Abstract
In the process of screening cell-type-specific genes, we identified juxtanodin (JN), an oligodendroglial protein featuring a putative C-terminal actin-binding domain. At the cellular level, JN in the rat CNS colocalized with 2′,3′-cyclic nucleotide-3′-phosphodiesterase (CNPase), a cytoskeleton-related oligodendroglial protein. In the myelin sheath, JN was found mainly in the abaxon and the lateral few terminal loops. Its apposition to the myelinated axon, through the latter, defined an axonal subregion, herewith termed juxtanode, at the Ranvier node-paranode junction. During forebrain ontogenesis, JN expression paralleled that of MBPs but lagged behind CNPase. Juxtanodin transfection promoted arborization of cultured OLN-93 cells and augmented endogenous CNPase expression and transport to the process arbors of cultured primary oligodendrocyte precursors. These results reveal JN as a cytoskeleton-related oligodendroglial protein that delineates the juxtanode and might serve oligodendrocyte motility, differentiation, or myelin-axon signaling. Functionally, JN may be involved in CNS myelination and/or specialization of the node of Ranvier.
Keywords: myelin-axon interaction, oligodendrocyte, node of Ranvier, cytoskeleton
Oligodendroglia are highly specialized myelin-forming cells of the CNS. Adequate myelination serves to insulate and accelerate the propagation of action potentials. Abnormalities in myelin formation/maintenance may underlie diverse neurological disorders, ranging from multiple sclerosis to schizophrenia (1, 2). Matched with highly specialized functions and unique architecture, various oligodendrocyte/myelin-selective molecules, such as myelin basic protein (MBP), myelin-associated glycoprotein, and 2′,3′-cyclic nucleotide-3′-phosphodiesterase (CNPase), have been isolated and characterized (1, 3). It is likely that many more are yet to be revealed. The identification and characterization of such molecules will probably help elucidate molecular mechanisms of myelination and dys- or demyelinating diseases.
The unique architecture of the myelin sheath entails specialized, yet elusive, cytoskeletal mechanisms of myelin-forming cells. Here, we report the molecular features, cellular expression, and functional roles of juxtanodin (JN), a previously unidentified cytoskeleton-related oligodendrocyte-specific protein.
Materials and Methods
Identification and Characterization of the mRNA. Cell-type-specific CNS genes were sought out by in situ hybridization histochemistry (ISH). Briefly, cDNA clones (n = 1,500) from a rat brain cDNA library (Invitrogen) were sequenced and compared by blast searches against National Center for Biotechnology Information nucleotide and protein nonredundant databases (4). For the unannotated cDNA sequences (n = 274), expression of the mRNAs in the CNS was mapped by ISH using digoxigenin-labeled riboprobes. A 3.6-kb cDNA clone with a predicted ORF encoding 282 amino acid residues was thereby identified, and the gene was subsequently named juxtanodin (JN). A search of EST databases revealed another cDNA sequence encoding a truncated JN of 268 residues (JN268).
Generation of Fusion Proteins and JN Antibody. The DNA fragments encoding residues 1-170 and 1-282 of deduced JN were cloned into pET41a (Novagen) to produce GST-JN170 and His-tagged JN. GST and GST-JN170 were purified by using Bulk and RediPack GST Purification modules (Amersham Pharmacia, Piscataway, NJ). Polyclonal antibody against GST-JN170 was developed in New Zealand White rabbits and affinity purified with GST-JN170-Sepharose 4B.
Mammalian Expression Plasmids and Transfection of Cell Culture. The spontaneously transformed, microfilament-containing OLN-93 cells (5) were cultured at 37°C in DMEM supplemented with 10% FCS. Primary oligodendrocyte precursor (OLP) cells were prepared from the optic nerves of 5- to 7-day-old rats (6, 7) in the facilities at the Department of Clinical Research, Singapore General Hospital (Singapore).
To elucidate the functional roles of JN and its putative C-terminal actin-binding domain (as reported by National Center for Biotechnology Information reverse-position-specific blast), the nucleotide sequences encoding JN (282 amino acid residues), JN268, and JN residues 1-247, 1-141, and 101-282 were subcloned into pXJ40 (8) with a FLAG tag at the 5′ end of the inserts, generating the constructs for FLAG-tagged JN, JN268, JN247, JN141, and JNc, respectively. Empty pXJ40 and pXJ40-BNIP-S served as controls (9). Cultured cells were transfected with the recombinant plasmids by using GIBCO Lipofectamine 2000 (Invitrogen). Forty-eight hours later, the cells were solubilized for Western blotting or fixed with 3% paraformaldehyde for immunocytochemistry.
Immunocytochemistry, Electron Microscopy, and Multiple Labeling. Adult rats (n = 18, body weight ≈200 g) were deeply anesthetized with Nembutal (100 mg/kg of body weight, i.p.) and transcardially perfused with saline, followed by 3% paraformaldehyde (plus 0.1% glutaraldehyde for immunoelectron microscopy) in 0.1 M phosphate buffer (pH 7.4). The brain and spinal cord were dissected, postfixed, and sectioned with a cryostat (for light microscopy) or a vibratome (for electron microscopy). All procedures involving experimental animals were approved by the Ethics Committee at the National University of Singapore.
