Abstract
FN3K (fructosamine 3-kinase) is a mammalian enzyme that catalyses the phosphorylation of fructosamines, which thereby becomes unstable and detaches from proteins. The homologous mammalian enzyme, FN3K-RP (FN3K-related protein), does not phosphorylate fructosamines but ribulosamines, which are probably formed through a spontaneous reaction of amines with ribose 5-phosphate, an intermediate of the pentose–phosphate pathway and the Calvin cycle. We show in the present study that spinach leaf extracts display a substantial ribulosamine kinase activity (approx. 700 times higher than the specific activity of FN3K in erythrocytes). The ribulosamine kinase was purified approx. 400 times and shown to phosphorylate ribulose-ε-lysine, protein-bound ribulosamines and also, with higher affinity, erythrulose-ε-lysine and protein-bound erythrulosamines. Evidence is presented for the fact that the third carbon of the sugar portion is phosphorylated by this enzyme and that this leads to the formation of unstable compounds decomposing with half-lives of approx. 30 min at 37 °C (ribulosamine 3-phosphates) and 5 min at 30 °C (erythrulosamine 3-phosphates). This decomposition results in the formation of a 2-oxo-3-deoxyaldose and inorganic phosphate, with regeneration of the free amino group. The Arabidopsis thaliana homologue of FN3K/FN3K-RP was overexpressed in Escherichia coli and shown to have properties similar to those of the enzyme purified from spinach leaves. These results indicate that the plant FN3K/FN3K-RP homologue, which appears to be targeted to the chloroplast in many species, is a ribulosamine/erythrulosamine 3-kinase. This enzyme may participate in a protein deglycation process removing Amadori products derived from ribose 5-phosphate and erythrose 4-phosphate, two Calvin cycle intermediates that are potent glycating agents.
Keywords: Amadori products, erythrose 4-phosphate, protein phosphorylation, protein repair, ribose 5-phosphate, ribulosamine/erythrulosamine 3-kinase
Abbreviations: DTT, dithiothreitol; erythruloselysine, erythrulose-ε-lysine; FN3K, fructosamine 3-kinase; FN3K-RP, fructosamine 3-kinase-related protein; N-α-t-Boc-L-lysine, N-α-t-butoxycarbonyl-L-lysine; PEG, poly(ethylene glycol); ribuloselysine, ribulose-ε-lysine
INTRODUCTION
FN3K (fructosamine 3-kinase) is a recently identified mammalian enzyme that phosphorylates fructosamines on their third carbon [1,2]. Fructosamines are formed by the spontaneous reaction of glucose with a primary amine, followed by an Amadori rearrangement. This process, known as glycation, affects proteins and low-molecular-mass compounds [3,4]. Phosphorylation of fructosamines on their third carbon leads to their destabilization [2] and shedding from proteins. FN3K is thereby responsible for deglycation, a new protein repair mechanism that has been shown to take place in human erythrocytes [5].
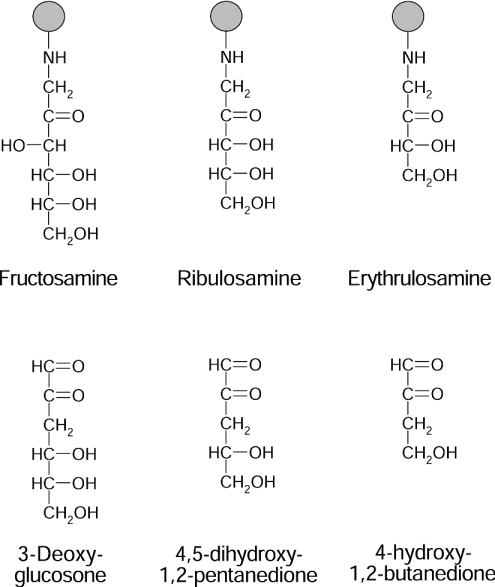
The identification of FN3K-RP (FN3K-related protein), an enzyme that does not phosphorylate fructosamines, but ribulosamines (Figure 1) and, with a 30 times lower affinity, psicosamines (Amadori compounds formed from D-ribose and D-allose respectively), indicated that deglycation also applies to ketoamines derived from sugars other than glucose [6]. The fact that FN3K-RP may remove ribulosamines and psicosamines from proteins was indeed demonstrated in human erythrocytes (one of the richest sources of FN3K-RP and FN3K) incubated with ribose or allose [7]. However, the question remains of how substrates for FN3K-RP form in vivo, particularly because the concentration of free ribose appears to be very low in tissues (G. Delpierre and E. V. Schaftingen, unpublished work) and because allose is a nonphysiological sugar in mammals. What makes this problem particularly intriguing is that FN3K-RP seems to be a more ancient enzyme compared with FN3K, being for instance present in fish, which apparently do not contain FN3K [8]. Phylogenetic studies indicate that the gene duplication event that led to present-day FN3K and FN3K-RP in mammals and birds took place after the fish radiation, and that the rate of evolution of the FN3K gene was faster than that of the FN3K-RP gene, consistent with the acquisition of a new function for FN3K, which phosphorylates fructosamines in addition to ribulosamines and psicosamines [8].
Figure 1. Structures of some ketoamines and of the decomposition product of their 3-phospho-derivatives.
Psicosamines are the C-3-epimers of fructosamines.
It has been proposed [6,8] that ribulosamines could arise indirectly through the reaction of amines with ribose 5-phosphate or ADP-ribose, two extremely potent glycating agents [9,10], followed by the (most probably enzymatic) removal of the phosphate or ADP group bound to C-5 of the ribulose moiety. These ‘pathways’ are still hypothetical at this stage, owing to the difficulty of demonstrating processes that take place at low rates and with low stoichiometries. However, the tissue distribution of FN3K-RP, especially the finding that its activity and those of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase are particularly low in skeletal muscle, provides indirect support for the role of ribose 5-phosphate as a glycating agent in vivo [8].
Ribose 5-phosphate is not only an intermediate of the pentose phosphate pathway, but also of the Calvin cycle. If our hypothesis that FN3K-RP serves to deglycate ribulosamines originating from ribose 5-phosphate is correct, then probably plants might also contain a ribulosamine kinase. The purpose of the present study was to check for the presence of such an enzyme in plants and characterize its function.
