combinations as listed in the Figures using the Effectene transfection reagent according to the manufacturer's instructions (Qiagen, Crawley, West Sussex, U.K.). After 48 h, cell lysates were prepared using the lysis buffer (50 mM Tris, pH 7.8, 150 mM NaCl, 0.1%, v/v, Nonidet P40 and 1%, v/v, protease inhibitor cocktail; Sigma). Extracts were used immediately where required.
GST pull-down method
BL21 cells were used to express the GST-tagged 80K-H constructs using a standard procedure given by Amersham Biosciences (Little Chalfont, Bucks., U.K.). BL21 cells overexpressing GST-tagged 80K-H constructs were lysed using Bugbuster (Novagen, Madison, WI, U.S.A.) and clarified extracts were incubated with glutathione beads (Amersham Biosciences). After several washes with PBS, the glutathione bead–80K-H complex was exposed to cell extracts prepared from cos-1 cells overexpressing HA-tagged PKCζ constructs. After 4 h of constant agitation at 4 °C, complexes were washed three times with PBS and then resuspended in a small volume of PBS.
Transfection into Cho cells
Cho (Cho-K1) cells were purchased from A.T.C.C. (A.T.C.C. number CCL-61; Manassas, VA, U.S.A.). These cells were not overexpressing the insulin receptor. Cho cells were cultured in Ham's medium supplemented with 10% (v/v) foetal bovine serum and 1% (v/v) glutamine at 37 °C in 5% CO2 atmosphere. Cho cells were made to stably express Myc-tagged GLUT4 by standard methods using Polyfect (Qiagen) as the transfection reagent. Transient transfection into Cho-mycGLUT4 cells of pcDNA4 Xpress-tagged 80K-H constructs was also performed using Polyfect. The transient transfection efficiency was determined in two ways. First, green fluorescent protein and fluorescence microscopy were used. This indicated a transient transfection efficiency of >75%. Secondly, the transient transfection efficiency was estimated for a construct actually used, namely Xpress-tagged 80K-H 248, and from the movement of GLUT4 out of the LDM (low-density microsome) fraction triggered by this construct in the absence of insulin. This indicated a transient transfection efficiency of >85% (compare lanes or scale bars 1 and 5 in Figure 5b). Thus the transient transfection efficiency was high.
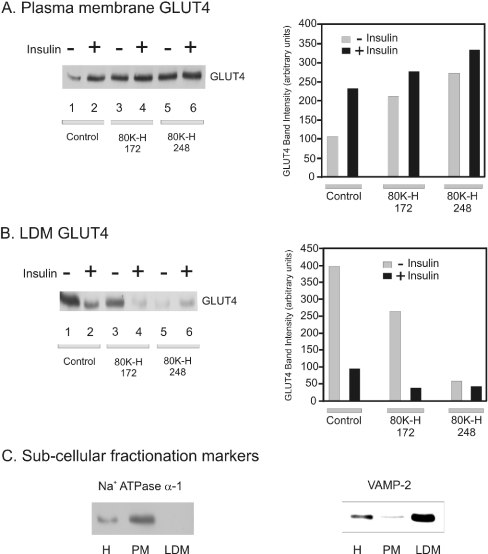
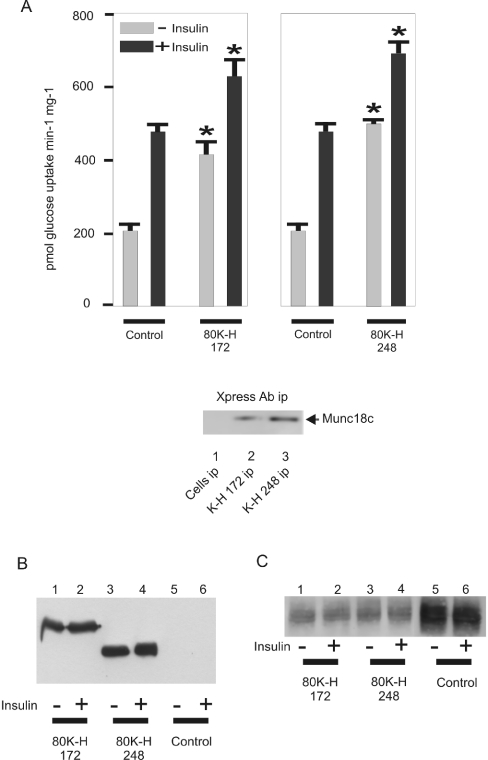
Figure 5. Expression of 80K-H constructs mimics the action of insulin in triggering GLUT4 translocation to the plasma membrane.
Cho cells expressing the Myc-tagged GLUT4 were transiently transfected with the indicated Xpress-tagged 80K-H constructs. Cells were serum-starved for 16 h and then incubated in the presence or absence of 100 nM insulin for 20 min. Plasma membrane and LDM fractions were prepared. (A) Plasma membrane fractions were probed for Myc to detect Myc-tagged GLUT4. Intensity values were calculated from densitometric scanning of the immunoblots. The graph shows the average data from two experiments. (B) LDM fractions were probed for Myc to detect Myc-tagged GLUT4. Intensity values were calculated from densitometric scanning of the immunoblots. The graph shows the average data from two experiments. (C) Equal amounts of homogenate (H), plasma membrane (PM) and LDM fraction were probed for either the plasma membrane marker Na+-ATPase α-1 or the GLUT4 vesicle marker VAMP-2. Na+-ATPase α-1, a plasma membrane marker, was abundant in the plasma membrane fraction and absent from the LDM fraction. VAMP-2, which is predominantly localized to the GLUT4 vesicle and thus is a marker of LDM, was abundant in the LDM fraction.
2-Deoxyglucose transport assay
Essentially the same method was used as described previously [12] but after several modifications. Cho-mycGLUT4 cells were serum-starved for 16 h before the assay. Cells in 22 mm dishes were washed once with 0.5 ml of prewarmed PBS and then once with prewarmed Krebs–Ringer buffer (10 mM Hepes, pH 7.4, 136 mM NaCl, 4.7 mM KCl and 1.25 mM MgSO4). Cells were then incubated in 0.5 ml of the same buffer in the presence or absence of 100 nM insulin for 20 min at 37 °C. A 2-deoxyglucose solution (550 μM cold 2-deoxy-D-glucose and 2.84 μM 2-deoxy-D-[3H]glucose from Amersham Biosciences) was added at 2 μCi/well. The assay was stopped after 5 min at 37 °C by the addition of 0.5 ml of Krebs–Ringer buffer containing 2 M cold 2-deoxy-D-glucose. Cells were washed three times with ice-cold PBS and extracted into lysis buffer (62.5 mM Tris, pH 7.5, and 1%, v/v, SDS). Duplicate aliquots of the extract were counted in Optiphase Hi-Safe 3 scintillant. Further aliquots were taken for the determination of protein concentration and analysis of Myc- and Xpress-tagged constructs by immunoblotting. Non-carrier uptake was measured for each condition by adding 50 μM cytochalasin B, 5 min before insulin stimulation, and the counts incorporated (typically <15% of total uptake) were subtracted from the total values.
