Abstract
Silencing of gene expression by small interfering RNAs (siRNAs) is rapidly becoming a powerful tool for genetic analysis and represents a potential strategy for therapeutic product development. However, there are no reports of systemic delivery for siRNAs toward treatment of bone-metastatic cancer. Accordingly, we report here that i.v. injection of GL3 luciferase siRNA complexed with atelocollagen showed effective reduction of luciferase expression from bone-metastatic prostate tumor cells developed in mouse thorax, jaws, and/or legs. We also show that the siRNA/atelocollagen complex can be efficiently delivered to tumors 24 h after injection and can exist intact at least for 3 days. Furthermore, atelocollagen-mediated systemic administration of siRNAs such as enhancer of zeste homolog 2 and phosphoinositide 3′-hydroxykinase p110-α-subunit, which were selected as candidate targets for inhibition of bone metastasis, resulted in an efficient inhibition of metastatic tumor growth in bone tissues. In addition, upregulation of serum IL-12 and IFN-α levels was not associated with the in vivo administration of the siRNA/atelocollagen complex. Thus, for treatment of bone metastasis of prostate cancer, an atelocollagen-mediated systemic delivery method could be a reliable and safe approach to the achievement of maximal function of siRNA.
Keywords: bone metastasis, prostate cancer
RNA interference (RNAi) induced by small interfering RNA (siRNA) has recently emerged as a powerful technique that is capable of suppressing expression of individual genes with a high degree of specificity (1). The technique has been used for studies of gene function in vivo, primarily in mice. The first demonstration of RNAi-mediated repression in an adult animal showed effective repression of a luciferase reporter gene after hydrodynamic transfection of siRNA expression plasmids into mouse liver (2, 3). Subsequent studies have delivered siRNA by various methods, including viral vector-mediated delivery (4, 5) and lipid-based delivery (6, 7). A more recent study showed that chemically modified siRNAs can silence an endogenous gene after i.v. injection in mice (8). These findings provide hope for using RNAi technology in disease control.
Many studies have used siRNAs as an experimental tool to dissect the cellular pathways that lead to uncontrolled cell proliferation and cancer. To develop siRNAs for cancer therapy, several researchers have investigated them in animal models (9-13). However, reports of RNAi-delivery strategies for bone-metastatic cancer are very limited. For example, in advanced prostate cancer, the sites most frequently affected by metastasis are the bones and regional lymph nodes. Patients with these metastases suffer pain and low limb edema, making it extremely important to explore avenues of treating such bone metastases.
We previously demonstrated the efficacy of atelocollagen for delivery of nucleotides, such as plasmid DNA and antisense oligonucleotides, in vitro and in vivo (14-19). Recently, we also reported that atelocollagen complexed with siRNA is resistant to nucleases and is efficiently transduced into cells, thereby allowing long-term gene silencing (20). Furthermore, intratumor injection of atelocollagen complexed with siRNA against fibroblast growth factor 4 mRNA showed efficient inhibition of tumor growth in an orthotopic xenograft model of a human nonseminomatous germ cell tumor (20). Another group reported that radiolabeled siRNA mixed with atelocollagen existed in the tumors for at least a week and remained intact and that the vascular endothelial growth factor siRNA with atelocollagen dramatically suppressed tumor angiogenesis and tumor growth in a PC-3 s.c. xenograft model (21). Thus, for local administration of siRNA, an atelocollagen-based nonviral delivery method could be a reliable approach to achieve the maximal function of siRNA in vivo. In addition, an atelocollagen complex can be delivered for i.v. injection as nanoparticles, making systemic delivery of siRNA possible. A recent report showed the potential for atelocollagen-mediated systemic antisense therapeutics for treating inflammatory disease (19).
In this study, noninvasive optical imaging technologies were used to facilitate the detection of metastatic lesions and the effects of synthetic siRNAs on tumor regression. The results indicate that systemic administration of atelocollagen complexed with siRNA into a mouse model of bone metastasis demonstrated effective gene silencing and tumor regression in bone-metastatic lesions. Furthermore, we also showed that atelocollagen-mediated systemic delivery of siRNA did not cause any side effects. Thus, systemic delivery of a siRNA/atelocollagen complex may have therapeutic potential in the treatment of advanced prostate cancer with bone metastasis.
Materials and Methods
Atelocollagen. Atelocollagen is a highly purified type I collagen of calf dermis with pepsin treatment (Koken, Tokyo). A collagen molecule has an amino acid sequence called a telopeptide at both N and C termini, which confers most of the collagen's antigenicity. Atelocollagen obtained by pepsin treatment is low in immunogenicity because it is free from telopeptides (22), and it is used clinically for a wide range of purposes, including wound healing and vessel prosthesis and as a bone cartilage substitute and haemostatic agent (16).
