Abstract
Molecular mechanisms underlying prostate and urothelial development remain unclear. This situation presents major limitations in identifying the cell type(s) and molecular events involved in the development of prostate and bladder cancer. It has been shown that mice lacking the basal cell marker p63 present several epithelial defects, including epidermis and prostate buds agenesis and urothelial abnormalities. Here, we use the p63–/– mouse as a tool to define cell lineages in the prostate epithelium and urothelium. By complementing p63–/– blastocysts with p63+/+ β-galactosidase (β-gal)-positive ES cells, we show that secretory cells of the prostate originate from p63-positive basal progenitor cells. Importantly, our urogenital sinus transplantation studies demonstrate that p63 prevents intestinal differentiation of the urogenital sinus endoderm and is therefore required to maintain commitment to the prostate cell lineage. Finally, in contrast with the prostate findings, analysis of the urothelium from rescued p63–/– chimeras shows that umbrella (superficial) cells can develop and be maintained independently from p63-positive basal and intermediate cells.
Keywords: animal model, development, stem cell, urothelium, mouse model
Unraveling the means by which the development and renewal of normal tissues occurs is an essential step for the elucidation of the mechanisms underlying the development of pathological processes, including cancer. Indeed, the discovery of hematopoietic stem cells has led to considerable progress toward the identification of the cell types and the molecular pathways implicated in the development of hematopoietic malignancies (1, 2). Although epithelial stem cells have been identified in both the skin and intestine, how the prostate epithelium and the urothelium are formed and maintained is unknown, and the existence of stem cells in these epithelia is controversial. It has been proposed that, in both the prostate epithelium and the urothelium, the basal cells represent or include the progenitor/stem cells (3–6). However, because both prostate secretory cells and urothelial umbrella (superficial) cells have the ability to divide, they may represent independent cell lineages with self-renewal capacities (7–10). Thus, the clarification of the developmental hierarchy among the different epithelial cell types that constitute the prostate and the urothelium is crucial for the future identification of the cell of origin of genitourinary malignancies, including prostate and bladder cancer.
It has been shown that p63 is a marker of basal cells (11) and is required for normal development of several epithelial tissues, including squamous epithelia, urothelium, and mammary, salivary, lacrymal, and prostate glands (12–14). To investigate whether p63-positive basal cells are the progenitor/stem cells of the prostate epithelium and urothelium, we rescued the developmental abnormalities of the p63–/– mice by injecting p63+/+ ROSA26 (β-gal-positive) ES cells into blastocysts isolated from p63+/– crosses. Information regarding cell lineages in the prostate epithelium and urothelium were obtained by assessing the contribution of the p63–/– cells to these epithelia. In addition, postnatal development of p63–/– urogenital sinus (UGS) was addressed by transplantation studies as well.
Materials and Methods
Generation of Chimeras. p63+/– female mice were superovulated and mated to p63+/– male mice. Blastocysts [3.5 days postcoitum (dpc)] were isolated from plugged females, injected with ROSA26 ES cells (gift from Stuart H. Orkin, Children's Hospital, Boston), and transferred to foster mothers.
Analysis of Chimeras. Tissue samples were embedded in OCT compound (Sakura Finetek Japan, Tokyo) and frozen in cold isopentane. X-gal histochemistry was performed on frozen tissue sections as described in ref. 15. Preliminary analysis of the prostate of ROSA26 adult mice demonstrated that β-gal is ubiquitously expressed only in the ventral prostate, whereas subsets of epithelial cells in the dorsolateral and anterior prostate are consistently β-gal negative (M.M.P. and S.S., unpublished data). As a consequence, only the ventral prostate was evaluated in the present study. Bladder and prostate specimens were analyzed by performing multiple sections throughout the entire organ. Immunohistochemistry for p63 was done by using the 4A4 monoclonal antibody (11); immunostaining for CK5 and CK14 was carried out with polyclonal antibodies (Covance). Laser microdissection was performed with the Molecular Machines & Industries instrument. Genotyping of microdissected cells was performed by real-time PCR by using the SYBR Green PCR Master Mix and Applied Biosystems PRISM 7700 Sequence Detector (both from Applied Biosystems). Primers specific for the WT and p63–/– allele were constructed by using PRIMER EXPRESS 2.0 (Applied Biosystems). When the efficiencies of amplification of the WT and p63–/–alleles were compared, they were approximately equal.
