Abstract
Ophiocordyceps sinensis, also known as DongChong XiaCao (DCXC) in China, is a complex of the parasitic fungus Hirsutella sinensis and its caterpillars host living in extreme alpine environments on the Qinghai-Tibetan Plateau. Wild resources of O. sinensis are threatened by over-harvesting due to its perceived high medicinal value. In recent years, numerous studies have pointed out that endofungal bacteria play an important role in fungal spore germination and zygote formation. In this sense, studying the endofungal bacteria of O. sinensis is of great interest regarding the conservation of this species. In this study, we investigated the community structure (abundance and rare sub-communities) and function of the soil-mycelial-sclerotia-stromata continuum endofungal bacteria in O. sinensis from the Qilian Mountain region of the Tibetan Plateau by using amplicon and macro-genome sequencing technologies. Based on the results, rare taxa exhibited more differences among the components, and enrichment and co-occurrence network analyses revealed that abundant taxa played a more important role. We further found that endofungal bacteria in external mycelial cortices have unique community structures and functions. In particular, they play an important role in material cycling, potentially providing essential nutrients during the lifecycle of O. sinensis. We successfully isolated 52 endofungal bacterial strains using high-throughput isolation techniques, some of them were undetected by high-throughput sequencing. We systematically investigated the structure and function of endofungal bacteria of the O. sinensis, providing a solid foundation for the cultivation and conservation of wild resources of this species at an industrial scale.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-025-03793-z.
Keywords: Qinghai-Tibet Plateau, Ophiocordyceps Sinensis, Microbial community and function, External mycelial cortices, Cultivation
Introduction
Ophiocordyceps sinensis (syn. Cordyceps sinensis), also termed DongChong XiaCao (DCXC) in China, is a complex of the parasitic fungus Hirsutella sinensis and its caterpillar host of the genus Thitarodes [1]. The lifecycle of O. sinensis can be divided into three stages: the infection of anamorph mycelia, sclerotium formation in the caterpillar, and the formation of the stromata [2]. The O. sinensis is a highly valued traditional Chinese medicine for treating chronic kidney disease. Recent studies have isolated various active components from the O. sinensis, with anti-inflammatory, antioxidant, and antitumor activities [3, 4]. Due to the strict symbiosis of O. sinensis with its host and its harsh living environment (mainly in alpine meadows of the Qinghai-Tibetan plateau (QTP) and surrounding areas at an elevation of 4,000 m), its resources are limited [5]. Because of its high medicinal and economic value, its wild resources are subject to over-collection and, therefore, threatened [6]. Because of this, O. sinensis has been included in the China Biodiversity Red List [7]. Although initial progress has been made in the cultivation of O. sinensis, there are still many constraints to industrial production [6].
Many studies point to the important role of endofungal microbiome in fungal fruit bodies. These studies mainly focused on the community structure of the endofungal microbiome of fungal fruit bodies and found that endofungal microbiome are essential in fungal spore germination, mycelial growth, and even zygote formation (sterile environments can limit the formation of zygotes) [8, 9]. Studies on O. sinensis have also revealed that endofungal microbiome play an essential role in fungal infection as well as sclerotia and stroma formation [10–13]. Up to now, over 600 fungal strains have been isolated, and over 200 novel bioactive metabolites have been isolated from these fungal strains [3, 14, 15]. However, studies on the isolation of endofungal bacteria and the functions of related strains are scarce. The traditional method for isolating endofungal bacteria from fungal fruit bodies is the plate dilution [16]. Although many endofungal bacteria have been isolated using this method, it is time-consuming and laborious. In recent years, new methods have been developed, such as the in situ culture method, termed “isolation chip method” [17] and the high-throughput isolation and identification technology, which combines bacterial culture and high-throughput sequencing technology to improve the isolation efficiency [18]. These novel techniques can be promising in the isolation of O. sinensis endofungal bacteria.
With the continuous advancement of sequencing technology and cost reduction, recent studies have attempted to study the community structure of endofungal microbiome of O. sinensis by high-throughput sequencing (HTS). These studies divided the O. sinensis into three parts: stromata, sclerotia, and external mycelial cortices covering the larvae’s surface (external mycelial cortices) and found that the microbial community structure of the external mycelial cortices was significantly different from that within the stromata and sclerotia and the adhering soil [11, 19–21]. Among the fungal community, Ascomycota and Basidiomycota are dominant phyla [11, 22], whereas Proteobacteria, Acidobacteria, Bacteroidetes, Actinobacteria, and Firmicutes are dominant bacterial phyla [20, 23]. In another study, the O. sinensis collected from different sites did not significantly differ in terms of their endofungal microbiome community structure [20]. Another study observed differences in the endofungal microbiome community structure of wild and artificially grown O. sinensis [3]. In recent years, studies on the community structure of endofungal bacteria in O. sinensis have revealed that the most abundant bacterial phyla in the O. sinensis external mycelial cortices and adjacent soils are also those most widely distributed (e.g., Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria) [23]. Zhang et al. (2021) also found that a few high-abundance taxa dominated the endofungal microbiome community of Cordyceps militaris [24]. Under natural conditions, communities are generally dominated by a few highly abundant taxa [25]. Many studies have focused on high-abundance taxa when investigating the microbial community structure. However, with the continuous progress of sequencing and analysis technologies, a growing number of studies reported that taxa with extremely low abundances (rare taxa, accounting for less than 0.01% of relative abundance) also play an essential ecological role [26–29]. This makes it necessary to study the community structure of rare O. sinensis endofungal microbiome taxa. Previous studies also used amplicon sequencing data to predict microbial functions. Based on the results, the microbes in the external mycelial cortices play essential roles in material recycling (exchange of nutrients between microorganisms and the environment within the cotyledons) and larvae decomposition [6, 20]. However, these authors mainly used 16S and ITS data and existing genomic data to make functional predictions, which greatly limits the results. Therefore, it is currently only possible to predict the functions of microbes with important roles in human health and industrial production [30]. In this sense, functional studies of O. sinensis endofungal microbiome based on metagenome sequencing are necessary.
In this study, we focus on the structure of endofungal bacteria communities (including abundant and rare taxa) inhabiting different components of O. sinensis (stromata, sclerotia, external mycelial cortices, and the soil in the relative alpine meadow (Fig. 1a) of the Qilian Mountains on the northeastern QTP. To this end, we employed metagenomic sequencing techniques to study the microbial community structure and functions of the endofungal bacteria. In addition, we used high-throughput cultivation and identification method to isolate endofungal bacteria from various parts of O. sinensis. The objectives were as follows: (1) To determine whether the bacterial distribution patterns of abundant and rare taxa of endofungal bacterial in the O. sinensis differ among different components of the O. sinensis. While the focus of this study is on endofungal microbes, analyzing bacterial communities from the surrounding soil is critical to understanding the microbial recruitment process. Soil bacteria represent a potential source of endofungal microbes, and comparing these communities provides insights into microbial selection, enrichment, and functional roles within the fungal environment.; (2) to investigate the differences in endofungal bacteria functions among the different components of O. sinensis via metagenomic sequencing; (3) to evaluate the potential of high-throughput isolation and identification techniques in isolating endofungal bacteria, with a focus on determining whether this method can capture a broader diversity of bacterial strains, including those not detectable by amplicon sequencing. Given the lack of studies employing traditional methods to isolate similar endofungal bacteria, direct comparisons were not feasible; however, this study will provides valuable insights into the capabilities and potential applications of this novel method.