Cell Lines. The bioluminescent human prostate carcinoma cell line PC-3M-luc-C6 (Xenogen, Alameda, CA) was cultured in Eagle's minimum essential medium (Invitrogen) supplemented with 10% heat-inactivated FBS (Equitech-Bio, Kerrville, TX), nonessential amino acids (Sigma-Aldrich), l-glutamine (ICN), 1 mM sodium pyruvate (Sigma-Aldrich), MEM vitamin solution (Sigma-Aldrich), and 200 μg/ml zeocin (Invitrogen). The cells were maintained in vitro at 37°C in a humidified atmosphere of 5% CO2.
siRNA Preparation. Synthetic 21-nt RNAs were purchased from Dharmacon Research (Lafayette, CO) in deprotected, desalted, and annealed form. The sequence for GL3 siRNA is reported in ref. 23. The sequence of human enhancer of zeste homolog 2 (EZH2) siRNA was 5′-GGA AAG AAC GGA AAU CUU AdTdT-3′ and 3′-dTdTC CUU UCU UGC CUU UAG AAU-5′, and human phosphoinositide 3′-hydroxykinase p110-α-subunit (p110-α) siRNA was 5′-GGU UAA AGA UCC AGA AGU AdTdT-3′ and 3′-dTdTC CAA UUU CUA GGU CUU CAU-5′. The nonspecific control siRNA duplex was also purchased from Dharmacon Research.
In Vivo Imaging of siRNA Delivery in Mice with Bone-Metastatic Tumors. Animal experiments in the present study were performed in compliance with the guidelines of the Institute for Laboratory Animal Research at the National Cancer Center Research Institute. Eight- to 9-week-old male athymic nude mice (CLEA Japan, Osaka) were anesthetized by exposure to 1-3% isoflurane on day 0 and subsequent days. On day 0 of the experiments, to generate an experimental metastasis model, the anesthetized animals were injected with 3 × 106 PC-3M-luc-C6 cells suspended in 100 μl of sterile Dulbecco's PBS into the left heart ventricle (24, 25). For in vivo imaging, the mice were administered d-luciferin (150 mg/kg, Promega) by i.p. injection. Ten minutes later, photons from animal whole bodies were counted by using the IVIS imaging system (Xenogen) according to the manufacturer's instructions. Data were analyzed by using livingimage 2.50 software (Xenogen). A successful intracardiac injection was indicated by day 0 images that showed a systemic bioluminescence distributed throughout the animal, and only those mice evidencing a satisfactory injection were continued in the experiment. The development of subsequent metastasis was monitored twice a week in vivo by bioluminescent imaging.
For preparing the siRNA/atelocollagen complex, equal volumes of atelocollagen (0.1% in PBS at pH 7.4) and siRNA solution were combined and mixed by rotating for 20 min at 4°C. The final concentration of atelocollagen was 0.05%. Four weeks after tumor injection, individual mice (from cohorts containing five animals) were injected with 200 μl of atelocollagen containing 25 μg of luciferase GL3 siRNA, atelocollagen alone, siRNA alone, or nonspecific siRNA/atelocollagen by i.v. tail vein injection. Tumor growth was not affected by these treatments. To control for mouse-to-mouse variability, the bioluminescence ratio for each mouse was normalized by dividing by the 1-day-posttreatment/pretreatment ratio of luciferase intensity for that mouse.
Detection of siRNA in Tumor Tissues or Normal Tissues by RNase Protection Assay. To show siRNA delivery in tumor tissues, 10-week-old male athymic nude mice were inoculated s.c. with 3 × 106 PC-3M-luc-C6 cells suspended in 50 μl of sterile Dulbecco's PBS. After 8 days, when a tumor reached a volume of 50-100 mm3, tumor-bearing mice (from cohorts containing three animals) were injected with 200 μl of 0.05% atelocollagen containing 25 μg of luciferase GL3 siRNA, atelocollagen alone, or siRNA alone by i.v. tail vein injection. The mice were killed 1 and 3 days after treatment of siRNA/atelocollagen complexes, and total RNA was extracted from a tumor and selected mice tissues by using ISOGEN (Nippon Gene, Tokyo). The RNase protection probe was made with a mirVana microRNA Probe Construction Kit (Ambion, Austin, TX). The cRNA probe specific for the antisense strand of GL3 siRNA was generated by using T7 RNA polymerase and 32P-labeled UTP. Total RNAs were used in an RNase protection assay using the mirVana miRNA Detection Kit (Ambion) per the manufacturer's protocol. Protected fragments were separated by electrophoresis in 15% polyacrylamide 8 M urea gels. The gels were exposed to x-ray films for 30 min, and the films were then scanned and analyzed by using nih image software. GL3 siRNA levels were corrected for wet tissue weights.
Atelocollagen-Mediated siRNA Transfection and Tumor Growth Assay in Vitro. The EZH2 siRNA or p110-α siRNA complexed with atelocollagen (final concentration = 0.008%) was prefixed to a six-well plate (37.5 pmol of siRNA/250 μl per well) according to the method described in refs. 15 and 20. The cultured PC-3M-luc-C6 cells were plated into the complex-prefixed plate at 5 × 104 cells per well. Bioluminescence from PC-3M-luc-C6 cells highly correlated to the total number of cells (26). For monitoring the inhibition of cell growth, the cells were lysed (n = 3) on days 2, 4, and 6 and then analyzed for luciferase activity (Bright-Glo Luciferase Assay System, Promega). Inhibition of luciferase production was normalized to the level of vehicle-treated cells.