Membrane fractionation
Cho-mycGLUT4 cells were fractionated using a procedure outlined previously [13].
Immunoprecipitation
3T3-L1 adipocytes were obtained and cultured as described previously [14,15]. L6 cells were purchased from A.T.C.C. (A.T.C.C. number CRL1458). L6 cells were cultured in Dulbecco's modified Eagle's medium (1 g/l glucose from Gibco Laboratories, Paisley, Renfrewshire, Scotland, U.K.) containing 10% (v/v) myoclone foetal calf serum (Gibco Laboratories) at 37 °C in the presence of 5% CO2 till they reached confluency, allowed to differentiate and the L6 myotubes were used 5 days after confluency. Extracts from L6 myotubes, 3T3-L1 adipocytes or Cho cells were diluted in lysis buffer [50 mM Tris, pH 7.5, 150 mM NaCl and 1% protease and phosphatase inhibitor cocktails (Sigma)] to give a 500 μl final volume (500 μg). Co-immunoprecipitation was performed by incubating 500 μg of cell lysate with a polyclonal PKCζ antibody (5 μl; Sigma), monoclonal Xpress antibody (5 μl; Invitrogen) or a monoclonal munc18c antibody (10 μl; BD Biosciences, Cowley, Oxford, U.K.) and Protein G/Protein A beads (50:50, 20 μl; Sigma). After 5 h of continuous gentle agitation at 4 °C, the beads were collected by pulse spin and then washed three times with lysis buffer, after which they were resuspended in PBS.
Immunoblotting
Immunoprecipitates and extracts were resolved by SDS/PAGE [14,15] and transferred on to a nitrocellulose filter. The membranes were probed with various primary antibodies followed by incubation with horseradish peroxidase-conjugated secondary antibodies as appropriate. Primary antibodies [cmyc (NEB), monoclonal HA (NEB), GST (NEB), Xpress (Invitrogen), 80K-H (BD Biosciences), PKCζ (Sigma)] were used according to the manufacturer's instructions. Blots were developed with the ECL® system according to the manufacturer's instructions (Amersham Biosciences). The 80K-H antibody was validated by immunoblotting against various overexpressed proteins (munc18c, PKCζ and 80K-H) produced in bacteria. A signal was only found with overexpressed 80K-H (results not shown). Immunoblotting against cell extracts containing various amounts of 80K-H showed a concentration curve (results not shown). Overexpression of 80K-H in cells increased the signal strength of the 80K-H band when compared with control cells (results not shown).
In vitro kinase assays
In vitro kinase assays were performed using HA-tagged full-length PKCζ, which is kinase-active, and an HA-tagged PKCζ construct lacking the C-terminal tail (PKCζ–CB), which is kinase-dead [10]. Lysates from cos-1 cells were prepared using the lysis buffer as described above. Cells were serum-starved for at least 24 h and stimulated with 15% (v/v) serum for 15 min before extraction. GST alone, GST-tagged 80K-H full-length protein and GST-tagged 80K-H 248 were overexpressed in BL21 and coupled with glutathione beads. cos-1 cell extracts and glutathione bead complexes were treated as described above for the GST pull-down assay, except after the final wash, the complexes were washed twice more with kinase buffer lacking ATP. In vitro kinase assays were performed at 30 °C for 30 min in a buffer containing 50 mM Tris (pH 7.5), 10 mM MgCl2, 12.5 μM ATP, 5 μCi of [γ-32P]ATP, phosphatidylserine (200 μg/ml) and diacylglycerol (40 μg/ml 1,2-dioctanoyl-sn-glycerol). Assay conditions were linear. The reactions were stopped by the addition of 5×Laemmli sample buffer and subsequent boiling for 3 min. Samples were then subjected to SDS/PAGE (10% polyacrylamide). The gel was stained with Coomassie Brilliant Blue and the GST-tagged band was excised for scintillation counting.
RESULTS
Detection of proteins binding to PKCζ
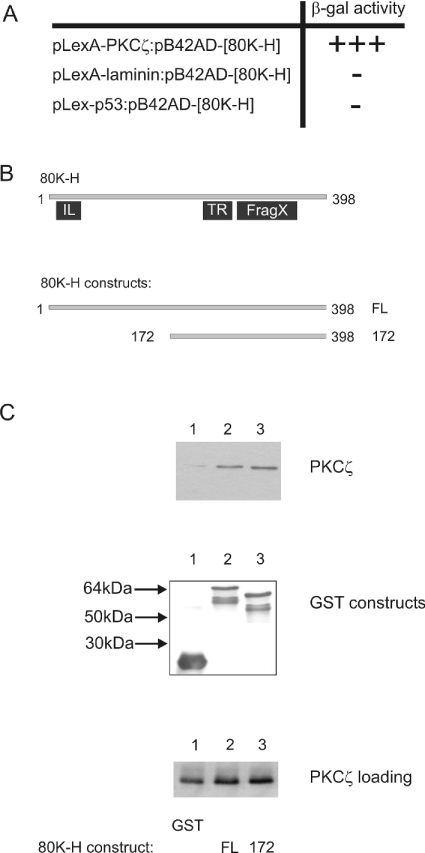
To identify proteins capable of interacting with PKCζ, we employed the LexA yeast two-hybrid system to screen a human brain cDNA library. Full-length human PKCζ (this PKCζ had an N-terminal extension of 37 amino acids and is described by Hodgkinson and Sale in [11]) was fused to the LexA DNA-binding domain of the ClonTech pLexA vector and subsequently used in an interactor hunt with a brain cDNA library. Screening of 5×106 transformants yielded 129 Leu+LacZ+ colonies. Sequencing of the clones yielded various proteins that have putative functions in vesicle transport, control of the cell cycle and apoptosis. In the present study, we focused solely on one protein, 80K-H, since this protein has been poorly characterized. Experiments performed in yeast showed robust β-galactosidase activity when PKCζ and 80K-H were co-expressed. When laminin or p53 were substituted for PKCζ in the pLexA plasmid, no β-galactosidase activity was seen (Figure 1A). Thus the interaction between 80K-H and PKCζ was a true positive.
Figure 1. PKCζ interacts with 80K-H in yeast and in vitro.