Grafting Experiments. Urogenital sinuses isolated from p63–/– and p63+/+ or p63+/– 18.5 dpc embryos were transplanted under the renal capsule of syngenic mice and analyzed 3 months after transplantation. p63–/– embryos were identified on the basis of the developmental abnormalities (i.e., limb agenesis), and the genotype was subsequently confirmed by PCR. Immunohistochemistry was performed with polyclonal antibodies to Nkx3.1 (kindly provided by C. Abate-Shen, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, Piscataway, NJ) and lysozyme (Zymed) and monoclonal antibodies to Cdx2 (gift of Biogenex, San Ramon, CA) and uroplakin III (Research Diagnostics). Immunostaining for CK14 and p63 was carried out as described above.
Results and Discussion
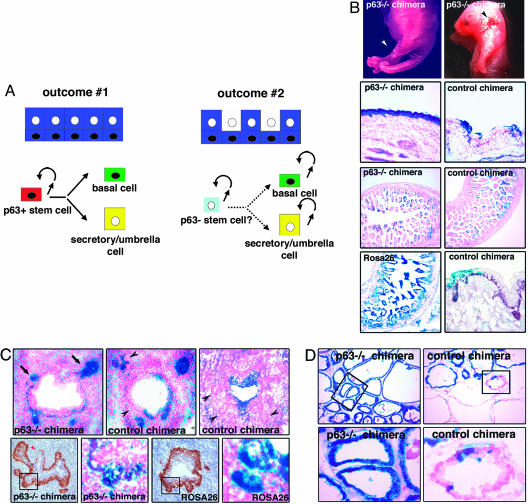
Complementation of p63–/– blastocysts with p63+/+ ROSA26 ES cells was used to study the role of p63-positive cells in the development of genitourinary organs. In this animal model, information regarding cell lineages in the prostate epithelium and urothelium can be obtained by assessing the relative contribution of p63+/+ and p63–/– cells to these epithelia. Two main outcomes are predicted. In the first scenario (Fig. 1A, outcome 1), both basal and secretory/umbrella cell compartments consist exclusively of p63+/+ (β-gal-positive) cells. This result would imply that both cell compartments require p63 for development and, therefore, derive from a common p63-positive basal stem cell. The second possibility is that p63–/– (β-gal-negative) cells contribute to the secretory/umbrella cell compartment (Fig. 1A, outcome 2). This outcome would lead to the conclusion that secretory/umbrella cells develop independently from p63-positive cells.
Fig. 1.
Blastocyst complementation of p63–/– mice. (A) Models of epithelial development based on predicted outcomes. In outcome 1, only p63+/+ β-gal-positive (blue) cells are detected in the prostate epithelium or urothelium of p63–/– chimeras. This result indicates that p63-negative cells originate from p63-positive progenitor cells. In outcome 2, both p63+/+ β-gal-positive (blue) and p63–/– β-gal-negative (white) cells are present in the prostate secretory or urothelial umbrella cell compartments. This result suggests that p63-positive and p63-negative cells either represent completely independent cell lineages with self-renewal capacity or derive from a common p63-negative stem cell. (B) Analysis of 18.5 dpc chimeras. Partially rescued p63–/– chimeras are identified by the presence of limb abnormalities including limb truncation (Top Left, white arrowhead) and split appearance of the paw (Top Right). Focal areas of skin ulceration are also observed (Top Right, black arrowhead). Only p63+/+ β-gal-positive cells constitute the epidermis of p63–/– chimeras (Top Middle Left, ×100), whereas p63–/– β-gal-negative epithelial cells are present in the intestine (Bottom Middle Left, ×100). The intestinal epithelium and epidermis of control chimeras show similar levels of chimerism (Top Middle Right and Bottom Middle Right, ×100). All intestinal epithelial cells of a ROSA26 control mouse are β-gal positive (Bottom Left, ×100). p63 expression is detected in β-gal-negative epidermal cells of control chimeras (Bottom Right, ×100). (C) X-gal staining and p63 immunostaining in the prostate of 18.5 dpc chimeras. Upper (×200) shows that, in p63–/– chimeras, prostate buds are only populated by p63+/+ β-gal-positive cells (arrow). A variable distribution of β-gal-negative (black arrowheads) and -positive cells is observed in control chimeras. Lower (alternating ×200 and ×400) shows p63 expression and X-gal staining in serial sections from a p63–/– chimera and a ROSA26 embryo. In the p63–/– chimera, the p63-positive basal cell layer is discontinuous (red arrowhead) and p63–/– β-gal-negative cells are present in the UGS epithelium but not in prostate buds (as also shown in Upper Left). The developing prostate and the UGS epithelium are entirely β-gal positive in the ROSA26 embryo. (D) X-gal staining in the prostate of adult chimeras. Only p63+/+ β-gal-positive cells are detected in the prostate epithelium of p63–/– chimeras (Left, ×100 and ×400), whereas both β-gal-positive and -negative cells are present in control chimeras (Right, ×100 and ×400).
