Abstract
Although bioavailability of NO in the coronary circulation is commonly evaluated by acetylcholine (ACh)-induced vasodilation, a change in plasma NO concentration and its relation to the flow response after injection of ACh are still unknown. Thus, we directly measured the concentration of NO in the coronary sinus by using a catheter-type NO sensor for coronary sinus. An NO-sensitive sensor was located and fixed in a 4-Fr catheter with a soft tip for protection of vascular wall. After calibration with an NO-saturated pure water, the catheter-type NO sensor was located in the coronary sinus in anesthetized dogs. The coronary flow velocity (CFV) was measured with a Doppler guide wire. Intracoronary injection of ACh (0.4 and 1.0 μg/kg) increased plasma NO concentration in a dose-dependent manner (3–10 nM). Although ACh increased CFV by 95%, there was no significant difference between the two ACh doses. After ACh, the peak value of plasma NO concentration was observed significantly later than CFV. NG-methyl-l-arginine (NO synthase inhibitor) decreased basal NO concentration by 3 nM and suppressed the ACh-induced NO synthesis with no significant change in average peak velocity. We conclude that production of NO in the coronary circulation can be evaluated in the coronary sinus. Although ACh increases both CFV and NO concentration, CFV dose not reflect NO concentration in terms of magnitude and time course. Direct measurement of plasma NO concentration by the catheter-type NO sensor is useful to evaluate bioavailability of NO in the coronary circulation.
Keywords: nitric oxide synthase, nitric oxide-selective sensor, acetylcholine, coronary blood flow, endothelial function
Nitric oxide (NO) released by endothelial cells is formed from l-arginine by nitric oxide synthase (NOS) (1). NO release is augmented by mechanical stimulation such as shear stress (2–6), pulsatile flow (7, 8), and axial strain (9). Such mechanical stresses, which are produced by the periodic myocardial contraction and relaxation, directly influence the coronary blood vessels, acting as an overwhelmingly dominant factor of the coronary blood flow regulation (10–12). Under the stimulatory influences by the mechanical stresses, coronary endothelial cells produce NO on a beat-to-beat basis (13). In the coronary circulation, endothelium-derived NO plays fundamental roles in the regulation of blood flow, exerting a tonic vasodilator influence at rest and with increasing myocardial metabolism (14).
However, bioavailability of NO is diminished in the subjects with a wide range of cardiac risk factors and pathologic conditions, such as aging, gender, smoking, hypertension, hypercholesterolemia, diabetes mellitus, hyperhomocystinemia, heart failure, infections, and polymorphisms of the endothelial NOS (15–23). Importantly, endothelial dysfunction, characterized by decreased bioavailability of NO, is a predictor of cardiovascular risk and outcome (24–28).
Several techniques have been used to evaluate the endothelial function or bioavailability of NO (29). Coronary endothelial function is most commonly assessed by intracoronary injection of acetylcholine (ACh), which stimulates release of NO and coronary artery dilation. In patients with risk factors for atherosclerosis and in those with coronary artery disease, infusion of ACh results in a diminished vasodilatory response or paradoxical vasoconstriction (30–35). In spasm arteries of patients with coronary spastic angina, activity of NO is deficient (28, 29). Although endothelium-dependent vasodilation has been commonly evaluated by intracoronary injection of ACh, there are still many uncertainties remaining in relation to the bioavailability of NO. First, it is unclear whether factors that result in endothelial dysfunction also lead to abnormalities in the functions of vascular smooth muscle cells. Namely, individuals with decreased endothelium-independent vasodilation response will have decreased endothelium-dependent vasodilation. Secondly, besides NO, other endothelium-derived relaxing and constricting factors such as prostacyclin, endothelium-derived hyperpolarizing factors, and endothelin might contribute to the vascular tone after injection of ACh through direct or indirect effects mediated by the changes in coronary blood flow and pressure patterns. Therefore, to evaluate the endothelial function and the bioavailability of NO in the coronary circulation, direct measurement of NO concentration in the coronary circulation would be of great value and use.
In prior years, it has been considered that NO, once released from vascular endothelial cells to blood stream, is immediately oxidized or inactivated by dissolved oxygen, oxyhemoglobin and/or oxygen radical species (36, 37). However, growing experimental and clinical evidence suggests that NO remain active in blood stream, causing remote vasodilatory response (38, 39). Accordingly, in this study, to measure the plasma concentration of NO produced in the coronary circulation, we developed a catheter-type NO sensor applicable to the coronary sinus. Here, we report the presence of NO in the coronary sinus at a resting condition and the real-time changes in NO concentration when endogenous NO production is stimulated by ACh or an NO donor is infused. Based on the simultaneous measurement of plasma NO concentration in the coronary sinus and coronary blood flow velocity, the relationship between NO concentration and coronary flow response to ACh will be discussed.