(A) The yeast reporter strain was co-transfected with 80K-H and various LexA constructs. Transformant yeast were assayed for growth on leucine-deficient medium and for β-galactosidase acitivity after five days. +++, Leu+LacZ+phenotype showing robust β-galactosidase activity and represents a two-hybrid interaction; –, no interaction as shown by no β-galactosidase activity. (B) 80K-H is a 398 amino acid-long protein. A homology search shows weak homology to the IL (ILWEQ) structure that is thought to possess F-actin binding properties, TR (tropomyosin) and cytoplasmic fragile X-interacting family domains (FragX) as shown in (B). The 80K-H constructs 80K-H 172 comprise amino acids 172–398. (C) cos-1 cells were transiently transfected with PKCζ (expressed as HA fusion). The 80K-H constructs, expressed as GST fusions, were coupled with glutathione beads. Cell extracts were incubated with the glutathione bead complexes, and after extensive washing, proteins were separated by SDS/PAGE, transferred on to a nitrocellulose membrane, and probed for HA to detect co-precipitated PKCζ (top panel) or GST to check for loading of the GST-tagged 80K-H constructs (middle panel). Aliquots of the lysate were also analysed for levels of expression of the HA-tagged PKCζ using HA antibody (bottom panel). The results are representative of four independent experiments.
PKCζ and 80K-H interact in vitro
To validate further the interaction between 80K-H and PKCζ seen in the yeast two-hybrid screen, GST pull-down assays were performed. For this, purified GST-tagged full-length 80K-H (80K-H FL) was coupled with glutathione beads and incubated with extracts of cos-1 cells transfected with HA-tagged PKCζ. Complexes were washed to remove non-specific binding, separated by SDS/PAGE and immunoblotted with anti-HA antibodies to test for precipitation of HA-tagged PKCζ. As shown in Figure 1C (top panel, lane 2), PKCζ associated with full-length 80K-H. That this association was not a result of non-specific binding is evident from the observation that the extracts of PKCζ, when incubated with GST, do not cause precipitation of PKCζ (Figure 1C, top panel, lane 1). The GST-tagged 80K-H deletion mutant, 80K-H 172, was also found to associate with PKCζ (Figure 1C, top panel, lane 3), showing that the N-terminal portion of 80K-H was not required for the interaction to occur.
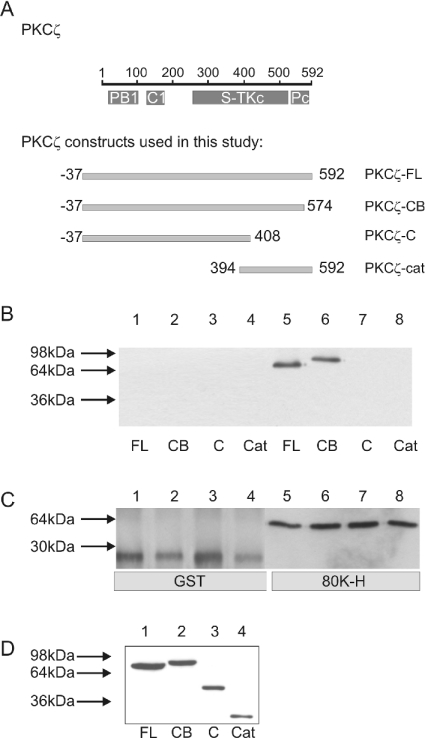
To determine which domain(s) of PKCζ were required for binding to 80K-H, cos-1 cells were transfected with various HA-tagged deletion mutants of PKCζ (see Figure 2A). Lysates were incubated with purified GST-tagged 80K-H FL coupled with glutathione beads. Complexes were immunoblotted with anti-HA antibodies to test for the precipitation of HA-tagged PKCζ constructs. A strong band was observed with full-length PKCζ and PKCζ-CB (Figure 2B, lanes 5 and 6). PKCζ-C and PKCζ-cat failed to bind (Figure 2B, lanes 7 and 8), despite the similar level of protein expression in all transfected cells (Figure 2D). These results are consistent with 80K-H binding to the kinase core of PKCζ or perhaps binding to a domain encompassing the N- and C-terminal regions of PKCζ if they come together in the three-dimensional structure.
Figure 2. Interaction of 80K-H with various PKCζ constructs in vitro.
PKCζ contains several domains, namely PB1, C1 that binds to diacylglycerol, a kinase domain (S-TKc) and PKC terminal domain (Pc) as shown in (A). cos-1 cells were transiently transfected with the HA-tagged PKCζ constructs shown in (A). Full-length 80K-H, expressed as a GST fusion protein, was coupled with glutathione beads (80K-H). GST bound to glutathione beads was used as a control (GST). Cell extracts were incubated with the glutathione bead complexes, and after extensive washing, proteins were separated by SDS/PAGE, transferred on to a nitrocellulose membrane and probed for HA to detect co-precipitated PKCζ constructs (B) or probed for GST to check for loading of the full-length GST-tagged 80K-H (C). Aliquots of the lysate were also analysed for levels of expression of the HA-tagged PKCζ constructs using HA antibody (D). The results are representative of two independent experiments.
Endogenous 80K-H, PKCζ and munc18c form a complex
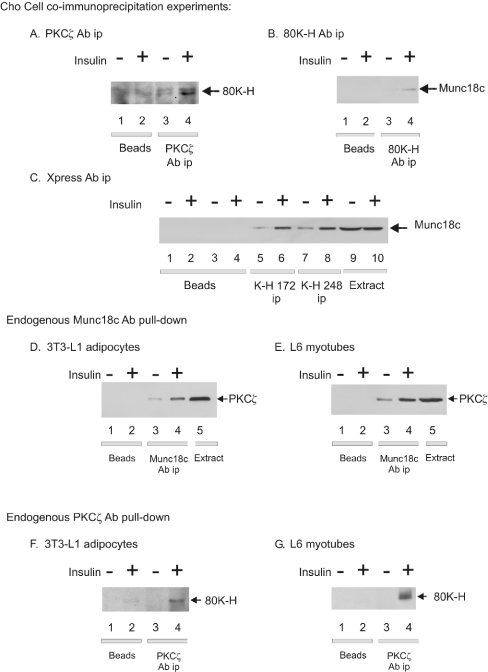
It was important to test whether the interaction between 80K-H and PKCζ occurred with the endogenous proteins in vivo. This was initially performed in insulin-sensitive Cho cells. Cells (untransfected) were lysed and the lysates were incubated with a PKCζ antibody to precipitate endogenous PKCζ. The immunoprecipitates were then probed with an 80K-H antibody. As shown in Figure 3(A), precipitation of endogenous PKCζ yielded the co-immunoprecipitation of endogenous 80K-H in Cho cells. Control experiments omitting the PKCζ antibody confirmed the absence of 80K-H (Figure 3A). Stimulation of Cho cells with insulin significantly increased the association between 80K-H and PKCζ by approx. 3-fold (Figure 3A). This was validated by immunoprecipitating endogenous 80K-H with the 80K-H antibody and blotting with the PKCζ antibody. This confirmed the association between endogenous 80K-H and endogenous PKCζ which, again, was observed to be markedly increased by insulin stimulation (results not shown). The observation that the binding between 80K-H and PKCζ was markedly increased by insulin, provides excellent evidence that the association between the two proteins was specific. Moreover, regulation of the binding by insulin provides a mechanism for delivery of an insulin signal beyond PKCζ.