As a first step, we assessed the feasibility of this model. Fifty-six chimeras were generated and analyzed at 18.5 dpc. In keeping with the male genotype of the ROSA26 ES cells, 90% of the embryos were males. Two male p63–/– ROSA26 chimeras (herein referred to as p63–/– chimeras) in which developmental defects were partially rescued by the injection of the p63+/+ ES cells were identified by the presence of focal areas of skin ulceration and a variety of defects of forelimbs and hindlimbs (Fig. 1B Top). Intriguingly, one of these abnormalities closely resembled the ectrodactyly observed in patients affected by the ectrodactyly-ectodermal dysplasia-cleft lip/palate syndrome, which is caused by heterozygous mutations in the p63 gene (16). Among the remaining chimeras, 50 appeared normal at external examination and 4 presented the characteristic p63–/– phenotype.
The role of p63-positive cells as progenitor/stem cells can be assessed only if p63 function is cell autonomous. To determine whether p63 function is cell autonomous, we next assessed the relative contribution of β-gal-positive (p63+/+) cells versus β-gal-negative (from original blastocyst) cells to the chimeric embryos by performing X-gal histochemistry. The level of chimerism (percent of β-gal-positive cells) was determined both in organs that require p63 for development (e.g., the epidermis) and in organs/tissues that are p63 independent (e.g., the intestinal epithelium). In the two partially rescued p63–/– embryos, meticulous microscopic examination revealed that the epidermis was exclusively populated by β-gal-positive (p63+/+) cells, whereas the level of chimerism in the intestinal epithelium was significantly lower (30–45%) (Fig. 1B Left Top Middle and Left Bottom Middle). This result indicated that p63 function is cell autonomous. In addition, our analysis demonstrated that most chimeras (79%) originated from p63+/+ or +/– blastocysts (herein referred to as control chimeras). Indeed, these mice showed similar level of chimerism in the epidermis and the intestinal epithelium (Fig. 1B Right Top Middle and Right Bottom Middle). Importantly, the detection of p63 expression in the β-gal-negative cells of the epidermis of these chimeras excluded the possibility that any of them represented p63–/– blastocysts in which the p63+/+ ROSA26 cells had abolished the developmental defects through a non-cell-autonomous p63 function (Fig. 1B Bottom Right). As a whole, these data demonstrate that p63 is required in a cell-autonomous manner for the development of epithelial tissues. Interestingly, the pattern of X-gal staining observed in the partially rescued p63–/– mice was also detected in six additional chimeras that did not show any developmental abnormalities at external examination. This finding indicated that these chimeras corresponded to p63–/– embryos in which the injection of p63+/+ ES cells had led to the complete rescue of both limb and skin defects. As a whole, these results prove the feasibility of the model.
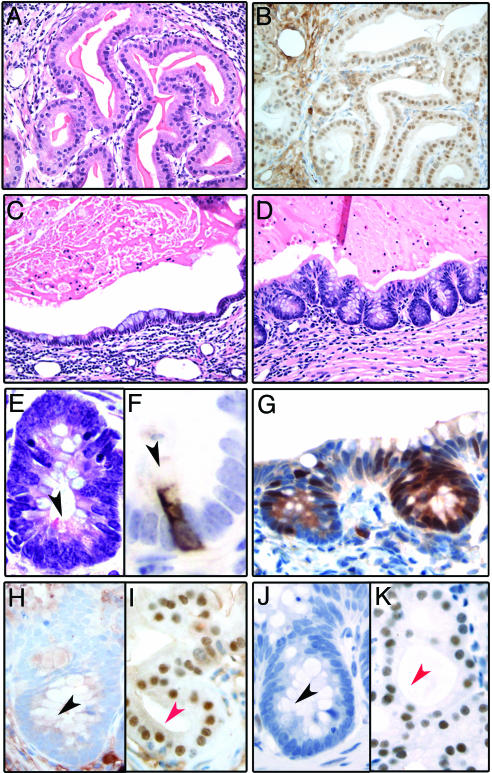
We next addressed the role of p63 in the development of organs derived from the UGS, including the prostate and bladder. We had previously shown that the p63–/– mice present abnormalities in the urogenital tract, including the absence of prostate buds and the presence of a pseudostratified columnar epithelium replacing the stratified epithelium that normally lines the UGS (13). As a first step, we analyzed the postnatal development of the p63–/– UGS by transplantation under the renal capsule of recipient male mice. In line with findings from Kurita et al. (17), we observed that p63–/– UGS grafts consisted of glandular structures lined in part by mucous-producing cells and luminal prostatic epithelium (Fig. 2A and C), as demonstrated by expression of Nkx3.1 (18) and androgen receptor (AR) (Fig. 2 B, I, and K). In keeping with Cunha's group, we found that cells with basal phenotype were consistently absent and that rare mucinous cells expressed the prostate marker Nkx3.1 (data not shown). Importantly however, extensive histologic examination of the mucinous epithelium also revealed in all cases (6 of 6) the