The following antibodies were used (mouse monoclonal antibody from Sigma, unless otherwise noted): anti-JN (1:100, rabbit polyclonal antibody, in-house produced), anti-CNPase (1:500, Chemicon), anti-FLAG (1:200), anti-glial fibrillary acidic protein (1:1,000, Chemicon), anti-MBP (1:1,000, goat polyclonal antibody, Santa Cruz Biotechnology), anti-neurofilament 200 (1:1,000), anti-OX42 (1:50, Harlan Sera-lab, Sussex, U.K.), anti-pan sodium channel (NavP, 1:200), and anti-potassium channel Kv1.2 (1:300, Upstate Biotechnology, Lake Placid, NY). In vitro transcription of digoxigenin-labeled riboprobes, ISH (probe concentration, 0.2 μg/ml), immunofluorescence (IF), immunoperoxidase (avidin-biotinylated peroxidase complex method), and immunoelectron microscopy followed protocols described in refs. 10 and 11. For simultaneous IF double/triple labeling, bound primary antibodies were revealed by appropriate secondary antibodies conjugated to either Alexa Fluor 568 or Alexa Fluor 488 (1:400, Invitrogen). For sequential double-labeling, IF signals for the first antigen were documented before immunoperoxidase for the second antigen and Luxol fast blue counterstaining (0.1% in 95% ethanol, overnight at 50°C) for the visualization of immunonegative cell arbors. Unexpected cross reactivity in double/triple labeling could be ruled out, based on control experiments in which one of the primary antibodies was omitted.
Immunoprecipitation and Northern and Western Blot Analyses. Rats were killed by the injection of Nembutal (100-150 mg/kg of body weight, i.p.), and the tissues were dissected and homogenized. For immunoprecipitation, solubilized proteins were precleared by protein A-agarose (Amersham Pharmacia) and incubated with 5 μg of JN antibody or rabbit IgG (control) followed by 25 μl of protein A-agarose (overnight at 4°C). Immunoprecipitated proteins were separated by SDS/PAGE. For Northern blot, poly(A)+ RNAs from various tissues were separated on 1% agarose/formaldehyde gel and transferred onto charged nylon membrane (PerkinElmer). Overnight hybridization with digoxigenin-labeled JN riboprobe (15 ng/ml) was followed by washes and detection with alkaline phosphatase-labeled anti-digoxigenin antibody and CDP-Star reagent (Roche, Basel, Switzerland). For Western blot, samples were separated on SDS/PAGE and transferred to PolyScreen poly(vinylidene difluoride) membrane (PerkinElmer). The primary antibody labeling was detected with alkaline-phosphatase-conjugated secondary antibodies and CDP-Star reagent.
Data Analyses. Immunofluorescent and immunoelectron microscopic preparations were analyzed by using a laser scanning confocal microscope (Fluoview FV500, Olympus, Tokyo) and an electron microscope (Philips EM208S, FEI, Eindhoven, The Netherlands), respectively. Molecular masses of protein or mRNA bands on the blots were estimated by using the program genetools (Syngene, Cambridge, U.K.). Integrated OD was calculated by multiplying the mean OD by the area (mm2) of the positive band above background by using the program imagej 1.33u (National Institutes of Health, Bethesda). OD was defined on 8-bit black/white images with gray level 255 (white) as 0 OD and gray level 0 (black) as 2.708 OD.
Results
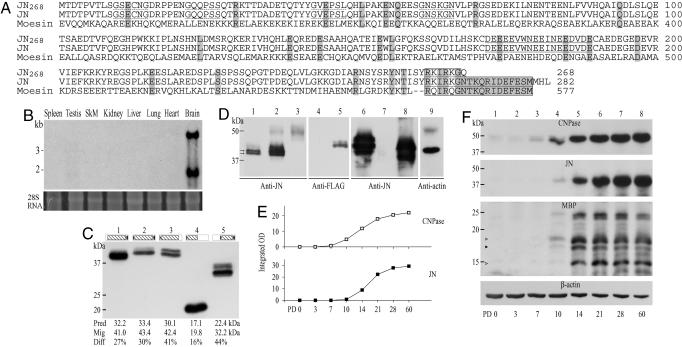
Molecular Features of JN. On multitissue Northern blots, the JN cRNA probe detected two major transcripts of 4.0 kb and 1.9 kb in rat brain poly(A)+ RNA. No hybridization signal was found in poly(A)+ RNAs of the other seven tissues examined (Fig. 1B). The 3.6-kb JN cDNA clone had a predicted ORF of 846 bp and a 5′ untranslated region of 80 bp with two in-frame stop codons.
Fig. 1.