Fig. 1.
Diagram of external mycelial cortices, stromata, and sclerotia of O. sinensis. Distribution of the sequencing number of different components (external mycelial cortices, stromata, and sclerotia) and communities (b whole community, c abundant sub-community, and d rare sub-community) in the O. sinensis. Bacteria enriched in various components of O. sinensis as demonstrated by LEfSe analysis (e OTU level, f bacterial phylum level)
Methods
Overall, 20 O. sinensis specimens (5 for high-throughput sequencing and 15 for the isolation of endofungal bacteria) were collected throughout the study area (99.09604°N,38.60455°E, 4,578 m above sea level). The dominant plant species at the sampling sites were Kobresia humilis, Elymus nutans, Stipa aliena, Festuca ovina, Polygonum viviparum, and Leontopodium japonicum. The 50-year means of temperature and rainfall in the area were − 2.93℃ and 399.8 mm, respectively [31]. The lifecycle of O. sinensis consists of the following stages: (1) spore release and germination, (2) infection and internal growth, (3) overwintering, (4) stromata formation, and (5) spore production [32]. The O. sinensis was divided into two parts (sclerotia and stromata), and the external mycelial cortices were carefully stripped from the fruit bodies (Fig. 1a). We sterilized the surface of O. sinensis to eliminate the influence of exohyphal microorganisms associates on the experimental results as follows: firstly, the sections were rinsed five times with sterilized water, after which the samples were immersed in 70% ethanol for 30 s, then in 5% sodium hypochlorite for 5 min, and finally rinsed five times in sterile water, and the final rinsed sterile water was inoculated on R2A medium for overnight incubation at 28 ℃ to verify the sterilization effect. The sterilization method was confirmed to be effective, as no microbial growth was observed on the R2A medium following overnight incubation at 28 °C with the final rinsed sterile water. Five soil samples were collected at a depth of 0–15 cm from a distance of 30 cm with O. sinensis as the center point and were used for sequencing to compare microbial communities in the soil with those in O. sinensis components and sieved through a 2-mm screen; roots and stones were removed manually. All samples were transferred to the laboratory in self-sealing bags and stored at −20 °C. The total DNA from each sample was extracted using a fast DNA SPIN Kit for Soil (MP Biomedicals, California), following the manufacturer´s recommendations. Amplicons (V3–V4 of bacterial 16S) were sequenced using the primers 341 F (CCT ACG GGN GGC WGC AG) and 805R (GAC TAC HVG GGT ATCT AAT CC) at Sangon Biotech Co. The PCR products were purified using EasyPure Quick Gel Extraction Kits (TransGen Biotech, Beijing, China), and libraries were constructed using the TruSeqTM DNA Sample Prep Kit (Illumina, San Diego, CA). Amplicon sequencing data were analyzed using USEARCH v. 10 and Qiime2, with the following steps: (1) Primers and low-quality (shorter than 200 bp or with a quality score (Q) < 30) sequences were removed using USEARCH, and forward and reverse reads were combined into a single sequence; (2) sequences were clustered into operational taxonomic units (OTUs) based on a 97% clustering threshold; (3) the taxonomy of the OTUs was analyzed using the RDP Classifier against the SILVA database (release 138. for bacteria). The definitions of abundant (with relative abundance > 0.1%) and rare taxa (with relative abundance < 0.01%) followed previous studies [26, 33]. The amplicon sequencing data were submitted to GenBank under the accession number PRJNA1045735.
For the metagenomic sequencing, sclerotia, stromata, and external mycelial cortices of O. sinensis were included. The low-quality raw metagenomics sequences were filtered using fastp (v. 0.23.1) based on a minimum Q Score of 20 and a minimum sequence length of 50 bp. We employed IDBA-UD v. 1.1.1 to assemble clean reads into scaffolds with default parameters. Scaffolds longer than 500 bp were used to predict open reading frames (ORFs) in MetaGeneMark (v. 0.43). After clustering at 95% similarity, the ORFs were used to construct a nonredundant gene catalog through MMseqs2 [34]. This catalog was searched against the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (release 80.1) by DIAMOND (v. 0.922.123) [34], with an E-value < 1e−10 to annotate the predicted genes to clusters of KEGG Orthology (KO) groups. The metagenome sequencing data were submitted to GenBank under the accession numbers PRJNA1045735.
For high-throughput isolation and identification of bacterial strains, we used the techniques developed by Zhang et al. (2021) [18], including the determination of dilution concentrations, two rounds of PCR, and the determination of the barcode for each plate and well, library construction, high-throughput sequencing, and analysis of the data obtained using the online platform Culturome (https://github.com/YongxinLiu/ Culturome) and a GUI web server (http://bailab.genetics.ac.cn/culturome/). The high-throughput sequencing data were submitted to GenBank under the accession numbers PRJNA1047261, and for isolated bacterial strains were OR855850-OR855902.
The α-diversity indices of the microbial communities were calculated in R (http://www.r-project.org.) with the vegan package [35]. One-way analysis of variance (ANOVA) was performed to test the significance of the differences between rare and abundant taxa, using the software package SPSS v. 26.0. The software STAMP (v. 2.1.3) was employed to evaluate the relative abundances of abundant and rare taxa, and also the abundances of KO functional categories for different groups, based on the Kruskal–Wallis test (p < 0.01) (using the Benjamini-Hochberg false discovery rate (FDR) correction). Based on the OTU level, the bacterial networks were analyzed using the online integrated Network Analysis Pipeline (iNAP, http://mem.rcees.ac.cn:8081/). Pair-wise relationships among the OTUs were determined by SparCC analysis at a SparCC correlation coefficient > 0.3 and a significance level of p > 0.05. The total number of edges (connections between nodes) and the degrees of co-occurrence (the number directly related to nodes) were used to deduce the network topology. Network modular analysis was conducted in Gephi with default parameters. The top 10 hubs with high degree and low closeness centrality values were identified, and the networks were visualized using Gephi (v. 0.10.0) [36]. To test for significant KO functional category enrichment in different parts of O. sinensis, ternary plots were constructed using the ggtern package [37] in the vcd package in R, and the heatmaps were constructed using the pheatmap package (v. 1.0.12) in R.