presence of areas in which the epithelium lining the glands closely resembled colonic epithelium (Fig. 2D). These areas, representing on average 5% of the mucinous epithelium, showed the presence of crypt-like structures in which both goblet cells and lysozyme-positive Paneth cells (19) were easily identified (Fig. 2 E and F). The intestinal differentiation of this epithelium was further substantiated by Cdx2 expression, a marker exclusively expressed in tissues of gastrointestinal derivation (20, 21) (Fig. 2G). Cdx-2 positivity was restricted to the crypt-like structures and was consistently negative in the WT UGS grafts (data not shown). Notably, the Cdx-2-positive cells were consistently negative for the prostate secretory cell markers Nkx3.1 and AR (Fig. 2 H and J). Thus, in the endodermally derived UGS, p63 appears to inhibit differentiation toward other endodermally derived epithelia outside the urogenital tract, such as the intestinal epithelium. In addition, p63 is necessary for differentiation of prostate basal cells but not of secretory cells. Although this experiment addresses the differentiation potential of p63–/– cells in a nonphysiological setting, it is not informative with respect to the origin of prostate secretory cells during normal prostate development.
Fig. 2.
Analysis of p63–/– UGS grafts. (A) Hematoxylin and eosin stained section from a p63–/– graft showing glands lined by prostatic secretory cells (×200). (B) Prostatic luminal differentiation in p63–/– grafts is confirmed by Nkx3.1 immunoreactivity (×200). (C and D) Hematoxylin and eosin stained sections from a p63–/– graft showing mucous-producing cells (×200). These cells are either organized in a monolayer lining the glandular lumina (C) or form crypt-like structures that closely resemble colonic mucosa (D). The mucous cells in the crypts have the typical appearance of goblet cells. (E and F) High magnification of a crypt-like structure demonstrates the presence of Paneth cells, characterized by supranuclear eosinophilic secretory granules (E, black arrowhead, ×600) and positivity for lysozyme (F, black arrowhead, ×1,000). (G) Cdx2 is selectively expressed in the epithelial component of the p63–/– graft showing morphologic features of intestinal differentiation (×400X). (H–K) Nuclear immunoreactivity for Nkx3.1 (H and I) and AR (J and K) is absent in the mucinous epithelium showing features of intestinal differentiation (H and J, black arrowhead) and present in the prostate secretory cells (I and K, red arrowhead, ×600).
To test whether p63-positive basal cells are dispensable for the development of secretory cells during normal prostate organogenesis, p63–/– chimeras were analyzed. Cells that populate prostate buds at 18.5 dpc display a basal cell phenotype (4). Thus, we performed immunohistochemistry for basal keratins (CK5 and CK14) to reliably detect prostate buds in the chimeras. We were able to identify prostate buds in seven of the eight p63–/– chimeric male mice (data not shown). Remarkably, prostate buds were exclusively populated by p63+/+ (β-gal-positive) cells, whereas the UGS epithelium was composed of both p63–/– and p63+/+ cells (Fig. 1C). Within the UGS epithelium p63+/+ cells were found in contact with the basement membrane and p63–/– cells lined the lumen. Taken together, our results confirm that p63 is necessary for prostate buds formation and indicate that p63 expression is required in all of the cells that constitute the prostate at early stage of development.
The reliability of these results depends on the demonstration that the six putative p63–/– chimeras lacking external defects originated, in fact, from p63–/– blastocysts. This demonstration is based on the two following observations. First, the total percentage of chimeras derived from p63–/– blastocysts (21%) is very close to the rate of p63–/– blastocysts expected from p63+/– crosses (25%). Second, and most importantly, the basal cell layer of the UGS epithelium was discontinuous in three of the six putative p63–/– chimeras (Fig. 1C Bottom Left), proving that these mice derived from p63–/– embryos in which the UGS defects were incompletely rescued.