EXPERIMENTAL
Materials
DEAE-Sepharose, Q-Sepharose and Sephacryl S-200 were from Amersham Biosciences (Little Chalfont, Bucks., U.K.). Biogel P2 and AG 50W-X4 (100–200 mesh) were from Bio-Rad Laboratories (Hercules, CA, U.S.A.) and Dowex 1-X8 (200 mesh; Cl− form) was from Acros Organics (Geel, Belgium). N-α-t-Boc-L-lysine (N-α-t-butoxycarbonyl-L-lysine) was from Novabiochem (Laüfelfingen, Switzerland) and [1-14C]D-ribose from American Radiolabeled Chemicals (St. Louis, MO, U.S.A).
Preparation of ketoamines
Ribuloselysine (ribulose-ε-lysine) and erythruloselysine (erythrulose-ε-lysine) were synthesized from D-ribose, D-erythrose and N-α-t-Boc-L-lysine essentially as described previously for fructoselysine [5], except that the sugar concentration was 80 mM (D-ribose) and 20 mM (D-erythrose), and that the incubation was performed for 4 h at 50 °C and 1 h at 40 °C respectively. After the removal of the t-Boc protecting group, the products were purified by cation-exchange chromatography and gel-filtration [5]. Paper chromatography in ethylacetate/pyridine/water (12:5:4) [6] showed a unique spot in the case of ribuloselysine and a major spot representing approx. 75% of total sugar in the case of erythruloselysine. The titre of the preparations was first determined by measuring the reducing sugar [11] with glucose as a standard. More precise values, corresponding to 90 and 75% of the titres determined by assaying the reducing sugars, were later obtained with end-point assays using spinach leaf ketoamine kinase and [γ-32P]ATP, followed by separation of the reaction products from unreacted [γ-32P]ATP by anion-exchange chromatography (see below). These new titres were used to compute the values of Michaelis constant (Km) and the rate at infinite concentration of substrate (Vmax).
For the synthesis of [14C]ribuloselysine, 15 μCi of [1-14C]D-ribose and 8 mg of N-α-t-Boc-L-lysine were incubated in 0.8 ml of methanol for 6 h at 50 °C. The sample was then processed as for the unlabelled compound. The yield with respect to radioactivity was approx. 50% (after purification) and the purity was 90%, as assessed by the extent of phosphorylation by human recombinant FN3K [1] or spinach leaf ketoamine kinase.
Fructoselysine and psicoselysine were synthesized as described previously [5,12]. Syntheses of α-glycated amino acids (ribuloseglycine, ribulosevaline and ribuloseleucine) were performed as for ribuloselysine except that the incubation was done under reflux for 2 h. Lysozyme glycated with different sugars was prepared as described previously [6]. For the preparation of lysozyme glycated with erythrose, the sugar concentration was 100 mM, the incubation time was 4 days and the degree of glycation was 4 mol/mol lysozyme.
Purification of ketoamine kinase from spinach leaves
All purification steps were performed at 4 °C and the preparation was stored at −70 °C between steps. Protein concentration was estimated either by measuring absorbance (A280) or by the Bradford assay [13] using bovine γ-globulin as a standard. Spinach leaves (125 g), washed with deionized water, were homogenized in a Waring Blendor with 125 ml of an ice-cold solution containing buffer A [50 mM Hepes (pH 7.1), 2.5 mM DTT (dithiothreitol), 1 μg/ml leupeptin and 1 μg/ml antipain]. The homogenate was filtered through three layers of cheesecloth and the resulting filtrate was centrifuged at 11000 g for 20 min. PEG [poly(ethylene glycol)] 6000 (2%, w/v; Merck, Darmstadt, Germany) was added to the supernatant (200 ml) and the preparation was mixed for 30 min and then centrifuged for 30 min at 11000 g. The PEG concentration of the resulting supernatant was brought to 25% and the preparation was centrifuged after 30 min. The pellet was resuspended in 50 ml of buffer A and centrifuged for 10 min at 11000 g. The supernatant was diluted twice with buffer B [25 mM Hepes (pH 7.1), 2 mM DTT, 2 μg/ml leupeptin and 2 μg/ml antipain] and loaded on to a 25 cm3 DEAE-Sepharose column equilibrated with the same buffer. The column was washed with 50 ml of buffer B and proteins were eluted with a NaCl gradient (0–500 mM in 2×100 ml of buffer B). The fractions containing ribulosamine kinase activity were pooled, brought to 100 ml with buffer C (25 mM glycine, pH 9, 10 mM NaCl, 2 mM DTT, 2 μg/ml leupeptin and 2 μg/ml antipain) and applied to a 20 cm3 Q-Sepharose column equilibrated with the same buffer. The gel was washed with 50 ml of buffer C and proteins were eluted with a 10–400 mM NaCl gradient in 2×100 ml of buffer C. The most active fractions were pooled and concentrated to 2 ml in a 10 ml of Amicon cell equipped with an YM-10 membrane. This sample was loaded on to a 1.8 cm×55 cm Sephacryl S-200 column equilibrated with 25 mM Hepes (pH 7.1), 100 mM NaCl, 2 mM DTT, 4 μg/ml leupeptin and 4 μg/ml antipain. Fractions containing ribulosamine kinase activity were pooled, supplemented with 0.1 mg/ml BSA and stored at −70 °C before use. For the determination of the molecular mass, the Sephacryl S-200 column was calibrated with pyruvate kinase (Mr=237000), lactate dehydrogenase (Mr=144000), glycerol 3-phosphate dehydrogenase (Mr=68000) and cytochrome c (Mr=12500).
Expression and purification of the Arabidopsis thaliana FN3K homologue
The A. thaliana FN3K homologue was produced in Escherichia coli as an N-terminal fusion protein with His6 tag. The coding region was PCR-amplified with Pwo polymerase, using clone U 19045 obtained from the Arabidopsis Biological Resource Center (http://www.arabidopsis.org) as a template, a 5′-primer (GTTCATATGGCTGCAATGAGTGAG) containing the putative ATG codon in an NdeI site (in italics) and a 3′-primer (CCCTAGATCTCTTAAGCTTTGAGCATC) containing the putative stop codon flanked by a BglII site (in italics). The PCR product was subcloned into pBlueScript and the sequence was verified. An NdeI–BglII fragment was then removed from pBlueScript and inserted into pET-15b expression vector, which was used to transform E. coli BL21(DE3) pLysS cells [14].