Results
Based on the high-throughput sequencing results, 1,694,889 16S rRNA sequences were obtained from 20 samples, and 4,561 OTUs were identified. There was no significant distribution trend in the number of sequences in the whole community and the abundant sub-community in different components (Fig. 1b, c). However, in the rare sub-community, we found a decreasing trend in the number of sequences in the soil-mycelial-sclerotia-stromata continuum (49,500, 23,671, 13,149, and 4,447 sequences each) (Fig. 1d). Only 122 abundant OTUs (2.70%) were generated, but they accounted for 72.54% of the relative abundance; 2,980 rare bacterial OTUs (65.3%) accounted for 9.61% of the total relative abundance (supplemental material).
At the phylum level, the whole community and the abundant sub-community were similar in terms of species composition, i.e., Proteobacteria dominated all samples, followed by Acidobacteria, Verrucomicrobia, and Bacteroidetes (Fig. 1b, c). Regarding the rare taxa, there was a homogeneous distribution of endofungal bacterial species without clearly dominant phyla (Fig. 1d). At the OTU level, we found nine OTUs enriched in soil (belonging to Proteobacteria (3), Acidobacteria (4), and Verrucomicrobia (2)) and six OTUs enriched in the sclerotia (all Proteobacteria), stromata (Proteobacteria), and external mycelial cortices (Proteobacteria) (Fig. 1e). Those 15 OTUs were all obtained from the abundant sub-community. Most bacterial phyla (Acidobacteria, Verrucomicrobia, Actinobacteria, Chloroflexi, Nitrospirae, and Parcubacteria, among others) were enriched in the soil (Fig. 1e). In contrast, four bacterial phyla (Bacteroidetes, Planctomycetes, Candidate_division_WPS_1, Armatimonadetes) were enriched in the external mycelial cortices. Only Proteobacteria were enriched in the stromata (Fig. 1f). Most OTUs (over 97%) were shared among the different parts of the whole community and the abundant sub-community (Fig. S1a, b). A different situation was observed for the rare sub-community, which showed more unique OTUs among the components (Fig. S1c). For example, 344, 250, 88, and 20 rare taxa OTUs were unique in the soil, sclerotia, external mycelial cortices, and stromata, respectively. In terms of α-diversity, we found no significant differences between soil and external mycelial cortices and between sclerotia and stromata in the whole and abundant communities. However, the α-diversity indices of the external mycelial cortices were significantly different compared to the sclerotia and stromata in the rare sub-community (Fig. 2a, b, c). In the abundant sub-community, such differences were rarely observed. We found similar results for ß-diversity (NMDS and ANOSIM analysis); in the abundant sub-community, there was a closer relationship among the different components (R = 0.63, p < 0.001) (Fig. 2d, e, f).
Fig. 2.
The α (Chao1, observed OTUs, ACE, Shannon, and Simpson index) in each component (external mycelial cortices, stromata, and sclerotia) and communities (a whole community, b abundant sub-community, and c rare sub-community) of O. sinensis. Non-metric multidimensional scaling (NMDS) ordinations of Bray-Cutis dissimilarity and Anosim (Analysis of similarities) in each component (external mycelial cortices, stromata, and sclerotia) and communities (d whole community, e abundant sub-community, and f rare sub-community) of O. sinensis
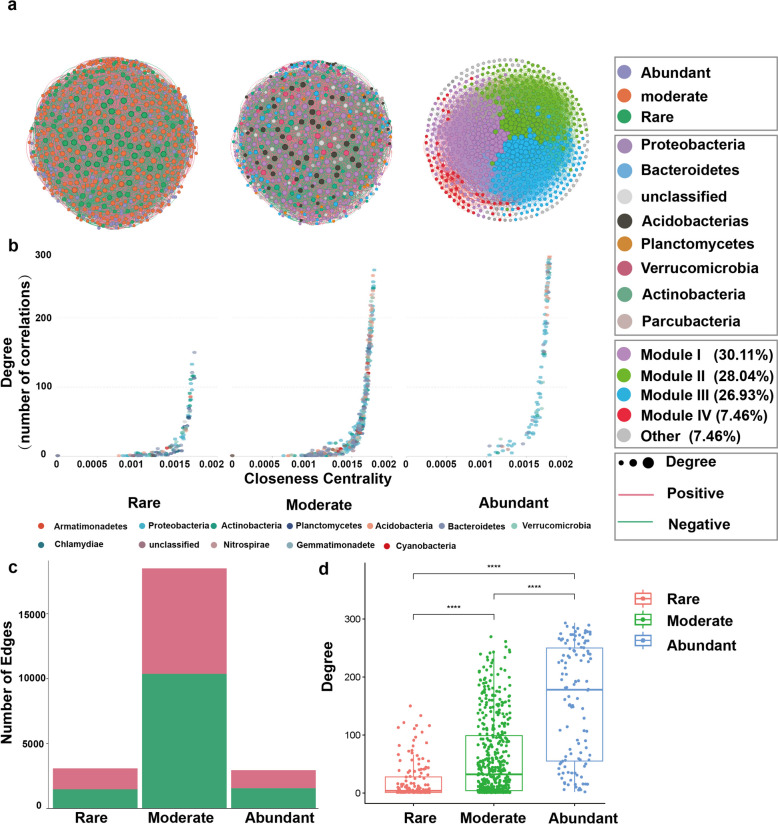
Microbial co-occurrence network analysis showed 724 nodes, of which 150 belonged to the rare sub-community, 458 to the moderate sub-community, and 116 to the abundant sub-community (Fig. 3a and Table S1). Degree (average 21 (rare), 61 (moderate), and 157 (abundant)) and closeness centrality (mean 0.0011 (rare), 0.0014 (moderate), 0.0016 (abundant)) of the rare sub-community were significantly lower than those of the moderate and abundant sub-communities. In addition, the moderate and abundant sub-communities had higher numbers of edges (Fig. 3b, d and Table S1). The opposite pattern was found for the rare sub-community, where 1,453 positive edges and 1,640 negative edges were detected (Fig. 3c and Table S1). For the abundant sub-community; these nodes were mainly from Proteobacteria and Acidobacteria (Table S2). We also examined the top 10 hubs of the rare and moderate sub-communities separately. The top 10 hubs were more diverse in the rare sub-community, with Bacteroidetes, Proteobacteria, Actinobacteria, and Planctomycetes (Table S2). The bacterial co-occurrence network was divided into four main modules, with Module #1 accounting for 33.39%, Module #2 accounting for 25.41%, Module #3 accounting for 21.38%, and Module #4 accounting for 3.62% of the whole network. These modules were mainly composed of moderate taxa; in terms of species, the main taxon was Proteobacteria. Also, there were more abundant than rare taxa (except in Module #2) (Fig. 3a and Table S2).
Fig. 3.