Figure 3. Insulin-regulated associations between endogenous 80K-H, PKCζ and munc18c in vivo.
Cells were serum-starved for 24 h and then incubated for 5 min in the presence or absence of 100 nM insulin. The indicated proteins were immunoprecipitated from cell lysates (0.5 mg of protein) and the immunoprecipitates were washed extensively. Proteins in the immunocomplex were Western blotted with the indicated antibody. Control immunoprecipitations containing an equal amount of cell lysate and Protein A/G beads but omitting the antibodies were also performed. (A) Cho cells. Endogenous PKCζ was immunoprecipitated and the immunoprecipiates were Western blotted with an 80K-H antibody. The results are representative of two independent experiments. 80K-H ran as a doublet in Cho cells for an unknown reason. (B) Cho cells. Endogenous 80K-H was immunoprecipitated and the immunoprecipitates were Western-blotted with a munc18c antibody. The results are representative of two independent experiments. (C) Cho cells overexpressing Xpress-tagged 80K-H 172 and 248 constructs. Xpress-tagged proteins were immunoprecipitated and the immunoprecipitates were Western-blotted with a munc18c antibody. Lanes 9 and 10 contain cell extract (1% of immunoprecipitation input). The results are representative of two independent experiments. (D, E) 3T3-L1 adipocytes or L6 myotubes. Endogenous munc18c was immunoprecipitated and the immunoprecipitates were Western blotted with a PKCζ antibody. Lane 5 shows the cell extract (1% of immunoprecipitation input). The results are representative of two independent experiments. Similar results were obtained in Cho cells. (F, G) 3T3-L1 adipocytes or L6 myotubes. Endogenous PKCζ was immunoprecipitated and the immunoprecipitates were Western-blotted with an 80K-H antibody. The results are representative of two independent experiments.
As 80K-H is related to the vesicle transport protein VASAP-60, we wished to determine whether 80K-H interacted with any of the proteins known to be involved in GLUT4 vesicle translocation. To test this, endogenous VAMP-2 and endogenous syntaxin-4 were first immunoprecipitated from Cho cells and blotted with the 80K-H antibody. No association between endogenous VAMP-2 or endogenous syntaxin-4 and 80K-H was detected. Next, the interaction of 80K-H with munc18c was evaluated. For this, endogenous 80K-H was immunoprecipitated from Cho cells and blotted with a munc18c antibody. As shown in Figure 3(B), precipitation of endogenous 80K-H yielded the co-immunoprecipitation of endogenous munc18c. The amount of co-immunoprecipitated munc18c was markedly increased by prior stimulation of the cells with insulin. Control experiments omitting the 80K-H antibody confirmed the absence of munc18c (Figure 3B). The reverse experiment of immunoprecipitating endogenous munc18c and blotting for 80K-H confirmed the insulin controlled association between endogenous 80K-H and endogenous munc18c in vivo (results not shown). Insulin regulation of the complex formation between 80K-H and munc18c provides excellent evidence for the formation of the complex being specific. Additionally, the experiments showing that VAMP-2 and syntaxin-4 did not significantly interact with 80K-H, act as further controls such that the interaction between 80K-H and munc18c was specific.
Next the interaction between two truncated 80K-H constructs and munc18c was tested. These constructs were chosen because they were used in the studies described below for investigating the role of 80K-H in GLUT4 vesicle translocation. The 80K-H constructs were 80K-H 172 and 80K-H 248, which lack the first 171 and 247 amino acids respectively. Xpress-tagged constructs were expressed in Cho cells and immunoprecipitated with Xpress antibody. Immunoprecipitates of each contained munc18c with the level of co-immunoprecipitated munc18c significant in the basal state and further enhanced by prior stimulation with insulin (Figure 3C). Immunoprecipitates of 80K-H 248 possessed higher levels of munc18c compared with those of 80K-H 172. This was not due to higher expression of 80K-H 248 because 80K-H 248 was expressed at similar levels as 80K-H 172 (e.g. Figure 4B). These results support those above showing an insulin-controlled interaction between 80K-H and munc18c. Additionally, they show that the N-terminal portion of 80K-H was not required for the interaction to take place.
Figure 4. Expression of 80K-H constructs mimics the action of insulin in stimulating glucose uptake.
Cho cells expressing the Myc-tagged GLUT4 were transiently transfected with the indicated Xpress-tagged 80K-H constructs. Cells were serum-starved for 16 h and then incubated in the presence (black bar) or absence (grey bar) of 100 nM insulin for 20 min and the rates of 2-deoxy-D-[3H]glucose uptake were determined. (A) Results are expressed as the means±S.E.M. for four to eight independent experiments. Results significantly different from control values or to the corresponding bars in the other panel are marked with an asterisk. The inset of (A) shows that 80K-H 172 and 80K-H 248 bound significant munc18c in the absence of insulin. For this, Xpress-tagged 80K-H constructs were immunoprecipitated from cells incubated in the absence of insulin and the immunoprecipitates were Western-blotted with a munc18c antibody as described in the legend to Figure 3. Cell fractions were also directly immunoblotted for Xpress to detect Xpress-tagged 80K-H constructs (B) and Myc to detect Myc-tagged GLUT4 (C).
As we had demonstrated that 80K-H forms a complex with both PKCζ and munc18c, it was logical to test if all the three proteins came together to form a complex. It was also important to determine whether the interactions observed above occurred in insulin's major target cell types. For this, 3T3-L1 adipocytes and L6 myotubes, which are known to be good models of insulin signalling in fat and muscle cells, were employed. Endogenous munc18c was immunoprecipitated from 3T3-L1 adipocytes (Figure 3D) and L6 myotubes (Figure 3E) and the immunoprecipiates blotted with a PKCζ antibody. This showed that PKCζ co-immunoprecipitated (Figures 3D and 3E) in each case. Stimulation of 3T3-L1 adipocytes and L6 myotubes with insulin significantly increased the amount of co-immunoprecipitated PKCζ by approx. 3-fold (Figures 3D and 3E). Control experiments omitting the munc18c antibody confirmed the absence of PKCζ (Figures 3D and 3E). The reverse experiment of immunoprecipitating endogenous PKCζ and blotting for munc18c confirmed the insulin–controlled association between endogenous PKCζ and endogenous munc18c in vivo (results not shown). We next tested whether the interaction seen between PKCζ and 80K-H in Cho cells also occurred in 3T3-L1 adipocytes and L6 myotubes. Immunoprecipitates of endogenous PKCζ from 3T3-L1 adipocytes and L6 myotubes were indeed found to contain 80K-H with prior insulin stimulation significantly increasing the amount of co-immunoprecipitated 80K-H by approx. 5-fold (Figures 3F and 3G). Thus these results confirm those obtained in Cho cells. The results also confirm that 80K-H is significantly expressed in adipocyte and muscle cell types in line with Northern-blot results [23]. In summary, the results show that insulin triggers interactions in vivo between endogenous PKCζ, 80K-H and munc18c. The interactions were highly insulin-regulated and provide excellent evidence that they were specific. Moreover, insulin regulation of the interactions provides a mechanism for delivery of an insulin signal to munc18c.
