Abstract
The hypertrophic Gq-protein-coupled receptor agonist PE (phenylephrine) activates protein synthesis. We showed previously that activation of protein synthesis by PE requires MEK [MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal-regulated kinase) kinase] and mTOR (mammalian target of rapamycin). However, it remained unclear whether ERK activation was required and which downstream components were involved in activating mTOR and protein synthesis. Using an adenovirus encoding the MKP3 (MAPK phosphatase 3) to inhibit ERK activity, we demonstrate that ERK is essential for the activation of protein synthesis by PE. Activation and phosphorylation of S6K1 (ribosomal protein S6 kinase 1) and phosphorylation of eIF4E (eukaryotic initiation factor 4E)-binding protein (both are mTOR targets) were also inhibited by MKP3, suggesting that ERK is also required for the activation of mTOR signalling. PE stimulation of cardiomyocytes induced the phosphorylation of TSC2 (tuberous sclerosis complex 2), a negative regulator of mTOR activity. TSC2 was phosphorylated only weakly at Thr1462, but phosphorylated at additional sites within the sequence RXRXX(S/T). This differs from the phosphorylation induced by insulin, indicating that MEK/ERK signalling targets distinct sites in TSC2. This phosphorylation may be mediated by p90RSK (90 kDa ribosomal protein S6K), which is activated by ERK, and appears to involve phosphorylation at Ser1798. Activation of protein synthesis by PE is partially insensitive to the mTOR inhibitor rapamycin. Inhibition of the MAPK-interacting kinases by CGP57380 decreases the phosphorylation of eIF4E and PE-induced protein synthesis. Moreover, CGP57380+rapamycin inhibited protein synthesis to the same extent as blocking ERK activation, suggesting that MAPK-interacting kinases and regulation of mTOR each contribute to the activation of protein synthesis by PE in cardiomyocytes.
Keywords: extracellular-signal-regulated kinase (ERK), hypertrophy, Mnk, mTOR, myocyte, protein synthesis
Abbreviations: ARVC, adult rat ventricular cardiomyocytes; eIF, eukaryotic initiation factor; ERK, extracellular-signal-regulated kinase; 4E-BP1, eIF4E-binding protein 1; GFP, green fluorescent protein; GSK, glycogen synthase kinase; HEK-293 cells, human embryonic kidney 293 cells; IEF, isoelectric focusing; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; MKP3, MAPK phosphatase 3; Mnk, MAPK-interacting kinase; MOI, multiplicity of infection; mTOR, mammalian target of rapamycin; p90RSK, 90 kDa ribosomal protein S6 kinase; PE, phenylephrine; PI3K, phosphoinositide 3-kinase; PKB, protein kinase B; S6K, ribosomal protein S6 kinase; TSC, tuberous sclerosis complex
INTRODUCTION
Cardiac hypertrophy is a major risk factor for heart failure and is characterized by increased mass of cardiac muscles, especially of the interventricular septum and left ventricular free wall. The cardinal feature of cardiac hypertrophy is increased protein synthesis leading to increased protein content and cell size [1]. Gq/G11-linked (e.g. α1-adrenergic) receptors play a crucial role in mediating these effects. Indeed, overexpression of these receptors or the downstream mediator Gαq induced a hypertrophic response [2]. PE (phenylephrine), an α1-receptor agonist, elicits hypertrophic effects in cardiomyocytes and stimulates protein synthesis [3–5].
The present study addresses the signalling events that activate protein synthesis in response to PE and elicit phosphorylation of regulatory components of the translational machinery. We have used adult cardiomyocytes rather than neonatal cardiomyocytes since there are significant differences between signalling connections in adult versus neonatal cells [6].
Previous studies indicated the importance of Ras/Raf/MEK [where MEK stands for MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal-regulated kinase) kinase] signalling in cardiac hypertrophy (reviewed in [7,8]). Our previous study showed that MEK signalling was critical for PE-activated protein synthesis and that this involves the activation of several components of the translational machinery that lie downstream of mTOR (mammalian target of rapamycin) [6,9,10]. Significant controversy remains regarding the role of ERK1/2 in the hypertrophic response (reviewed in [4]): for example, inhibition of ERK1/2 expression with antisense oligonucleotides decreased the hypertrophic response in neonatal cardiomyocytes [11]; however, ERK1/2 activation was not observed in the transgenic mice overexpressing Gαq [2]. The mTOR pathway is currently the focus of substantial interest: of particular relevance to hypertrophy is the fact that mTOR signalling is involved in controlling cell and animal growth [12,13]. Indeed, the mTOR inhibitor rapamycin attenuates cardiac hypertrophy in vivo [14,15]. Important recent developments have revealed how signalling from the insulin receptor activates mTOR: stimulation of PI3K (phosphoinositide 3-kinase) leads to phosphorylation/activation of Akt [also termed PKB (protein kinase B)]. Akt then phosphorylates the TSC (tuberous sclerosis complex) protein. TSC2 forms a complex with TSC1 that inhibits mTOR, by acting as a GAP (GTPase-activating protein) towards Rheb, a positive regulator of mTOR signalling [16,17]. Phosphorylation of TSC2 is proposed to alleviate this inhibition.
mTOR lies upstream of several regulators of mRNA translation. These include the S6Ks (40 S ribosomal protein S6 kinases) S6K1 and S6K2 [12]. mTOR also positively regulates the phosphorylation of the translational repressor 4E-BP1 [eIF4E (eukaryotic initiation factor 4E)-binding protein 1] [18], leading to its release from eIF4E, allowing the latter to form productive initiation complexes [19].
Other translation initiation factors are regulated independently of mTOR. eIF4E is also phosphorylated by the Mnks (MAPK-interacting kinases), which are activated by the classical MAPK (ERK) pathway [20]. The guanine nucleotide-exchange factor eIF2B, which is essential for recruitment of the initiator methionyl-tRNA to ribosomes, is regulated through both PI3K and MEK/ERK signalling (reviewed in [21]).
Our previous studies suggest that, in ARVC (adult rat ventricular cardiomyocytes), mTOR activity is activated by PE through signalling events that require MEK, implying novel connections between the MEK/ERK and mTOR pathways [6,9]. Interestingly, rapamycin substantially, but incompletely, inhibits PE-activated protein synthesis, suggesting that mTOR-dependent and -independent events are involved in the stimulation of protein synthesis by PE in ARVC. Previous reports suggested that ERK is not itself involved in the activation of S6K1 [22] and that S6K1 can be activated directly by MEK [23]. We therefore considered it important, first, to establish whether activation of mTOR signalling by PE in ARVC is indeed mediated by MEK in an ERK1/2-independent manner. To address this, we exploited an adenoviral vector encoding MKP3 (MAPK phosphatase 3). MKP3 is a dual-specific phosphatase that dephosphorylates the activation loop of ERK1/2 with very high specificity [24,25]. Thus we are able to inhibit ERK1/2 signalling without affecting the activity of other pathways. Secondly, given that PE does not activate protein synthesis through the PI3K/Akt pathway [9], which is involved in the phosphorylation of TSC2 in response to insulin stimulation of cells [26], we considered it important to establish whether activation of mTOR signalling by PE involved the phosphorylation of TSC2. Lastly, we wished to study the additional events involved in the mTOR-independent activation of protein synthesis by PE.
The present results show that activation of ERK1/2 is essential for the stimulation of mTOR signalling and protein synthesis by PE. Importantly, we show that the signalling downstream of ERK1/2 to stimulate protein synthesis involves at least two distinct events. Our results suggest that PE stimulates the MEK-dependent phosphorylation of TSC2, implicate p90RSK (90 kDa S6K) as a potential TSC2 kinase and indicate a role for the Mnks in PE-activated protein synthesis.
MATERIALS AND METHODS
Reagents
The adenovirus expressing GFP (green fluorescent protein) was kindly provided by Dr C. Sutherland (University of Dundee, Dundee, U.K.). Anti-phospho-Akt substrate (serine/threonine), anti-phospho-TSC2 (Ser939 and Thr1462), anti-phospho-GSK3α/β (glycogen synthase kinase 3α/β) (Ser21/Ser9), anti-ERK1/2, anti-phospho-p38 MAPK (Thr180/Tyr182), anti-phospho-Akt (Thr309), anti-phospho-ERK1/2 (Thr202/Tyr204) and anti-eIF4E antibodies were purchased from Cell Signaling Technology (Hitchin, Herts., U.K.). Anti-GSK3β antibodies were obtained from BD Transduction Laboratories (Oxford, U.K.). The anti-4E-BP1 antibodies were a gift from Dr A. Thomas (Utrecht, The Netherlands). Anti-TSC2 antibodies, activated Akt, p90RSK1 and activated ERK2 were provided by the Division of Signal Transduction Therapy, School of Life Sciences, University of Dundee. Propranolol was obtained from Sigma (Poole, Dorset, U.K.). The Mnk inhibitor, originally described by Novartis as CGP57380 [27], was synthesized by Dr R. Marquez (University of Dundee, U.K.). All other materials were obtained as described previously [9,28].
Cell isolation and culture
ARVC were isolated by perfusion and cultured as described previously [9] from adult male Sprague–Dawley rats (250–300 g). Cells were incubated overnight before treatments and collection of lysates. Cells infected with adenovirus were cultured for 48 h before treatments and collection of lysates.
Preparation of cell lysates
Details of treatments are presented in the Figure legends. Cells were harvested as described previously [9]. Cells used for the analysis of TSC2 phosphorylation were lysed in TNE extraction buffer (10 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1%, v/v, Triton X-100 and 1 mM dithiothreitol).