The co-occurrence networks of the different components in the O. sinensis. a, the nodes are colored according to sub-communities, bacterial phylum, and modules. Node size is proportional to the number of connections (degree). b, comparison of node-level topological features in Fig. 3a (degree and closeness centrality) demonstrating the high degree and closeness centrality for the hub taxa. The edges (c) and degree (d) distribution of bacterial taxa in the different sub-communities
The NMDS ordination of the KO functional categories indicated that the functional position of the external mycelial cortices was significantly different from that of the sclerotia and stromata. At the same time, there was no significant difference in the sclerotia and stromata (Fig. 4a). The results of the metagenome sequencing of different O. sinensis components (external mycelial cortices, sclerotia, and stromata) showed that most KO functional categories were enriched in the external mycelial cortices (576). In contrast, the KO functional categories enriched in the sclerotia (117) were few, and no KO functional categories were found enriched in the stromata (Fig. 4b). The categories “Cancer: overview”, “Cancer: specific types”, “Cardiovascular disease” affiliated to “Human Diseases”, “Cell motility”, “Cellular community - prokaryotes” affiliated to “Cellular Processes”, “Endocrine system”, “Environmental adaptation”, “Excretory system”, “Immune system” affiliated to “Organismal Systems”, and “Metabolism of cofactors and vitamins”, “Metabolism of other amino acids” affiliated to “Metabolism” were only enriched in the external mycelial cortices. In contrast, the categories “Infectious disease: bacterial” and “Infectious disease: bacterial” affiliated to “Human Diseases” were only enriched in the sclerotia (supplemental material).
Fig. 4.
Functional profiles of microbiomes in the O. sinensis. a, Nonmetric multidimensional scaling (NMDS) ordination of KEGG Orthology. b Ternary plot of KEGG functional categories. The size of each circle represents its relative abundance (weighted average), and the position represents its relative abundance with respect to each component. Colored circles KEGG functional categories represent enriched in one component compared with the others (blue in the mycelial, cortices, green in the sclerotia, orange in the stromata, and gray is not significantly enriched). c The comparison of KEGG pathways between different components based on the Kruskal-Wallis H test
The relative abundances of metabolism-related pathways such as “Metabolic pathways”, “Biosynthesis of secondary metabolites”, “Microbial metabolism in diverse environments”, and “Carbon metabolism” were significantly higher in the external mycelial cortices than in the other two components (Fig. 4c). The relative abundances of disease-related pathways such as “Amyotrophic lateral sclerosis“, “Huntington disease“, and “Carbon metabolism” were significantly higher in the sclerotia and the stromata (Fig. 4c). We further investigated the distribution of C, N, and P cycle-related genes in different parts of O. sinensis. The abundances of carbon fixation-related functional genes (e.g., pccA and smtA) and carbon degradation-related genes (e.g., cdh and abfa) were significantly higher in the external mycelial cortices compared to the other compartments (Fig. S2). Similarly, nitrate reduction genes (e.g., narG and narH), nitrogen assimilation genes (nasA and nasB), and denitrification genes (e.g., nirS and nosZ) showed the highest abundances in the external mycelial cortices (Fig. S3). Genes related to organic P mineralization (e.g., phnX and ugpQ), P transport system (e.g., phnC and phnE), and inorganic P solubilization (gcd) also showed the highest abundances in the external mycelial cortices (Fig. S4).
High-throughput isolation and identification processes involved 80 96-well plates, with a total 864 wells; based on the rarefaction curve, the number of wells was sufficient (Fig. 5a). The results of species annotation after high-throughput sequencing showed that the 279 ASVs belonged to 4 bacterial phyla, 7 orders, 16 classes, 42 families, and 82 genera (Fig. 5b). Proteobacteria, Actinobacteria, Firmicutes, and Bacteroidetes were the dominant phyla. The dominant classes were Actinobacteria, α-Amastigotes, and γ-Amastigotes, and the dominant genera were Nocardioides, Pseudomonas, and Arthrobacter. In addition, 4 bacterial families and 29 bacterial genera were not detected in the high-throughput sequencing (from the environmental samples).
Fig. 5.
The results of bacterial isolation based on High-throughput cultivation and identification method. a Rarefaction curve of ASVs based on the number of wells containing bacteria. The curve reaches the plateau stage, indicating that a sufficient number of plates were used for high-throughput bacterial isolation. B The phylogenetic tree showing the taxonomic distribution of cultivated bacteria. The colored inner ring represents the bacterial strains successfully isolated on plate media
We isolated 52 bacterial strains from those wells, which belonged to 4 phyla, 7 orders, 13 classes, 17 families, 26 genera, and 40 species (Table S4). When comparing these results to those obtained via high-throughput sequencing, we isolated all bacterial phyla and classes; in particular, 81.2% of the orders, 40.5% of the families, and 31.7% of the genera were isolated. Additionally, the isolated genera Caballeronia, Dyella, Galbitalea, Microterricola, Paenarthrobacter, Plantibacter, Rhodopseudomonas, and Tardiphaga were not detected via high-throughput sequencing (from the environmental samples). The dominant phyla were Sphingobacteriia, Actinomycetia, Alphaproteobacteria, and Gammaproteobacteria, and the dominant classes were Sphingobacteriia, Actinomycetia, Alphaproteobacteria, and Gammaproteobacteria. The dominant genera were Pedococcus (16 strains), Pseudomonas (6 strains), Paenarthrobacter (5 strains), and Tardiphaga (5 strains). At the species level, the dominant species were Pedobacter steynii (7), Tardiphaga robiniae (5), Pseudomonas umsongensis (3), Pedobacter nototheniae (3), and Paenarthrobacter nicotinovorans (3). Strain T1P1C2 is a suspected new species (98.45% similarity to the type species in the database) (Table S4).
Discussion
Previous studies on the structure of environmental microbial communities tended to study abundant sub-communities. Nevertheless, taxa with extremely low relative abundances, which have been neglected in previous studies, also play essential roles in the microbial community [33]. In the present study, we examined the abundant and rare sub-communities in the different components of O. sinensis. The bacterial phylum Proteobacteria was dominant in the whole and abundant sub-communities of all compartments (consistent with the findings of other studies based on different environments) [29, 38], which is also consistent with other findings on the endofungal bacterial community of the O. sinensis [11, 19, 20, 23]. Proteobacteria show high abundances and a wide range of distribution in various environments, mainly due to their wide ecological niche width and because they are less restricted by dispersal limitation than those of the rare taxa [26, 39]. In the rare sub-community, we found a more homogeneous distribution pattern masked by high-abundance species. The same phenomenon was found when analyzing α and β diversity, i.e., compared to whole and abundant sub-communities, we found more differences in the rare sub-community among the different components. According to a previous study, the number of rare taxa sequences from the soil-mycelial-sclerotia-stromata continuum is progressively decreasing as the soil provides the bacteria with the relative optimum of moisture and nutrients [24]. Also, the endofungal bacteria of the O. sinensis need to be tolerant to the antimicrobial substances produced by the fungus while coping with limited nutrient levels, which results in lower relative abundances and numbers of sequences compared to those of the soil [22, 24]. This distribution pattern is more similar to the two-step recruitment model of plant microorganisms [40–43] and different from the amplification-selection model [44], which may be mainly due to the difference in the sequencing strategy (the former is based on the relative abundance, while the latter is based on the absolute abundance), and it is necessary to adopt the absolute abundance-based approach for further studies on the distribution pattern of O. sinensis and endosymbiotic microorganisms. The responses of the microbial communities from the different components to the changes in the biotic and abiotic factors in the soil-root continuum also differ, which gives insights into further studies on the selection of microorganisms by hosts, the responses of microbial communities to changes in environment factors, and the assembly processes of the corresponding communities in the soil-mycelial-sclerotia-stromata continuum.