Prostate secretory cells appear in the prostate epithelium only a few days after birth (4). As a result, the analysis of the chimeric embryos does not permit to ascertain whether p63 is required for the development of secretory cells. Indeed, secretory cells could derive either from p63-positive basal cells within the buds or from p63-negative cells within the UGS epithelium, which subsequently colonize the prostate. To discriminate between these two possibilities, 140 additional chimeras were generated and analyzed at 7 weeks of age, when the prostate epithelium is fully differentiated. One chimera showed minor limb abnormalities, including split paw and digit fusion, indicating incomplete abolishment of the p63–/– phenotype (Fig. 5 A and B, which is published as supporting information on the PNAS web site). This and five additional chimeras presented the pattern of staining characteristic of rescued p63–/– mice by X-gal histochemistry (Fig. 5 C–F). The p63–/– genotype of the original blastocyst was further confirmed in the six putative p63–/– chimeras by genotyping β-gal-negative hepatocytes isolated by laser capture microdissection (Fig. 6, which is published as supporting information on the PNAS web site). Histochemical analysis of the prostate epithelium of the adult p63–/– rescued mice consistently showed the absence of β-gal-negative p63–/– cells in all six animals (Fig. 1D Left). In contrast, in 96% (23 of 24) of control chimeras displaying comparable levels of chimerism (40–85%), the prostate epithelium consisted of both β-gal-positive and -negative cells (Fig. 1D Right). Only in a single control chimera showing the highest level of chimerism (85%) was the prostate epithelium entirely β-gal positive (data not shown). The consistent absence of β-gal-negative cells in the prostate epithelium of p63–/– rescued mice and the significantly higher frequency of this feature in p63–/– as compared to control chimeras (Fisher's exact test, P < 0.0001) leads to the conclusion that p63 is required for the development of both basal and secretory cells during normal prostate organogenesis. Notably, UGS transplantation studies show that p63–/– cells are able to differentiate into prostate secretory cells but not basal cells. Thus, the requirement for p63 in the development of secretory cells during normal prostate organogenesis implies that secretory cells derive from p63-positive basal cells (Fig. 1 A, outcome 1).
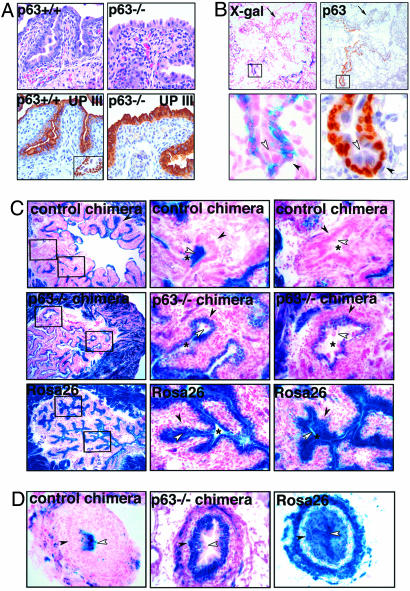
The urothelium consists of basal, intermediate, and umbrella cells. Basal and intermediate cells express p63, whereas umbrella cells are p63-negative and selectively express Uroplakin III (Fig. 3A Left) (11, 22). Previous reports indicate that the urothelium of p63–/– mice is abnormal (12) and suggest that p63 is required for urothelial differentiation (12, 23). Here, we show that the urothelium of 18.5 dpc p63 –/– embryos contains a single layer of cells that resemble urothelial umbrella cells and express Uroplakin III (Fig. 3A Right). The observation that cells with morphological and immunophenotypic features of umbrella cells can form in absence of p63-positive basal and intermediate cells suggests that basal/intermediate cells do not represent the progenitor/stem cells of the urothelium. To further test this hypothesis, we analyzed the relative contribution of p63–/– versus p63+/+ cells to the urothelium of the chimeras. Remarkably, we observed that in both p63–/– embryonic (n = 8) and adult (n = 6) rescued chimeras, p63–/– cells were able to contribute to the urothelial umbrella cell compartment, whereas only p63+/+ cells populated the basal and intermediate layers (Fig. 3 B–D). Specifically, the contribution of p63+/+ cells to the umbrella cells ranged from 0% to 15% in the p63–/– chimeras. Thus, p63–/– cells are able to form fully differentiated and functional umbrella cells of the urothelium. Moreover, p63-positive cells are not required for the significant turnover of umbrella cells, which occurs during both embryonic and postnatal bladder growth (24–26). In line with these results, we found that in some control chimeras, the levels of chimerism in the basal/intermediate and the umbrella cell compartments were significantly different (Fig. 7, which is published as supporting information on the PNAS web site), suggesting that the two cell compartments are not hierarchically related. These findings are in contrast with the typical columnar arrangement of β-gal-positive cells seen in squamous epithelia (Fig. 1B), which documents the vertical cell differentiation occurring in these tissues. Taken together, our data indicate that urothelial umbrella cells develop independently from p63-positive basal and intermediate cells (Fig. 1 A, outcome 2).