80K-H constructs mimic the action of insulin in stimulating glucose uptake
To investigate the role of the 80K-H in signalling glucose uptake, we generated a Cho cell line expressing Myc-tagged GLUT4 (abbreviated to Cho-mycGLUT4 cells). Such cells are known to show insulin-stimulated glucose transport and to share many similarities with 3T3-L1 adipocytes, a canonical cell line used to investigate insulin-stimulated glucose transport [16,17]. The cells were cultured in Dulbecco's modified Eagle's medium as this has been shown to be required for insulin-responsive trafficking [17]. We first generated Myc-tagged GLUT4 and confirmed its functionality using cos-1 cells. Thus expression of the Myc-tagged GLUT4 in cos-1 cells increased glucose uptake by 10-fold when compared with untransfected cells (results not shown). The Myc-tagged GLUT4 was then expressed in Cho cells. This resulted in a marked increase in insulin-stimulated levels of glucose transport compared with wild-type Cho cells (results not shown). The level of insulin-stimulated glucose uptake in the Cho-mycGLUT4 cells was approx. 2.5-fold (Figure 4A), which was in good agreement with the published literature on insulin-stimulated trafficking of GLUT4 to the plasma membrane of Cho cells [17]. Transient transfection of various Xpress-tagged 80K-H constructs was then performed to test if they modulated glucose uptake. We were not able to express 80K-H in Cho cells. The 80K-H constructs employed were 80K-H 172 and 80K-H 248. These constructs bound significant amounts of munc18c in the absence of insulin (Figure 4A). Overexpression of the 80K-H 172 construct mimicked the action of insulin in stimulating glucose uptake (Figure 4A). Overexpression of the 80K-H 248 construct consistently yielded a further increase of glucose uptake compared with that achieved with the 80K-H 172 construct. This was in agreement with our observation that the 80K-H 248 construct bound more effectively to munc18c than the 80K-H 172 construct (Figure 4A). Overexpression of the 80K-H 172 and 80K-H 248 constructs also increased glucose uptake in the presence of insulin. However, the effects were relatively less than in the absence of insulin. This was as expected because glucose transport in the presence of insulin and absence of the constructs was already near maximal with at least 70% of the available GLUT4 having translocated to the plasma membrane (Figure 5).
80K-H constructs mimic the action of insulin in increasing GLUT4 translocation
To test if the results observed in the glucose transport assay reflected translocation of GLUT4, the distribution of Myc-tagged GLUT4 was determined in Cho-mycGLUT4 cells. As expected, in control cells, insulin increased the amount of plasma membrane associated Myc-tagged GLUT4 (Figure 5A, left panel, lanes 1 and 2; and right panel, bars 1 and 2) and caused a concomitant decrease in the level of Myc-tagged GLUT4 in the LDM fraction (Figure 5B, left panel, lanes 1 and 2; and right panel, bars 1 and 2). Expression of the 80K-H 172 construct mimicked the action of insulin in increasing the amount of Myc-tagged GLUT4 in the plasma membrane (Figure 5A, left panel, lanes 2 and 3; and right panel, bars 2 and 3) with associated decreases in the levels of Myc-tagged GLUT4 in the LDM fraction (Figure 5B, left panel, lanes 1 and 3; and right panel, bars 1 and 3). Next, the 80K-H construct 80K-H 248 which binds munc18c more strongly than 80K-H 172 was tested. Overexpression of the 80K-H 248 construct consistently had a greater effect on translocation of the Myc-tagged GLUT4 from the LDM to the plasma membrane than that observed in response to expression of the 80K-H 172 construct (Figures 5A and 5B, left panels, lanes 3 and 5; and right panels, bars 3 and 5). In the presence of insulin and absence of the 80K-H constructs, most of the available GLUT4 had already translocated to the plasma membrane (Figures 5A and 5B, left panels, lanes 1 and 2; and right panels, bars 1 and 2). Thus, although 80K-H 172 and 80K-H 248 did increase GLUT4 translocation from the LDM to the plasma membrane in the presence of insulin, the effects were relatively moderate as expected (Figures 5A and 5B, left panels, lanes 2, 4 and 6, and right panels, bars 2, 4 and 6). These results fully support the experiments in which glucose uptake was measured.
Membrane localization of 80K-H
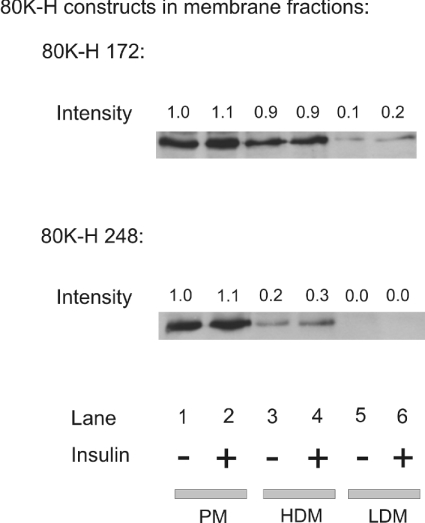
In Cho-mycGLUT4 cells expressing Xpress-tagged 80K-H constructs, the 80K-H constructs were found to be located predominantly in the plasma membrane, placing them in the correct location to interact with PKCζ and munc18c (Figure 6). Insulin stimulation did not alter the distribution of the 80K-H constructs between the plasma membrane, LDM fractions and high-density microsome fractions (Figure 6). No 80K-H construct was detected in the cytoplasm (results not shown).
Figure 6. Membrane localization of 80K-H.
Cho cells expressing the Myc-tagged GLUT4 were transiently transfected with the indicated Xpress-tagged 80K-H constructs. Cells were serum-starved for 16 h and then incubated in the presence or absence of 100 nM insulin for 20 min. Plasma membrane, HDM and LDM fractions were prepared. Cell fractions were probed for Xpress to detect Xpress-tagged 80K-H 172 or 80K-H 248. Fold and intensity values were calculated from densitometric scanning of the immunoblots with the value of lane 1 taken to be 1.0. The results are representative of two independent experiments.