Expression of the FN3K homologue was performed at 16 °C for 18 h in 50 ml of Luria–Bertani medium containing 100 mg/l ampicillin and 25 mg/l chloramphenicol. The bacterial extract was prepared as described previously [12]. After centrifugation, the supernatant was gently mixed for 20 min at 4 °C with 0.5 ml of Talon® resin equilibrated with buffer D (25 mM Hepes, pH 7.4, 0.5 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml antipain and 300 mM NaCl). The mixture was then transferred on to a column. After washing of the column with 20 ml of buffer D and 10 ml of buffer D containing 10 mM imidazole, the His-tagged protein was eluted with 150 mM imidazole in 5×2 ml of buffer D. The fractions containing A. thaliana FN3K homologue, as determined by SDS/PAGE analysis, were supplemented with 10% (w/v) glycerol and stored at −70 °C.
Measurement of enzymatic activities
All assay media contained 25 mM Hepes (pH 7.1), 1 mM DTT, 1 mM MgCl2 and 1 mM EGTA (assay buffer) and the incubation temperature was 30 °C unless otherwise indicated. Ribuloselysine kinase activity was assayed by the conversion of [14C]ribuloselysine into [14C]ribuloselysine-phosphate in assay buffer (final volume: 40 μl) containing 2 mM ATP-Mg, 8000 c.p.m. [14C]ribuloselysine and the indicated concentration of unlabelled ribuloselysine. The incubation was stopped by the addition of 100 μl of ice-cold 10% (w/v) HClO4. After neutralization with KHCO3, the salts were removed by centrifugation and the supernatant was diluted 6-fold in 10 mM Mes (pH 6). The sample (1 ml) was applied on to an AG 50W-X4 column (Na+ form, 1 ml), which was then washed with 2 ml of the dilution buffer. The flowthrough and washing fractions, containing the phosphorylated [14C]ribuloselysine, were counted for radioactivity.
Phosphorylation of erythruloselysine and other unlabelled ketoamines was assayed through the conversion of [γ-32P]ATP in assay buffer containing 500000 c.p.m. of [γ-32P]ATP, 100 μM ATP-Mg and the indicated concentration of ketoamine. The incubation (40 μl) was stopped by dilution in 1 ml of the ice-cold buffer E containing 10 mM Mes (pH 6) and 200 mM NaCl, and immediate application of the samples on Dowex 1-X8 columns equilibrated with buffer E. The columns were washed with 4 ml of buffer E to elute erythruloselysine-phosphate and inorganic phosphate (see below). Phosphorylation of glycated lysozyme was performed as described previously [1].
Preparation and analysis of the phosphorylation products
Ribuloselysine-phosphate was prepared by incubating 1 μmol ribuloselysine in 1 ml of assay buffer containing 2 mM ATP-Mg (plus 106 c.p.m. of [γ-32P]ATP to facilitate the identification of the phosphorylation product) and 20 m-units of partially purified spinach leaf ribulosamine kinase. After 30 min of incubation at 30 °C, the reaction mixture was loaded on to a Dowex 1-X8 column (1 ml), which was washed with 1 ml of water to elute the phosphorylated (and unphosphorylated) ribuloselysine, separating them from unreacted ATP, which remained bound to the column. Fractions containing the product of interest (2 ml) were then applied on to an AG 50W-X4 column (Na+ form, 1 ml) equilibrated with 10 mM Mes (pH 6) and the column was washed with the same buffer to elute ribuloselysine-phosphate, while unreacted ribuloselysine was retained in the column. The purification was pursued by gel-filtration on Biogel P2 equilibrated with water to separate ribuloselysine-phosphate from salts. A single peak of radioactivity was observed and the richest fraction was analysed by electrospray ionization tandem MS, performed as described previously [15]. Erythruloselysine-phosphate was similarly prepared except that the incubation was performed at 18 °C with 0.1 μmol erythruloselysine and 10 m-units of enzyme in a volume of 0.3 ml.
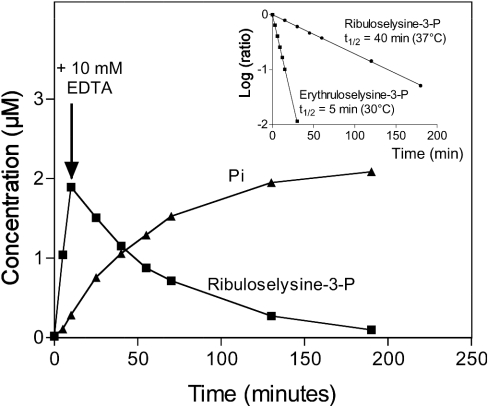
To study the stability of the phosphorylated ribuloselysine, we incubated [14C]ribuloselysine (400000 c.p.m.) in 0.2 ml of assay buffer containing 2 mM ATP-Mg and 1 m-unit of partially purified spinach leaf ribulosamine kinase. After 10 min of incubation at 37 °C, HClO4 was added to a final concentration of 1 M and the incubation was prolonged at 37 °C. At the indicated times, the reaction medium was then loaded on to an AG 50W-X4 column (H+ form) equilibrated with water, and the column was washed with 3 ml of water. The flowthrough and washing fractions were counted for radioactivity. The same experiment was performed to study the degradation of the compound at neutral pH, but 10 mM EDTA was added instead of HClO4. After the indicated times (shown in Figure 5), 30 μl of samples were diluted in 1 ml of 10 mM HCl and analysed by chromatography on AG 50W-X4 columns as explained above. The stability of 32P-labelled phosphorylation products was also studied. For this purpose, we performed the same incubation as described above but with unlabelled ribuloselysine (1 mM) or erythruloselysine (0.1 mM), 10 μM ATP-Mg and 2×106 c.p.m. of [γ-32P]ATP. In the case of erythruloselysine, the incubation was performed at 30 °C and the time before adding HClO4 or EDTA was decreased to 6 min. The reaction media (30 μl) were diluted in 1 ml of 10 mM Mes (pH 6) and applied on to Dowex 1-X8 columns equilibrated with the same buffer. The columns were washed with 3 ml of Mes buffer to elute ketoamine-phosphates and then with 4 ml of 200 mM NaCl in the same buffer to elute inorganic phosphate.