Methods
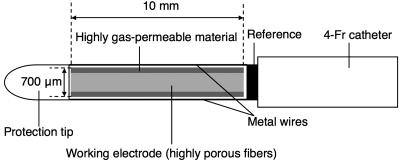
A Catheter Type of NO Sensor. An NO sensor (amiNO-700 XL, Innovative Instruments, Tampa, FL; 700 μm in diameter at the detection tip) was mounted in a 4-Fr catheter (1,200 mm long; Hirakawa Hewtech, Tokyo) and fixed with silicon adhesive. A soft protection tip of polyurethane was attached at the edge of the detection tip to protect the vessel wall, and two metal wires were also attached along the detection tip to protect the detection tip (Fig. 1). Oxidative current of NO was monitored by using an NO monitor (model inNO-T, Innovative Instruments). Each sensor was calibrated by using an NO-saturated pure water as described in ref. 40. Briefly, NO-saturated pure water was prepared by bubbling pure NO gas in oxygen-free pure water. Using a gas-tight syringe, 5 μl was injected into a well stirred saline solution (50 ml) in which an NO sensor was immersed (final NO concentration: 190 nM).
Fig. 1.
Structure of a catheter-type NO sensor for measurement in coronary sinus.
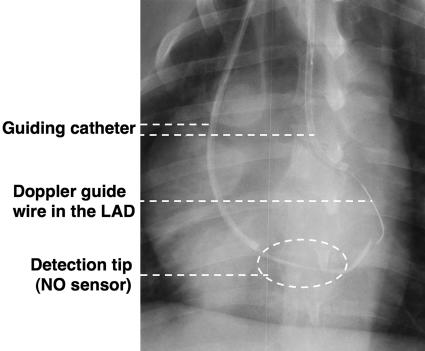
Animal Preparation. This study conformed to the Guideline on Animal Experiments of Kawasaki Medical School and the Guideline for Care and Use of Laboratory Animals published by the National Institutes of Health. Adult mongrel dogs (n = 7) were initially sedated with ketamine (200 mg i.m.) and anesthetized with pentobarbital sodium (30 mg/kg i.v.). Their weights ranged from 20 to 29 kg (25 ± 5 kg). Each dog was heparinized by injecting 100 units/kg of heparine. Animals were ventilated by using a respirator pump (model VS-600, Instrumental Development, Pittsburgh). The NO sensor was located in the coronary sinus through a 7-Fr JR catheter (Bright Chip, Cordis, Miami) from the right jugular vein, and a Doppler guide wire (FloWire, JoMed, Rancho Cordova, CA) was located in the left anterior descending artery through another 7-Fr JR catheter inserted in the right carotid artery under cinefluorography (Fig. 2).
Fig. 2.
X-ray photo showing the location of NO sensor. The detection tip of the NO sensor (dotted circle) was located in the coronary sinus through a 7-Fr catheter from the right jugular vein. The Doppler guide wire was inserted in the left anterior descending (LAD) artery.
Experimental Protocol. We continuously monitored the plasma NO concentration in the coronary sinus by the NO sensor, phasic coronary blood flow by the Doppler guide wire in the left anterior descending artery, and aortic pressure by a strain gauge pressure transducer (model TP-400T, Nihon Kohden, Tokyo). We measured average peak velocity (APV) as coronary flow velocity.
We injected saline (vehicle) and ACh of 0.4 μg/kg and 1.0 μg/kg from the left coronary artery (LCA) for 20 sec. In each bolus, each solution was diluted to 5 ml with saline. Next, we administrated NG-methyl-l-arginine (l-NAME, 10 μg/kg per min) into the LCA for 20 min to inhibit NO synthesis. We then repeated ACh injection at two doses (0.4 μg/kg and 1.0 μg/kg). Finally, we injected an NO donor, isosorbide dinitrate (ISDN, 0.5 mg).
Statistical Analysis. All data were expressed as mean values ± SEM. Statistical analyses for comparison of changes in plasma NO concentration, coronary flow velocity, and systemic hemodynamic variables by ACh before and after l-NAME were conducted by paired t test. A probability value of <0.05 was considered statistically significant.