Quantitative RT-PCR. Total RNA was extracted from PC-3M-luc-C6 cells by using ISOGEN and treated with DNase I (Takara Shuzo, Otsu, Japan). Five micrograms of total RNA was used to produce cDNAs with oligo(dT) 12 primer by superscript III RNA polymerase (Invitrogen). cDNA was diluted 5-fold and used for quantitative PCR. For quantitation, aliquots of 5 μl of cDNA samples were subjected to quantitative PCR in 50-μl reactions by using Platinum Quantitative PCR SuperMix-UDG (Invitrogen) and Assays-on-Demand TaqMan primers/probe sets (Applied Biosystems) specific for human EZH2, p110-α, and GAPDH. Reactions were carried out by using the Applied Biosystems PRISM 7700 Sequence Detection System. The reactions were incubated at 50°C for 2 min and then heated to 95°C for 2 min, followed by 45 cycles of 30 s at 95°C, 15 s at 60°C, and 20 s at 72°C. Human EZH2 and p110-α expression levels were normalized to GAPDH levels.
Analysis of siRNA/Atelocollagen Treatment for Bone-Metastatic Prostate Cancer. Mice were inoculated with PC-3M-luc-C6 cells into the left cardiac ventricle on day 0 as described above. The EZH2, p110-α, and nonspecific control siRNA (50 μg) with or without 0.05% atelocollagen in a 200-μl volume were injected into the mouse tail vein on days 3, 6, and 9 postinoculation. Each experimental condition included eight animals per group. The development of subsequent metastasis was monitored twice a week in vivo by bioluminescent imaging for 4 weeks. To control for mouse-to-mouse variability, the bioluminescence ratio for each mouse was normalized by dividing by the before/after treatment ratio of luciferase intensity for that mouse. At the end of the experiment on day 28, to confirm the presence of neoplastic cells, selected tissues were excised from the mice at necropsy. Tissues were fixed in 4% formaldehyde-PBS(-), embedded in paraffin, cut into 5-mm sections, and stained with hematoxylin/eosin.
Monitoring of IFN Induction in Mice Treated with Atelocollagen-Mediated siRNA. Eight-week-old male athymic nude mice were injected with nonspecific control siRNA (50 μg) with 0.05% atelocollagen in a 200-μl volume by i.v. tail vein injection. Each experimental condition included four animals per group. The positive control group was injected with poly(I:C) (Amersham Pharmacia Biosciences). To measure serum cytokine levels, blood was harvested from mice 2 h after injection by cardiac puncture. IL-12 (p40) and IFN-α levels (R & D Systems) were measured by ELISA according to the manufacturer's instructions.
Statistical Analysis. The results are given as mean ± SD. Statistical analysis was conducted by using the analysis of variance with the Bonferroni correction for multiple comparisons. P ≤ 0.05 was considered a significant difference.
Results
Efficient Delivery of Atelocollagen-Mediated Luciferase siRNA in Bone-Metastatic Regions. To increase the potential for bone metastasis from PC-3M-luc-C6 cells, we injected the cells into the left ventricle of the heart (25). Mice with successful intracardiac injection of PC-3M-luc-C6 cells on day 0 were imaged twice a week for up to 4 weeks. In all mice, early indications of metastasis to various tissues were observed within 1 week after cell injection (data not shown). Four weeks after tumor injection, the observed patterns of metastasis indicated lesions developing in the thorax, jaws, and/or legs of the mice (Fig. 1A). To test whether atelocollagen-mediated siRNA systemic delivery is valid for a gene silencing effect on the metastatic sites, the animals were treated with atelocollagen alone, a nonspecific control siRNA/atelocollagen complex, a luciferase GL3 siRNA alone, or a luciferase GL3 siRNA/atelocollagen complex i.v. In mice receiving the luciferase siRNA/atelocollagen complex, bioluminescence was inhibited by 80-90% in the whole body, including the bone metastases, when compared with before treatment (Fig. 1). In contrast, the bioluminescent signals of most of the metastatic sites in the mice treated with atelocollagen alone or the control siRNA/atelocollagen complex had increased. Treatment with luciferase siRNA alone either had no effect or slightly suppressed photon emission from the tumor cells. After the imaging analysis, tissues expressing bioluminescence were excised from the mice at necropsy. Subsequent histopathology analysis confirmed micrometastases in the lung, dental pulp, tibia, femur, and other soft tissues (data not shown). Thus, our results indicate that siRNA can be delivered by using atelocollagen and can thereby inhibit gene expression in a specific manner in metastatic sites, including bone metastases.
Fig. 1.