Figure 5. Stability of the putative ribuloselysine 3-phosphate and erythruloselysine 3-phosphate.
Ribulose-lysine was phosphorylated with the spinach leaf enzyme and [γ-32P]ATP. After 10 min, the reaction was stopped by adding 10 mM EDTA and the incubation was prolonged at 37 °C for the indicated times. The products (ribuloselysine 3-phosphate and inorganic phosphate) were analysed by anion-exchange chromatography. The same experiment was performed with erythruloselysine, but at 30 °C and using shorter incubation times. The inset shows the results expressed as the decimal logarithm of the ratio of the concentration of ketoamine-phosphates at the indicated times on their concentration when EDTA was added.
To prepare the quinoxaline derivatives of the degradation products of ribuloselysine and erythruloselysine 3-phosphate, 0.2 μmol of each ketoamine was incubated for 4 h at 37 °C (ribuloselysine) or 30 °C (erythruloselysine) in 0.1 ml of assay buffer containing 2 mM ATP-Mg and 1 m-unit of spinach leaf enzyme. The reaction was stopped by the addition of one volume of acetic acid. o-Phenylenediamine was added to a final concentration of 1 mM and the mixture was incubated for 30 min at 50 °C. The quinoxaline derivatives were purified by reverse-phase HPLC with detection at 320 nm, and the major peak was analysed by MS (see above).
RESULTS
Identification of a ketoamine kinase in spinach leaves
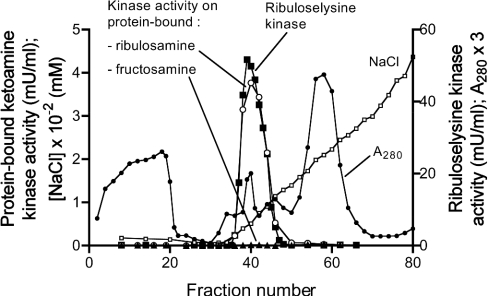
To check for the presence of a ketoamine kinase in plants, a PEG pellet derived from a spinach leaf extract was applied on DEAE-Sepharose and the phosphorylation of lysozyme glycated with glucose or ribose was tested in the fractions of this column. As shown in Figure 2, a peak of enzyme phosphorylating protein-bound ribulosamines, but not protein-bound fructosamines was eluted with the salt gradient. This kinase also phosphorylated free [14C]ribuloselysine, which was used to monitor the enzymatic activity in further purification steps, i.e. chromatography on Q-Sepharose at pH 9 and gel filtration on Sephacryl S-200. Ribulosamine kinase was eluted from the latter column with an apparent Mr of 25000. Using this procedure, the enzyme was purified approx. 400 times to a specific activity of 174 units/g of protein and with a yield of 6% (Table 1). The preparation still contained several polypeptides at this purification stage, as indicated by SDS/PAGE analysis (results not shown), but was free of ATPase activity (which could potentially interfere in assays using radiolabelled ATP).
Figure 2. Chromatography of a spinach leaf extract on DEAE-Sepharose.
A 25% PEG pellet resuspended in buffer B was applied to a DEAE-Sepharose column. Protein was eluted with a linear NaCl gradient and fractions of 5 ml (1–25) or 3 ml (26–80) were collected. Ketoamine kinase activity was assayed using lysozyme glycated with glucose (▲) or ribose (○) and [γ-32P]ATP, or with [14C]ribuloselysine and unlabelled ATP (■). A280 was also measured.
Table 1. Purification of spinach leaf ketoamine kinase.
| Step | Volume (ml) | Protein (g) | Total activity (m-unit)* | Specific activity (unit/g) | Purification | Yield (%) |
|---|---|---|---|---|---|---|
| Leaf extract | 200 | 6.1 | 2819 | 0.5 | 100 | |
| 25% PEG pellet | 50 | 2.9 | 1590 | 0.5 | 1 | 56 |
| DEAE-Sepharose | 17 | 0.12 | 873 | 7 | 15 | 31 |
| Q-Sepharose | 10 | 0.01 | 342 | 34 | 74 | 12 |
| Sephacryl S-200 | 7 | 0.001 | 183 | 174 | 379 | 6 |
* One unit of enzyme is the amount that catalyses the phosphorylation of one μmol of ribuloselysine/min in the presence of 100 μM ribuloselysine.
Substrate specificity of the spinach leaf enzyme
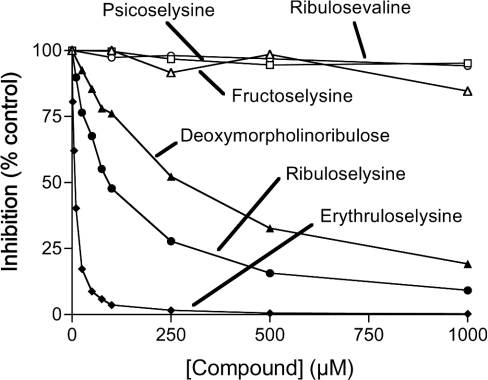
The purified enzyme phosphorylated free ribuloselysine with a Km of 119 μM and a Vmax of 398 μmol·min−1·(g of protein)−1. To identify other substrates of the enzyme, we tested whether several unlabelled low-molecular-mass ketoamines could inhibit the phosphorylation of [14C]ribuloselysine by competing with this substrate (Figure 3). Neither fructosamines (fructoselysine and deoxymorpholinofructose) nor psicoselysine affected the enzymatic activity at concentrations up to 1 mM, whereas two ribulosamines, deoxymorpholinoribulose and unlabelled ribuloselysine, exerted 50% inhibition at 250 and 100 μM respectively. Even more potent inhibition was observed with the tetrose derivative, erythruloselysine (50% inhibition at 5 μM). No inhibition was observed with ribulosamines bound to the α-amino groups of glycine, valine or leucine, or with D-ribulose or D-erythrulose (all tested at 1 mM).
Figure 3. Inhibition of the phosphorylation of [14C]ribuloselysine by different unlabelled ketoamines.
The phosphorylation of [14C]ribuloselysine by spinach leaf ribulosamine kinase was tested in the presence of different concentrations of the indicated compounds. Deoxymorpholinofructose, ribuloseglycine, ribuloseleucine, ribulose and erythrulose were also devoid of effect (results not shown). Results expressed are means for two experimental values.