In this study, the results of the lefse analysis showed that most OTUs were enriched in the soil, and these OTUs were all from the abundant sub-community. Those OTUs enriched in the different components of O. sinensis were annotated to bacterial phyla such as Proteobacteria, Acidobacteria, Verrucomicrobia and Firmicutes. Those bacteria have essential functions in the decomposition of materials, the prevention and control of pathogens, and the promotion of plant growth [45–47]. Therefore, the bacteria enriched in the different components of O. sinensis may play essential roles in the infestation of the fungus and the decomposition of the insects; they also maintain the stability of the microbial community. The unique OTUs were almost all from the rare community, suggesting that abundant taxa play a fundamental function in each community. This explains why they were enriched and shared by each component. Rare taxa provide a high species diversity and play an important role when the community faces perturbations. According to previous studies, a significant portion of the rare taxa in a microbial community will be rapidly transformed into abundant taxa under extreme environmental changes, such as rising temperatures, increased freeze-thaw cycles, altered precipitation patterns, and shifts in soil nutrient dynamics can lead to significant internal changes, such as disruptions in water content, osmotic pressure, and nutrient availability within the O. sinensis [29, 33, 48].
According to the alpha diversity data, there were significant differences between mycelial cortices and the other two components of O. sinensis in the rare sub-communities, suggesting that the microbial community in the mycelial cortices is unique in terms of structure and functions. There were no differences between the sclerotia and stromata because these two components were initially of the same structure [24]. Although previous studies point out that there are no differences in alpha diversity among the different O. sinensis components [6, 22], these studies only considered the whole bacterial community and ignored the rare taxa, and the differences may have been masked by those taxa.
Microbial co-occurrence networks are now widely used in microbial community ecology studies to analyze the potential interactions among microbes and to reveal the underlying ecological processes [49]. Previous studies in various environments have shown that moderate and rare taxa play a more critical role in the network than generally assumed [29, 50, 51]. In the microbial co-occurrence network of this study, we found that although moderate (458) and rare (150) taxa possessed higher numbers of nodes, abundant taxa had significantly higher degrees than the other two taxa, whereas all hubs were also from abundant taxa. This suggests that abundant taxa may play a more critical role in maintaining the stability of the topology of the co-occurrence network (taxa with many connections, potentially are key players in the microbial community). We also analyzed the top 10 hubs in the rare taxa and found that their species diversity was higher. The abundant sub-community consists mainly of Proteobacteria and Acidobacteria, which are the most important components of the endophytic bacterial community of both plant and fungal fruit bodies and perform the most fundamental ecological functions, e.g., some Proteobacteria synthesize phytohormones (e.g., growth factors.) [52], some Proteobacteria produce antibiotics to inhibit the growth of phytopathogenic bacteria [53]. Acidobacteria are involved in the decomposition of organic matter in the soil and in nutrient cycling, and in particular, they play an important role in carbon cycling [54]. we conclude that in the soil-mycelial-sclerotia-stromata continuum, abundant taxa play a fundamental role in maintaining the base function (including nutrient cycling, stress tolerance, symbiotic interaction, etc.) and stability of the community. In contrast, different rare taxa are selected in different components according to the environmental conditions and functional needs, for example, the rare taxa uniquely associated with the external mycelial cortices of o. sinensis include families such as Xanthomonadaceae, Verrucomicrobiaceae, Planctomycetaceae, Rhodospirillaceae, Chitinophagaceae, and Anaerolineaceae. Although present in low relative abundance, these taxa are likely to perform critical ecological functions within the microbial community. The Xanthomonadaceae contributes to nutrient cycling through its roles in organic matter degradation and secondary metabolite production [55]. Likewise, Verrucomicrobiaceae is involved in polysaccharide degradation, aiding in the breakdown of complex organic materials in the environment surrounding o. sinensis [56].
Microbial community ecology is not only the study of microbial distribution patterns and influencing factors but, more importantly, the study of the functions of the microbial community [57]. Previous studies on the functions of endofungal bacteria in the O. sinensis used amplicon data. These studies pointed out that the functions of O. sinensis endofungal bacteria are mainly “metabolic pathways” as well as “environmental information processing“, and that “membrane transport” helps O. sinensis acclimatization to the plateau environment [20]. These studies also found a higher abundance of degradation pathways in the external mycelial cortices, indicating an essential role in host invasion. However, amplicon-based predictions of microbial functions are limited by primer preferences and the abundance of reference genomes in the database and, thus, may not provide a realistic picture of community functions [58]. In the present study, we used metagenome sequencing to investigate the microbial community functions in different compositions of O. sinensis, and our findings were similar to those regarding the endofungal bacterial community composition of O. sinensis. The functions of microbial communities in the external mycelial cortices differed from those in the sclerotia and stromata. Numerous KO functional categories were only enriched in the external mycelial cortices, suggesting that these components have a richer functionality than the other two components. Further analysis showed that the relative abundances of C, N, and P cycle-related genes were highest in the external mycelial cortices, which indicated that the bacterial community in this component may provide essential nutrients for O.sinensis during infestation, colonization, and reproduction. These results have not been reported in previous prediction-based studies. This may provide an additional way for O. sinensis to obtain nutrients other than only from decomposing larvae, ensuring that O. sinensis can cope with the harsh environment of the QTP, including extreme temperatures, low oxygen levels, and limited availability of essential nutrients. Under these conditions, the ability of the associated microbial community to utilize a diverse range of nutrient sources likely serves as a critical adaptive strategy [59]. Similar to previous studies, many of the KO functional categories enriched in the sclerotia and stromata were designated as disease categories, indicating that endofungal bacteria are involved in the infestation of the host by O. sinensis, this result is similar to some previous studies.