Immunoprecipitation of TSC2
TSC2 was immunoprecipitated from lysates using 5 μg of anti-TSC2 [for the analysis of endogenous TSC2 in ARVCs or HEK-293 cells (human embryonic kidney 293 cells)] or anti-FLAG antibodies (for TSC2 overexpressed in HEK-293 cells). Antibodies were prebound to Protein G–Sepharose. Immunoprecipitates were washed and resuspended in 25 μl of 1×SDS sample buffer [29,30] for the analysis of phosphorylation of TSC2 in cardiomyocytes or HEK-293 cells or in kinase buffer A (20 mM Hepes, pH 7.4, 50 mM KCl, 10 mM MgCl2 and 5% glycerol) for the in vitro phosphorylation experiments with TSC2 overexpressed in HEK-293 cells.
SDS/PAGE and immunoblotting
SDS/PAGE and Western blotting were performed as described previously [29,30]. Blots for S6K1 and 4E-BP1 were performed as described in [9,29,30]. Briefly, analysis of S6K1 was performed on a 10% (w/v) acrylamide/0.1% methylene bisacrylamide gel, whereas 4E-BP1 was analysed using gels containing 13.5% acrylamide and 0.36% methylene bisacrylamide. Analysis of TSC2 was performed using 7.5% (w/v) polyacrylamide/0.1% methylene bisacrylamide SDS/polyacrylamide gels run for 1.5 h at 200 V. All other resolving gels contained 12.5% acrylamide and 0.1% methylene bisacrylamide. TSC2 protein gels were transferred to an Immobilon-P membrane at 100 V for 1.5 h at 4 °C.
m7GTP–Sepharose chromatography and IEF (isoelectric focusing)
To assess the phosphorylation state of eIF4E, m7GTP–Sepharose affinity chromatography was performed by the method of Vries et al. [29]. Briefly, cardiomyocyte lysates containing 500 μg of protein were incubated with 25 μl of a slurry of m7GTP–Sepharose 4B, diluted 1:2 with Sepharose 4B, at 4 °C for 2 h with constant rotation. Purified eIF4E was then separated by IEF, using Ampholines over the pH range 3.5–10, and transferred on to an Immobilon-P membrane. eIF4E was detected using anti-eIF4E polyclonal antibodies.
In vitro S6K1 assays
The activities of S6K1 were assessed by the technique described previously [6]. Briefly, S6K1 was immunoprecipitated for 2 h at 4 °C from 250 μg of ARVC lysate using 5 μg of S6K1 antibody (DSTT, University of Dundee) prebound to Protein G–Sepharose. Immunoprecipitates were washed and resuspended in a volume of 10 μl of kinase buffer B (50 mM Hepes, pH 7.6, 50 mM β-glycerophosphate, 1 mM EDTA, 1 mM EGTA and 1 mM dithiothreitol). The activities of the immunoprecipitates were then assayed against an S6 peptide substrate as described previously [31].
Incorporation of radioactive label into protein
Rates of protein synthesis were assessed by a standard procedure, i.e., by measuring the incorporation of 35S-labelled methionine into newly synthesized trichloroacetic acid-precipitable material, as described previously [9,28]. Briefly, cardiomyocytes were stimulated with insulin or PE as described for 1.5 h before the addition of 5 μCi/ml [35S]methionine for a further 30 min. Cells were washed and harvested in lysis buffer as described above. Protein content was determined by the Bradford method [32] and equal amounts of protein were loaded on to Whatman 3MM paper filters before precipitation with 10% (w/v) trichloroacetic acid. Incorporation of label was assessed by scintillation counting.
In vitro treatment of TSC2 with activated ERK2, Akt and p90RSK1
HEK-293 cells were transfected by the calcium phosphate method [33] with pRK7-TSC1 and pRK7-TSC2 (wild-type or SATA [Ser939→Ala/Thr1462→Ala] or Ser1798→Ala). Cells were cultured overnight and serum-starved for a minimum of 8 h. TSC2 was immunoprecipitated from lysates as described above. The in vitro phosphorylation experiments were performed using FLAG-tagged TSC1 and TSC2 expressed in HEK-293 cells and kinase buffer A. Immunoprecipitates were incubated at 30 °C for the appropriate time period in the presence of 100 μM ATP, 1 mM MgCl2, 0.01 unit of activated ERK2, Akt or p90RSK1 and, where appropriate, 1 μCi of [γ-32P]ATP. Samples were run on an SDS/polyacrylamide gel as described above. Either the gels were transferred on to Immobilon-P membrane and Western blotted with antisera as indicated in the Figures or (for radiolabelling experiments) the gels were fixed and stained and then subjected to autoradiography. The SATA and Ser1798→Ala mutants of TSC2 were created using the Quik Change® system (Stratagene, Cambridge, U.K.).
Site-directed mutagenesis of TSC2
The SATA mutant of TSC2 was kindly provided by Dr A. Tee. The Ser1798→Ala mutant of TSC2 was created using the Quik Change® system with the SATA mutant as a template.
Statistical analyses
Data from the S6K1, protein synthesis assays and Western blots were classified according to treatment and adenovirus groups. Due to variation in basal S6K1 activity and incorporation of the radioactive label between experiments, statistical analyses were performed on data from individual replicate experiments. Data presented are the averages of three individual replicate experiments ±S.E.M. Data were analysed for statistically significant differences by two-way ANOVA using a data analysis package in Microsoft Excel. Tukey's honestly significant difference (HSD) method (P<0.01 or P<0.05) was then employed to identify significant differences between groups. Densitometric analysis of Western blots was performed using NIH ImageJ 1.32j software (http://rsb.info.nih.gov/ij/). Where phospho-specific blots were analysed, data were normalized to total protein blots before comparison. Statistical analysis was by two-way ANOVA for MKP3 experiments or by one-way ANOVA for TSC2 and eIF4E blots.
RESULTS
β-Adrenergic signalling does not interfere with the activation of protein synthesis by PE
We have previously used the α1-adrenergic agonist PE in our studies on the control of protein synthesis in ARVC. However, we were concerned that PE might also activate β-adrenergic signalling in cardiomyocytes, which could inhibit protein synthesis and interfere with the responses we were studying [34,35]. Therefore, to investigate if α-adrenergic activation might affect the activation of protein synthesis by PE (through the α1-adrenergic pathway), cells were treated with the β-blocker propranolol before stimulation with PE. Pretreatment of cells with 20 μM propranolol did not significantly increase PE-induced protein synthesis (Figure 1A). PE induced a 1.8-fold increase in the incorporation of radioactive label. In the presence of propranolol, PE elicited a slightly greater increase in protein synthesis. However, this difference was not statistically significant (P>0.05). Therefore all further experiments were performed in the absence of propranolol, as in our earlier work. In contrast, the α1-adrenergic antagonist prazosin (2 μM) inhibited the activation of protein synthesis by >95% (results not shown).
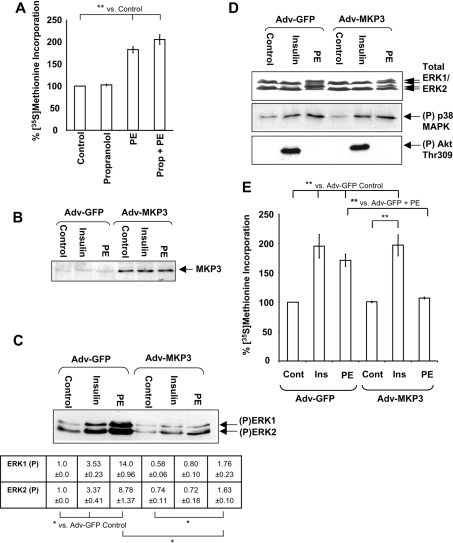
Figure 1. Overexpression of MKP3 blocks PE-induced activation of protein synthesis.
(A) Overnight cultures of cardiomyocytes were pretreated with 20 μM propranolol for 45 min before stimulation with 10 mM PE for 1.5 h. Protein synthesis was assayed as described in the Materials and methods section. Results are expressed as percentage change in [35S]methionine incorporation relative to the Adv-GFP control cells (±S.E.M.), which is set at 100%. Results are representative of three replicate experiments using separate preparations of ARVC, and each experiment comprised three replicates. S.E.M. is calculated from the averages of the three independent replicate experiments. **P<0.01 as indicated. (B–E) Freshly isolated ARVC were infected with Adv-GFP or Adv-MKP3 as indicated at an MOI of 100 pfu/cell. (B) Cells were treated with 10 nM insulin or 10 μM PE or DMSO (vehicle; control cells) for 5 min as indicated and then harvested. Lysates were analysed by SDS/PAGE/immunoblotting, as described in the Materials and methods section, and probed with anti-MKP3 antibody. (C) Cells were treated with 10 μM PE or 10 nM insulin for 5 min and harvested. Lysates were subjected to SDS/PAGE and immunoblots were analysed for phosphorylated ERK1/2 with anti-phospho-ERK1/2. Arrows indicate phosphorylated forms of ERK1 and ERK2. Densitometry was performed as described in the Materials and methods section, with ERK phospho-blots normalized to total ERK protein levels (determined by Western blotting, D) before comparison. Numbers are absolute values indicating fold change (±S.E.M.) in phosphorylated ERK1 and ERK2 relative to the Adv-GFP control sample. *P<0.05 as indicated. (D) Top panel: immunoblots were probed with an antibody for ERK1/2 to confirm equal loading. The middle and bottom panels show immunoblots that were analysed for phosphorylated p38 MAPK using anti-phospho-(Thr180/Tyr182) p38 MAPK or phosphorylated (Thr309) Akt antibodies respectively. Blots are representative of three independent replicate experiments using separate preparations of ARVC. (E) Cells were stimulated with PE (10 μM) or insulin (10 nM) for 1.5 h. Protein synthesis was assayed and results are presented as in (A).
MKP3 almost completely ablates PE-induced phosphorylation of ERK1/2 without affecting other signalling pathways
To study the role of ERK in the activation of protein synthesis and the translational machinery by PE, we employed an adenovirus encoding either the ERK-specific MAPK phosphatase MKP3 or, as negative control, GFP (Adv-GFP). In preliminary experiments, an MOI (multiplicity of infection) of 100 pfu (plaque-forming units) was found to give maximum suppression of ERK1/2 activity without affecting cell viability, and this MOI was used in all subsequent experiments. ARVC infected with Adv-MKP3 express MKP3 protein at much higher levels than control cells (Figure 1B). After 48 h infection with the adenovirus, at least 85% of the cells remained rod-shaped and viable as assessed by Trypan Blue exclusion (results not shown).