80K-H is phosphorylated
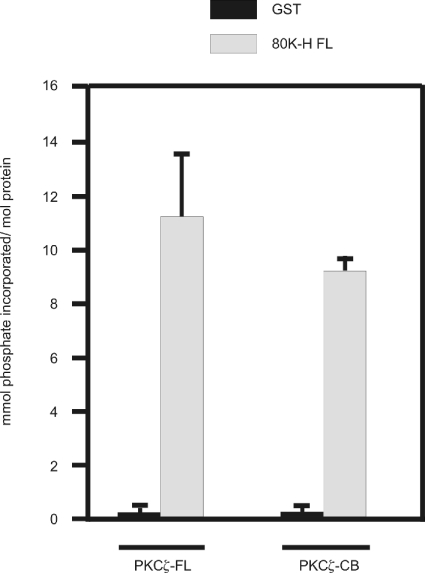
We next considered whether 80K-H was a substrate for PKCζ. For this, GST-tagged 80K-H protein was first incubated with extracts of cells overexpressing either kinase-active PKCζ or PKCζ-CB, which we have shown previously to be kinase-dead, to allow binding of 80K-H to PKCζ or PKCζ-CB [10]. The GST-tagged 80K-H FL protein was then pulled down, extensively washed and incubated in the presence of [γ-32P]ATP. Use of kinase-active PKCζ showed that 80K-H was significantly phosphorylated (Figure 7, bar 2). Comparable phosphorylation was obtained with kinase-dead PKCζ (Figure 7, bar 4). These results show that 80K-H is phosphorylated but that PKCζ is not directly responsible. It is well known that PKCζ binds other kinases and delivers them to target sites [10,32].
Figure 7. 80K-H is phosphorylated in vitro.
GST-tagged 80K-H or GST was coupled with glutathione beads and exposed to cell extracts of cos-1 cells transiently transfected with full-length HA-tagged PKCζ (kinase active) or HA-tagged PKCζ-CB (kinase-dead). After extensive washing, complexes were incubated in the presence of [γ-32P]ATP. Samples were resolved by SDS/PAGE (10% polyacrylamide) and the gel was stained with Coomassie Brilliant Blue. The band corresponding to the GST construct was excised and incorporation of 32P was determined by scintillation counting. Results are the means±S.E.M. for four independent experiments.
DISCUSSION
80K-H was originally identified in 1989 as a protein that can be phosphorylated by a crude PKC mixture in vitro [18,19]. However, the biological function of 80K-H has been poorly characterized [20–23]. 80K-H is closely related to VASAP-60, a protein known to regulate vesicle trafficking [9]. Consequently, we tested whether 80K-H is involved in vesicle trafficking. Results herein identify 80K-H as a protein involved in vesicle trafficking, notably enhancing glucose transport through GLUT4.
One of the major effects of insulin is to promote the influx of glucose into fat and muscle cells. This is achieved by the activation of a signalling cascade and the trafficking of intracellular vesicles containing the glucose transporter GLUT4 to the plasma membrane. Several kinases involved in insulin-stimulated glucose transport have been discovered, namely PKCζ/λ [2–4,6–8] and PKB [6–8]. Two candidate substrates for PKB are AS160 [33] and PIKfyve [34]. CAP-Cbl has also been implicated in insulin-stimulated glucose transport [6–8]. GLUT4 vesicle trafficking is similar to that seen in synaptic vesicles. The GLUT4 vesicle contains the v-SNARE [SNAP (soluble N-ethylmaleimide-sensitive fusion protein attachment protein) receptor] VAMP-2, which binds to the t-SNARE complex containing syntaxin-4 and SNAP-23 found in the plasma membrane [6–8]. Several proteins have been proposed that act to regulate the interaction between syntaxin-4 and VAMP-2. These proteins include synip [24] and munc18c [25–29]. munc18c binds to syntaxin-4, and since this association is stronger than that between VAMP-2 and syntaxin-4 [25–29], it acts as a clamp preventing the GLUT4 vesicle binding to the plasma membrane in the basal state. Insulin stimulation decreases the binding of munc18c to syntaxin-4, thereby enabling the GLUT4 vesicle to dock with the plasma membrane. Displaced munc18c may also be involved in fusing the GLUT4 vesicle to the plasma membrane [6,29]. In addition, the protein TUG has been proposed to sequester and retain GLUT4 intracellularly with insulin releasing the tether [30]. Despite this work, there has been no direct link established with the signalling cascade initiated by insulin. We performed a yeast two-hybrid screen, with PKCζ as bait, in the hope of identifying such a link. The yeast two-hybrid screen identified 80K-H as an interactor of PKCζ. The interaction between 80K-H and PKCζ was confirmed by GST pull-down assays. This approach showed that the GST-tagged 80K-H deletion mutant, 80K-H 172, also associated with PKCζ, showing that the interaction did not require the N-terminal portion of 80K-H. To confirm finally the physiological nature of the interaction, the endogenous proteins were shown to interact in vivo in Cho cells, 3T3-L1 adipocytes and L6 myotubes. Moreover, the interaction was increased by 3–5-fold when the cells were stimulated with insulin. This showed that the interaction was insulin-regulated and provided further very good evidence that the interaction was specific. Considering, as already mentioned, that the vesicle trafficking protein VASAP-60 is homologous with 80K-H, we were interested to see if 80K-H interacted with any of the proteins known to be involved in the movement of GLUT4 vesicles. Endogenous 80K-H was observed to immunoprecipitate with endogenous munc18c from Cho cells, 3T3-L1 adipocytes and L6 myotubes. The amount of the co-immunoprecipitating protein was markedly enhanced by prior stimulation of the cells with insulin. Insulin regulation of the complex formation between 80K-H and munc18c provides excellent evidence that the formation of the complex was specific. Moreover, neither syntaxin-4 nor VAMP2 interacted significantly with 80K-H, providing further controls that the complex formation between 80K-H and munc18c was specific. Endogenous munc18c was observed to immunoprecipitate with endogenous PKCζ. Again, the amount of the co-immunoprecipitating protein was significantly enhanced by approx. 3-fold by insulin. Thus insulin triggers interactions in vivo between endogenous PKCζ, 80K-H and munc18c. These interactions were highly insulin-regulated, providing excellent evidence that the interactions were specific. Moreover, insulin regulation of the interactions provides a mechanism for delivery of an insulin signal to munc18c.
To investigate whether 80K-H was involved in GLUT4 vesicle trafficking, Cho cells stably expressing GLUT4 were employed. Such cells have been used in various studies to investigate the mechanism whereby insulin stimulation promotes GLUT4 translocation to the plasma membrane [13,16,17]. Cho cells, similar to 3T3-L1 adipocytes, possess GLUT4 storage vesicles that translocate to the plasma membrane in response to insulin stimulation. Two truncated constructs of 80K-H were used, namely 80K-H 172 and 80K-H 248. These constructs bound significant munc18c in the absence of insulin (Figure 4). Overexpression of these 80K-H constructs mimicked the action of insulin in stimulating both glucose uptake and translocation of Myc-tagged GLUT4 (Figures 4 and 5). The level of effect was proportional to the ability of the constructs to bind munc18c because the 80K-H 248 construct, which associated with munc18c more efficiently than the 80K-H 172 construct, consistently yielded greater increases. These results provide strong evidence that 80K-H is involved in GLUT4 vesicle translocation to the plasma membrane.