The endofungal bacteria of fungal fruit bodies play an essential role in promoting fungal mycelial growth, spore germination, and fruit body formation [60]. Metagenomic approaches (16S amplicons and shotgun sequencing) have been used to study microbial communities and functions. These direct sequencing methods can rapidly obtain information on the composition and functions of microbial communities, along with genome-wide information, without the need for pure microbial cultures [61]. Recently, metagenomic sequencing technology has led to significant advances in understanding the composition and function of endofungal microbiome communities of fungal fruit bodies, including Agaricus pseudolutosus [13], Russula decolorans [62], Amylocystis lapponica [63], and entomopathogenic fungi such as O. sinensis [19, 23] and O. highlandensis [64]. However, those methods are limited by the complexity of the community, the primer set bias, the biomass, the sequencing technology, and the reference databases [65]. The isolation and culture of environmental microorganisms is an indispensable core resource in studying the functions and mechanisms of single microbial strains [18]. However, although many new bacterial species have been isolated using the traditional method (gradient dilution method), and some species not obtained by high-throughput sequencing can be obtained at the same time [65], this method also has its shortcomings. In particular, it is time-consuming and labour-intensive, the fast-growing species will often cover up the slow-growing ones, and sanger sequencing requires pure cultures [18]. In this study, we performed high-throughput isolation techniques to isolate and characterize O. sinensis endofungal bacteria, obtained a total of 279 ASVs, and successfully isolated and identified 52 bacterial strains from them. A large proportion of bacteria not detected via high-throughput sequencing, and potentially new bacterial species were also obtained, demonstrating the high efficiency of this method in isolating endofungal bacteria bacteria. This method has advantages over the gradient dilution method and other newly developed methods for cultureomics (e.g., isolation chip), such as not requiring additional equipment and eliminating duplicate picking of single colonies belonging to the same ASV, significantly improving efficiency. However, this method also has shortcomings. For example, it is not suitable for culturing bacterial strains that cannot grow in liquid medium, and the low success rate of transferring strains from liquid to solid medium in 96-well plates (only 52 bacterial strains were successfully obtained from 279 ASVs in this study) is also a major challenge that needs to be overcome urgently. Based on the results obtained in this study, the combination of high-throughput sequencing and cultureomics approaches is essential in future studies to obtain complete information on microbial communities in the environment.
Conclusion
In this study, we systematically investigated the structure and functions of endofungal bacteria communities in O. sinensis using amplicon and metagenome sequencing technologies. Bacterial diversity in the rare sub-community showed greater differences in the soil-mycelial-sclerotia-stromata continuum. We found that the structure and functions of the bacterial communities in the external mycelial cortices differed significantly from those of the other components. Bacteria in external mycelial cortices may play an essential role in the infestation and colonization of H. sinensis. In addition, we isolated 52 bacterial strains annotated to 40 bacterial species (including one potentially new bacterial species) using a high-throughput isolation method, which will lay a solid foundation for future microbe-insect interaction studies and the large-scale cultivation of O. sinensis, with the overall aim of protecting O. sinensis habitats.
Supplementary Information
Acknowledgements
This study was financially supported by Qinghai Provincial Science and Technology Major Project [No. 2023-SF-A5], West Light Foundation, Chinese Academy of Sciences, the Special fund for Qilian Mountain National Park [QHTX-2020-004], Long-term National Scientific Research Base of Qilian Mountain National Park, xining 810000, Qinghai, China.
Authors’ contributions
ContributionsNa Li and Jiani Li conceived this study. Rui Xing designed the research. Zhilin Feng and Zhihua Wu performed the experiments. Qingo gao and Jiuli Wang analyzed experimental data. Na Li, Jiani Li, and Rui Xing wrote the paper together. Shilong Chen review and editing. Na Li and Jiani Li contributed equally to this work and should be considered co-first authors. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by Qinghai Provincial Science and Technology Major Project [No. 2023-SF-A5], West Light Foundation, Chinese Academy of Sciences, the Special fund for Qilian Mountain National Park [QHTX-2020-004], Long-term National Scientific Research Base of Qilian Mountain National Park, xining 810000, Qinghai, China.
Data availability
The datasets generated during the current study are available in the GenBank under the accession numbers PRJNA1047261 and OR855850-OR855902.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Na Li and Jiani Li contributed equally to this work.
References
- 1.Zhou X-W, Li L-J, Tian E-W. Advances in research of the artificial cultivation of Ophiocordyceps sinensis in China. Crit Rev Biotechnol. 2014;34(3):233–43. 10.3109/07388551.2013.791245. [DOI] [PubMed] [Google Scholar]
- 2.Zhong X, Gu L, Wang H, Lian D, Zheng Y, Zhou S, et al. Profile of Ophiocordyceps sinensis transcriptome and differentially expressed genes in three different mycelia, sclerotium and fruiting body developmental stages. Fungal Biol. 2018;122(10):943–51. 10.1016/j.funbio.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Guo H, Du Z, Liu XZ, Che Y, Ye X. Ecology-based screen identifies new metabolites from a Cordyceps-colonizing fungus as cancer cell proliferation inhibitors and apoptosis inducers. Cell Prolif. 2009;42(6):838–47. 10.1111/j.1365-2184.2009.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yue K, Ye M, Zhou Z, Sun W, Lin X. The genus cordyceps: a chemical and pharmacological review. J Pharm Pharmacol. 2013;65(4):474–93. 10.1111/j.2042-7158.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 5.Dai Y, Wu C, Yuan F, Wang Y, Huang L, Chen Z, et al. Evolutionary biogeography on Ophiocordyceps sinensis: an indicator of molecular phylogeny to geochronological and ecological exchanges. Geosci Front. 2020;11(3):807–20. 10.1016/j.gsf.2019.09.001. [Google Scholar]
- 6.Sun T, Zou W, Luo R, Li C, Zhang C, Yu H. Compositional and functional diversities of core microbial communities in wild and artificial Ophiocordyceps sinensis. Int Microbiol. 2023. 10.1007/s10123-023-00333-5. [DOI] [PubMed] [Google Scholar]
- 7.Feng K, Peng X, Zhang Z, Gu S, He Q, Shen W, et al. iNAP: an integrated network analysis pipeline for microbiome studies. iMeta. 2022;1(2): e13. 10.1002/imt2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noble R, Dobrovin-Pennington A, Hobbs PJ, Pederby J, Rodger A. Volatile C8 compounds and pseudomonads influence primordium formation of Agaricus bisporus. Mycologia. 2009;101(5):583–91. 10.3852/07-194. [DOI] [PubMed] [Google Scholar]
- 9.Bonfante P, Anca IA. Plants, mycorrhizal fungi, and bacteria: a network of interactions. Annu Rev Microbiol. 2009;63:363–83. 10.1146/annurev.micro.091208.073504. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Wang Z, Liu L, Xia Y, Cao Y, Yin Y. Analysis of the intestinal Microflora in Hepialus gonggaensis larvae using 16S rRNA sequences. Curr Microbiol. 2008;56(4):391–6. [DOI] [PubMed] [Google Scholar]
- 11.Zhang CB, Ren CH, Wang YL, Wang QQ, Wang YS, Weng QB. Uncovering fungal community composition in natural habitat of Ophiocordyceps sinensis using high-throughput sequencing and culture-dependent approaches. BMC Microbiol. 2020;20(1):331. 10.1186/s12866-020-01994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu-Ling LI, Yi-Sang Y, Zong-Hao Z, Xin L, Hai-Feng XU, Shao-Li MA, et al. Synergy of fungal complexes isolated from the intestines of Hepialus lagii larvae in increasing infection potency. J Fungal Res. 2016;14(02):96–112. 10.13341/j.jfr.2014.1067.