The results described above suggested that erythruloselysine was a good substrate for the spinach leaf enzyme. However, measuring the phosphorylation of this erythrulosamine was made difficult by the fact that no radiolabelled tetrose is commercially available and that the erythrulosamine-phosphate formed by the enzyme spontaneously broke down with a half-life of a few minutes at 30 °C (see below). Phosphorylation of free erythruloselysine was therefore quantified by measuring the conversion of [γ-32P]ATP into compounds that are eluted from anionexchangers with 200 mM NaCl (sum of erythrulosamine-phosphate and inorganic phosphate), whereas higher salt concentrations are needed to elute ATP. Using this method, the spinach leaf enzyme was found to phosphorylate erythruloselysine with a Km of 8 μM and a Vmax of 325 μmol·min−1·g of protein−1 (Table 2), i.e. a catalytic efficiency approx. 12 times higher than that observed with ribuloselysine. The same method, applied to other compounds (all at 1 mM), indicated that ribuloseglycine, ribulosevaline and ribuloseleucine were phosphorylated at 5–10% of the rate observed with 1 mM ribuloselysine, whereas no detectable phosphorylation (<1% of the rate observed with ribuloselysine) was observed with fructoselysine, deoxymorpholinofructose, D-ribulose and D-erythrulose.
Table 2. Kinetic properties of plant ketoamine kinases.
Data are the means for two values. The preparations of enzyme used were purified spinach leaf enzyme and recombinant A. thaliana FN3K/FN3K-RP homologue expressed in E. coli.
| Spinach leaf enzyme | A. thaliana FN3K homologue | |||
|---|---|---|---|---|
| Km (μM) | Vmax (μmol·min−1·g−1) | Km (μM) | Vmax (μmol·min−1·g−1) | |
| Ribuloselysine | 119 | 398 | 135 | 685 |
| Erythruloselysine | 8 | 325 | 25 | 749 |
| Lysozyme glycated with ribose | 659 | 72 | 274 | 43 |
| Lysozyme glycated with erythrose | 279 | 39 | 104 | 20 |
The ability of the spinach leaf enzyme to phosphorylate protein-bound ketoamines was tested by measuring the incorporation of 32P from [γ-32P]ATP into lysozyme glycated with different sugars. The purified enzyme did not phosphorylate protein-bound fructosamines, psicosamines or ribulosamine 5-phosphates, but did act on protein-bound ribulosamines and erythrulosamines (results not shown). In both cases, we determined the stability of the reaction product by stopping the incubation with EDTA to chelate Mg2+, an indispensable cofactor for kinases, and pursuing the incubation at neutral pH and 30 °C or 37 °C. Half-lives of 25 min at 37 °C and 5 min at 30 °C were observed with the phosphorylation products of protein-bound ribulosamines and erythrulosamines respectively (results not shown). Owing to the lability of the phosphorylation products, the kinetic constants were determined at 30 °C with short incubation times (30 s and 2 min in the case of erythrulosamines and ribulosamines respectively). Lower apparent Km and Vmax values were observed with protein-bound erythrulosamines when compared with protein-bound ribulosamines (Table 2).
Characterization of the phosphorylation products of ribuloselysine and erythruloselysine
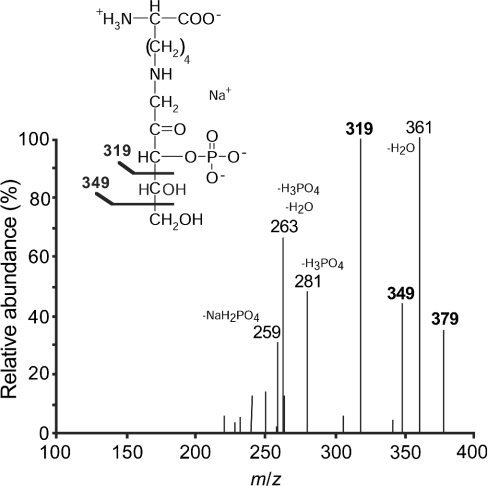
To identify the carbon phosphorylated by the spinach leaf enzyme, the phosphorylation products of ribuloselysine and erythruloselysine were partially purified by ion-exchange chromatography and gel-filtration, and analysed by MS. As expected for phosphorylated ribuloselysine, the mass spectrum indicated a major deprotonated molecular ion [M−H]− at m/z 357 with its sodium adduct [M−2H+Na]− at m/z 379. Fragmentation of this latter species led to anions at m/z 349 and 319 (Figure 4), corresponding to the loss of C-5 and C-4–C-5 of the sugar moiety respectively. Fragments were also clearly detected at m/z 361 (loss of water), 281 (loss of H3PO4), 263 (loss of H3PO4 and water) and 259 (loss of NaH2PO4). In the case of erythruloselysine-phosphate (results not shown), fragmentation of the m/z 349 ion corresponding to the deprotonated molecular ion of its monosodium form [M−2H+Na]−, yielded anions with m/z 331 (loss of water), 319 (loss of C-4), 251 (loss of H3PO4) and 229 (loss of NaH2PO4).
Figure 4. Tandem MS analysis of the phosphorylation product of ribuloselysine by spinach leaf ribulosamine-kinase.
The fragmentation spectrum of the deprotonated, monosodium molecular ion at m/z 379 is shown. The structure of ribuloselysine 3-phosphate and the assignment of the main peaks are also shown.
The stability of both compounds was also tested at acidic and neutral pH. For this purpose, [32P]ribuloselysine-phosphate and [32P]erythruloselysine-phosphate were prepared by incubating the spinach leaf enzyme with the appropriate ketoamines and [γ-32P]ATP. Incubation of these products in 1 M HClO4 at 37 °C for 1 h did not result in any significant decomposition of the radiolabelled compounds, as indicated by the fact that the radioactivity was still completely retained on cation-exchangers at acidic pH and 0 °C (results not shown). In contrast, both products were unstable at neutral pH, degrading with half-lives of 40 min at 37 °C and 5 min at 30 °C in the case of the pentose and tetrose derivatives respectively (Figure 5). In both cases, the radioactive product resulting from the degradation could be extracted quantitatively as a phosphomolybdic complex in butyl acetate [16], indicating that it was inorganic phosphate (results not shown). Furthermore, experiments with [14C]ribuloselysine-phosphate, labelled on its sugar moiety, showed that degradation of this compound led to a sugar derivative that was no longer retained on cation-exchangers at acidic pH, indicating that the bond between the ε-amino group of lysine and C-1 of the sugar moiety had been disrupted.