Monitoring luciferase inhibition in vivo with bioluminescent imaging. (A) Representative images of nude mice injected with 3 × 106 PC-3M-luc-C6 cells suspended in 100 μl of sterile Dulbecco's PBS into the left ventricle of the heart. Four weeks after tumor injection, each animal was administered i.v. with 200 μl of 0.05% atelocollagen solution, 25 μg of luciferase GL3 siRNA, GL3 siRNA/atelocollagen complex, or nonspecific siRNA/atelocollagen complex. (B) Normalized fold change (1 day posttreatment/pretreatment) of bioluminescence emitted from whole body of mice. Data represent the mean ± SD (n = 4). *, P < 0.001 versus other experimental groups.
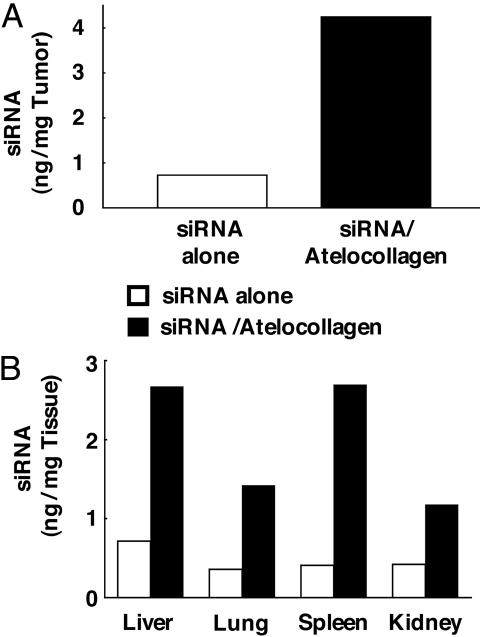
Enhanced Delivery of siRNA into Tumors by Atelocollagen. The efficacy of delivery of siRNA into tumors was evaluated. Athymic nude mice were inoculated s.c. with 3 × 106 PC-3M-luc-C6 cells and injected i.v. with luciferase siRNA/atelocollagen, luciferase siRNA alone, or atelocollagen alone. We assessed the delivery of siRNA 1 day after the i.v. administration. As shown in Fig. 2A and also in Fig. 7, which is published as supporting information on the PNAS web site, a significant amount of siRNA was detected in tumors with atelocollagen-mediated delivery (4.3 ng of siRNA/mg of tumor weight). In contrast, i.v. injection of siRNA alone (0.7 ng/mg of tumor weight) was less efficient compared with atelocollagen-mediated delivery. We also assessed the delivery of luciferase siRNA in several tissues, such as liver, lung, spleen, and kidney. As shown in Fig. 2B, a relatively high amount of siRNA was detected in tissues from mice administered with the siRNA/atelocollagen complex compared with mice with siRNA alone. In addition, siRNA delivered with atelocollagen existed intact for at least 3 days (data not shown). Taken together, these results suggest that the systemic injection of the siRNA/atelocollagen complex allows a more efficient delivery of siRNA into tumors than siRNA alone and causes siRNA to be retained for a longer period therein.
Fig. 2.
Distribution of siRNA delivered with atelocollagen in tumor tissues and normal tissues. The nude mice were inoculated s.c. with 3 × 106 PC-3M-luc-C6 cells. Once tumors had reached 50-100 mm3, tumor-bearing mice were injected with 200 μl of 0.05% atelocollagen containing 25 μg of luciferase GL3 siRNA or siRNA alone by i.v. tail vein injection. The mice were killed 1 day after treatment with siRNA/atelocollagen complexes, and total RNA was extracted from a tumor (A) and selected tissues (B). Detection of luciferase GL3 siRNA was performed by RNase protection assay. GL3 siRNA levels were corrected for wet tissue weights.
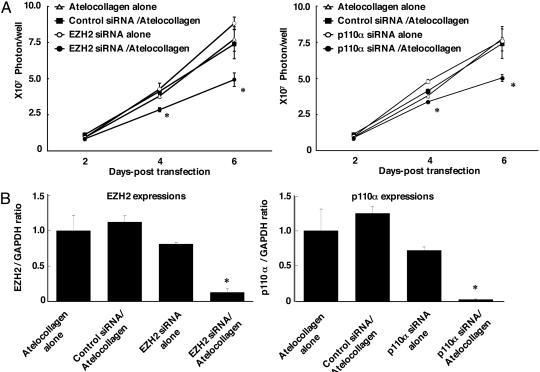
Atelocollagen-Mediated siRNA Transfer Allows Efficient Inhibition of PC-3M-luc Cell Growth in Vitro. To screen target genes for showing growth inhibition of PC-3M-luc cells, EZH2 and p110-α were selected as target genes. The atelocollagen-mediated siRNA reverse cell transfection method was used. The cultured PC-3M-luc-C6 cells were plated into a siRNA/atelocollagen complex-prefixed plate. For monitoring cell growth, we analyzed luciferase activity. Inhibition of cell growth was observed on PC-3M-luc cells treated with EZH2 and p110-α siRNA/atelocollagen complexes (Fig. 3A). Inhibition of mRNA levels of targets was also shown (Fig. 3B). These results revealed that EZH2 and p110-α may be the target of inhibition of the metastasis of PC-3M-luc cells.
Fig. 3.