Treatment of Adv-GFP-infected cardiomyocytes with 10 μM PE induced 14- and 8.8-fold increase in the phosphorylation of ERK1 and ERK2 respectively when compared with untreated cells (P<0.05; Figure 1C). In contrast, 10 nM insulin caused only a 3-fold increase in ERK1/2 phosphorylation relative to untreated cells (P<0.05). Basal ERK1/2 phosphorylation was decreased in untreated ARVC overexpressing MKP3, although these effects were not statistically significant (P>0.05). In contrast, MKP3 expression markedly decreased PE- and insulin-induced ERK1/2 phosphorylation compared with the Adv-GFP cells (P<0.05). A small residual increase in ERK1/2 phosphorylation in Adv-MKP3-infected cells was still seen after stimulation with PE, although the PE-stimulated levels of phosphorylated ERK1/2 were not significantly higher than those observed in the Adv-GFP-infected control cells. Separate immunoblots using an antibody that detects ERK1/2 irrespective of their phosphorylation state indicated equal loading levels (Figure 1D, top panel).
Although MKP3 demonstrates high specificity for ERK1/2, it has been reported to dephosphorylate p38 MAPK and JNK (c-Jun N-terminal kinase) [25,36]. However, expression of MKP3 did not affect either the basal level of p38 MAPK phosphorylation in ARVC or the slight activation caused by PE [6] (Figure 1D), indicating that MKP3 does not dephosphorylate p38 MAPK within ARVC. PE does not activate JNK in ARVC [6], and indeed even arsenite fails to activate it [37]. Akt phosphorylation, which is stimulated by insulin, was unaffected by MKP3, further illustrating the specificity of MKP3 (Figure 1D).
MKP3 blocks the activation of protein synthesis by PE but not by insulin
In Adv-GFP-infected ARVC, treatment with PE significantly increased protein synthesis to 175% of control (Figure 1E) and insulin induced a 2-fold increase (P<0.01), in line with previous findings [6,9]. MKP3 completely blocked the PE-induced activation of protein synthesis (P<0.01), whereas insulin-induced protein synthesis remained unaffected (Figure 1E). Thus MKP3 completely ablates PE-induced protein synthesis in an agonist-specific manner. This suggests that activation of protein synthesis by PE may require activation of ERK as well as the upstream kinase MEK [9]. Since MKP3 had no effect on insulin-stimulated protein synthesis, these results also indicate that ERK activation is not required for this response. This is in agreement with the observation that MKP3 did not impair the ability of insulin to activate Akt (PKB) (Figure 1D) and with our earlier finding that the activation of protein synthesis by insulin in ARVC involves PI3K/Akt signalling [28].
Inhibition of ERK1/2 inhibits the phosphorylation and activation of S6K1 and the phosphorylation of 4E-BP1
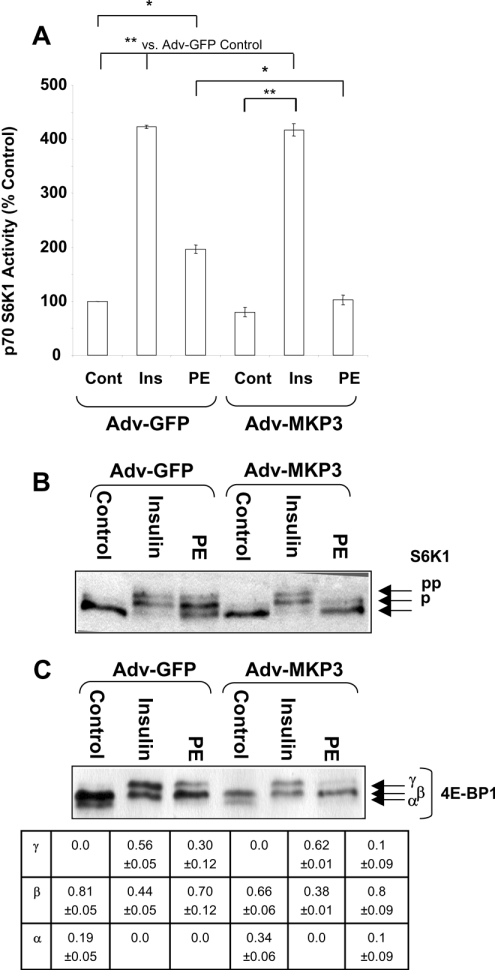
PE treatment of ARVC results in the phosphorylation and activation of S6K1 and increased phosphorylation of 4E-BP1 by an MEK-dependent mechanism [9]. The effects of MKP3 expression on PE- and insulin-induced changes in S6K1 activity were assessed. The data are presented in Figure 2(A) as percentage change in activity relative to Adv-GFP control samples. In ARVC expressing GFP, S6K1 was activated approx. 4-fold by insulin (P<0.01%) and 2-fold by PE (P<0.05%). Expression of MKP3 completely blocked the activation of S6K1 by PE, whereas its activation by insulin was unaffected (Figure 2A). Activation of S6K1 involves its phosphorylation at multiple sites [12]. An immunoblot for S6K1 demonstrated that insulin and PE induced its phosphorylation in Adv-GFP-infected cells, whereas MKP3 expression blocked S6K1 phosphorylation induced by PE, but not by insulin (Figure 2B).
Figure 2. Overexpression of MKP3 ablates PE-induced phosphorylation of downstream targets of mTOR.
Isolated cardiomyocytes were infected with adenoviruses, treated and harvested as for Figure 1. (A) Extracts were processed for the determination of S6K1 activity as described in the Materials and methods section. Assays were conducted in duplicate and corrected for incorporation in the absence of substrate. Results are expressed as percentage increase relative to Adv-GFP control samples (±S.E.M.), which is set at 100%. Results are representative of three replicate experiments using separate preparations of ARVC. S.E.M. is calculated from the averages of the three independent replicate experiments. *P<0.05 or **P<0.01 for the indicated comparisons. (B, C) Cells were treated as for Figure 1, but lysates were processed for SDS/PAGE and immunoblotting. (B) Immunoblot showing mobility shift of S6K1 induced by PE or insulin. Immunoblots were probed with anti-total S6K1; p and pp denote increasingly phosphorylated forms. (C) Immunoblot of 4E-BP1. The α, β and γ forms (in order of increasing phosphorylation) are indicated by arrows. Densitometry was performed as described in the Materials and methods section. Values indicate the fraction of 4E-BP1 in each isoform (±S.E.M.). Blots are representative of three replicate experiments using separate preparations of ARVC.
4E-BP1 exists in differentially phosphorylated forms and three species are distinguishable on SDS/PAGE; the most highly phosphorylated form, the γ-isoform, does not bind eIF4E and is therefore inactive in repressing translation [9]. In unstimulated cardiomyocytes, 4E-BP1 exists in the less phosphorylated α/β species (80% in β-form and 20% in α-form; Figure 2C). Stimulation with PE or insulin caused a shift of 4E-BP1 to more phosphorylated forms, with the loss of the α-form and the appearance of the γ-isoform. MKP3 overexpression induced a slight increase in the amount of 4E-BP1 in the β-form (66%) and an increase in the α-isoform (34%) compared with Adv-GFP control cells. Although the cause of this is unclear, it has been reported previously that adenovirus infection can induce the phosphorylation of 4E-BP1 [38]. MKP3 overexpression substantially decreased the PE-induced increase in 4E-BP1 phosphorylation. A small amount of the γ-form of 4E-BP1 remained; however, it is important to note that proportion of inactive γ-isoform decreased from 0.3 in Adv-GFP+PE sample to 0.1 in Adv-MKP3+PE sample. In contrast, MKP3 did not affect the insulin-induced 4E-BP1 phosphorylation. Therefore the activation of MEK alone is not sufficient to activate mTOR signalling in response to PE in ARVC. These results also demonstrate that the effects of PE on S6K1 and 4E-BP1 require ERK1/2 activation, whereas responses to insulin do not.