JN's molecular features and expression profile in the postnatal forebrains. (A) Alignment and comparison of JN and JN268 with rat moesin, a typical ERM protein. Boxed sequences at the C termini denote the putative actin-binding domain. Identical residues are shaded, low-complexity regions are underlined, and potential N-myristoylation sites (reported by scanning Prosite patterns) are underlined by dots. (B) Multitissue Northern blot hybridized with JN-specific riboprobe. Two JN transcripts were revealed in the brain but not in the other seven tissues (SkM, skeletal muscle). (Bottom) The strip shows the SYBR gold staining of 28S rRNA on the nylon membrane. (C) Western blots, comparing the mobility of native brain JN (lane 1) with FLAG-tagged JN, JN247, JN141, and JNc (lanes 2-5). Framed schematic diagrams at the top represent the respective proteins. The filled triangles on the left sides of the diagrams stand for the N-terminal FLAG tag. The filled ellipses on the right sides of the diagrams denote the putative C-terminal actin-binding domain of JN. Differences (Diff) between predicted (Pred) molecular masses and actual migrations (Mig) of the proteins are shown below the blots. (D) Western blots, showing molecular analyses of JN. Labels below the blots indicate antibodies used for detections. The blot samples are from brain (lane 1), immunoprecipitates of rat brain lysate by the anti-JN antibody (lanes 2 and 9) or by preimmune rabbit serum (lane 3), 293T cells transfected by empty pXJ40 plasmid (lanes 4 and 7) or by FLAG-tagged JN (lanes 5 and 6), and His-tagged JN expressed by E. coli (lane 8). (E) Line graphs, showing relative levels of JN (Upper) or CNPase (Lower) immunoreactivities in the PD 0, 3, 7, 10, 14, 21, 28, and 60 rat forebrains. See Materials and Methods for quantification of positive bands on Western blots. (F) Western blots, comparing expression of CNPase, JN, MBPs, and actin in the PD 0, 3, 7, 10, 14, 21, 28, and 60 rat forebrains. Each lane was loaded with 20 μg of solubilized proteins.
The deduced peptide JN consisted of 282 amino acid residues (predicted Mr = 32,226; pI = 4.64) with high contents of glutamic acids (14.89%, 42 of 282) and serines (9.57%, 27 of 282). blast searches against National Center for Biotechnology Information nonredundant nucleotide and protein databases yielded no positive hits, except for an unnamed mRNA sequence predicted by automated genome computation (GenBank accession no. NM_001008311, 100% identity with JN), a deduced human KIAA1189 protein (284 amino acid residues, 58% amino acid identity with JN), and an unnamed mouse protein inferred from a cDNA sequence (accession no. NP_084248, 235 amino acid residues, 64% amino acid identity with JN). EST databases presented a cDNA clone encoding a truncated JN consisting of 268 amino acid residues (JN268; Fig. 1 A).
In comparison with characterized proteins, the JN C terminus showed homology to the actin-binding domain of the ezrin-radixin-moesin (ERM) proteins. Fig. 1 A compares JN and JN268 with moesin (NCBI Protein Data Bank accession no. AAB61666). The boxed residues denote the homologous region among the three. Unlike the ERM proteins, however, JN exhibited no detectable plasma-membrane-binding FERM (band four-point-one ERM homology) domain. Instead, a scan of the Prosite database of protein families and domain patterns (12) reported four potential N-myristoylation sites near the JN N terminus (at residues 10-15, 22-27, 43-48, and 61-66; underlined by dots in Fig. 1 A).
On Western blots of adult rat brain lysate, anti-JN antibody, but not preimmune or GST-JN170 preabsorbed serum, detected a 41-kDa major band that occasionally appeared as a closely spaced doublet (double arrows in Fig. 1D, lane 1). A band at the same position was also obtained from immunocomplexes of rat brain lysate precipitated with the JN antibody (Fig. 1D, lane 2) but not with the preimmune rabbit serum (Fig. 1D, lane 3). Actin was coimmunoprecipitated with JN by the JN antibody (Fig. 1D, lane 9). FLAG-tagged JN (by transfected 293T cells) or His-tagged JN (by transformed BL21 Escherichia coli), as detected by JN antibody, migrated to ≈43 kDa on Western blot (Fig. 1D, lanes 6 and 8). The position of the former was confirmed by the anti-FLAG antibody (lane 5). No FLAG or JN signal was revealed on the blots of cells transfected with empty pXJ40 vector (Fig. 1D, lanes 4 and 7).
The above immunoblotting results showed a discrepancy between the calculated molecular mass (32.2 kDa) and the actual migration of JN on SDS/PAGE. Further tests attributed this atypical migration of JN largely to its residues 101-247. As shown in Fig. 1C (lanes 2-5), in vitro expression of FLAG-tagged JN, JN247, JN141, and JNc yielded products that, on Western blots, measured heavier than predicted by 30%, 41%, 16%, and 44%, respectively.
Expression Profile of JN in the Developing Forebrain. In the developing forebrain, JN immunoreactivity emerged at about the same time as major MBP isoforms but lagged behind CNPase expression by ≈2-3 days. As shown in Fig. 1 (E and F), JN signal was undetected at postnatal day (PD) 0 through PD 7, remained very weak at PD 10, rapidly increased for the period PD 14-PD 21, and approximated the adult level by PD 28. Similarly, faint MBP signals appeared at PD 10. The 17-kDa MBP gradually diminished after peaking at PD 14 (indicated by a filled arrowhead in Fig. 1F). Some other MBP isoforms quickly reached plateau expression by PD 14-PD 21 and stayed high thereafter (indicated by open arrowheads in Fig. 1F). Significant CNPase signal, on the other hand, was discerned as early as PD 7 and increased gradually to about the adult level at PD 28.