The EZH2 and p110-α siRNA inhibit PC-3M-luc-C6 cell proliferation and suppress EZH2 and p110-α expression. (A) Inhibition of PC-3M-luc-C6 cell proliferation. For monitoring the inhibition of cell growth, cells were lysed on days 2, 4, and 6 and then analyzed for luciferase activity. (B) The effects of siRNA transfection on expression of EZH2 and p110-α mRNA. EZH2 and p110-α mRNA expression levels were measured by quantitative PCR. Data represent the mean ± SD (n = 3). *, P < 0.05 versus cells treated with atelocollagen alone.
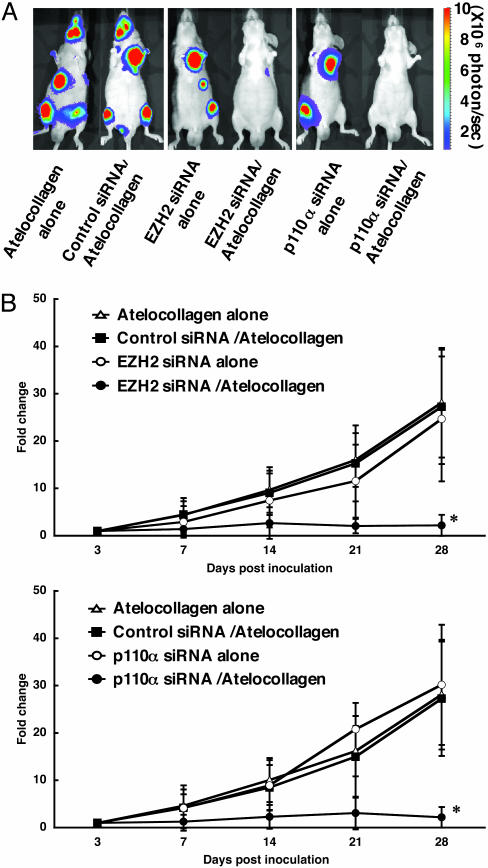
Inhibition of Metastatic Tumor Growth in Bone Tissues in Animals with Atelocollagen Complex. To assess the inhibition of bone metastasis by the atelocollagen-mediated siRNA delivery system, EZH2 and p110-α siRNA/atelocollagen complexes were administered i.v. into mice on days 3, 6, and 9 of postintracardiac ventricle injection of PC-3M-luc cells. The development of bone metastasis was monitored in vivo by bioluminescent imaging. At the end of the experiment on day 28, mice treated with atelocollagen alone and the control siRNA/atelocollagen complex-treated group showed high metastasis in the thorax, jaws, and/or legs (Figs. 4A and 5A). Total luminescence from all tumors was determined at different times posttreatment for each mouse. As seen in Fig. 4B, there was an increase in luminescence in mice treated with atelocollagen alone, the control siRNA/atelocollagen complex, EZH2 siRNA alone, and p110-α siRNA alone, whereas the EZH2 siRNA/atelocollagen-treated and p110-α siRNA/atelocollagen-treated groups had no increase in luminescence during the same observation period. There were significant differences between the EZH2- and p110-α siRNA/atelocollagen-treated groups and the other three experimental groups on day 28 (P < 0.05). Histopathological analysis revealed that metastasis of PC-3M-luc-C6 cells in the dental pulp was significantly inhibited by the EZH2 and p110-α siRNA/atelocollagen complexes (Fig. 5B). Therefore, the atelocollagen-mediated systemic delivery of siRNA could be a unique strategy for inhibition of bone-metastatic prostate tumor growth in vivo.
Fig. 4.
Inhibition of metastatic tumor growth in bone tissues by the atelocollagen-mediated siRNA delivery system. Mice were inoculated with PC-3M-luc-C6 cells into the left cardiac ventricle on day 0. The EZH2, p110-α, and nonspecific control siRNAs (50 μg) with or without 0.05% atelocollagen in a 200-μl volume were injected into the mouse tail vein on days 3, 6, and 9 postinoculation. Each experimental regimen comprised eight animals. (A) Representative images of nude mice at the end of the experiment on day 28. (B) Normalized fold change (posttreatment/pretreatment) of bioluminescence emitted from whole body of mice. Data represent the mean ± SD (n = 8). *, P < 0.05 versus other experimental groups.
Fig. 5.
Confirmation of prostate cancer bone metastasis by ex vivo imaging and histopathology. (A) The isolated organs from mice (Fig. 4) were reimaged. The bioluminescent signals were detected from the bone region in the jaw and leg of mice treated with atelocollagen alone, EZH2 siRNA/atelocollagen, and p110-α siRNA/atelocollagen, respectively. (B) Hematoxylin/eosin-stained sections of the dental pulp from the same mice that were imaged in Fig. 4. Arrow marks the carcinomatous micrometastasis. (Scale bars, 100 μm.)