PE induces the phosphorylation of TSC2 in an MEK-dependent manner
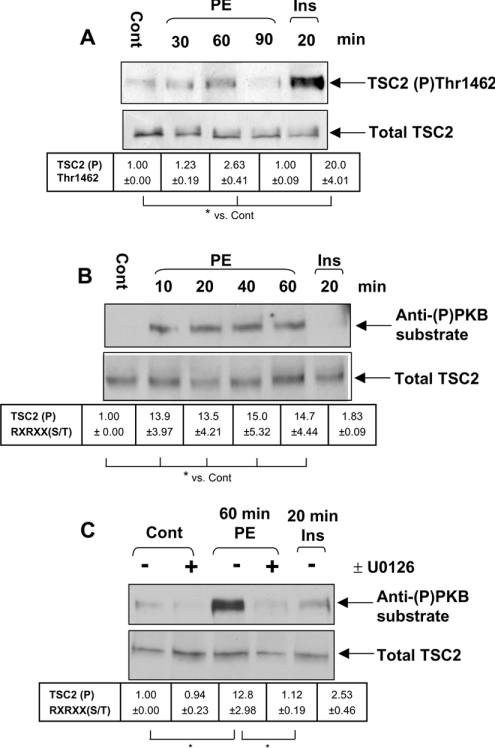
The TSC1/2 complex, which negatively regulates mTOR signalling [17,26], is a candidate for involvement in the activation of protein synthesis by hypertrophic agents such as PE. Therefore the effects of PE on TSC2 were studied. Insulin induced the phosphorylation of TSC2 at the Akt site Thr1462 (Figure 3A), with a 20-fold increase in the signal compared with untreated cells; PE also increased phosphorylation of this site, but much less when compared with insulin, with only a 2–3-fold increase in signal intensity (Figure 3A). We also used an antibody designed to recognize phosphorylated serine/threonine residues in the sequence RXRXX(S/T): the so-called ‘anti-phospho-PKB(Akt) substrate antibody’. PE induced a 15-fold increase in phosphorylation of TSC2 at a site or sites detected by this antibody, whereas insulin did not (Figure 3B). Phosphorylation at these sites occurred by 10 min and was maintained for at least 60 min (Figure 3B). Importantly, pretreatment of cells with U0126 (MEK inhibitor) almost completely blocked their phosphorylation (Figure 3C). It is surprising that this antibody did not detect the phosphorylation of TSC2 induced by insulin, as the reported Akt sites appear to be in a suitable sequence context for recognition by this reagent [39]: the batches of this antibody do not seem to detect efficiently the phosphorylation of the Akt sites in TSC2. The data thus show that PE induces the phosphorylation of TSC2 at different sites from insulin and this occurs in a MEK-dependent manner. These presumably lie in sequences of the form RXRXX(S/T) and are potential targets for phosphorylation by p90RSK, a downstream effector of ERK that has a similar, but distinct, substrate specificity to Akt (PKB) [40]. Attempts to study the phosphorylation of TSC2 further by in vivo radiolabelling in ARVC stimulated with PE or insulin proved inconclusive due to low levels of TSC2 in cardiomyocytes and their intolerance to the phosphate-free medium. An alternative approach to identifying the sites in TSC2 targeted by MEK/ERK signalling would be to express TSC2, or express selected mutants with altered phosphorylation sites, in ARVC using adenoviral vectors. Co-expression with TSC1 would be required to stabilize TSC2. However, the cDNAs for TSC1 and TSC2 are both large and, despite repeated attempts, we were not able to create adenoviral vectors encoding these proteins. We have therefore used an alternative approach, also employed by other authors [41], in which TSC1/2 are expressed in HEK-293 cells, and the effects of specific stimuli and signalling inhibitors are explored.
Figure 3. Treatment of ARVC with PE induces the phosphorylation of TSC2.
Isolated ARVC were cultured overnight and, where indicated, treated with 10 nM U0126 for 45 min before stimulation with either PE (10 μM) or insulin (10 nM) for the time periods indicated. Cells were pretreated with U0126 or with DMSO as a control. Cells were stimulated with 10 μM PE or 10 nM insulin for the time periods indicated and harvested as described above. Endogenous TSC2 was immunoprecipitated from 1 mg of lysate and analysed by SDS/PAGE and immunoblotting as described in Materials and methods section. Blots are representative of three independent replicate experiments using separate preparations of ARVC. (A) Immunoblot showing the level of TSC2 phosphorylated at Thr1462, assessed using a phospho-specific antibody. (B, C) Immunoblots showing levels of TSC2 phosphorylated at serine/threonine residues within RXRXX(S/T) motifs (using the so-called ‘anti-(P)PKB substrate antibody’). (A–C) Lower panels indicate total levels of TSC2 used to assess loading. Densitometry was conducted as described in the Materials and methods section. Phosphoblots were normalized to total protein levels before comparison. Values indicate fold change in the amount of TSC2 phosphorylated at the different sites relative to control (±S.E.M.). *P<0.05 as indicated.
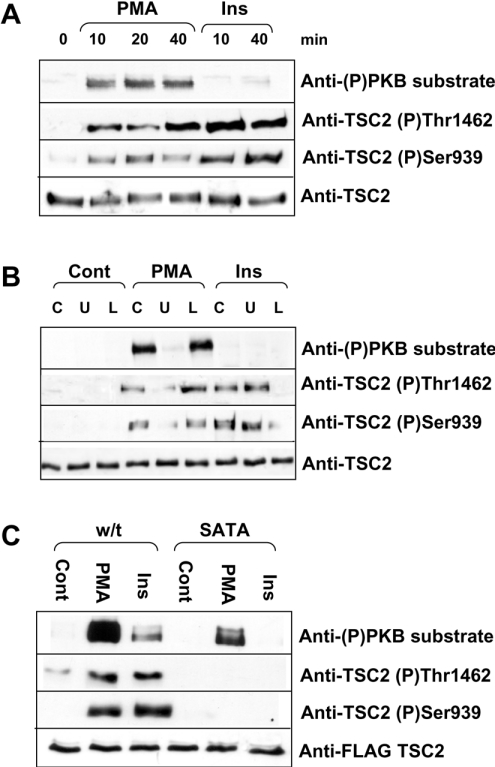
PMA also regulates sites in TSC2 distinct from those affected by insulin
As shown in Figure 4(A), stimulation of HEK-293 cells by PMA, which activates ERK but not Akt [42] resulted in a marked increase in the phosphorylation of the endogenous TSC2 detected using the anti-phospho-PKB substrate antibody. In contrast, insulin (which activates Akt but has almost no effect on ERK [42]) did not increase the signal seen with this antibody. Insulin did elicit a marked increase in the signal seen with the (P)Thr1462 and (P)Ser939 antisera, indicating that it does induce phosphorylation of TSC2 at the sites reported previously [39]. A smaller increase using the site-specific TSC2 (P)Thr1462 and (P)Ser939 antisera was also observed after PMA treatment (Figure 4A), suggesting that PMA may also regulate these sites, although it was also possible that these antisera might cross-react with other sites in TSC2.
Figure 4. PMA induces the phosphorylation of TSC2.
(A–C) Immunoblots showing phosphorylation of TSC2 in response to PMA or insulin stimulation of HEK-293 cells. Topmost panels show TSC2 phosphorylated at serine/threonine residues within the sequence RXRXX(S/T). Second panels of (A–C) show TSC2 phosphorylated at Thr1462 and third panels show TSC2 phosphorylated at Ser939. The bottommost panels show total TSC2 as a loading control. (A) HEK-293 cells were treated with 1 μM PMA or 100 nM insulin for the time periods shown (min). (B) Cells were pretreated with 10 nM U0126 (U) or 30 μM LY294002 (L) for 45 min before stimulation with 1 mM PMA or 100 nM insulin for 20 min. (C) FLAG-tagged TSC1 and either wild-type (w/t) or SATA mutant TSC2 were overexpressed in HEK-293 cells. Cells were stimulated with 1 μM PMA or 100 nM insulin for 20 min. Cell lysates were harvested as described in the Materials and methods section. Endogenous TSC2 (A, B) or overexpressed, FLAG-tagged TSC1/2 (C) were immunoprecipitated from cell lysates and analysed by Western blotting as described in the Materials and methods section. Blots are representative of three independent experiments.
The ability of PMA to increase the phosphorylation of the endogenous TSC2 at sites recognized by all three antisera was blocked by the MEK inhibitor U0126, consistent with a role for MEK/ERK signalling in mediating this effect (Figure 4B), but was not affected by the PI3K inhibitor LY294002. Conversely, the ability of insulin to increase phosphorylation at Ser939 and Thr1462 was blocked by LY294002 but not by U0126, consistent with a role for PI3K, and thus probably for Akt, in these effects (Figure 4B).
To assess whether PMA did affect Ser939 and Thr1462, we made use of a mutant of TSC2 in which both sites are mutated to alanine residues (‘SATA’ mutant). This was expressed in HEK-293 cells along with TSC1. In some cases, wild-type TSC2 was used instead of the SATA mutant. As shown in Figure 4(C), no signal was seen with the anti-(P)Ser939 or -(P)Thr1462 antisera after treatment of cells with PMA or insulin. This indicates that these antisera do not recognize other sites in TSC2 that are targets for phosphorylation in response to either PMA or insulin. Importantly, PMA still elicited an increase in the signal for the anti-phospho-PKB substrate antibody (Figure 4C), showing (i) that this antibody recognizes additional sites in TSC2 other than Ser939 and Thr1462 and (ii) that PMA enhances phosphorylation at such sites, which presumably lie in sequence contexts of the form RXRXX(S/T) (see also below).
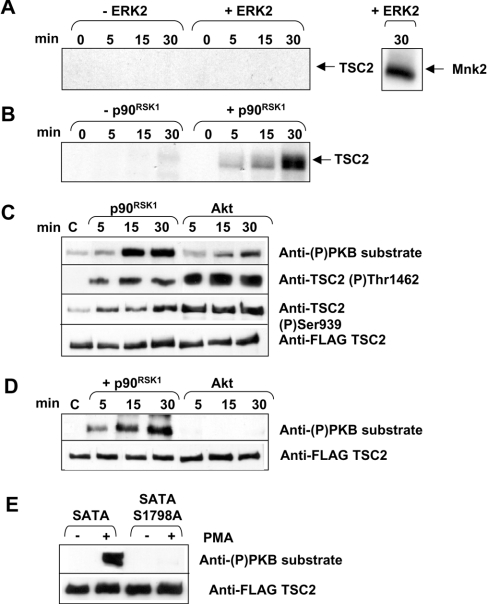
Activated p90RSK1, but not ERK2, phosphorylates TSC2 in vitro
Given the above findings, we asked whether ERK2 or p90RSK1 could phosphorylate TSC2 in vitro. Since TSC2 is only expressed at low levels in ARVC, it was necessary to overexpress FLAG-tagged TSC2 as a source of material for these studies. We therefore co-expressed FLAG-tagged TSC1 and TSC2 in HEK-293 cells. TSC1 and TSC2 were immunoprecipitated from the lysates using anti-FLAG. Immunoprecipitates were incubated with [γ-32P]-ATP and activated ERK2 or p90RSK1. ERK2 did not phosphorylate TSC2; however, it did phosphorylate Mnk2, used as a positive control (Figure 5A). In contrast, p90RSK1 strongly phosphorylated TSC2 (Figure 5B), suggesting that p90RSK may mediate the MEK-dependent phosphorylation of TSC2 induced by PE. Unfortunately, no specific inhibitors of p90RSK family kinases are yet available and we are unable to address directly the role of p90RSK in the phosphorylation of TSC2 in ARVC.
Figure 5. Activated p90RSK1 phosphorylates TSC2 in vitro.