- 13.Xing R, Zhang H-C, Gao Q-b, Zhang F-q, Chi X-F, Chen S-l. Bacterial communities associated with mushrooms in the Qinghai-Tibet Plateau are shaped by soil parameters. Int Microbiol. 2023;26(2):231–42. 10.1007/s10123-022-00286-1. [DOI] [PubMed] [Google Scholar]
- 14.Guo H, Sun B, Gao H, Chen X, Liu S, Yao X, et al. Diketopiperazines from the cordyceps-colonizing fungus Epicoccum nigrum. J Nat Prod. 2009;72(12):2115–9. 10.1021/np900654a. [DOI] [PubMed] [Google Scholar]
- 15.Yongjie Z, Bingd S, Shu Z, Mu W, Xingzhong L, Wenfeng G, et al. Mycobiotal investigation of natural Ophiocordyceps sinensis based on culture-dependent investigation分离自冬虫夏草可培养真菌的多样性研究. 菌物学报. 2010;29(4):518–27. [Google Scholar]
- 16.Lewis WH, Tahon G, Geesink P, Sousa DZ, Ettema TJG. Innovations to culturing the uncultured microbial majority. Nat Rev Microbiol. 2021;19(4):225–40. 10.1038/s41579-020-00458-8. [DOI] [PubMed] [Google Scholar]
- 17.Berdy B, Spoering AL, Ling LL, Epstein SS. In situ cultivation of previously uncultivable microorganisms using the ichip. Nat Protoc. 2017;12(10):2232–42. 10.1038/nprot.2017.074. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Liu Y-X, Guo X, Qin Y, Garrido-Oter R, Schulze-Lefert P, et al. High-throughput cultivation and identification of bacteria from the plant root microbiota. Nat Protoc. 2021;16(2):988–1012. 10.1038/s41596-020-00444-7. [DOI] [PubMed] [Google Scholar]
- 19.Xia F, Liu Y, Guo M-Y, Shen G-R, Lin J, Zhou X-W. Pyrosequencing analysis revealed complex endogenetic microorganism community from natural DongChong XiaCao and its microhabitat. BMC Microbiol. 2016;16(1):196. 10.1186/s12866-016-0813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia F, Zhou X, Liu Y, Li Y, Bai X, Zhou X. Composition and predictive functional analysis of bacterial communities inhabiting Chinese cordyceps insight into conserved core microbiome. BMC Microbiol. 2019;19(1):105. 10.1186/s12866-019-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia F, Liu Y, Shen GR, Guo LX, Zhou XW. Investigation and analysis of microbiological communities in natural Ophiocordyceps sinensis. Can J Microbiol. 2015;61(2):104–11. 10.1139/cjm-2014-0610. [DOI] [PubMed] [Google Scholar]
- 22.Xia F, Chen X, Guo M-Y, Bai X-H, Liu Y, Shen G-R, et al. High-throughput sequencing-based analysis of endogenetic fungal communities inhabiting the Chinese cordyceps reveals unexpectedly high fungal diversity. Sci Rep. 2016;6(1): 33437. 10.1038/srep33437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang RH, Wang XL, Su JH, Li Y, Jiang SP, Gu F, et al. Bacterial diversity in native habitats of the medicinal fungus Ophiocordyceps sinensis on Tibetan Plateau as determined using Illumina sequencing data. FEMS Microbiol Lett. 2014;362(5). 10.1093/femsle/fnu044. [DOI] [PubMed]
- 24.Zhang XM, Tang DX, Li QQ, Wang YB, Xu ZH, Li WJ, et al. Complex microbial communities inhabiting natural cordyceps militaris and the habitat soil and their predicted functions. Antonie Van Leeuwenhoek. 2021;114(4):465–77. 10.1007/s10482-021-01534-6. [DOI] [PubMed] [Google Scholar]
- 25.Grime JP. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J Ecol. 1998;86(6):902–10. [Google Scholar]
- 26.Zheng W, Zhao Z, Lv F, Wang R, Wang Z, Zhao Z, et al. Assembly of abundant and rare bacterial and fungal sub-communities in different soil aggregate sizes in an apple orchard treated with cover crop and fertilizer. Soil Biol Biochem. 2021;156: 108222. 10.1016/j.soilbio.2021.108222. [Google Scholar]
- 27.Hou J, Wu L, Liu W, Ge Y, Mu T, Zhou T, et al. Biogeography and diversity patterns of abundant and rare bacterial communities in rice paddy soils across China. Sci Total Environ. 2020;730: 139116. 10.1016/j.scitotenv.2020.139116. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Yang J, Yu Z, Wilkinson DM. The biogeography of abundant and rare bacterioplankton in the lakes and reservoirs of China. ISME J. 2015;9(9):2068–77. 10.1038/ismej.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Z, Ma Y, Feng T, Kong X, Wang Z, Zheng W, et al. Assembly processes of abundant and rare microbial communities in orchard soil under a cover crop at different periods. Geoderma. 2022;406: 115543. 10.1016/j.geoderma.2021.115543. [Google Scholar]
- 30.Sun S, Jones RB, Fodor AA. Inference-based accuracy of metagenome prediction tools varies across sample types and functional categories. Microbiome. 2020;8(1):46. 10.1186/s40168-020-00815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing R, Deng Y-f, Yao Y, Gao Q-b, Zhang F-q, Wang J-l, et al. Fine-scale genetic diversity and genet dynamics of the fairy ring fungus Floccularia luteovirens on the Qinghai–Tibet plateau. Fungal Ecol. 2022;60: 101194. 10.1016/j.funeco.2022.101194. [Google Scholar]
- 32.Zhang Y, Li E, Wang C, Li Y, Liu X. Ophiocordyceps sinensis, the flagship fungus of China: terminology, life strategy and ecology. Mycology. 2012;3(1):2–10. 10.1080/21501203.2011.654354. [Google Scholar]
- 33.Jiao S, Lu Y. Abundant fungi adapt to broader environmental gradients than rare fungi in agricultural fields. Glob Change Biology. 2020;26(8):4560–20. 10.1111/gcb.15130. [DOI] [PubMed] [Google Scholar]
- 34.Steinegger M, Söding J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat Biotechnol. 2017;35(11):1026–8. 10.1038/nbt.3988. [DOI] [PubMed] [Google Scholar]
- 35.Simpson GL, Solymos P, Stevens M, Wagner H. Vegan: community ecology package. Time Int. 2015;1997(6):15–7. [Google Scholar]
- 36.Bastian M, Heymann S, Jacomy M. Gephi: an open source software for exploring and manipulating networks. In: Proceedings of the Third International Conference on Weblogs and Social Media, ICWSM 2009, San Jose, California, USA, May 17–20, 2009. 2009.