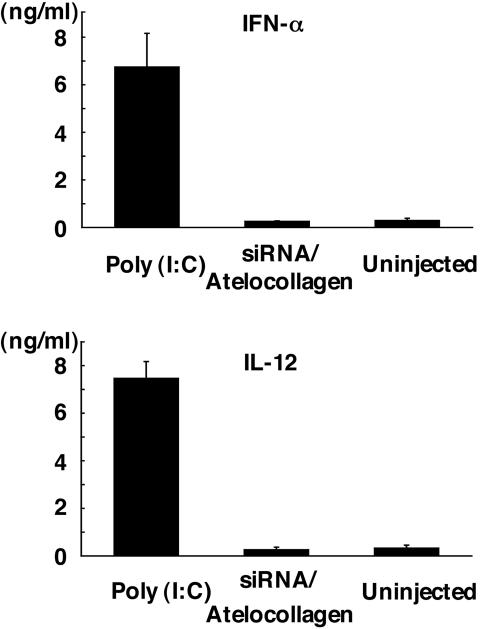
Absence of IFN Response to Atelocollagen-Mediated siRNA Delivery System. To test whether the atelocollagen-mediated siRNA systemic delivery has the possibility of inducing IFN responses in mice, the plasma levels of IFN-α and IL-12 in mice exposed to the siRNA/atelocollagen complex or poly(I:C) by i.v. injection were measured by ELISA (Fig. 6). As observed with IFN-α and IL-12, the siRNA/atelocollagen complex failed to elicit IFN-α and IL-12 responses, whereas poly(I:C) induced a strong response. These results show that it is possible to administer a siRNA/atelocollagen complex without inducing nonspecific turning on of genes, leading to an immune response.
Fig. 6.
Absence of IFN response to the atelocollagen-mediated siRNA delivery system. Nude mice were injected with nonspecific control siRNA (50 μg) with 0.05% atelocollagen in a 200-μl volume by i.v. tail vein injection. The positive control group was injected with poly(I:C). Serum was collected 2 h postinjection; IFN-α and IL-12 levels were determined by ELISA. Data represent the mean ± SD (n = 4).
Discussion
Our findings indicate that siRNA can be delivered to bone-metastatic lesions by atelocollagen-mediated systemic injection. Furthermore, we also showed that an atelocollagen-mediated siRNA delivery system can be used to silence endogenous genes involved in metastatic tumor cell growth.
At present, the main obstacle to the development of therapeutic products using RNAi technologies is a suitable delivery method. Viral delivery systems are efficient but cause concerns over serious side effects (27). Cationic lipid complexes also can be effective siRNA delivery agents (6). However, lipid delivery of synthetic siRNAs can reportedly induce immune activation in vivo (28). An important consideration for siRNA-mediated inhibition of gene expression is whether the observed effects are specific and not due to nonspecific “off-target” effects (29) and are free from potential IFN responses (30). Heidel et al. (31) showed that it is possible to administer naked, synthetic siRNAs to mice and down-regulate an endogenous or exogenous target without inducing an IFN response. In our experiments, in agreement with Heidel et al. (31), injection of siRNA/atelocollagen did not induce an IFN response or IL-12. Therefore, our atelocollagen-based siRNA delivery method can be varied to minimize the potential for an off-target effect of siRNA.
Successful application of RNAi as a therapeutic method requires an efficient and suitable delivery system that can target a restricted cell population in vivo. The prolonged circulation time of high-molecular-weight macromolecules enables them to use the vascular abnormalities of solid tumor tissues, a phenomenon called the enhanced permeability and retention (EPR) effect (32, 33). This EPR effect is attributed to anatomical and pathophysiological alterations such as increased vascular density due to neoangiogenesis, impaired lymphatic recovery, and lack of smooth muscle layer in solid tumor vessels. The EPR effect facilitates extravasation of polymeric drugs more selectively at tumor tissues, and this selective targeting to solid tumor tissues may lead to superior therapeutic benefits with fewer systemic adverse effects. In our experiments, siRNA/atelocollagen complexes showed greater selective accumulation in tumor tissues, compared with normal tissues, possibly due to an EPR mechanism. Although further analysis is required, our atelocollagen-mediated siRNA delivery method could possess the potential for selective targeting to tumor tissues.
The major risk faced by patients with prostate cancer is the development of metastatic disease. Although genes associated with metastatic prostate cancer can be identified readily by screening techniques [e.g., gene arrays (34)], the validation and characterization of these genes will require sophisticated animal models and gene delivery systems. Expression microarray studies identified EZH2 as a gene overexpressed in hormone-refractory metastatic prostate cancer, and it has been found that patients with clinically localized prostate cancers that express EZH2 have a worse prognosis than those who do not express the protein (34). In addition, knocking down the EZH2 protein in PC3 invasive prostate cancer cells by using RNAi technology inhibited proliferation of cells in vitro. In contrast, catalytic subunits of the phosphatidylinositol 3-kinase p110-α regulate a variety of cellular responses such as survival, proliferation, and cell migration (35). In this report, our data demonstrate that expression of EZH2 and p110-α are involved in tumor growth in metastatic osseous sites. It has been reported that EZH2 and p110-α protein are also elevated in breast cancer (36, 37). Therefore, EZH2 and p110-α siRNA/atelocollagen complexes may also have therapeutic potential for inhibiting the growth of breast cancer in bone-metastatic sites. Although EZH2 and p110-α siRNA efficiently inhibited proliferation of PC-3M-luc cells, further work will be required to develop a siRNA therapy that induces the cytocidal effect specific to prostate cancer cells.