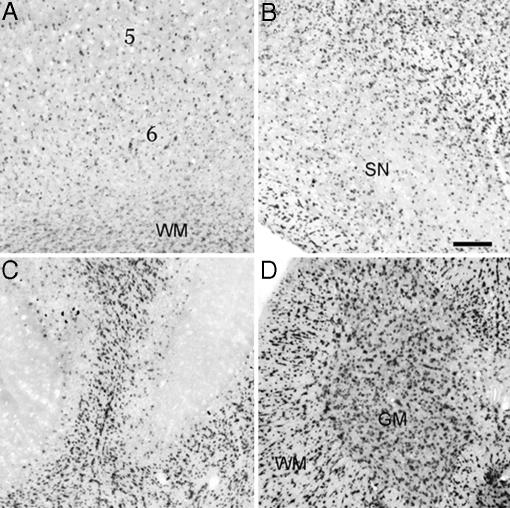
Cellular Expression of JN in the CNS. By using ISH, JN mRNA was detected in the perikarya of glia-like cells throughout the CNS. Positive cell bodies appeared generally small and scattered in the gray matter, and they were more numerous, morphologically more variable, and often queued along the direction of axon passage in the white matter (Fig. 2). Overall, the spinal cord showed the highest expression levels and the densest JN-positive signals, followed by the brainstem, cerebellum, thalamus, and hypothalamus. In the cerebral and cerebellar cortices, positive cells were scarce superficially but increased markedly toward deeper layers (Fig. 2). No significant JN labeling was detected in spinal dorsal roots (data not shown).
Fig. 2.
JN mRNA expression in the CNS, as revealed by ISH in the deep layers of frontal cerebral cortex (A), ventral midbrain region including the substantia nigra (B), cerebellar cortex (C), and cervical spinal cord (D). 5 and 6, layers V and VI of frontal cerebral cortex; GM, gray matter; SN, substantia nigra; WM, white matter. (Scale bar: 0.2 mm.)
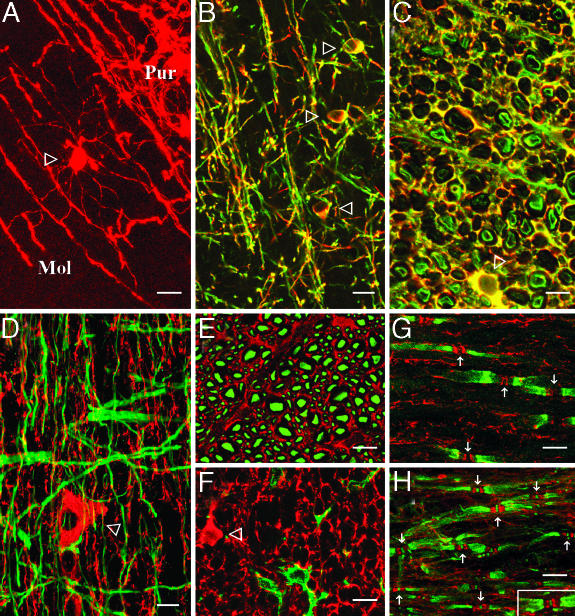
Distribution of JN immunoreactive cell bodies in the CNS agreed with that of the mRNA. Additionally, immunopositive signals along axons/fiber tracts were observed throughout the CNS, especially in the white matter (Fig. 3 A-H). On cross sections of fiber tracts, such as those in the spinal white matter, JN-immunoreactive profiles were seen around the neurofilament-200-positive axons and, often, separated from the latter by a negative zone (Fig. 3E). At the cellular level, JN colocalized with CNPase in all CNS regions examined. Subcellularly, JN distribution overlapped that of CNPase substantially but not completely. On cross section of fiber tracts of cervical spinal white matter, for example, CNPase often labeled two concentric rings, corresponding, probably, to the abaxonal and adaxonal layers of the myelin sheath. JN coexisted with CNPase mostly in the abaxonal (outer) ring and, at times, a small part of the adaxonal (inner) ring (Fig. 3C). No significant JN colocalization was found with glial fibrillary acidic protein in astrocytes, OX42 in microglia, or neurofilament 200 in neurons (Fig. 3 D-F).
Fig. 3.
Confocal photomicrographs showing JN distribution in the CNS. (A) JN immunofluorescence in the cerebellar cortex. Note the JN-positive oligodendrocyte at the border between molecular (Mol) and Purkinje cell (Pur) layers. (B-F) Double immunofluorescence of JN with CNPase (B and C), GFAP (D), neurofilament 200 (E), or OX42 (F) in layers III and IV of frontal cortex (B), cross sections (C, E, and F), or longitudinal section (D) of cervical spinal white matter. (G) Double immunofluorescence of JN and Kv1.2 on longitudinal section of spinal white matter. Arrows point to the presumptive positions of the nodes of Ranvier. (H) Triple staining of JN (red), Kv1.2 (green), and NavP (green) on a longitudinal section of spinal white matter. Arrows point to examples of the NavP clusters at the nodes of Ranvier. (Inset) An example from another microscopic field. Note the JN-enriched bands/dots flanking the node of Ranvier and between the strongly Kv1.2-positive juxtaparanodes. In all panels, single-labeling would appear red (JN) or green (others). Yellow indicates double-labeling. Open arrowheads denote cell bodies of oligodendrocytes. (Scale bars: 10 μm.)