Fig. 3.
Analysis of the urothelium. (A) Comparison of bladder urothelium in p63–/– and control littermates (18.5 dpc), showing the presence of a single layer of cells with ample eosinophilic cytoplasm in p63–/– embryos. Similarly to umbrella cells of wild-type mice, these cells express Uroplakin III (UP III). p63-positive basal and intermediate cells of wild-type urothelium are shown in Inset. (Magnification: ×200X). (B) X-gal staining and p63 immunostaining in the bladder of a partially rescued p63–/– chimera (18.5 dpc), showing partial absence of the basal and intermediate cell layers (arrows). In the rescued epithelium, p63-positive basal and intermediate cell layers are exclusively formed by p63+/+ β-gal-positive cells. The umbrella cell layer consists of p63–/– β-gal-negative cells. Lower represents higher magnification (×400) of the marked regions in top panels (×40). (C) Comparison of X-gal staining of the bladder epithelium of p63–/– chimeras, control chimeras, and ROSA26 mice at 7 weeks. Center and Right are higher magnifications (×200) of marked regions in Left (×40). The asterisk indicates the bladder lumen, the black arrowhead indicates basal/intermediate cells, and the open arrowhead indicates umbrella cells. In control chimeras, both the basal/intermediate and the umbrella cell layers are occupied by β-gal-positive and -negative cells. In p63–/– chimeras, only p63+/+ β-gal-positive cells are present in the basal and intermediate cell layers. Approximately 85% of the cells that constitute the umbrella cell layer are p63–/– (β-gal negative). All urothelial cells of ROSA26 mice display β-gal activity. (D) X-gal staining of the urothelium lining the ureters (×200). Similarly to the bladder urothelium, p63–/– β-gal-negative cells are present in the umbrella layer of p63–/– chimeras. In the control chimera, β-gal-positive umbrella cells are observed above β-gal-negative basal and intermediate cells. The ureter of ROSA26 mice is entirely β-gal positive.
The results from this study constitute an important advance in the understanding of the biology of epithelial organs of the genitourinary tract, including the prostate, bladder, and ureters.
We have previously demonstrated that p63–/– mice present defects in prostatic bud development. Here, we show that when such developmental defects are abolished by complementing p63–/– blastocysts with p63+/+ ES cells, only p63+/+ cells compose the normal prostate epithelium. Although we cannot exclude that very rare p63–/– cells contribute to the secretory cell compartment, these results indicate that the overwhelming majority of the secretory cells derive from p63-positive cells.
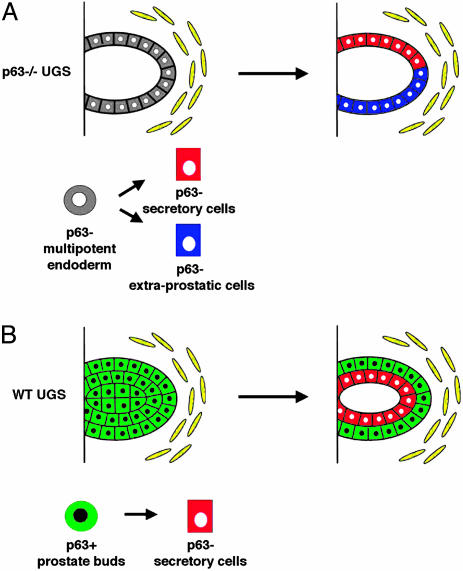
Our transplantation studies and those performed by others show that the p63–/– UGS develops into glandular structures lined by both mucous-producing cells and prostate secretory cells. Although Kurita et al. (17) examined the expression of prostate specific markers and the activation of the Src-Ras-MAPK pathway in the mucinous epithelium, extraprostatic differentiation was not addressed. Importantly, we demonstrate that the mucinous epithelium of p63–/– grafts focally presents unequivocal morphologic and molecular features of intestinal differentiation. Although both prostate and intestinal epithelia derive from the endoderm, it is well known that intestinal epithelium does not require p63 for normal development. Taken together, our findings support a model in which, in the absence of p63, the UGS endoderm retains its multipotentiality and has the ability to form both prostate secretory cells and intestinal epithelium (Fig. 4A). Thus, although p63 is not necessary for prostate secretory cells differentiation, its expression is required in multipotent UGS endodermal cells to restrict development to the prostate cell lineage. Indeed, during normal organogenesis, prostate buds are solely formed by p63-positive progenitor cells, which are committed to the prostate cell lineage and give rise exclusively to the prostatic epithelium (Fig. 4B). Importantly, therefore, our findings uncover a previously uncharacterized role for p63 in regulating cell fate determination in the UGS endoderm.