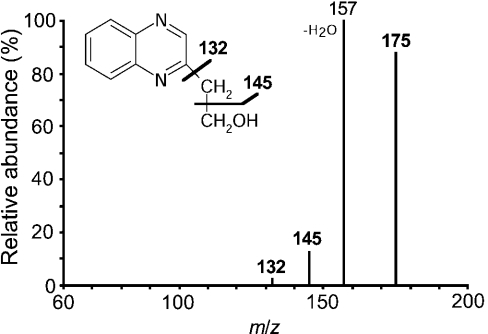
It was previously shown that on degradation of fructoselysine 3-phosphate, the sugar moiety is liberated as 3-deoxyglucosone (Figure 1) [2]. To test the possibility that α-dicarbonyl compounds were also liberated from ribuloselysine 3-phosphate and erythruloselysine 3-phosphate, the reaction mixture obtained after a 4 h incubation of spinach leaf FN3K-RP with the appropriate ketoamine and ATP was incubated with o-phenylenediamine, an agent that complexes dicarbonyl compounds to form quinoxaline derivatives. In both cases, the formation of a quinoxaline derivative was indicated by the appearance of a compound with an absorption peak at approx. 320 nm [17] in amounts (calculated using methylglyoxal as a standard) that nearly matched the amounts of inorganic phosphate, another degradation product. Both quinoxaline derivatives were purified by HPLC, appearing in the chromatograms as major peaks, which were analysed by MS. In the case of the pentose derivative (results not shown), a major protonated molecular ion [M+H]+ at m/z 205 was detected in the mass spectrum; this latter underwent, on fragmentation, losses of m/z 18 (water), 36 (two molecules of water), 48 (water and CHOH), 60 (2×CHOH) and 73 (dihydroxypropyl radical), best explained by a quinoxaline derivative formed from 4,5-dihydroxy-1,2-pentanedione (Figure 1). For the product formed from erythruloselysine 3-phosphate (Figure 6), the mass spectrum indicated a major protonated molecular ion [M+H]+ at m/z 175, which yielded fragments corresponding to losses of m/z 30 (CHOH), 18 (water) and 43 (hydroxyethyl radical), best explained by a hydroxyethylquinoxaline structure resulting from the condensation of o-phenylenediamine with 4-hydroxy-1,2-butanedione (Figure 1).
Figure 6. Tandem MS analysis of the quinoxaline derivative of the erythruloselysine-phosphate degradation product.
Erythruloselysine was incubated for 4 h at 30 °C with spinach leaf ketoamine 3-kinase and ATP, and the resulting mixture was treated with o-phenylenediamine. The quinoxaline derivative was purified by HPLC and analysed by tandem MS. The fragmentation spectrum of the protonated molecular ion at m/z 175 and the structure of hydroxyethylquinoxaline are shown.
Database analysis and properties of the A. thaliana homologue of FN3K/FN3K-RP
Database searches indicated that the A. thaliana genome comprises only one gene homologous to FN3K and FN3K-RP. A cDNA sequence encoding an Oryza sativa homologue is also available in the databanks. As shown in Figure 7, the last approx. 290 residues of these plant proteins share approx. 37% sequence identity with human FN3K and FN3K-RP. Interestingly, the N-terminus is poorly conserved between the two plant enzymes, which suggests that it may serve as a targeting signal for an organelle. Predictions made with the TargetP server (http://www.cbs.dtu.dk/services/TargetP/) for the A. thaliana and O. sativa proteins gave scores of 0.473 and 0.941 for a chloroplast targeting peptide, 0.394 and 0.297 for a mitochondrial transit peptide and 0.149 and 0.009 for a signal peptide respectively. This suggests that the enzyme is quite probably targeted to the chloroplast, at least in O. sativa. High scores (≥0.9) for a chloroplast-targeting peptide were also obtained with protein sequences derived from expressed sequence tags of Hordeum vulgare, Sorghum bicolor and Zea mays.
Figure 7. Alignment of human FN3K and FN3K-RP with homologous proteins from A. thaliana and O. sativa.
Conserved residues are indicated in bold. The following sequences are shown: A. thaliana (At) NP_191667; O. sativa (Os) AK066948; human FN3K (FHs) Q9H479; human FN3K-RP (RHs) Q9HA64.
A vector driving the expression of the processed form of the A. thaliana homologue of FN3K/FN3K-RP in fusion with an N-terminal His6 tag was prepared and used to express this protein in E. coli. Incubation of the cells at 16 °C allowed the expression of a partially soluble protein, with the expected molecular mass (∼33000), as indicated by SDS/PAGE analysis. This protein, which was purified by chromatography on Talon® column to near-homogeneity (results not shown), catalysed the phosphorylation of ribuloselysine and erythruloselysine (Table 2), but did not act on fructoselysine. It also phosphorylated lysozyme glycated with ribose or with erythrose (Table 2), but not with glucose or allose (results not shown).
DISCUSSION
Catalysed reaction and identity
The spinach leaf enzyme characterized in the present work phosphorylates ribulosamines and erythrulosamines on the third carbon of their sugar moiety, as indicated by the following observations. MS analysis of the phosphorylation product of ribuloselysine allows one to exclude phosphorylation on C-4 or C-5, since a fragment of m/z 60, corresponding to C-4 and C-5 can be lost independently of the phosphate group. Similarly, the loss of an m/z 30 fragment excludes C-4 as the phosphorylated carbon in erythruloselysine-phosphate. Phosphorylation of C-2 in ribuloselysine-phosphate can be excluded because furanosyl-phosphates are extremely acid-labile [18,19], whereas the phosphorylation product of ribuloselysine was acid-stable. C-2 is also unlikely to be phosphorylated in erythruloselysine, because the sugar moiety of this compound is too short to form a hemiketal. Finally, phosphorylation on the third carbon best accounts for the spontaneous conversion of the ribuloselysine-phosphate and erythruloselysine-phosphate into inorganic phosphate and 2-oxo-3-deoxyaldoses. A similar reaction has been shown to occur with fructoselysine 3-phosphate, which is converted into lysine, inorganic phosphate and 3-deoxyglucosone [2]. The absence of a hydroxyl group on C-3 of the degradation product is the stigma of phosphate elimination from this carbon.