Confocal microscopy and multiple immunofluorescence further revealed JN-enriched narrow bands or dots flanking the nodes of Ranvier. Fig. 3G shows such examples on a longitudinal section of spinal white matter. Between the Kv1.2-positive juxtaparanodes of adjacent myelin sheaths, two narrow bands of intense JN reactivity were found adjoining a JN-negative zone. Triple staining of JN, Kv1.2, and NavP established the latter zone as the node of Ranvier (with NavP clusters) and ascertained its close relation with the flanking JN bands (Fig. 3H).
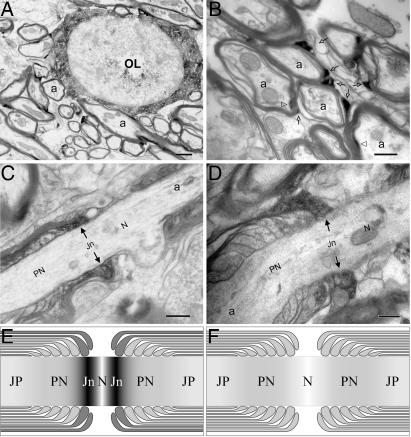
Immunoelectron microscopy confirmed the presence of JN in oligodendroglial perikarya and processes (Fig. 4A). Although the immunoreactivities appeared clustered, JN′s relation to specific organelles or the cytoplasmic membrane could not be established. Of the myelin sheath, strong signals were observed in the outermost cytoplasm-containing layer (abaxon or outer loop/”tongue”). To a lesser extent, JN was also present in the cytoplasm-containing inner layer lining the compact myelin sheath (adaxon or inner loop/tongue; Fig. 4 A and B). Of the terminal loops, only those lateral few (mostly numbers 1-3) adjoining the node of Ranvier showed clear deposits of JN immunoreactivity that, on occasion, could be seen associated with the loop membrane or submembranous layer (Fig. 4 C and D). Medial terminal loops further away from the node appeared to be devoid of significant JN immunoreactivity, as were the compact lamellae of the myelin sheath.
Fig. 4.
Electron micrographs, showing JN immunoreactivity in oligodendrocyte and myelin sheaths. (A and B) Electron micrographs of the cerebellum, showing JN in the cytoplasm of an oligodendrocyte (A), in the abaxons (open arrows), and, occasionally, in the adaxons (open arrowheads) but not in the compact layers of the myelin sheath (B). (C and D) On longitudinal sections of myelinated axons in cervical spinal cord (C) or corpus callosum (D), JN immunoreactivity was observed in the lateral few terminal loops (filled arrows) abutting the node of Ranvier. The axonal segment surrounded by the JN-positive lateral terminal loops is herewith defined as the juxtanode. (E and F) Schematic diagrams comparing the suggested (E) with the conventional (F) domains of myelinated axon. The internode is not shown. a, axon; Jn, juxtanode; JP, juxtaparanode; N, node of Ranvier; OL, oligodendrocyte; PN, paranode. (Scale bars: 1.0 μm in A, 0.5 μm in B, and 0.3 μm in C and D.)
Based on its distribution and site of apposition with the myelinated axon, we named this protein juxtanodin and propose a juxtanode domain of myelinated axon at the Ranvier node-paranode junction (Fig. 4E, Jn). Fig. 4 E and F compares the proposed with the conventional segmental divisions of myelinated axon and myelin sheath.
JN Promotes Arborization of Cultured Oligodendrocytes. OLN-93 cells, under normal culture conditions, showed no JN immunoreactivity. At low to medium densities, the majority were bipolar in morphology, although some displayed more than two primary cellular processes. In general, the cell bodies and processes were smooth on the surface and gave rise to only a few branches.
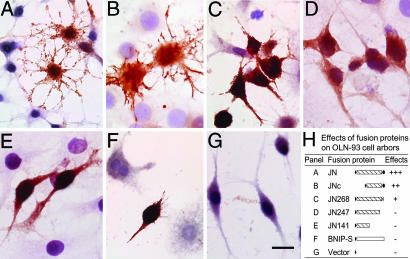
At 48 h after being transfected, ≈5-10% of cells in the culture expressed the extrinsic protein, as determined by immunocytochemistry. In comparison with neighboring untransfected cells in the same culture, JN- and JNc-transfected OLN-93 cells exhibited not only longer and more numerous primary processes but also significantly finer process arbors with many filopodium-like prickles from the processes or directly from the cell bodies (Fig. 5 A and B). Some transfected cells also gave rise to lamellopodium-like protrusions. JN268 transfection produced similar but much weaker effects. Transfection by JN247, JN141, empty pXJ40 vector, or the JN-unrelated BNIP-S (9), however, did not induce clear morphological change in OLN-93 processes (Fig. 5).
Fig. 5.