Fig. 4.
Models of UGS development in p63–/– and WT mice. (A) In the absence of p63, the UGS endoderm maintains the capacity to differentiate toward multiple lineages and develops glandular structures lined by both prostate luminal cells and extraprostatic (i.e., intestinal) epithelium. (B) During normal prostate organogenesis, prostate buds are composed of p63-positive basal progenitor cells, which give rise to all of the cell types that compose the mature prostate epithelium. p63 expression is required in these progenitor cells to restrict their differentiation potential to the prostate cell lineage.
The demonstration that p63-positive cells represent prostate progenitor cells during development suggests that basal cells of the adult prostate, which are p63 positive, might function as adult stem cells. However, our experiments cannot rule out that once developed, secretory cells are maintained by a process of self-duplication.
It is currently believed that the basal layer of the urothelium contains a pool of stem cells that are necessary to replace the umbrella cells that are lost within the lumen. This hypothesis is mainly based on studies performed on urothelial cells in culture (5, 27, 28). Our results in vivo challenge this view and support a model in which umbrella cells develop and are maintained independently from p63-positive basal and intermediate cells. Although we cannot exclude that umbrella cells can form as a result of more than one differentiation program involving more than one progenitor cell population, our data suggest that umbrella and basal/intermediate cells are either entirely independent lineages capable of self-renewal or are maintained by a common p63-negative progenitor cell.
Although our experiments address the development of the prostate epithelium and urothelium, this animal model can be also used to evaluate the role of basal cells in the regeneration of these epithelia after the induction of cell injury in vivo. Additionally, this system may be used to assess cell lineages in other epithelial organs that require p63 for development (e.g., bronchial and mammary epithelia).
Supplementary Material
Acknowledgments
We thank Stuart H. Orkin, David M. Livingston, Myles Brown, and Ramesh A. Shivdasani for critical reading of the manuscript. This work was supported by grants from the Department of Defense and the National Cancer Institute [Specialized Program of Research Excellence (SPORE) in RenalCancer], the Hershey Prostate Cancer/Survivor Walk Award, CaPCURE Award, and an award from Istituto Dermopatico dell'Immacolata (Rome) (to S.S.); and grants from the National Cancer Institute (R01, P01, and Specialized Program of Research Excellence in Prostate Cancer) and the Gelb Center for genitourinary oncology at the Dana–Farber Cancer Institute, a Novartis Investigator grant, and a CaPCURE Award (to M.L.).
Author contributions: S.S., P.W.K., W.R.S., and M. Loda designed research; S.S., M.M.P., M. Lindauer, J.W.H., C.G., S.D., and P.M. performed research; F.M. contributed new reagents/analytic tools; S.S., C.G., and M. Loda analyzed data; and S.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: UGS, urogenital sinus.