Results and Discussion
Calibration of Sensors. The basic performance of the integrated catheter-type NO sensors was reported in our preliminary study (40). The NO sensor showed no noticeable change in response to oxygen, ACh, and solution mixing, indicating high specificity to NO. The mean sensitivity of the seven sensors used in the present study was 366 ± 122 pA/nM. In our previous study, prototype catheter-type NO sensors for the measurement in aorta, which used the same sensor as that used in this study, showed the sensitivity of 498 ± 40 pA/nM (seven sensors) (41). The mean sensitivity of the NO sensor for the coronary sinus was slightly lower than that of the NO sensor for the aorta. This difference may be attributable to the decrease in the surface area of the detection tip due to the soft protection tip.
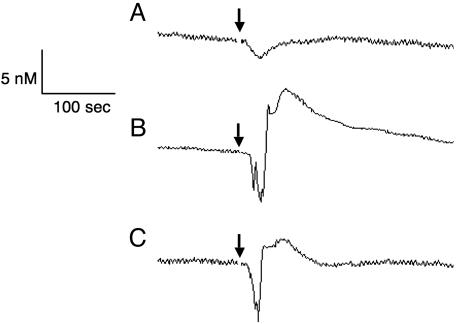
Evaluation of Coronary NO Production in the Coronary Sinus. Plasma NO concentration was successfully measured in the coronary sinus by the catheter-type NO sensor in all dogs studied without any harmful complications. Fig. 3 shows representative tracings of the plasma NO concentration in the coronary sinus after injection of saline, ACh, and ACh after l-NAME. Intracoronary injection of saline (vehicle) caused only a small fluctuation in the plasma NO concentration (Fig. 3A). Plasma NO concentration increased sharply after intracoronary injection of ACh. ACh of 0.4 μg/kg increased the plasma NO concentration by 5.7 nM (Fig. 3B). The ACh-induced increase in NO concentration was attenuated after the treatment with l-NAME (Fig. 3C). The acute increase in plasma NO concentration by ACh was observed in all dogs studied. Malinski and colleagues characterized the time courses of NO release in response to shear stress (42) and bradykinin (43) by using a polymeric porphyrin/Nafion-coated carbon fiber microsensor. In the cultured endothelial cells, shear stress caused by laminar flow of perfusate (0.02–1 Pa) induced NO release 3 to 5 sec after the initiation of flow (42). Bradykinin-induced NO was detected 8 sec after the addition of bradykinin (43). Although the basic performance of the NO sensor and the experimental condition are different between their and our studies, the rapid response of the endothelial cells to shear stress and agonists was consistently observed in their in vitro study and in this in vivo study. Vallance et al. measured the ACh-stimulated increase in the endogenous NO level in vivo in the human hand vein (44) by using their handmade NO sensor (45) and observed an increase of ≈130 nM. The difference in the agonist-stimulated increase in plasma NO concentration between their report and this study may be attributed to the following factors. In their study (44), ACh was infused anterogradely into the hand vein at 10–15 mm proximal to the end of the catheter to which the sensor was inserted. Because of the relatively short distance between the injection site and the measuring site, NO scavenging and trapping effects by hemoglobin and other blood components were much less than that in this study. The difference in the material and architecture of the two sensors also could provide the different performance of the sensors, e.g., selectivity to NO (40, 45).
Fig. 3.
Typical tracings of the plasma NO concentration in the coronary sinus. (A) Saline (vehicle) was injected at the arrow. (B) ACh of 0.4 μg/kg was injected at the arrow. (C) ACh of 0.4 μg/kg was injected at the arrow after inhibition of NO synthesis by l-NAME. The injection of saline caused only transient fluctuation but no increase in NO concentration. In contrast, ACh increased NO concentration markedly. l-NAME attenuated the increase of NO concentration by ACh.
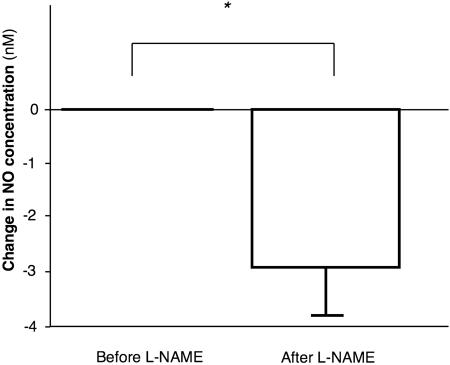
Part of the NO released into the blood stream might be subjected to oxidative decomposition and to nitrosative chemistry as in the previous study on the systemic circulation (46). Nevertheless, a substantial portion of the NO produced in the coronary circulation was transported to the venous outflow tract, i.e., the coronary sinus. It should be noticed that under resting condition, NO was present in the coronary sinus at a measurable concentration (Fig. 4). l-NAME decreased the basal NO concentration by 3.1 ± 0.8 nM (P < 0.05 vs. before l-NAME). Preservation of NO in the flowing blood is concordant with the previous in vivo investigation with authentic NO, which revealed the capability of plasma to transport NO in its free form along the forearm vascular tree (47).