Effects of JN expression on OLN-93 arborization, as revealed by immunoperoxidase by using the anti-JN (A-E) or anti-FLAG (F and G) antibody. The preparations were lightly counterstained with cresyl violet to visualize untransfected cells. JN-transfected OLN-93 cells exhibited longer and more numerous processes and finer process arbors (A). JNc transfection produced similar but weaker effects (B). Fewer morphological changes were seen in cells transfected with JN268 (C). No enhanced process arborization was seen when cells were transfected with JN247 (D), JN141 (E), BNIP-S (F), or empty pXJ40 vector (G). (H) Summary of the transfection results in cultured OLN-93 cells. In schematic diagrams representing the fusion proteins, the filled triangles on the left stand for the N-terminal FLAG tag, and the filled ellipses on the right denote the putative C-terminal actin-binding domain of JN. (Scale bar: 20 μm.)
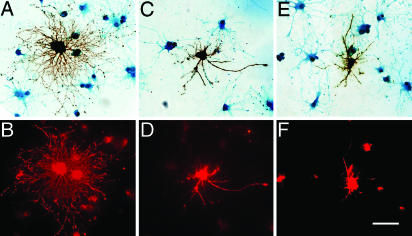
JN Promotes CNPase Trafficking to the Site of Action in Primary OLP Cells. Primary OLP cells from 5- to 7-day-old rat optic nerve did not yet exhibit significant intrinsic JN immunoreactivity at the stage of 7-9 days in culture. JN transfection elicited more extensive arborization and branching of the cells, in comparison with the untransfected cells and cells transfected with JN247 or JN141 (Fig. 6 A, C, and E). Moreover, JN transfection up-regulated CNPase expression and facilitated CNPase transport to the process arbors. As a result, the transfected OLPs showed markedly higher levels of CNPase immunoreactivity, especially in their processes (Fig. 6 A and B). The untransfected surrounding OLPs exhibited CNPase signals, mainly in the perikarya (Fig. 6B). Similar effects, although less prominent, were observed in cells transfected with JNc (data not shown). Transfection with JN247 and JN141 produced increasingly weaker, but still observable, facilitatory effects on CNPase expression and trafficking (Fig. 6 C-F).
Fig. 6.
Photomicrographs, showing the effects of JN (A and B), JN247 (C and D), or JN141 transfection (E and F) on primary OLP process ramification (A, C, and E) and CNPase expression and transport to the process arbors (B, D, and F). The cells were sequentially processed for CNPase immunofluorescence (red in B, D, and F), JN immunoperoxidase (brownish black in A, C, and E), and Luxol fast blue counterstaining (blue in A, C, and E). (Scale bar: 50 μm.)
Discussion
This study identified JN as a cytoskeleton-related oligodendroglial protein. Of CNS myelinated axons, JN distribution in the node-abutting lateral terminal loops of myelin sheath morphologically defined a juxtanode region between the paranode and the node of Ranvier. JN emergence during CNS ontogenesis paralleled that of major MBPs and coincided with the onset of myelination. JN transfection induced extensive process outgrowth and filopodium-like-protrusion formation in cultured OLN-93 cells and enhanced endogenous CNPase expression and transport to the site of action at the process arbors of primary OLPs.
JN: An Oligodendroglia Marker of a Previously Unidentified Protein Family. As evidenced by our double-labeling results, JN was a specific marker for oligodendroglia in the CNS. In contrast to CNPase, neither JN mRNA nor protein was detected in the Schwann cells of spinal dorsal roots, suggesting its absence in the peripheral nervous system and, therefore, higher expression specificity.
Although exhibiting an ERM-like C-terminal actin-binding domain, JN lacked the N-terminal membrane-binding FERM domain found in all known ERM proteins (13). Hence, it might not belong to the ERM protein family. It is unknown whether and how the potential N-myristoylation sites near the JN N terminus, as predicted by Prosite patterns, could serve a similar membrane-binding function.
The existence of JN isoforms is supported by our Northern blot results and by blast searches of EST cDNA databases. It is worth noting that JN268 exhibited diminished arborization-promoting capability, as compared with JN. Shortening the putative actin-binding domain in the former may also affect subcellular localization of the protein.
The atypical mobility of CNS native JN on SDS/PAGE was reproduced by in vitro expressed JN. It remains unclear how this unusual feature of the protein arose. Our data indicated a greater contribution by the middle fragment of JN. Of particular interest, in this regard, was the high content of glutamic acids in the fragment (18.49% by frequency), especially in the region of low complexity (residues 172-189).
JN, Actin Cytoskeleton, and Myelin Sheath. The unique architecture of the myelin sheath calls for dedicated oligodendroglial cytoskeletal machinery in the CNS (14, 15). Tubulin, actin, and associated proteins have been well documented in oligodendrocytes, but the molecular mechanisms underlying the motility of the oligodendroglia/myelin sheath remain poorly understood (16). The following evidence supports a role of JN in binding/regulating oligodendroglial cytoskeleton: (i) JN showed substantial colocalization in the myelin sheath with CNPase, a known cytoskeleton-related oligodendroglial protein (17-19). (ii) The JN C terminus resembled the well characterized actin-binding domain of ERM proteins. (iii) JN expression promoted arborization and CNPase trafficking to the process arbors of cultured primary OLPs. (iv) Actin was coimmunoprecipitated with JN.