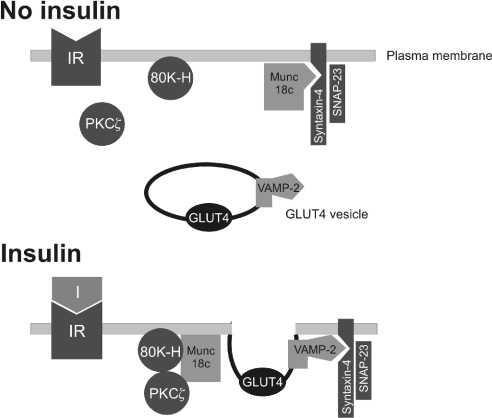
Because 80K-H associates with munc18c, the simplest mechanism for the action of 80K-H is that it functions by releasing the clamp action of munc18c on syntaxin-4, thereby allowing VAMP-2 to bind. Displaced munc18c may also be involved in fusing the GLUT4 vesicle to the plasma membrane. A hypothetical model illustrating this is shown in Figure 8. It is also possible that the 80K-H constructs acted by inhibiting the internalization of GLUT4 or the trafficking of GLUT4 from the endosomal system into specialized GLUT4 storage vesicles. Although, in the model of Figure 8, PKCζ, 80K-H and munc18c are shown to bind each other in a complex, the precise interactions between the proteins require elucidation. For example, although it seems probable that the complex formed is a ternary complex between PKCζ, 80K-H and munc18c, our results do not exclude a set of binary interactions between PKCζ, 80K-H and munc18c. Our results also raise the possibility that PKCζ functions to deliver 80K-H to munc18c. This would depend on PKCζ acting through its well-known ability to exert its effects through binding other proteins [10,31,32].
Figure 8. Hypothetical model showing insulin-triggered formation of a PKCζ–80K-H–munc18c complex and how this may trigger GLUT4 translocation to the plasma membrane.
The model shows how the complex formation may decrease the clamping action of munc18c, thereby allowing VAMP-2 to bind syntaxin-4 and deliver GLUT4 to the plasma membrane. Displaced munc18c may also be involved in fusing the GLUT4 vesicle to the plasma membrane. Although PKCζ, 80K-H and munc18c are shown to bind each other, the precise interactions that result in the complex formation require elucidation. Other accessory proteins are omitted for simplicity.
Although 80K-H was significantly phosphorylated, PKCζ did not appear to be directly involved. Nonetheless, the phosphorylation of 80K-H opens up other avenues for its regulation.
In conclusion, we have identified a physiological interaction between PKCζ, 80K-H and munc18c that is insulin-regulated and that 80K-H is a protein involved in vesicle transport. These results identify 80K-H as a new player in insulin-triggered GLUT4 translocation. 80K-H may provide a key link between the insulin signalling cascade and GLUT4 vesicle transport.
Acknowledgments
This work was supported by Diabetes UK (London, U.K.).
References
- 1.Zhou G., Wooten M. W., Coleman E. S. Regulation of atypical zeta-protein kinase C in cellular signalling. Exp. Cell Res. 1994;214:1–11. doi: 10.1006/excr.1994.1227. [DOI] [PubMed] [Google Scholar]
- 2.Farese R. V. Function and dysfunction of aPKC isoforms for glucose transport in insulin-sensitive and insulin-resistant states. Am. J. Physiol. Endocrinol. Metab. 2002;283:E1–E11. doi: 10.1152/ajpendo.00045.2002. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay G., Standaert M. L., Sajan M. P., Kanoh Y., Miura A., Braun U., Kruse F., Leitges M., Farese R. V. Protein kinase C-lambda knockout in embryonic stem cells and adipocytes impairs insulin-stimulated glucose transport. Mol. Endocrinol. 2004;18:373–383. doi: 10.1210/me.2003-0087. [DOI] [PubMed] [Google Scholar]
- 4.Bandyopadhyay G., Sajan M. P., Kanoh Y., Standaert M. L., Quon M. J., Lea-Currie R., Sen A., Farese R. V. PKC-zeta mediates insulin effects on glucose transport in cultured preadipocyte-derived human adipocytes. J. Clin. Endocrinol. Metab. 2002;87:716–723. doi: 10.1210/jcem.87.2.8252. [DOI] [PubMed] [Google Scholar]
- 5.Sajan M. P., Bandyopadhyay G., Kanoh Y., Standaert M. L., Quon M. J., Reed B. C., Dikic I., Farese R. V. Sorbitol activates atypical protein kinase C and GLUT4 glucose transporter translocation/glucose transport through proline-rich tyrosine kinase-2, the extracellular signal-regulated kinase pathway and phospholipase D. Biochem. J. 2002;362:665–674. doi: 10.1042/0264-6021:3620665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster L. J., Klip A. Mechanism and regulation of GLUT-4 vesicle fusion in muscle and fat cells. Am. J. Physiol. Cell Physiol. 2000;279:C877–C890. doi: 10.1152/ajpcell.2000.279.4.C877. [DOI] [PubMed] [Google Scholar]
- 7.Watson R. T., Kanzaki M., Pessin J. E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- 8.Elmendorf J. S. Signals that regulate GLUT4 translocation. J. Membr. Biol. 2002;190:167–174. doi: 10.1007/s00232-002-1035-3. [DOI] [PubMed] [Google Scholar]
- 9.Brule S., Rabahi F., Faure R., Beckers J. F., Silversides D. W., Lussier J. G. Vacuolar system-associated protein-60: a protein characterized from bovine granulosa and luteal cells that is associated with intracellular vesicles and related to human 80K-H and murine beta-glucosidase II. Biol. Reprod. 2000;62:642–654. doi: 10.1095/biolreprod62.3.642. [DOI] [PubMed] [Google Scholar]
- 10.Hodgkinson C. P., Sale E. M., Sale G. J. Characterization of PDK2 activity against protein kinase B gamma. Biochemistry. 2002;41:10351–10359. doi: 10.1021/bi026065r. [DOI] [PubMed] [Google Scholar]
- 11.Hodgkinson C. P., Sale G. J. Regulation of both PDK1 and the phosphorylation of PKC-zeta and -delta by a C-terminal PRK2 fragment. Biochemistry. 2002;41:561–569. doi: 10.1021/bi010719z. [DOI] [PubMed] [Google Scholar]
- 12.Sale E. M., Atkinson P. G., Arnott C. H., Chad J. E., Sale G. J. Role of ERK1/ERK2 and p70S6K pathway in insulin signalling of protein synthesis. FEBS Lett. 1999;446:122–126. doi: 10.1016/s0014-5793(99)00193-3. [DOI] [PubMed] [Google Scholar]
- 13.Lim S.-N., Bonzelius F., Low S. H., Wille H., Weimbs T., Herman G. A. Identification of discrete classes of endosome-derived small vesicles as a major cellular pool for recycling membrane proteins. Mol. Biol. Cell. 2001;12:981–995. doi: 10.1091/mbc.12.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnott C. H., Sale E. M., Miller J., Sale G. J. Use of an antisense strategy to dissect the signalling role of protein-tyrosine phosphatase alpha. J. Biol. Chem. 1999;274:26105–26112. doi: 10.1074/jbc.274.37.26105. [DOI] [PubMed] [Google Scholar]