Fig. 4.
Change in baseline plasma NO concentration by l-NAME (10 μg/kg per min for 20 min). *, P < 0.05.
The biochemical half-life of NO in the plasma layer has been calculated to be in the range of 100–500 sec (48), considering the concentration of dissolved oxygen in blood (≈150–250 μM) and assuming NO concentration of the nanomolar range as measured in this study. Such estimated biochemical half-life of NO is much longer than the transit time of blood in the coronary capillary vessels where the most of the endothelial cells, the predominant source of NO synthesis, locates. The capillary transit time has been reported to be ≈0.8 sec in rabbits (49) and 0.4 sec in pigs (50). Accordingly, the presence of NO produced by the coronary blood vessels in the coronary sinus is reasonable from the viewpoints of the biochemical half-life of NO and the kinetics of coronary circulation.
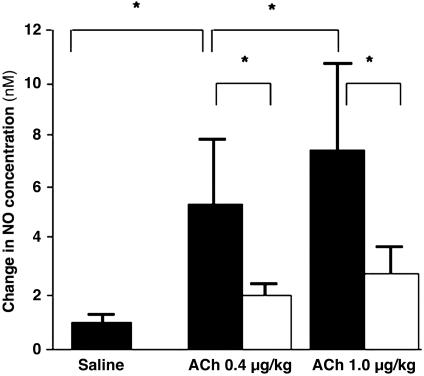
ACh increased plasma NO concentration in a dose-dependent manner (Fig. 5). The increased NO concentration by ACh was significantly greater than that by saline (Fig. 5). After l-NAME, the increase in NO concentration by ACh was attenuated significantly (1.9 ± 0.6 nM for 0.4 μg/kg, 2.8 ± 0.8 nM for 1.0 μg/kg, P < 0.05; P < 0.05 vs. before l-NAME; Fig. 5). Although the dose of l-NAME used in this study (10 μg/kg per min for 20 min) was reported to inhibit NO synthesis greatly (51, 52), administration of this dose could not achieve complete inhibition of NO production by ACh. We believe that under in vivo circumstances with stimulatory mechanical stresses, it may be extremely difficult to completely abolish the NO production by using the competitive inhibitors of NOS. Accordingly, determination of the contribution of the endothelium-derived hyperpolarizing factor to the vasodilation based on the NOS-inhibitor/indomethacin-resistant response to ACh needs careful consideration. Stoen et al. (53) reported that by using the femoral artery rings from young piglets, an NO inactivator, oxyhaemoglobin, abolished the NOS-inhibitor/indomethacin-resistant relaxation to ACh. They stated that ACh-induced vasodilation of the femoral artery of young piglets may depend entirely on NO. Although in their study NO concentration was not measured after NOS inhibition, incomplete inhibition of NOS and/or NO derived from nonenzymatic control might contribute to the NOS-inhibitor/indomethacin-resistant relaxation by ACh. Their study may also support the above-mentioned caution.
Fig. 5.
ACh-induced change in NO concentration and the effect of NOS inhibition. Plasma NO concentration was increased by ACh in a dose-dependent manner (0.4 and 1.0 μg/kg). l-NAME suppressed the increase of NO concentration by ACh significantly. (Filled bars, before l-NAME; open bars, after l-NAME; *, P < 0.05.)