FLAG-tagged TSC1 and TSC2 were immunoprecipitated from HEK-293 cells and immunoprecipitates were incubated with 1 μCi of [γ-32P]ATP and 0.01 unit of activated ERK2 (A) or p90RSK1 (B) for the time periods indicated. Samples were separated by SDS/PAGE. Phosphorylated proteins were visualized by autoradiography of the dried gel. The autoradiograph is representative of two independent replicate experiments. (C) Same as (A, B) except that reactions were performed with unlabelled ATP and with Akt where indicated, and analysis was by SDS/PAGE or Western blotting with the indicated antisera. The FLAG blot serves as a loading control. (D) Same as (C) except that the SATA mutant TSC2 was used (where Ser939 and Thr1462 have both been mutated to alanine). (E) HEK-293 cells were transfected with vectors for FLAG-tagged wild-type or SATA/SATA plus Ser1798→Ala mutants of TSC2 (and TSC1). Cells were treated with PMA (1 μM, 20 min) where shown; the TSC2 was immunoprecipitated and subjected to Western-blot analysis using the indicated antisera.
To study further the phosphorylation of TSC2 by p90RSK1, we incubated TSC1/2 complexes derived from transfected HEK-293 cells with p90RSK1 or Akt in vitro, and used the phospho-specific antisera to gain further insight. As shown in Figure 5(C), incubation with p90RSK1 led to increased phosphorylation at sites recognized by the anti-phospho-PKB substrate antibody and, to a lesser extent, by the anti-(P)Ser939 and -(P)Thr1462 antibodies. Conversely, Akt elicited stronger signals with the anti-(P)Ser939 and -(P)Thr1462 and a weak signal with the anti-phospho-PKB substrate antibody. This may reflect some phosphorylation by Akt, under these in vitro conditions, of sites other than Ser939 and Thr1462 (sites which do not appear to be appreciably phosphorylated in response to insulin in vivo, Figure 4C). Thus, like PMA treatment in vivo, incubation with p90RSK1 in vitro results in the phosphorylation of TSC2 at Ser939 and Thr1462 and at additional sites detected by the anti-phospho-PKB substrate antibody. To confirm that sites other than Ser939 and Thr1462 are phosphorylated by p90RSK1 in vitro, we expressed the TSC2(SATA) mutant in HEK-293 cells (along with TSC1) and analysed its phosphorylation by p90RSK1 in vitro. As shown in Figure 5(D), p90RSK1 was still able to phosphorylate the TSC2(SATA) mutant in vitro at sites recognized by the anti-phospho-PKB substrate antibody, whereas Akt was no longer able to do so. This is consistent with our conclusion that p90RSK1 phosphorylates TSC2 at sites distinct from Akt. Inspection of the sequence of TSC2 reveals a number of serine/threonine residues in motifs of the form RXRXX(S/T) that could be sites for phosphorylation both by p90RSK and by epitopes recognized by the anti-phospho-PKB substrate antibody. We mutated several of these residues to alanines: Ser981, Thr993, Ser1130 and Ser1132. None of these mutations altered the reactivity seen with the anti-phospho-PKB substrate antibody. However, as shown in Figure 5(E), mutation of Ser1798 to alanine abolished the signal seen with this antibody after treatment of cells with PMA. These results indicate that Ser1798 is the site recognized by the anti-phospho-PKB substrate antibody, and that PMA regulates its phosphorylation, in agreement with the conclusions of [43,44]. The fact that this site is also phosphorylated by p90RSK1 in vitro [44] suggests, but does not prove, that its phosphorylation is mediated by members of the RSK group of kinases in vivo.
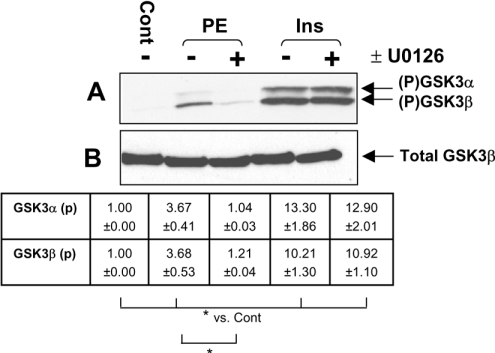
PE has only a small effect on the phosphorylation of GSK3αβ
Our previous studies suggested that there is a component of PE-induced protein synthesis that is MEK-dependent but rapamycin-insensitive. A potential candidate eIF2B, which is important in translational control [21], is phosphorylated and inhibited by GSK3 and activated in a rapamycin-insensitive manner (at least in response to insulin [45]). PE induced only a modest phosphorylation of GSK3αβ compared with the large effect of insulin (Figure 6). Furthermore, we could not detect changes in the phosphorylation of eIF2B in response to PE (results not shown). Interestingly, the effect of PE on GSK3 phosphorylation was blocked by U0126, whereas that of insulin was not. This could reflect an input from p90RSK to GSK3, as suggested by earlier studies [46,47]. The relatively modest effect of PE on GSK3αβ and the absence of a detectable change in eIF2B phosphorylation suggest that regulation of eIF2B is unlikely to be important in the activation of protein synthesis by PE.
Figure 6. PE induces only limited phosphorylation of GSK3α/β in ARVC.
Immunoblot showing levels of GSK3α/β phosphorylation in samples stimulated with PE (10 μM) or insulin (10 nM) for 5 min. Extracts were separated by SDS/PAGE and subject to immunoblotting as described in the Materials and methods section. (A) Where indicated (+), isolated ARVC were treated with 10 nM U0126 for 45 min before stimulation. Cells were then treated with PE (10 μM) or insulin (10 nM). Phosphorylated GSK3α/β were detected with anti-phospho-(Ser21/Ser9) GSK3α/β. (B) An immunoblot probed for total GSK3β (the major isoform in ARVC) demonstrated equal loading. Densitometry was conducted as described in the Materials and methods section. Values represent fold change in phosphorylated GSK3α/β relative to control sample (±S.E.M.). Signals from phospho-blots were normalized to total protein levels before comparison. *P<0.05 as indicated. Blots are representative of three independent replicate experiments using separate preparations of ARVC.
Inhibition of Mnk1/2 activity inhibits PE-activated protein synthesis additively with rapamycin
The eIF4E kinases Mnk1/2 interact with and are phosphorylated by ERK1/2 [48,49]. Thus it is possible that the Mnks contribute to the MEK-dependent activation of protein synthesis induced by PE. We therefore examined the effect of CGP57380, an inhibitor of Mnk1/2 [27], on PE-induced protein synthesis (Table 1). Our other studies have revealed that, within cells, this compound does not inhibit ERK or upstream signalling to ERK (M. Buxadé and C. G. Proud, unpublished work). It also lacks significant inhibitory activity in vitro against approx. 30 other kinases tested: particularly relevant ones tested include MSK1 and p90RSK1 that lie downstream of ERK, p38 MAPK α/β and JNK (M. Buxadé and C. G. Proud, unpublished work).
Table 1. Inhibition of Mnk1/2 decreases the rate of protein synthesis in cardiomyocytes.
Overnight cultures of ARVC were treated with U0126 (10 nM), rapamycin (100 nM), SB203580 (10 nM), SB202474 (10 nM), CGP57380 (40 μM) or a combination of rapamycin and CGP57380 (Rap+CGP) for 45 min. ARVC were stimulated with PE for 1.5 h. Protein synthesis was assayed as described in the Materials and methods section. Presented data are normalized to the control sample, and also to unstimulated cells in the presence of the appropriate inhibitor(s), to correct for any effect of the inhibitor on basal rates of protein synthesis. Data shown (±S.E.M.) were calculated from the averages of three independent replicate experiments. The three replicate experiments were performed each using separate preparations of ARVC and each experiment included triplicate determinations.
| Incorporation of [35S]methionine | |||
|---|---|---|---|
| Inhibitor | −/+ PE | % of control | % of control with inhibitor(s) |
| Control | − | 100.0 | 100.0 |
| U0126 | − | 94.1±2.2 | 100.0 |
| Rapamycin | − | 104.2±1.2 | 100.0 |
| SB203580 | − | 85.7±2.0 | 100.0 |
| SB202474 | − | 81.5±3.0 | 100.0 |
| CGP57380 | − | 87.4±2.6 | 100.0 |
| Rap+CGP | − | 77.1±4.9 | 100.0 |
| Control | + | 189.1±5.8* | 189.1±4.9 |
| U0126 | + | 111.2±3.3 | 118.2±3.3† |
| Rapamycin | + | 147.9±3.3** | 142.1±4.8 |
| SB203580 | + | 158.7±9.1* | 184.9±6.8 |
| SB202474 | + | 166.7±8.7* | 204.2±7.1 |
| CGP57380 | + | 145.2±1.7* | 166.5±5.7‡ |
| Rap+CGP | + | 94.3±5.5 | 122.7±1.4† |
* P<0.01 for comparisons between the indicated treatment and control without PE.
† P<0.01 for comparisons between the indicated sample and control+PE.
‡ P<0.05 for comparisons between the indicated sample and control+PE.
In parallel, we also re-examined the effects of other inhibitors. Since, in some cases, these compounds have small effects on basal protein synthesis, it is important to relate the data for PE-induced protein synthesis in the presence of the inhibitor to the rate of incorporation of radiolabel seen in the presence of inhibitor in cells that were not treated with PE. Both the raw data and the normalized values are presented in Table 1.
As we have reported earlier [9], activation of protein synthesis by PE was almost completely ablated by the inhibition of MEK (by U0126 or PD184352). Rapamycin treatment also attenuated the induction of protein synthesis, but less so than U0126 [9]. Pretreatment of cells with CGP57380 also significantly decreased the induction of protein synthesis by PE compared with PE alone (P<0.05%). Strikingly, treatment with both CGP57380 and rapamycin together inhibited the activation of protein synthesis by PE to the same extent as U0126 alone. This suggests that Mnk1/2 may mediate the mTOR-independent element of the activation of protein synthesis by PE.