- 15.Sale E. M., Atkinson P. G., Sale G. J. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J. 1995;14:674–684. doi: 10.1002/j.1460-2075.1995.tb07046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei M. L., Bonzelius F., Scully R. M., Kelly R. B., Herman G. A. GLUT4 and transferrin receptor are differentially sorted along the endocytic pathway in CHO cells. J. Cell Biol. 1998;140:565–575. doi: 10.1083/jcb.140.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogan J. S., McKee A. E., Lodish H. F. Insulin-responsive compartments containing GLUT4 in 3T3-L1 and CHO cells: regulation by amino acid concentrations. Mol. Cell. Biol. 2001;21:4785–4806. doi: 10.1128/MCB.21.14.4785-4806.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai K., Hirai M., Minoshima S., Kudoh J., Fukuyama R., Shimizu N. Isolation of cDNAs encoding a substrate for protein kinase C: nucleotide sequence and chromosomal mapping of the gene for a human 80K protein. Genomics. 1989;2:309–315. doi: 10.1016/0888-7543(89)90063-3. [DOI] [PubMed] [Google Scholar]
- 19.Harai M., Shimizu N. Purification of two distinct proteins of approximate Mr 80,000 from human epithelial cells and identification as proper substrates for protein kinase C. Biochem. J. 1990;270:583–589. doi: 10.1042/bj2700583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh K. C., Lim Y. P., Ong S. H., Siak C. B., Cao X., Tan Y. H., Guy G. R. Identification of p90, a prominent tyrosine-phosphorylated protein in fibroblast growth factor-stimulated cells, as 80K-H. J. Biol. Chem. 1996;271:5832–5838. doi: 10.1074/jbc.271.10.5832. [DOI] [PubMed] [Google Scholar]
- 21.Li Y. M., Mitsuhashi T., Wojciechowicz D., Shimizu N., Li J., Stitt A., He C., Bannerjee D., Vlassara H. Molecular identity and cellular distribution of advanced glycation endproduct receptors: relationship of p60 to OST-48 and p90 to 80K-H membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11047–11052. doi: 10.1073/pnas.93.20.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poirier O., Nicaud V., Vionnet N., Raoux S., Tarnow L., Vlassara H., Parving H. H., Cambien F. Polymorphism screening of four genes encoding advanced glycation end-product putative receptors. Association study with nephropathy in type 1 diabetic patients. Diabetes. 2001;50:1214–1218. doi: 10.2337/diabetes.50.5.1214. [DOI] [PubMed] [Google Scholar]
- 23.Gkika D., Mahieu F., Nilius B., Hoenderop J. G., Bindels R. J. 80K-H as a new Ca2+ sensor regulating the activity of the epithelial Ca2+ channel transient receptor potential cation channel V5 (TRPV5) J. Biol. Chem. 2004;279:26351–26357. doi: 10.1074/jbc.M403801200. [DOI] [PubMed] [Google Scholar]
- 24.Min J., Okada S., Kanzaki M., Elmendorf J. S., Coker K. J., Ceresa B. P., Syu L.-J., Noda Y., Saltiel A. R., Pessin J. E. Synip: a novel insulin-regulated syntaxin 4-binding protein mediating GLUT4 translocation in adipocytes. Mol. Cell. 1999;3:751–760. doi: 10.1016/s1097-2765(01)80007-1. [DOI] [PubMed] [Google Scholar]
- 25.Thurmond D. C., Ceresa B. P., Okada S., Elmendorf J. S., Coker K., Pessin J. E. Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3L1 adipocytes. J. Biol. Chem. 1998;273:33876–33883. doi: 10.1074/jbc.273.50.33876. [DOI] [PubMed] [Google Scholar]
- 26.Tamori Y., Kawanishi M., Niki T., Shinoda H., Araki S., Okazawa H., Kasuga M. Inhibition of insulin-induced GLUT4 translocation by Munc18c through interaction with syntaxin4 in 3T3-L1 adipocytes. J. Biol. Chem. 1998;273:19740–19746. doi: 10.1074/jbc.273.31.19740. [DOI] [PubMed] [Google Scholar]
- 27.Tellam J. T., Macaulay S. L., McIntosh S., Hewish D. R., Ward C. W., James D. E. Characterization of Munc-18c and syntaxin-4 in 3T3-L1 adipocytes. Putative role in insulin-dependent movement of GLUT-4. J. Biol. Chem. 1997;272:6179–6186. doi: 10.1074/jbc.272.10.6179. [DOI] [PubMed] [Google Scholar]
- 28.Hickson G. R., Chamberlain L. H., Maier V. H., Gould G. W. Quantification of SNARE protein levels in 3T3-L1 adipocytes: implications for insulin-stimulated glucose transport. Biochem. Biophys. Res. Commun. 2000;270:841–845. doi: 10.1006/bbrc.2000.2525. [DOI] [PubMed] [Google Scholar]
- 29.Spurlin B. A., Thomas R. M., Nevins A. K., Kim H. J., Kim Y.-J., Noh H.-L., Shulman G. I., Kim J. K., Thurmond D. C. Insulin resistance in tetracycline-repressible Munc18c transgenic mice. Diabetes. 2003;52:1910–1917. doi: 10.2337/diabetes.52.8.1910. [DOI] [PubMed] [Google Scholar]
- 30.Bogan J. S., Hendon N., McKee A. E., Tsao T.-S., Lodish H. F. Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature (London) 2003;425:727–733. doi: 10.1038/nature01989. [DOI] [PubMed] [Google Scholar]
- 31.Muller G., Ayoub M., Storz P., Rennecke J., Fabbro D., Pfizenmaier K. PKC zeta is a molecular switch in signal transduction of TNF-alpha, bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 1995;14:1961–1969. doi: 10.1002/j.1460-2075.1995.tb07188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moscat J., Diaz-Meco M. T. The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep. 2000;1:399–403. doi: 10.1093/embo-reports/kvd098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sano H., Kane S., Sano E., Miinea C. P., Asara J. M., Lane W. S., Garner C. W., Lienhard G. E. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 34.Berwick D. C., Dell G. C., Welsh G. I., Heesom K. J., Hers I., Fletcher L. M., Cooke F. T., Tavare J. M. Protein kinase B phosphorylation of PIKfyve regulates the trafficking of GLUT4 vesicles. J. Cell Sci. 2004;117:5985–5993. doi: 10.1242/jcs.01517. [DOI] [PubMed] [Google Scholar]