In conclusion, we have developed a technique to efficiently and safely deliver siRNA to a bone-metastatic lesion by an atelocollagen-mediated systemic injection and demonstrated specific inhibition of target gene expression. To our knowledge, our results present the first evidence that gene silencing by means of systemic delivery of siRNA/atelocollagen complexes may have therapeutic potential in the treatment of advanced prostate cancer with bone metastasis.
Supplementary Material
Acknowledgments
We thank Ms. Ayako Inoue and Ms. Maho Kodama for their excellent technical work. This work was supported in part by a grant-in-aid for the Third-Term Comprehensive 10-Year Strategy for Cancer Control; Health Science Research grants for Research on the Human Genome and Gene Therapy from the Ministry of Health, Labour, and Welfare of Japan; a grant-in-aid for Scientific Research on Priority Areas (Cancer) from the Ministry of Education, Culture, Sports, Science, and Technology; and the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research of Japan.
Author contributions: F.T. and T.O. designed research; F.T., N.N., and Y.Y. performed research; Y.M., S.N., K. Honma, T.T., K. Hanai, and A.S. contributed new reagents/analytic tools; F.T., H.S., K. Hirai, N.N., and Y.Y. analyzed data; and F.T. and T.O. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: siRNA, small interfering RNA; RNAi, RNA interference; EZH2, zeste homolog 2; p110-α, phosphoinositide 3′-hydroxykinase p110-α-subunit.
References
- 1.Hannon, G. J. & Rossi, J. J. (2004) Nature 431, 371-378. [DOI] [PubMed] [Google Scholar]
- 2.McCaffrey, A. P., Meuse, L., Pham, T. T., Conklin, D. S., Hannon, G. J. & Kay, M. A. (2002) Nature 418, 38-39. [DOI] [PubMed] [Google Scholar]
- 3.Lewis, D. L., Hagstrom, J. E., Loomis, A. G., Wolff, J. A. & Herweijer, H. (2002) Nat. Genet. 32, 107-108. [DOI] [PubMed] [Google Scholar]
- 4.Brummelkamp, T. R., Bernards, R. & Agami, R. (2002) Cancer Cell 2, 243-247. [DOI] [PubMed] [Google Scholar]
- 5.Xia, H., Mao, Q., Paulson, H. L. & Davidson, B. L. (2002) Nat. Biotechnol. 20, 1006-1010. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen, D. R., Leirdal, M. & Sioud, M. (2003) J. Mol. Biol. 327, 761-766. [DOI] [PubMed] [Google Scholar]
- 7.Layzer, J. M., McCaffrey, A. P., Tanner, A. K., Huang, Z., Kay, M. A. & Sullenger, B. A. (2004) RNA 10, 766-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soutschek, J., Akinc, A., Bramlage, B., Charisse, K., Constien, R., Donoghue, M., Elbashir, S., Geick, A., Hadwiger, P., Harborth, J., et al. (2004) Nature 432, 173-178. [DOI] [PubMed] [Google Scholar]
- 9.Verma, U. N., Surabhi, R. M., Schmaltieg, A., Becerra, C. & Gaynor, R. B. (2003) Clin. Cancer Res. 9, 1291-1300. [PubMed] [Google Scholar]
- 10.Filleur, S., Courtin, A., Ait-Si-Ali, S., Guglielmi, J., Merle, C., Harel-Bellan, A., Clezardin, P. & Cabon, F. (2003) Cancer Res. 63, 3919-3922. [PubMed] [Google Scholar]
- 11.Duxbury, M. S., Ito, H., Benoit, E., Zinner, M. J., Ashley, S. W. & Whang, E. E. (2003) Biochem. Biophys. Res. Commun. 311, 786-792. [DOI] [PubMed] [Google Scholar]
- 12.Duxbury, M. S., Ito, H., Zinner, M. J., Ashley, S. W. & Whang, E. E. (2004) Oncogene 23, 1448-1456. [DOI] [PubMed] [Google Scholar]
- 13.Spankuch, B., Matthess, Y., Knecht, R., Zimmer, B., Kaufmann, M. & Strebhardt, K. (2004) J. Natl. Cancer Inst. 96, 862-872. [DOI] [PubMed] [Google Scholar]
- 14.Ochiya, T., Takahama, Y., Nagahara, S., Sumita, Y., Hisada, A., Itoh, H., Nagai, Y. & Terada, M. (1999) Nat. Med. 5, 707-710. [DOI] [PubMed] [Google Scholar]
- 15.Honma, K., Ochiya, T., Nagahara, S., Sano, A., Yamamoto, H., Hirai, K., Aso, Y. & Terada, M. (2001) Biochem. Biophys. Res. Commun. 289, 1075-1081. [DOI] [PubMed] [Google Scholar]