SB203580, an inhibitor of p38 MAPK (which can also activate the Mnks), did not significantly inhibit PE-induced protein synthesis (Table 1). SB202474, a negative control for SB203580 that does not bind p38 MAPK [50], also had no effect on PE-induced protein synthesis. This suggests that p38 MAPK plays little or no role in the activation of protein synthesis by PE in ARVC.
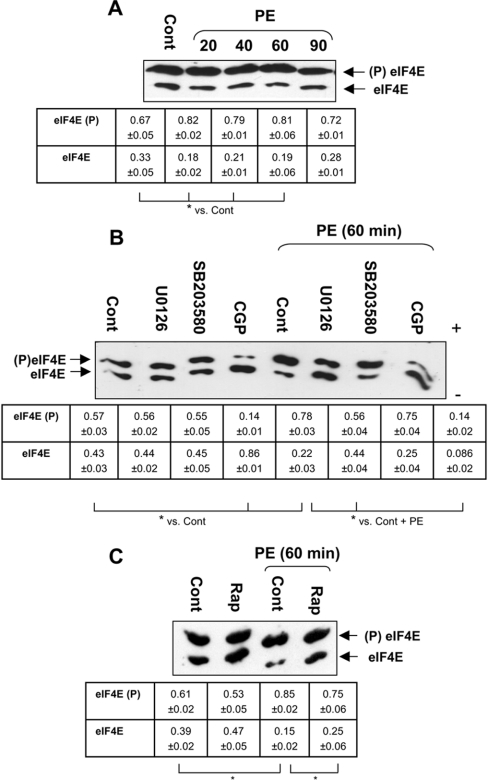
Inhibition of Mnk1/2 decreases eIF4E phosphorylation
Given the above results indicating a role for Mnks in PE-induced protein synthesis, we assessed the phosphorylation of eIF4E, their common substrate by IEF. In unstimulated ARVC, approx. 67% of eIF4E is already phosphorylated; PE significantly increases this phosphorylation (P<0.05), approx. 80% of eIF4E being phosphorylated after 20 min of PE treatment (Figure 7A). U0126, SB203580 or rapamycin had no significant effect on basal eIF4E phosphorylation (Figures 7B and 7C). U0126 prevented the PE-induced increase in eIF4E phosphorylation. In contrast, inhibition of p38 MAPK by SB203580 had no effect. CGP57380 almost completely eliminated eIF4E phosphorylation in unstimulated or PE-treated cells (Figure 7B), in agreement with the idea that Mnk1/2 are responsible for the phosphorylation of eIF4E in vivo [51,52]. These results are consistent with the idea that the effect of CGP57380 on the activation of protein synthesis by PE reflects a role for eIF4E phosphorylation. Rapamycin also inhibited PE-induced, but not basal, phosphorylation of eIF4E (Figure 7C). This may be because rapamycin inhibits PE-induced binding of eIF4E to eIF4G [9], which facilitates the phosphorylation of eIF4E by Mnks [53]. Levels of eIF4F complexes are very low in unstimulated ARVC [9], which may be the reason why rapamycin has little effect on eIF4E phosphorylation under this condition.
Figure 7. Phosphorylation of eIF4E in ARVC is inhibited by CGP57380.
Where indicated, ARVC were stimulated with 10 μM PE. The presence of signalling inhibitors is indicated. Lysates were collected and eIF4E was purified from them by affinity chromatography (m7GTP–Sepharose) and analysed by IEF as described in the Materials and methods section. (A) ARVC were stimulated with PE for the time periods shown. (B) ARVC were pretreated with signalling inhibitors as detailed in Table 1 before exposure to PE for 60 min. The polarity of the gel is also shown (+/−). (C) ARVC were pretreated with rapamycin, where indicated, as in Table 1 and then stimulated with PE, as above. (A–C) Arrows indicate the phosphorylated (upper band) and non-phosphorylated (lower band) forms of eIF4E. Blots are representative of three replicate individual experiments using separate preparations of ARVC. Densitometry was conducted as described in the Materials and methods section. Values represent the relative proportions of phosphorylated (top two rows of tables) and non-phosphorylated (lower two rows of tables) (±S.E.M.). *P<0.05 as indicated.
DISCUSSION
Our previous studies show that PE activates MEK/ERK signalling, leading to stimulation of protein synthesis and S6K1 and 4E-BP1 phosphorylation in ARVC [9]. These effects are blocked by inhibition of MEK. The present study demonstrates that ERK1/2 activation is also required for these effects since MKP3 overexpression inhibits ERK1/2 and completely blocks PE-induced activation of protein synthesis, impairs the phosphorylation and activation of S6K1 and decreases the phosphorylation of 4E-BP1. Our results indicate that phosphorylation of ERK1/2 by PE was not completely blocked by MKP3 (Figure 1C); however, it is worth noting that the level of activation in the presence of MKP3 is significantly lower than that seen in GFP-expressing cells; most importantly, PE-induced protein synthesis was completely ablated. Therefore any residual phospho-ERK1/2 was not sufficient to mediate the downstream effects on protein synthesis. The results presented here are consistent with those of a previous study [54] showing that overexpression of MKP1 decreased the hypertrophic response in neonatal cardiomyocytes and transgenic mice. However, the effect of MKP1 on protein synthesis was not assessed, and since MKP1 can dephosphorylate JNK and p38 MAPK in addition to ERK, the pathways mediating these effects are unclear. These results also support previous work that suggested a role for ERK1/2 in PE-induced hypertrophy [4,5]; however, they contradict the results of a separate study that found that transgenic mice overexpressing Gαq develop cardiac hypertrophy without ERK1/2 activation [2].
Inhibition of ERK by MKP3 also strongly impaired the activation of mTOR signalling (S6K1 and 4E-BP1) by PE. These results contrast with other findings suggesting that phosphorylation of S6K1 was dependent on MEK but not on ERK1/2 [22,23]. Ballou et al. [22] found that ERK2 does not phosphorylate S6K1 in vitro and there was no correlation between the level of ERK2 and S6K1 activity in vivo, suggesting that ERK2 is not the direct activating enzyme for S6K1. Furthermore, Lenormand et al. [23] reported that inhibition of ERK1 did not impair the activation of S6K1. The discrepancies between these previous studies and the present results may be due to differences in the stimuli or experimental approaches used or in the cell types examined. It is noteworthy, however, that the present study was performed using a primary cell type, in contrast with previous studies. Our results suggest that ERK1/2, or signalling downstream of them, activates mTOR. Indeed, a link between the MAPK and mTOR pathways has also recently been suggested by other studies [41].
Therefore we also examined the effect of PE on TSC2, a negative regulator of mTOR signalling. The results show that PE only induces a low level of phosphorylation of TSC2 at Thr1462, whereas insulin elicited a marked phosphorylation at this site. Interestingly, PE treatment induced MEK-dependent phosphorylation at serine/threonine residues within the sequences of the type RXRXX(S/T). As argued above, the effect of PE on TSC2 may be mediated through p90RSK, which can phosphorylate such motifs; indeed, we demonstrate that p90RSK, but not ERK2, can efficiently phosphorylate TSC2 in vitro. We provide evidence that, in vitro, p90RSK1 phosphorylates sites other than the previously identified Akt sites (Ser939 and Thr1462). These sites are detected by the so-called ‘anti-phospho-PKB substrate’ antibody. We show that this reactivity is lost when Ser1798 is mutated to an alanine when cells are treated with PMA. These results agree with a recent study that also identified Ser1798 in TSC2 as a target for p90RSK1 in response to PMA stimulation [44]. One important technical point to emerge from these studies is that, at least for preparation of the antibody we have used, the commercial anti-phospho-PKB substrate antiserum does not efficiently recognize the Akt sites in TSC2.
Our results suggest that TSC2 can be phosphorylated in a MEK-dependent/PI3K-independent manner in response to PE. This agrees with previous work [41], which found that, in HEK-293 cells, TSC2 was phosphorylated in response to PMA in a PKC/ERK-dependent but PI3K-independent manner. This is consistent with our finding that PE and insulin induce phosphorylation at different sites within TSC2. Further research, beyond the scope of this study, is required to identify these sites and explore their effects on TSC2 function. Phosphorylation of TSC2 (e.g. by p90RSK) may alleviate its inhibition of mTOR in a manner similar to its phosphorylation by Akt, although the methods to test this are not yet available. It seems probable that the effects of PE and PMA on TSC2 in vivo involve phosphorylation of Ser1798 at the extreme C-terminus of TSC2. This is based on the facts that (i) p90RSK1 phosphorylates TSC2 in vitro at a site(s) that is detected by the anti-phospho-PKB substrate antibody; (ii) in vivo, PE or PMA also increase the signal seen with this antibody; and (iii) mutation of Ser1798 to an alanine abolished the PMA-induced phosphorylation in cells. Our results also indicate that PMA and MEK signalling also elicit phosphorylation of TSC2 at Ser939 and Thr1462, albeit, probably, to a lesser extent than insulin/Akt. The results for Ser1798 agree with the recent report by Roux et al. [44]. When the present paper was under revision, Ballif et al. [43] provided results suggesting that Ser1364 in TSC2 is also phosphorylated in response to PMA. The fact that this event was blocked by bisindolylmaleimide was interpreted as indicating that it is catalysed by PKC: however, this compound also inhibits a number of other protein kinases, including p90RSKs and related enzymes, so caution must be exercised in interpreting its effects [55].
The above data concern mTOR signalling, which is known to regulate cell growth. However, our results suggest that there is an element of the PE-induced protein synthesis that is MEK-dependent but rapamycin-insensitive. What events are involved in this? We first investigated GSK3/eIF2B signalling. However, we observed only a very small change in GSK3α/β phosphorylation in response to PE and saw no change in eIF2B phosphorylation. Thus it appears unlikely that the GSK3/eIF2B pathway plays a significant role in the activation of protein synthesis by PE. However, GSK3 is a negative regulator of cardiac hypertrophy [4] and may play a role in other facets of the hypertrophic response, e.g. through the transcription factor NFAT (nuclear factor of activated T-cells) [56].