The stability of ketoamine 3-phosphates at neutral pH is highly dependent on the size of their sugar moiety, since half-lives of 8 h, 25 min and <5 min have been observed for derivatives of hexoses, pentoses and tetroses respectively ([2,7]; the present study). This suggests that the spontaneous degradation of ketoamine 3-phosphates proceeds from their linear conformation. The latter is the only possible conformation for erythrulosamines, and, on the other hand, a minor, nearly undetectable conformation of hexulosamines [2,6]. The proportion of the open-chain form is quite probably an intermediate in the case of ribulosamines, probably of the order of 20% by analogy with ribulose [20].
The finding that the recombinant A. thaliana homologue of FN3K/FN3K-RP has kinetic properties similar to those of the spinach leaf enzyme indicates that plant ketoamine 3-kinases are homologous to mammalian FN3K and FN3K-RP. The plant enzymes phosphorylate low-molecular-mass and protein-bound erythrulosamines and ribulosamines, but not fructosamines or psicosamines, indicating that they have a narrower specificity than their mammalian homologues, which use hexose, pentose [6] and tetrose (J. Fortpied and E. V. Schaftingen, unpublished work) derivatives as substrates. The common property of all enzymes of the FN3K family appears therefore to be, at least in eukaryotes (prokaryotes have not yet been tested), their ability to phosphorylate ribulosamines and erythrulosamines. This was presumably the function of the common ancestor of these proteins, the FN3K activity being a late acquisition of one of the two isoenzymes present in mammals and birds [8].
Physiological function
The answer to the question of the physiological function of plant ketoamine 3-kinase can only be tentative at this stage. At least two hypotheses can be formulated. The first one is that this enzyme catalyses one of the steps in the biosynthesis of a metabolite playing a physiological role in plants. However, there is no obvious candidate for such a metabolite. Furthermore, no known enzymatic pathway leads to the formation of a compound structurally similar to ribuloselysine or erythruloselysine.
The second hypothesis is that the plant enzymes serve to deglycate compounds with a primary amine and most particularly proteins that have reacted with ribose, erythrose or one of their derivatives. It is worth recalling that ribose 5-phosphate [9] and erythrose 4-phosphate (J. Fortpied and E. V. Schaftingen, unpublished work) are potent glycating agents that react with amines at rates approximately three orders of magnitude higher than glucose, owing to the fact that they are to a larger extent (ribose 5-phosphate), or even completely (erythrose 4-phosphate), in their acyclic form. Ribose 5-phosphate and erythrose 4-phosphate are expected to be most abundant where the Calvin cycle takes place, i.e. in chloroplasts, which are the predicted localization of plant ketoamine 3-kinase in several species. As plant ketoamine 3-kinase does not appear to act on ribulosamine 5-phosphates, we postulate that an unidentified phosphatase converts them in vivo into substrates of the FN3K/FN3K-RP homologue.
Another potent glycating agent that deserves consideration is ADP-ribose, which may form through hydrolysis of poly(ADP-ribose) or NAD+ [21,22]. ADP-ribulosamines could also potentially be converted into ribulosamines through removal of their ADP moiety. The higher catalytic efficiency of plant ketoamine 3-kinase on erythrulosamines as compared with ribulosamines fits better, however, with their involvement in correcting glycation initiated by Calvin cycle intermediates.
If this deglycation mechanism takes place, plants should contain 4,5-dihydroxy-1,2-pentanedione and/or 4-hydroxy-1,2-butanedione. Interestingly, Hauck et al. [23] while studying the formation of 4,5-dihydroxy-2,3-pentanedione, a precursor of the fruit flavour norfuraneol, isolated from tomatoes the quinoxaline derivative of another dihydroxypentanedione, which was present in 20-fold larger amounts than the norfuraneol precursor. The fact that on fragmentation, the quinoxaline derivative of this more abundant isomer lost a fragment of m/z 73 suggests that it corresponds to 4,5-dihydroxy-1,2-pentanedione. It would be of interest to confirm this structure and search also for the 4-hydroxy-1,2-butanedione derivative in plants. It would be also interesting to know more about enzymes that metabolize these two dicarbonyl compounds, most particularly since they are likely to react readily with arginine residues, like other dicarbonyl compounds such as methylglyoxal and 3-deoxyglucosone [24,25].
Spinach leaves have an approx. 700-fold higher ribulosamine kinase activity when compared with the specific activity of FN3K in human erythrocytes [1], one of the richest sources of FN3K (and FN3K-RP) in mammals [8]. This suggests that the glycation ‘stress’ in plants is increased compared with that caused by glucose in mammalian tissues. Plants may therefore be interesting models to identify other enzymes involved in deglycation, most particularly the hypothetical ribulosamine 5-phosphatase. Furthermore, plant ketoamine 3-kinase may be useful to tag protein-bound ribulosamines with [γ-32P]ATP, thereby facilitating their identification. FN3K has been similarly used to identify glycated sites in haemoglobin [26].
Acknowledgments
We thank Dr H. Batoko for providing the A. thaliana clone U 19045 and Dr G. Delpierre for helpful comments. This work was supported by the Directorate General – Higher Education and Scientific Research, French Community of Belgium, the Fund for Medical Scientific Research, the Interuniversity Attraction Poles Program, Belgian Science Policy and the European Foundation for the Study of Diabetes. J.F. is a Fellow of the Fonds pour l'Encouragement à la Recherche dans l'Industrie et dans l'Agriculture.