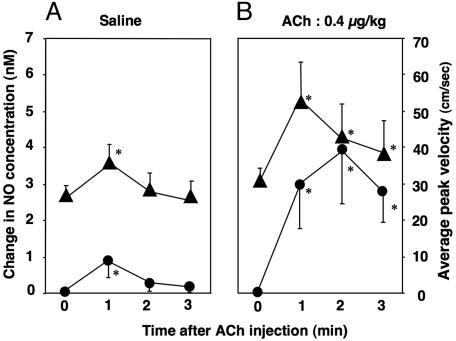
The saline injection caused some increase in APV associated with a slight increase in plasma NO concentration (Fig. 6A). The NO concentration and APV reached their peak values 1 min after the injection of saline and then decreased afterward. The similar time course of the APV and NO concentration after saline injection support the notion that the NO plays a primary role in the flow-induced vasodilation (8). APV 1 min after ACh injection was significantly greater than that for saline (Fig. 6B). The ACh-induced change in coronary blood flow can possibly be modified by the ACh-induced change in systemic hemodynamics. However, in this study, the intracoronary administration of ACh caused no significant changes in blood pressure and heart rate (Table 1).
Fig. 6.
Time courses of change in NO concentration (•) and APV (▴) after injection of saline and ACh. (A) Injection of saline increased the peak values of NO concentration and APV 1 min after the injection. (B) Injection of ACh also increased both NO concentration and APV at 1 min after ACh injection. NO concentration further increased and reached its peak value 2 min after ACh injection, indicating sustained NO production by ACh. (*, P < 0.05 vs. time 0.)
Table 1. Hemodynamic and NO data in this study.
| Control | After l-NAME | |
|---|---|---|
| Mean blood pressure, mmHg | 88 ± 12 | 92 ± 12 |
| Heart rate, bpm | 118 ± 15 | 120 ± 12 |
| Average peak velocity, cm/sec | ||
| Rest | 27.2 ± 2.0 | 27.2 ± 3.3 |
| ACh (0.4 μg/kg, i.c.) | 52.8 ± 5.0 | 30.2 ± 3.4* |
| ACh (1.0 μg/kg, i.c.) | 53.3 ± 10.2 | 33.5 ± 2.4* |
| NO concentration, nM | ||
| ACh (0.4 μg/kg, i.c.) | 5.0 ± 2.7 | 1.9 ± 0.6* |
| ACh (1.0 μg/kg, i.c.) | 7.3 ± 3.5 | 2.8 ± 0.8* |
bpm, beat per minute. *, P < 0.05 vs. control.
The plasma NO concentration increased in an ACh-dose-dependent manner before l-NAME (5.0 ± 2.7 nM for 0.4 μg/kg and 7.3 ± 3.5 nM for 1.0 μg/kg, P < 0.05; Fig. 5). However, there was no significant difference in APV between the two doses of ACh (52.8 ± 5.0 cm/sec for 0.4 μg/kg and 53.3 ± 10.2 cm/sec for 1.0 μg/kg, P = 0.67; Table 1). This finding implies the upper limit of the NO-induced vasodilatory influence. The discrepancy between the change in NO concentration and vasodilatory response to ACh should be taken into consideration when evaluating the bioavailability of NO based on the endothelium-dependent vasodilation. In the coronary circulation, NO contributes to vasodilation, but NO is not the only regulator of coronary blood flow (14). This notion is also illustrated in Fig. 6B, in which NO concentration further increased even after 2 min of ACh administration, whereas APV decreased from 1 min to 2 min. In fact, the peak value of the plasma NO concentration was observed significantly later than the peak of APV after injection of ACh for 0.4 μg/kg (98 ± 38 sec vs. 52 ± 21 sec, P < 0.05), indicating sustained NO production by intracoronary injection of ACh. Blood flow during ACh is mainly determined by the endothelium-dependent vasodilatory effect and the direct vasoconstrictive effect. There are many possible factors influencing these vascular effects during ACh besides NO, e.g., endothelium-derived hyperpolarizing factor, adenosine, ATP-dependent K+ channels, prostaglandins, local myogenic response, and endothelin. Thus, the blood flow response to ACh may not necessarily be the unique marker of the bioavailability of NO.
The difference in the time profiles between coronary flow velocity and NO concentration might be explained by the following factors. ACh increases both plasma NO concentration and blood flow. Then, the associated elevation of shear stress may further increase the plasma NO concentration by a positive feedback mechanism. Furthermore, flow-induced release of endogenous ACh from the endothelial cells (54), if any, may at least partially contribute to the sustained elevation of the plasma NO concentration.
It should be noticed that in this study, coronary flow velocity was measured in the left anterior descending artery, whereas plasma NO concentration was measured in the coronary sinus. Thus, coronary transit time might cause the delay of the change in NO concentration in the coronary sinus after intracoronary injection of ACh. However, as discussed above, capillary transit time is calculated to be ≈1 sec, minimizing its effect on the time difference between the peaks of flow velocity and plasma NO concentration. With increasing coronary blood flow, the blood volume in the coronary microvessels could be increased (55). This result may cause the decrease in NO concentration, obscuring the increase in NO production in the coronary circulation.