We also studied the role of Mnks, since they are regulated in an ERK-dependent manner. Pretreatment of cells with the Mnk inhibitor CGP57380 had a small inhibitory effect on both basal and PE-activated protein synthesis but, strikingly, this effect was additive to that of rapamycin. When used together, rapamycin and CGP57380 gave a level of inhibition similar to that observed on MEK inhibition. This suggests that the MEK-dependent effects of PE on protein synthesis are mediated by a combination of mTOR and the Mnks, and may suggest a role for phosphorylation of eIF4E, a well-characterized substrate of the Mnks. However, the changes in eIF4E phosphorylation are modest, especially given the high basal level, and it must be questionable whether they can really contribute to the substantial increase in protein synthesis. It is possible that there are other Mnk substrates whose phosphorylation changes more markedly and which (also) contribute to the activation of translation.
The level of eIF4E phosphorylation was therefore also analysed. In unstimulated ARVC, eIF4E is already substantially phosphorylated and this was decreased markedly by the Mnk inhibitor CGP57380, but not by inhibition of MEK or p38 MAPK. PE stimulation of ARVC induced an increase in eIF4E phosphorylation that was blocked by U0126. The observation that the high basal level of eIF4E phosphorylation in unstimulated ARVC is decreased by CGP57380 but not by U0126 appears to be initially surprising. However, this pattern matches the characteristics of Mnk2, which, unlike Mnk1, has high basal activity in cells that is blocked by CGP57380 but not by MEK inhibition [57] (J. Parra-Palau and C. G. Proud, unpublished work). It is possible that ARVC primarily express Mnk2 rather than Mnk1, whose basal activity is much lower. This is also consistent with the observation (Figure 7B) that the p38 MAPK inhibitor SB203580 had no effect on basal or PE-stimulated eIF4E phosphorylation since, unlike Mnk1, Mnk2 interacts with ERK but not p38 MAPK [49]. Unfortunately, Mnk1- and Mnk2-specific antibodies are not yet available for examining the expression levels of these proteins in ARVC.
Rapamycin also suppressed the PE-induced phosphorylation of eIF4E. This may be because inhibition of mTOR would inhibit binding of eIF4E to eIF4G, which is required for eIF4E phosphorylation; however, the effects of rapamycin and CGP57380 on the rate of protein synthesis were additive. It is possible that Mnk may regulate protein synthesis through mechanisms other than eIF4E, which are only affected by CGP57380. The observation that CGP57380 impairs PE-stimulated but not basal protein synthesis is also surprising. It may indicate that increased eIF4E phosphorylation is needed for the stimulation of protein synthesis (either to facilitate ribosomal scanning or for other reasons, as discussed in [51]). Alternatively, it may be that the Mnks have additional substrates, other than eIF4E, that are important for the activation of translation but not for its basal rate. The fact that rapamycin did not impair basal eIF4E phosphorylation is consistent with the absence of eIF4E/eIF4G binding under this condition [9], but suggests that in ARVC basal eIF4E phosphorylation is independent of the eIF4E/eIF4G interaction. The reasons for this are unclear.
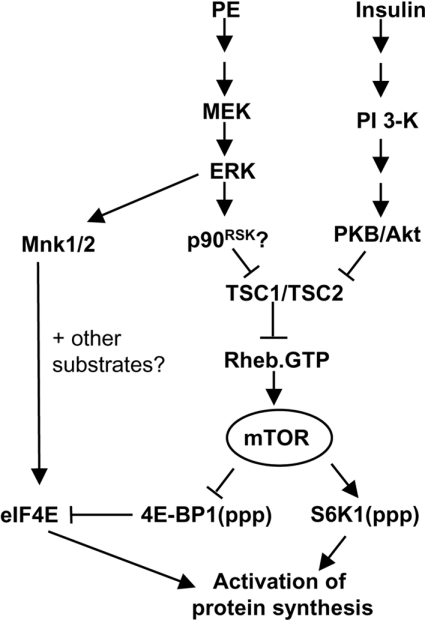
In summary, results of the present study substantially extend our understanding of the mechanisms by which PE activates protein synthesis in cardiomyocytes (Figure 8). Our results clearly indicate a key role for ERK itself in mediating the effects of PE on the downstream targets of mTOR, S6K1 and 4E-BP1, and in activating protein synthesis. The results also show that PE induces the phosphorylation of TSC2 at sites distinct from those affected by insulin (through Akt). It is probable, therefore, that phosphorylation and inactivation of TSC2 links ERK signalling to stimulation of mTOR, e.g. in response to PE. Given that insulin also activates ERK, albeit much less strongly than PE [6], the phosphorylation of TSC2 through MEK/ERK signalling may also contribute to the activation of mTOR by this stimulus. However, the lack of effect of MKP3 on insulin-stimulated protein synthesis suggests that the contribution is minor. Future studies will focus on the roles of kinases that lie downstream of ERK1/2 in the activation of protein synthesis and, particularly, on the roles of TSC1/2 and Rheb. Our results suggest that divergent signalling downstream of ERK (through Mnks and probably p90RSK/TSC2) mediates the MEK-dependent activation of protein synthesis by PE in adult cardiomyocytes. Our results also suggest that TSC1/2/Rheb signalling may play a key role in the hypertrophic effects of PE, consistent with their known roles in cell-growth control.
Figure 8. Diagram showing the signalling pathways and connections involved in the activation of protein synthesis in cardiomyocytes.
Insulin activates protein synthesis by the PI3K pathway, which involves the activation of PKB/Akt. However, the effects of PE are mediated through the classical MAPK pathway and results presented in this study indicate that ERK1/2 activity is required to activate protein synthesis and mTOR signalling. It is probable that a kinase downstream of ERK, such as p90RSK, phosphorylates TSC2, alleviating its inhibition of Rheb. This results in the activation of the mTOR pathway and S6 kinases and the inhibition of 4E-BP1 activity; both of these events activate protein synthesis. The Mnks also contribute to the activation of protein synthesis by PE, probably through the phosphorylation of eIF4E and perhaps of other substrates.
Acknowledgments
This work was funded by the British Heart Foundation (to C. G. P.), the National Research Service Award HL 10165 (to P. F. P.) and the National Institutes of Health (grant HL 22563; A. Sorokin).
References
- 1.Hannan R. D., Jenkins A., Jenkins A. K., Brandenburger Y. Cardiac hypertrophy: a matter of translation. Clin. Exp. Pharmacol. Physiol. 2003;30:517–527. doi: 10.1046/j.1440-1681.2003.03873.x. [DOI] [PubMed] [Google Scholar]
- 2.Sakata Y., Hoit B. D., Liggett S. B., Walsh R. A., Dorn G. W. Decompensation of pressure-overload hypertrophy in G alpha q-overexpressing mice. Circulation. 1998;97:1488–1495. doi: 10.1161/01.cir.97.15.1488. [DOI] [PubMed] [Google Scholar]
- 3.Hannan K. M., Brandenburger Y., Jenkins A., Sharkey K., Cavanaugh A., Rothblum L., Moss T., Poortinga G., McArthur G. A., Pearson R. B., et al. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol. Cell. Biol. 2003;23:8862–8877. doi: 10.1128/MCB.23.23.8862-8877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frey N., Olson E. N. Cardiac hypertrophy: the good, the bad, and the ugly. Annu. Rev. Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 5.Sugden P. H. Signaling in myocardial hypertrophy: life after calcineurin? Circ. Res. 1999;84:633–646. doi: 10.1161/01.res.84.6.633. [DOI] [PubMed] [Google Scholar]
- 6.Wang L., Gout I., Proud C. G. Cross-talk between the ERK and p70 S6 kinase (S6K) signaling pathways. MEK-dependent activation of S6K2 in cardiomyocytes. J. Biol. Chem. 2001;276:32670–32677. doi: 10.1074/jbc.M102776200. [DOI] [PubMed] [Google Scholar]
- 7.Sugden P. H., Clerk A. Activation of the small GTP-binding protein Ras in the heart by hypertrophic agonists. Trends Cardiovasc. Med. 2000;10:1–8. doi: 10.1016/s1050-1738(00)00038-4. [DOI] [PubMed] [Google Scholar]
- 8.Bueno O. F., Molkentin J. D. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ. Res. 2002;91:776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 9.Wang L., Proud C. G. Ras/Erk signaling is essential for activation of protein synthesis by Gq protein-coupled receptor agonists in adult cardiomyocytes. Circ. Res. 2002;91:821–829. doi: 10.1161/01.res.0000041029.97988.e9. [DOI] [PubMed] [Google Scholar]
- 10.Wang L., Proud C. G. Regulation of the phosphorylation of elongation factor 2 by MEK-dependent signalling in adult rat cardiomyocytes. FEBS Lett. 2002;531:285–289. doi: 10.1016/s0014-5793(02)03536-6. [DOI] [PubMed] [Google Scholar]
- 11.Glennon P. E., Kaddoura S., Sale E. M., Sale G. J., Fuller S. J., Sugden P. H. Depletion of mitogen-activated protein kinase using an antisense oligodeoxynucleotide approach downregulates the phenylephrine-induced hypertrophic response in rat cardiac myocytes. Circ. Res. 1996;78:954–961. doi: 10.1161/01.res.78.6.954. [DOI] [PubMed] [Google Scholar]
- 12.Avruch J., Belham C., Weng Q., Hara K., Yonezawa K. The p70 S6 kinase integrates nutrient and growth signals to control translational capacity. Prog. Mol. Subcell. Biol. 2001;26:115–154. doi: 10.1007/978-3-642-56688-2_5. [DOI] [PubMed] [Google Scholar]
- 13.Thomas G., Hall M. N. TOR signalling and the control of cell growth. Curr. Opin. Cell Biol. 1998;9:782–787. doi: 10.1016/s0955-0674(97)80078-6. [DOI] [PubMed] [Google Scholar]