References
- 1.Delpierre G., Rider M. H., Collard F., Stroobant V., Vanstapel F., Santos H., Van Schaftingen E. Identification, cloning, and heterologous expression of a mammalian fructosamine-3-kinase. Diabetes. 2000;49:1627–1634. doi: 10.2337/diabetes.49.10.1627. [DOI] [PubMed] [Google Scholar]
- 2.Szwergold B. S., Howell S., Beisswenger P. J. Human fructosamine-3-kinase: purification, sequencing, substrate specificity, and evidence of activity in vivo. Diabetes. 2001;50:2139–2147. doi: 10.2337/diabetes.50.9.2139. [DOI] [PubMed] [Google Scholar]
- 3.Hodge J. E. The Amadori rearrangement. Adv. Carbohyd. Chem. 1955;10:169–205. doi: 10.1016/s0096-5332(08)60392-6. [DOI] [PubMed] [Google Scholar]
- 4.Baynes J. W., Watkins N. G., Fisher C. I., Hull C. J., Patrick J. S., Ahmed M. U., Dunn J. A., Thorpe S. R. The Amadori product on protein: structure and reactions. Prog. Clin. Biol. Res. 1989;304:43–67. [PubMed] [Google Scholar]
- 5.Delpierre G., Collard F., Fortpied J., Van Schaftingen E. Fructosamine 3-kinase is involved in an intracellular deglycation pathway in human erythrocytes. Biochem. J. 2002;365:801–808. doi: 10.1042/BJ20020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collard F., Delpierre G., Stroobant V., Matthijs G., Van Schaftingen E. A mammalian protein homologous to fructosamine-3-kinase is a ketoamine-3-kinase acting on psicosamines and ribulosamines but not on fructosamines. Diabetes. 2003;52:2888–2895. doi: 10.2337/diabetes.52.12.2888. [DOI] [PubMed] [Google Scholar]
- 7.Collard F., Wiame E., Bergans N., Fortpied J., Vertommen D., Vanstapel F., Delpierre G., Van Schaftingen E. Fructosamine 3-kinase-related protein and deglycation in intact erythrocytes. Biochem. J. 2004;382:1–7. doi: 10.1042/BJ20040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delplanque J., Delpierre G., Opperdoes F. R., Van Schaftingen E. Tissue distribution and evolution of fructosamine 3-kinase and fructosamine 3-kinase related protein. J. Biol. Chem. 2004;279:46606–46613. doi: 10.1074/jbc.M407678200. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S. D., Pandey B. N., Mishra K. P., Sivakami S. Amadori product and age formation during nonenzymatic glycosylation of bovine serum albumin in vitro. J. Biochem. Mol. Biol. Biophys. 2002;6:233–242. doi: 10.1080/10258140290031554. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson E. L., Cervantes-Laurean D., Jacobson M. K. Glycation of proteins by ADP-ribose. Mol. Cell. Biochem. 1994;138:207–212. doi: 10.1007/BF00928463. [DOI] [PubMed] [Google Scholar]
- 11.Park J. T., Johnson M. J. A submicrodetermination of glucose. J. Biol. Chem. 1949;181:149–151. [PubMed] [Google Scholar]
- 12.Wiame E., Van Schaftingen E. Fructoselysine 3-epimerase, an enzyme involved in the metabolism of the unusual Amadori compound psicoselysine in Escherichia coli. Biochem. J. 2004;378:1047–1052. doi: 10.1042/BJ20031527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 14.Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 15.Delpierre G., Vanstapel F., Stroobant V., Van Schaftingen E. Conversion of a synthetic fructosamine into its 3-phospho derivative in human erythrocytes. Biochem. J. 2000;352:835–839. [PMC free article] [PubMed] [Google Scholar]
- 16.McClard R. W. Synthesis and purification of [1-32P]fructose-1,6-bisphosphate with high specific radioactivity. Anal. Biochem. 1979;96:500–503. doi: 10.1016/0003-2697(79)90612-2. [DOI] [PubMed] [Google Scholar]
- 17.Li R., Kenyon G. L. A spectrophotometric determination of alpha-dicarbonyl compounds and its application to the enzymatic formation of alpha-ketobutyrate. Anal. Biochem. 1995;230:37–40. doi: 10.1006/abio.1995.1434. [DOI] [PubMed] [Google Scholar]
- 18.Van Schaftingen E., Hue L., Hers H. G. Fructose 2,6-bisphosphate, the probable structure of the glucose- and glucagon-sensitive stimulator of phosphofructokinase. Biochem. J. 1980;192:897–901. doi: 10.1042/bj1920897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalckar H. M. The enzymatic synthesis of purine ribosides. J. Biol. Chem. 1947;167:477–486. [PubMed] [Google Scholar]
- 20.Wu J., Serianni A. S., Vuorinen T. Furanose ring anomerization: kinetic and thermodynamic studies of the D-2-pentuloses by 13C-NMR spectroscopy. Carbohyd. Res. 1990;206:1–12. doi: 10.1016/0008-6215(90)84001-b. [DOI] [PubMed] [Google Scholar]
- 21.Howard M., Grimaldi J. C., Bazan J. F., Lund F. E., Santos-Argumedo L., Parkhouse R. M., Walseth T. F., Lee H. C. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- 22.Uchida K., Suzuki H., Maruta H., Abe H., Aoki K., Miwa M., Tanuma S. Preferential degradation of protein-bound (ADP-ribose)n by nuclear poly(ADP-ribose) glycohydrolase from human placenta. J. Biol. Chem. 1993;268:3194–3200. [PubMed] [Google Scholar]
- 23.Hauck T., Hubner Y., Bruhlmann F., Schwab W. Alternative pathway for the formation of 4,5-dihydroxy-2,3-pentanedione, the proposed precursor of 4-hydroxy-5-methyl-3(2H)-furanone as well as autoinducer-2, and its detection as natural constituent of tomato fruit. Biochim. Biophys. Acta. 2003;1623:109–119. doi: 10.1016/j.bbagen.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Lo T. W., Westwood M. E., McLellan A. C., Selwood T., Thornalley P. J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994;269:32299–32305. [PubMed] [Google Scholar]
- 25.Konishi Y., Hayase F., Kato H. Novel imidazolone compound formed by the advanced Maillard reaction of 3-deoxyglucosone and arginine residues in proteins. Biosci. Biotech. Biochem. 1994;58:1953–1955. [Google Scholar]
- 26.Delpierrre G., Vertommen D., Communi D., Rider M. H., Van Schaftingen E. Identification of fructosamine residues deglycated by fructosamine-3-kinase in human hemoglobin. J. Biol. Chem. 2004;279:27613–27620. doi: 10.1074/jbc.M402091200. [DOI] [PubMed] [Google Scholar]