The physiological functions of CNPase have been attributed to its roles in regulating tubulin polymerization/distribution (17) and in myelin-axon interaction. CNPase also cross-links with actin (18, 19). Like JN, CNPase is found mainly in the cytoplasm-containing noncompact regions of the myelin sheath (20, 21). In CNPase1-knockout mice, axons undergo swelling and degeneration without significant abnormality of the myelin sheath (22). These findings suggest a CNPase role in axon support and myelin-axon interaction through as yet unknown mechanisms.
The exact relationship between JN and the cytoskeleton remains unclear. For the induction of oligodendroglial arborization, the C-terminal ERM-like actin-binding domain of JN appeared to be directly involved. Our transfection results indicated that the C-terminal 181 residues of JN were sufficient. In particular, the last 35 residues of JN, in which resides the potential ERM-like actin-binding domain, seemed to be indispensable. The ERM proteins function as conformationally regulated membrane-cytoskeleton crosslinkers in actin-rich surface structures, such as microvilli, microspikes, and membrane ruffles, by using their C-terminal F-actin-binding domain (the last 34 residues) and an N-terminal membrane-binding FERM domain (13). Cellular process extension and filopodium formation are known actin-filament-based processes (14, 15). Together, these findings suggest that JN most likely exerted its effects on cell motility and arborization by interacting with and/or influencing the actin cytoskeleton of oligodendroglia.
Apart from affecting actin cytoskeleton through the C-terminal domain, JN is likely to also function by other mechanisms. JN′s promotion of CNPase expression and transport, for example, was increasingly diminished, but not totally abolished, by truncation of the C-terminal 35 or even 141 amino acid residues. Future studies will be needed to clarify the cytoskeletal or other molecular elements responsible for these JN effects on CNPase.
JN and the Delineation of the Juxtanode. As revealed by confocal microcopy and JN immunoelectron microscopy, the conventional terminal (paranodal) loops of myelin sheath are not homogeneous in their molecular composition. The JN-positive lateral terminal loops neighboring the node of Ranvier clearly differ from those JN-negative medial terminal loops adjoining the juxtaparanode. Accompanying this molecular heterogeneity, functional disparity between the two is expected. Hence, the proposed distinction of juxtanode from the paranode may bear important functional implications. The former might, for example, be more related to myelin-axon interaction or to induction/sustaining of the node of Ranvier, whereas the latter may be more involved in the formation/maintenance of compact myelin lamellae.
JN, Myelination, and Specialization of the Node of Ranvier. The maturation of oligodendrocytes is characterized by process outgrowth and refinement, the programmed appearance and redistribution of certain marker molecules, such as CNPase and MBPs, and the formation of myelin sheath (1). Expression of JN in the developing forebrain lagged behind that of CNPase, paralleled those of major MBP isoforms, and coincided with the onset of myelination therein (1, 23). This order of expression-onset of CNPase, MBPs, and JN in vivo seemed to be preserved in cultured primary OLPs, in which JN did not appear before at least 10 days in culture (data not shown). Our immunohistochemical studies in the developing CNS also confirm this observation (data not shown). In vitro, JN transfection not only promoted process arborization but also increased CNPase expression and transport to the process arbors of cultured OLN-93/primary OLP cells. These data suggest structural integration of exogenous JN into the host-cell cytoskeleton, functional activity of the protein for intracellular trafficking/cellular motility, and coordinated maturational acceleration of cultured oligodendrocytes in response to the exogenous JN. Taken together, our results indicate an important role of JN in late-stage oligodendroglia maturation, in myelin/Ranvier node formation during CNS development, and in the maintenance and plasticity of related structures in the mature CNS.
In the peripheral nervous system (PNS), ERM proteins enriched in the perinodal Schwann cell microvilli are strongly implicated in inducing Ranvier node formation (24, 25). In the CNS, oligodendroglia are known to similarly direct nodal Na+ channel clustering but are devoid of ERM proteins (26). We hypothesize that JN serves a CNS function similar to that of the ERM proteins in the PNS, based on its putative ERM-like actin-binding domain, its influence on oligodendroglial-process outgrowth/branching to approach the putative target axons, and its selective positioning to the juxtanodal terminal loops next to the node. It remains unknown to what proteins/structures, if any, JN links the actin cytoskeleton and how these molecules relate to those downstream on the myelin-axon signaling cascade to influence node formation.
Acknowledgments
We thank Dr. B. L. Tang (National University of Sinapore) for providing antibodies, Dr. J. H. Tang for technical assistance, and Mr. Y. H. Ou Yang for reading the manuscript. This work was supported by Biomedical Research Council Grant 01/1/21/19/179 (to F.L.) and National University of Singapore Academic Research Fund Grant R181-000-052-112 (to F.L.).