References
- 1.Sawyers, C. L., Denny, C. T. & Witte, O. N. (1991) Cell 64, 337–350. [DOI] [PubMed] [Google Scholar]
- 2.Passegue, E., Jamieson, C. H., Ailles, L. E. & Weissman, I. L. (2003) Proc. Natl. Acad. Sci. USA 100, Suppl. 1, 11842–11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Marzo, A. M., Nelson, W. G., Meeker, A. K. & Coffey, D. S. (1998) J. Urol. 160, 2381–2392. [DOI] [PubMed] [Google Scholar]
- 4.Wang, Y., Hayward, S., Cao, M., Thayer, K. & Cunha, G. (2001) Differentiation (Berlin) 68, 270–279. [DOI] [PubMed] [Google Scholar]
- 5.Marceau, N. (1990) Lab. Invest. 63, 4–20. [PubMed] [Google Scholar]
- 6.Bonkhoff, H. & Remberger, K. (1996) Prostate 28, 98–106. [DOI] [PubMed] [Google Scholar]
- 7.Evans, G. S. & Chandler, J. A. (1987) Prostate 11, 339–351. [DOI] [PubMed] [Google Scholar]
- 8.English, H. F., Santen, R. J. & Isaacs, J. T. (1987) Prostate 11, 229–242. [DOI] [PubMed] [Google Scholar]
- 9.Messier, B. & Leblond, C. P. (1960) Am. J. Anat. 106, 247–285. [DOI] [PubMed] [Google Scholar]
- 10.Tsujimura, A., Koikawa, Y., Salm, S., Takao, T., Coetzee, S., Moscatelli, D., Shapiro, E., Lepor, H., Sun, T. T. & Wilson, E. L. (2002) J. Cell Biol. 157, 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang, A., Kaghad, M., Wang, Y., Gillett, E., Fleming, M. D., Dotsch, V., Andrews, N. C., Caput, D. & McKeon, F. (1998) Mol. Cell 2, 305–316. [DOI] [PubMed] [Google Scholar]
- 12.Yang, A., Schweitzer, R., Sun, D., Kaghad, M., Walker, N., Bronson, R. T., Tabin, C., Sharpe, A., Caput, D., Crum, C. & McKeon, F. (1999) Nature 398, 714–718. [DOI] [PubMed] [Google Scholar]
- 13.Signoretti, S., Waltregny, D., Dilks, J., Isaac, B., Lin, D., Garraway, L., Yang, A., Montironi, R., McKeon, F. & Loda, M. (2000) Am. J. Pathol. 157, 1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mills, A. A., Zheng, B., Wang, X. J., Vogel, H., Roop, D. R. & Bradley, A. (1999) Nature 398, 708–713. [DOI] [PubMed] [Google Scholar]
- 15.Gounari, F., Signoretti, S., Bronson, R., Klein, L., Sellers, W. R., Kum, J., Siermann, A., Taketo, M. M., von Boehmer, H. & Khazaie, K. (2002) Oncogene 21, 4099–4107. [DOI] [PubMed] [Google Scholar]
- 16.van Bokhoven, H. & McKeon, F. (2002) Trends Mol. Med. 8, 133–139. [DOI] [PubMed] [Google Scholar]
- 17.Kurita, T., Medina, R. T., Mills, A. A. & Cunha, G. R. (2004) Development (Cambridge, U.K.) 131, 4955–4964. [DOI] [PubMed] [Google Scholar]
- 18.Bhatia-Gaur, R., Donjacour, A. A., Sciavolino, P. J., Kim, M., Desai, N., Young, P., Norton, C. R., Gridley, T., Cardiff, R. D., Cunha, G. R., et al. (1999) Genes Dev. 13, 966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bry, L., Falk, P., Huttner, K., Ouellette, A., Midtvedt, T. & Gordon, J. I. (1994) Proc. Natl. Acad. Sci. USA 91, 10335–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suh, E. & Traber, P. G. (1996) Mol. Cell. Biol. 16, 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moskaluk, C. A., Zhang, H., Powell, S. M., Cerilli, L. A., Hampton, G. M. & Frierson, H. F., Jr. (2003) Mod. Pathol. 16, 913–919. [DOI] [PubMed] [Google Scholar]
- 22.Moll, R., Wu, X. R., Lin, J. H. & Sun, T. T. (1995) Am. J. Pathol. 147, 1383–1397. [PMC free article] [PubMed] [Google Scholar]
- 23.Urist, M. J., Di Como, C. J., Lu, M. L., Charytonowicz, E., Verbel, D., Crum, C. P., Ince, T. A., McKeon, F. D. & Cordon-Cardo, C. (2002) Am. J. Pathol. 161, 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erman, A., Jezernik, K., Stiblar-Martincic, D., Romih, R. & Veranic, P. (2001) Histochem. Cell Biol. 115, 309–316. [DOI] [PubMed] [Google Scholar]
- 25.Ayres, P. H., Shinohara, Y. & Frith, C. H. (1985) J. Urol. 133, 506–512. [DOI] [PubMed] [Google Scholar]
- 26.Jost, S. P. (1985) J. Anat. 143, 39–43. [PMC free article] [PubMed] [Google Scholar]
- 27.Mackillop, W. J., Bizarri, J. P. & Ward, G. K. (1985) Cancer Res. 45, 4360–4365. [PubMed] [Google Scholar]
- 28.Varley, C. L., Stahlschmidt, J., Smith, B., Stower, M. & Southgate, J. (2004) Am. J. Pathol. 164, 1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.