- 37.Hamilton NE, Ferry M. Ggtern: ternary diagrams using ggplot2. Journal of Statistical Software, Code Snippets. 2018;87(3):1–17. 10.18637/jss.v087.c03. [Google Scholar]
- 38.D Ainsworth T, Krause L, Bridge T, Torda G, Raina J-B, Zakrzewski M, et al. The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. ISME J. 2015;9(10):2261–74. 10.1038/ismej.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pester M, Bittner N, Deevong P, Wagner M, Loy A. A ‘rare biosphere’ microorganism contributes to sulfate reduction in a peatland. ISME J. 2010;4(12):1591–602. 10.1038/ismej.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards J, Johnson C, Santos-Medellín C, Lurie E, Podishetty NK, Bhatnagar S, et al. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci U S A. 2015;112(8):E911–920. 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durán P, Thiergart T, Garrido-Oter R, Agler M, Kemen E, Schulze-Lefert P, et al. Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell. 2018;175(4):973–83.e14. 10.1016/j.cell.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhuang W, Yu X, Hu R, Luo Z, Liu X, Zheng X, et al. Diversity, function and assembly of mangrove root-associated microbial communities at a continuous fine-scale. Npj Biofilms Microbiomes. 2020;6(1):52. 10.1038/s41522-020-00164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong Y, Sorensen PO, Zhu G, Jia X, Liu J, Shangguan Z, et al. Differential microbial assembly processes and co-occurrence networks in the soil-root continuum along an environmental gradient. iMeta. 2022;1(2): e18. 10.1002/imt2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Wang M, Xie X, Guo S, Zhou Y, Zhang X, et al. An amplification-selection model for quantified rhizosphere microbiota assembly. Sci Bull. 2020;65(12):983–6. 10.1016/j.scib.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Satola B, Wübbeler JH, Steinbüchel A. Metabolic characteristics of the species Variovorax paradoxus. Appl Microbiol Biotechnol. 2013;97(2):541–60. 10.1007/s00253-012-4585-z. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Wu P, Chen J, Yan H. Biodegradation of microcystin-RR by a new isolated Sphingopyxis sp. USTB-05. Chin J Chem Eng. 2010;18(1):108. 10.1016/S1004-9541(08)60330-4. [Google Scholar]
- 47.Santos PE-DL, Ro B-C, Caballero-Mellado J. Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Appl Environ Microbiol. 2001;67(6):2790–8. 10.1128/AEM.67.6.2790-2798.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiong C, He JZ, Singh BK, Zhu YG, Zhang LJEM. Rare taxa maintain the stability of crop mycobiomes and ecosystem functions. 2020. [DOI] [PubMed]
- 49.Faust K, Sathirapongsasuti JF, Izard J, Segata N, Gevers D, Raes J, et al. Microbial co-occurrence relationships in the human microbiome. PLoS Comput Biol. 2012;8(7): e1002606. 10.1371/journal.pcbi.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue Y, Chen H, Yang JR, Liu M, Huang B, Yang J. Distinct patterns and processes of abundant and rare eukaryotic plankton communities following a reservoir cyanobacterial bloom. ISME J. 2018;12(9):2263–77. 10.1038/s41396-018-0159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu M, Huang Q, Xiong Z, Liao H, Lv Z, Chen W, et al. Distinct responses of rare and abundant microbial taxa to in situ chemical stabilization of cadmium-contaminated soil. mSystems. 2021;6(5):e0104021–e. 10.1128/mSystems.01040-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costacurta A, Vanderleyden J. Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol. 1995;21(1):1–18. 10.3109/10408419509113531. [DOI] [PubMed] [Google Scholar]
- 53.Levy E, Gough FJ, Berlin KD, Guiana PW. Inhibition of Septoria tritici and other phytopathogenic fungi and bacteria by Pseudomonas fluorescens and its antibiotics. Plant Pathol. 1992;41(3):335–41. 10.1111/j.1365-3059.1992.tb02355.x. [Google Scholar]
- 54.Kalam S, Basu A, Ahmad I, Sayyed RZ, El-Enshasy HA, Dailin DJ, et al. Recent understanding of soil acidobacteria and their ecological significance: a critical review. Front Microbiol. 2020;11:11. 10.3389/fmicb.2020.580024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh VK, Kumar A. Secondary metabolites from endophytic fungi: production, methods of analysis, and diverse pharmaceutical potential. Symbiosis. 2023;90(2):111–25. 10.1007/s13199-023-00925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacoby RP, Koprivova A, Kopriva S. Pinpointing secondary metabolites that shape the composition and function of the plant microbiome. J Exp Bot. 2021;72(1):57–69. 10.1093/jxb/eraa424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Widder S, Allen RJ, Pfeiffer T, Curtis TP, Soyer OS. Challenges in microbial ecology: building predictive understanding of community function and dynamics. 2016. [DOI] [PMC free article] [PubMed]
- 58.Djemiel C, Maron P-A, Terrat S, Dequiedt S, Cottin A, Ranjard L. Inferring microbiota functions from taxonomic genes: a review. Gigascience. 2022;11:11. 10.1093/gigascience/giab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.StanLotter H, Fendrihan S. Adaption of microbial life to environmental extremes: novel research results and application. In: Adaption of microbial life to environmental extremes: novel research results and application. 2012. p. 1-282.
- 60.Pent M, Põldmaa K, Bahram M. Bacterial communities in boreal forest mushrooms are shaped both by soil parameters and host identity. Front Microbiol. 2017;8:8. 10.3389/fmicb.2017.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yue H, Yue W, Jiao S, Kim H, Lee Y-H, Wei G, et al. Plant domestication shapes rhizosphere microbiome assembly and metabolic functions. Microbiome. 2023;11(1):70. 10.1186/s40168-023-01513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koskinen J, Roslin T, Nyman T, Abrego N, Michell C, Vesterinen EJ. Finding flies in the mushroom soup: host specificity of fungus-associated communities revisited with a novel molecular method. Mol Ecol. 2019;28(2):190–202. 10.1111/mec.14810. [DOI] [PubMed] [Google Scholar]
- 63.Maurice S, Arnault G, Nordén J, Botnen SS, Miettinen O, Kauserud H. Fungal sporocarps house diverse and host-specific communities of fungicolous fungi. ISME J. 2021;15(5):1445–57. 10.1038/s41396-020-00862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li C, Tang D, Wang Y, Fan Q, Zhang X, Cui X, et al. Endogenous bacteria inhabiting the ophiocordyceps highlandensis during fruiting body development. BMC Microbiol. 2021;21(1):178. 10.1186/s12866-021-02227-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li S, Lian W-H, Han J-R, Ali M, Lin Z-L, Liu Y-H, et al. Capturing the microbial dark matter in desert soils using culturomics-based metagenomics and high-resolution analysis. Npj Biofilms Microbiomes. 2023;9(1):67. 10.1038/s41522-023-00439-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available in the GenBank under the accession numbers PRJNA1047261 and OR855850-OR855902.