- 16.Ochiya, T., Nagahara, S., Sano, A., Itoh, H. & Terada, M. (2001) Curr. Gene Ther. 1, 31-52. [DOI] [PubMed] [Google Scholar]
- 17.Hirai, K., Sasaki, H., Sakamoto, H., Takeshita, F., Asano, K., Kubota, Y., Ochiya, T. & Terada, M. (2003) J. Gene Med. 5, 951-957. [DOI] [PubMed] [Google Scholar]
- 18.Sano, A., Maeda, M., Nagahara, S., Ochiya, T., Honma, K., Itoh, H., Miyata, T. & Fujioka, K. (2003) Adv. Drug Delivery Rev. 55, 1651-1677. [DOI] [PubMed] [Google Scholar]
- 19.Hanai, K., Kurokawa, T., Minakuchi, Y., Maeda, M., Nagahara, S., Miyata, T., Ochiya, T. & Sano, A. (2004) Hum. Gene Ther. 15, 263-272. [DOI] [PubMed] [Google Scholar]
- 20.Minakuchi, Y., Takeshita, F., Kosaka, N., Sasaki, H., Yamamoto, Y., Kouno, M., Honma, K., Nagahara, S., Hanai, K., Sano, A., et al. (2004) Nucleic Acids Res. 32, e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takei, Y., Kadomatsu, K., Yuzawa, Y., Matsuo, S. & Muramatsu, T. (2004) Cancer Res. 64, 3365-3370. [DOI] [PubMed] [Google Scholar]
- 22.Stenzel, K. H., Miyata, T. & Rubin, A. L. (1974) Annu. Rev. Biophys. Bioeng. 3, 231-253. [DOI] [PubMed] [Google Scholar]
- 23.Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K. & Tuschl, T. (2001) Nature 411, 494-498. [DOI] [PubMed] [Google Scholar]
- 24.Arguello, F., Furlanetto, R. W., Baggs, R. B., Graves, B. T., Harwell, S. E., Cohen, H. J. & Frantz, C. N. (1992) Cancer Res. 52, 2304-2309. [PubMed] [Google Scholar]
- 25.Jenkins, D. E., Yu, S. F., Hornig, Y. S., Purchio, T. & Contag, P. R. (2003) Clin. Exp. Metastasis 20, 745-756. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins, D. E., Oei, Y., Hornig, Y. S., Yu, S. F., Dusich, J., Purchio, T. & Contag, P. R. (2003) Clin. Exp. Metastasis 20, 733-744. [DOI] [PubMed] [Google Scholar]
- 27.Scanlon, K. J. (2004) Anticancer Res. 24, 501-504. [PubMed] [Google Scholar]
- 28.Sioud, M. & Sorensen, D. R. (2003) Biochem. Biophys. Res. Commun. 312, 1220-1225. [DOI] [PubMed] [Google Scholar]
- 29.Jackson, A. L., Bartz, S. R., Schelter, J., Kobayashi, S. V., Burchard, J., Mao, M., Li, B., Cavet, G. & Linsley, P. S. (2003) Nat. Biotechnol. 21, 635-637. [DOI] [PubMed] [Google Scholar]
- 30.Bridge, A. J., Pebernard, S., Ducraux, A., Nicoulaz, A. L. & Iggo, R. (2003) Nat. Genet. 34, 263-264. [DOI] [PubMed] [Google Scholar]
- 31.Heidel, J. D., Hu, S., Liu, X. F., Triche, T. J. & Davis, M. E. (2004) Nat. Biotechnol. 22, 1579-1982. [DOI] [PubMed] [Google Scholar]
- 32.Greish, K., Fang, J., Inutsuka, T., Nagamitsu, A. & Maeda, H. (2003) Clin. Pharmacokinet. 42, 1089-1105. [DOI] [PubMed] [Google Scholar]
- 33.Satchi-Fainaro, R., Puder, M., Davies, J. W., Tran, H. T., Sampson, D. A., Greene, A. K., Corfas, G. & Folkman, J. (2004) Nat. Med. 10, 255-261. [DOI] [PubMed] [Google Scholar]
- 34.Varambally, S., Dhanasekaran, S. M., Zhou, M., Barrette, T. R., Kumar-Sinha, C., Sanda, M. G., Ghosh, D., Pienta, K. J., Sewalt, R. G., Otte, A. P., et al. (2002) Nature 419, 624-629. [DOI] [PubMed] [Google Scholar]
- 35.Katso, R., Okkenhaug, K., Ahmadi, K., White, S., Timms, J. & Waterfield, M. D. (2001) Annu. Rev. Cell Dev. Biol. 17, 615-675. [DOI] [PubMed] [Google Scholar]
- 36.Kleer, C. G., Cao, Q., Varambally, S., Shen, R., Ota, I., Tomlins, S. A., Ghosh, D., Sewalt, R. G., Otte, A. P., Hayes, D. F., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 11606-11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachman, K. E., Argani, P., Samuels, Y., Silliman, N., Ptak, J., Szabo, S., Konishi, H., Karakas, B., Blair, B. G., Lin, C., et al. (2004) Cancer Biol. Ther. 3, 772-775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.