- 14.Shioi T., McMullen J. R., Tarnavski O., Converso K., Sherwood M. C., Manning W. J., Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 15.McMullen J. R., Sherwood M. C., Tarnavski O., Zhang L., Dorfman A. L., Shioi T., Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 16.McManus E. J., Alessi D. R. TSC1-TSC2: a complex tale of PKB-mediated S6K regulation. Nat. Cell Biol. 2002;4:E214–E216. doi: 10.1038/ncb0902-e214. [DOI] [PubMed] [Google Scholar]
- 17.Manning B. D., Cantley L. C. Rheb fills a GAP between TSC and TOR. Trends Biochem. Sci. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Gingras A.-C., Raught B., Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence J. C., Abraham R. T. PHAS/4E-BPs as regulators of mRNA translation and cell proliferation. Trends Biochem. Sci. 1997;22:345–349. doi: 10.1016/s0968-0004(97)01101-8. [DOI] [PubMed] [Google Scholar]
- 20.Mahalingam M., Cooper J. A. Phosphorylation of mammalian eIF4E by Mnk1 and Mnk2: tantalizing prospects for a role in translation. Prog. Mol. Subcell. Biol. 2001;27:131–142. [PubMed] [Google Scholar]
- 21.Proud C. G. Regulation of eukaryotic initiation factor eIF2B. Prog. Mol. Subcell. Biol. 2001;26:95–114. doi: 10.1007/978-3-642-56688-2_4. [DOI] [PubMed] [Google Scholar]
- 22.Ballou L. M., Luther H., Thomas G. MAP2 kinase and 70K S6 kinase lie on distinct signalling pathways. Nature (London) 1991;349:348–350. doi: 10.1038/349348a0. [DOI] [PubMed] [Google Scholar]
- 23.Lenormand P., McMahon M., Pouysségur J. Oncogenic Raf-1 activates p70 S6 kinase via a mitogen-activated protein kinase-independent pathway. J. Biol. Chem. 1996;271:15762–15768. doi: 10.1074/jbc.271.26.15762. [DOI] [PubMed] [Google Scholar]
- 24.Camps M., Nichols A., Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- 25.Muda M., Theodosiou A., Rodrigues N., Boschert U., Camps M., Gillieron C., Davies K., Ashworth A., Arkinstall S. The dual specificity phosphatases M3/6 and MKP3 are highly selective for inactivation of distinct mitogen activated protein kinases. J. Biol. Chem. 1996;271:27205–27208. doi: 10.1074/jbc.271.44.27205. [DOI] [PubMed] [Google Scholar]
- 26.Manning B. D., Cantley L. C. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem. Soc. Trans. 2003;31:573–578. doi: 10.1042/bst0310573. [DOI] [PubMed] [Google Scholar]
- 27.Knauf U., Tschopp C., Gram H. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol. Cell. Biol. 2001;21:5500–5511. doi: 10.1128/MCB.21.16.5500-5511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L., Wang X., Proud C. G. Activation of mRNA translation by insulin in rat cardiomyocytes involves multiple rapamycin-sensitive steps. Am. J. Physiol. 2000;278:H1056–H1068. doi: 10.1152/ajpheart.2000.278.4.H1056. [DOI] [PubMed] [Google Scholar]
- 29.Vries R. G. J., Flynn A., Patel J. C., Wang X., Denton R. M., Proud C. G. Heat shock increases the association of binding protein-1 with initiation factor 4E. J. Biol. Chem. 1997;272:32779–32784. doi: 10.1074/jbc.272.52.32779. [DOI] [PubMed] [Google Scholar]
- 30.Diggle T. A., Moule S. K., Avison M. B., Flynn A., Foulstone E. J., Proud C. G., Denton R. M. Both rapamycin-sensitive and -insensitive pathways are involved in the phosphorylation of the initiation factor 4E binding protein (4E-BP1) in response to insulin in rat epididymal fat cells. Biochem. J. 1996;316:447–453. doi: 10.1042/bj3160447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moule S. K., Edgell N. J., Welsh G. I., Diggle T. A., Foulstone E. J., Heesom K. J., Proud C. G., Denton R. M. Multiple signalling pathways involved in the stimulation of fatty acid and glycogen synthesis by insulin in rat epididymal fat pads. Biochem. J. 1995;311:595–601. doi: 10.1042/bj3110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;77:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 33.Hall-Jackson C. A., Cross D. A., Morrice N., Smythe C. ATR is a caffeine-sensitive, DNA-activated protein kinase with a substrate specificity distinct from DNA-PK. Oncogene. 1999;18:6707–6713. doi: 10.1038/sj.onc.1203077. [DOI] [PubMed] [Google Scholar]
- 34.McLeod L. E., Wang L., Proud C. G. β-Adrenergic agonists increase phosphorylation of elongation factor 2 in cardiomyocytes without eliciting calcium-independent eEF2 kinase activity. FEBS Lett. 2001;489:225–228. doi: 10.1016/s0014-5793(01)02100-7. [DOI] [PubMed] [Google Scholar]
- 35.Fuller S. J., Sugden P. H. Acute inhibition of rat heart protein synthesis in vitro during β-adrenergic stimulation or hypoxia. Am. J. Physiol. 1988;255:E537–E547. doi: 10.1152/ajpendo.1988.255.4.E537. [DOI] [PubMed] [Google Scholar]
- 36.Groom L. A., Sneddon A. A., Alessi D. R., Dowd S., Keyse S. M. Differential regulation of the MAP,SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 1996;15:3621–3632. [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X., Proud C. G. p70 S6 kinase is activated by sodium arsenite in adult rat cardiomyocytes: roles for phosphatidylinositol 3-kinase and p38 MAP kinase. Biochem. Biophys. Res. Commun. 1997;238:207–212. doi: 10.1006/bbrc.1997.7273. [DOI] [PubMed] [Google Scholar]
- 38.Feigenblum D., Schneider R. J. Cap binding protein (eukaryotic initiation factor 4E) and 4E inactivating protein BP1 independently regulate cap dependent translation. Mol. Cell. Biol. 1996;16:5450–5457. doi: 10.1128/mcb.16.10.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manning B. D., Tee A. R., Logsdon M. N., Blenis J., Cantley L. C. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 40.Alessi D. R., Caudwell F. B., Andjelkovic M., Hemmings B. A., Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 41.Tee A. R., Anjum R., Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/Akt-dependent and -independent phosphorylation of tuberin. J. Biol. Chem. 2003;278:37288–37296. doi: 10.1074/jbc.M303257200. [DOI] [PubMed] [Google Scholar]
- 42.Herbert T. P., Kilhams G. R., Batty I. H., Proud C. G. Distinct signalling pathways mediate insulin and phorbol ester-stimulated eIF4F assembly and protein synthesis in HEK 293 cells. J. Biol. Chem. 2000;275:11249–11256. doi: 10.1074/jbc.275.15.11249. [DOI] [PubMed] [Google Scholar]
- 43.Ballif B. A., Roux P. P., Gerber S. A., Mackeigan J. P., Blenis J., Gygi S. P. Quantitative phosphorylation profiling of the ERK/p90 ribosomal S6 kinase-signaling cassette and its targets, the tuberous sclerosis tumor suppressors. Proc. Natl. Acad. Sci. U.S.A. 2005;102:667–672. doi: 10.1073/pnas.0409143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roux P. P., Ballif B. A., Anjum R., Gygi S. P., Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welsh G. I., Stokes C. M., Wang X., Sakaue H., Ogawa W., Kasuga M., Proud C. G. Activation of translation initiation factor eIF2B by insulin requires phosphatidylinositol 3-kinase. FEBS Lett. 1997;410:418–422. doi: 10.1016/s0014-5793(97)00579-6. [DOI] [PubMed] [Google Scholar]
- 46.Sutherland C., Cohen P. The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett. 1994;338:37–42. doi: 10.1016/0014-5793(94)80112-6. [DOI] [PubMed] [Google Scholar]
- 47.Sutherland C., Leighton I. A., Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem. J. 1993;296:15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukunaga R., Hunter T. Mnk1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921–1933. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waskiewicz A. J., Flynn A., Proud C. G., Cooper J. A. Mitogen-activated kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cocolakis E., Lemay S., Ali S., Lebrun J. J. The p38 MAPK pathway is required for cell growth inhibition of human breast cancer cells in response to activin. J. Biol. Chem. 2001;276:18430–18436. doi: 10.1074/jbc.M010768200. [DOI] [PubMed] [Google Scholar]
- 51.Scheper G. C., Proud C. G. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur. J. Biochem. 2002;269:5350–5359. doi: 10.1046/j.1432-1033.2002.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ueda T., Watanabe-Fukunaga R., Fukuyama H., Nagata S., Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol. Cell. Biol. 2004;24:6539–6549. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pyronnet S., Imataka H., Gingras A. C., Fukunaga R., Hunter T., Sonenberg N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits Mnk1 to phosphorylate eIF4E. EMBO J. 1999;18:270–279. doi: 10.1093/emboj/18.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bueno O. F., De Windt L. J., Lim H. W., Tymitz K. M., Witt S. A., Kimball T. R., Molkentin J. D. The dual-specificity phosphatase MKP-1 limits the cardiac hypertrophic response in vitro and in vivo. Circ. Res. 2001;88:88–96. doi: 10.1161/01.res.88.1.88. [DOI] [PubMed] [Google Scholar]
- 55.Davies S. P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antos C. L., McKinsey T. A., Frey N., Kutschke W., McAnally J., Shelton J. M., Richardson J. A., Hill J. A., Olson E. N. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. U.S.A. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheper G. C., Morrice N. A., Kleijn M., Proud C. G. The MAP kinase signal-integrating kinase Mnk2 is an eIF4E kinase with high basal activity in mammalian cells. Mol. Cell. Biol. 2001;21:743–754. doi: 10.1128/MCB.21.3.743-754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]