Author contributions: F.L. designed research; B.Z., Q.C., A.G., H.C., Y.G.C., J.P.B., and F.L. performed research; F.L. contributed new reagents/analytic tools; B.Z., B.C.L., E.A.L., and F.L. analyzed data; and F.L. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CNPase, 2′,3′-cyclic nucleotide-3′-phosphodiesterase; ERM, ezrin-radixin-moesin; FERM, band four-point-one ERM homology; ISH, in situ hybridization histochemistry; JN, juxtanodin; MBP, myelin basic protein; OLP, primary oligodendrocyte precursor; PD, postnatal day.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. DQ119821).
References
- 1.Baumann, N. & Pham-Dinh, D. (2001) Physiol. Rev. 81, 871-927. [DOI] [PubMed] [Google Scholar]
- 2.Stewart, D. G. & Davis, K. L. (2004) Int. Rev. Neurobiol. 59, 381-424. [DOI] [PubMed] [Google Scholar]
- 3.Pfeiffer, S. E., Warrington, A. E. & Bansal, R. (1993) Trends Cell Biol. 3, 191-197. [DOI] [PubMed] [Google Scholar]
- 4.Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W. & Lipman, D. J. (1997) Nucleic Acids Res. 25, 3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richter-Landsberg, C. & Heinrich, M. (1996) J. Neurosci. Res. 45, 161-173. [DOI] [PubMed] [Google Scholar]
- 6.Bögler, O. (1997) in Current Protocols in Neuroscience, eds. Crawley, J. N., Gerfen, C., McKay, R., Rogawski, M., Sibley, D. R. & Skolnick, J. (Wiley, New York), pp. 3.4.1-3.4.9.
- 7.Hu, Q. D., Ang, B. T., Karsak, M., Hu, W. P., Cui, X. Y., Duka, T., Takeda, Y., Chia, W., Sankar, N., Ng, Y. K., et al. (2003) Cell 115, 163-175. [DOI] [PubMed] [Google Scholar]
- 8.Manser, E., Huang, H. Y., Loo, T. H., Chen, X. Q., Dong, J. M., Leung, T. & Lim, L. (1997) Mol. Cell. Biol. 17, 1129-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou, Y. T., Soh, U. J., Shang, X., Guy, G. R. & Low, B. C. (2002) J. Biol. Chem. 277, 7483-7492. [DOI] [PubMed] [Google Scholar]
- 10.Liang, F., Hatanaka, Y., Saito, H., Yamamori, T. & Hashikawa, T. (2000) J. Comp. Neurol. 416, 475-495. [PubMed] [Google Scholar]
- 11.Ling, E. A., Kaur, C. & Wong, W. C. (1992) Histol. Histopathol. 7, 93-100. [PubMed] [Google Scholar]
- 12.Falquet, L., Pagni, M., Bucher, P., Hulo, N., Sigrist, C. J., Hofmann, K. & Bairoch, A. (2002) Nucleic Acids Res. 30, 235-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bretscher, A. (1999) Curr. Opin. Cell Biol. 11, 109-116. [DOI] [PubMed] [Google Scholar]
- 14.Badour, K., Zhang, J. & Siminovitch, K. A. (2003) Immunol. Rev. 192, 98-112. [DOI] [PubMed] [Google Scholar]
- 15.Schafer, D. A. (2004) Nature 430, 734-735. [DOI] [PubMed] [Google Scholar]
- 16.Richter-Landsberg, C. (2000) J. Neurosci. Res. 59, 11-18. [DOI] [PubMed] [Google Scholar]
- 17.Bifulco, M., Laezza, C., Stingo, S. & Wolff, J. (2002) Proc. Natl. Acad. Sci. USA 99, 1807-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Angelis, D. A. & Braun, P. E. (1996) J. Neurochem. 67, 943-951. [DOI] [PubMed] [Google Scholar]
- 19.Dyer, C. A. & Benjamins, J. A. (1989) J. Neurosci. Res. 24, 201-211. [DOI] [PubMed] [Google Scholar]
- 20.Trapp, B. D., Bernier, L., Andrews, S. B. & Colman, D. R. (1988) J. Neurochem. 51, 859-868. [DOI] [PubMed] [Google Scholar]
- 21.Braun, P. E., Sandillon, F., Edwards, A., Matthieu, J. M. & Privat, A. (1988) J. Neurosci. 8, 3057-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lappe-Siefke, C., Goebbels, S., Gravel, M., Nicksch, E., Lee, J., Braun, P. E., Griffiths, I. R. & Nave, K. A. (2003) Nat. Genet. 33, 366-374. [DOI] [PubMed] [Google Scholar]
- 23.Hamano, K., Takeya, T., Iwasaki, N., Nakayama, J., Ohto, T. & Okada, Y. (1998) Brain Res. Dev. Brain Res. 108, 287-293. [DOI] [PubMed] [Google Scholar]
- 24.Gatto, C. L., Walker, B. J. & Lambert, S. (2003) J. Cell Biol. 162, 489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melendez-Vasquez, C. V., Rios, J. C., Zanazzi, G., Lambert, S., Bretscher, A. & Salzer, J. L. (2001) Proc. Natl. Acad. Sci. USA 98, 1235-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan, M. R., Cho, M. H., Ullian, E. M., Isom, L. L., Levinson, S. R. & Barres, B. A. (2001) Neuron 30, 105-119. [DOI] [PubMed] [Google Scholar]