There was no significant difference in APV between before and after l-NAME administration (27.2 ± 2.0 cm/sec vs. 27.0 ± 3.3 cm/sec, P = 0.94). This conclusion is consistent with the previous report showing that NOS inhibition with arginine analogs resulted in small or no change in coronary blood flow at rest or when myocardial oxygen consumption was increased (14). Although these findings support the notion that NO is not the only determinant of coronary blood flow, some previous reports showed that inhibition of NOS decreased coronary venous oxygen tension (14), indicating the contribution of NO to the maintenance of oxygen supply to the myocardium at rest.
The increase in APV by ACh injection was suppressed by l-NAME (25.6 ± 3.0 cm/sec vs. 3.2 ± 0.7 cm/sec, P < 0.05). Traverse et al. (56) measured coronary blood flow and oxidative products of NO (nitrate + nitrite = NOx) in the coronary venous blood during the administration of ACh. Resting coronary blood flow was not significantly changed after the administration of l-arginine analog, but the increase in coronary blood flow to ACh was significantly reduced. NO (NOx) production in response to ACh was also reduced after adding an l-arginine analog. These findings coincide with ours, indicating that NO plays a pivotal role in the ACh-induced coronary vasodilation. Interestingly, they also noticed that a substantial number of animals they examined had negative arteriovenous NOx gradients between aorta and coronary sinus at rest (56). Recently, in vivo conversion of nitrite, which is the major pool of bioavailable NO, to NO has been proposed by enzymatic reduction with xanthine oxidoreductase, nonenzymatic disproportionation, or acidic reduction (57). Cosby et al. showed that nitrite-induced vasodilation in humans is associated with reduction of nitrite to NO by deoxyhemoglobin, providing a mechanism for hypoxia-regulated catalytic NO production by erythrocytes, or endothelial or tissue heme proteins (57). The reduction mechanism of nitrite to NO may explain the negative arteriovenous NOx gradients between aorta and coronary sinus. Although the reduction mechanism of nitrite to NO is beyond the scope of this study, we suggest the possibility that NOx is not a dependable measure of the production of NO in the coronary circulation.
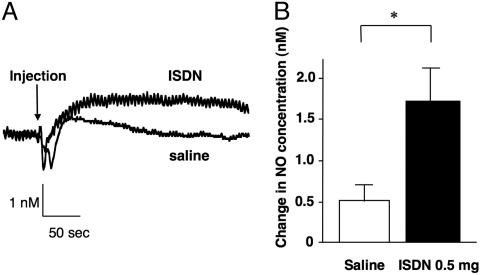
NO Production in the Coronary Circulation from NO Donor. Fig. 7 shows the typical tracings of NO concentration after injection of ISDN and saline. After a transient fluctuation after the injection of ISDN, NO concentration increased and sustained (Fig. 7A). This work shows the direct measurement of NO in response to ISDN in the coronary circulation. The increase in NO concentration by ISDN was significantly greater than that by saline (1.7 ± 0.4 nM vs. 0.5 ± 0.2 nM, P < 0.05; Fig. 7B). Glyceryl trinitrate (GTN) is another widely used coronary vasodilator that is characterized as the NO donor. Recently, different concentration-vasodilatory responses were reported for ISDN and GTN (58). GTN may apparently exert its vasodilatory activity independently of its NO-releasing properties, whereas ISDN exerts its activity by NO donation. Accordingly, we have chosen ISDN as the NO donor in this study. ISDN dilates angiographically normal coronary arteries in patients with hypertension, whereas flow-dependent dilation is lost (59). Therefore, ISDN may be considered to be a reasonable NO donor for the patients with the decreased bioavailability of NO, contributing to the physiological NO-mediated vasodilation. Future studies are required to establish a reasonable NO donation method to the patients with decreased bioavailability of NO. This catheter-type NO sensor for the coronary sinus will be a useful tool to evaluate the bioavailability of NO and the pharmacokinetics of the NO donors or NO modulators.
Fig. 7.
Change in NO concentration by ISDN. (A) NO concentration was increased and sustained by ISDN compared with saline. (B) The increase in NO concentration by ISDN was significantly larger than that by saline. (*, P < 0.05.)
Conclusion. NO produced in the coronary circulation persists in the coronary sinus at a measurable level at rest. Intracoronary injection of ACh increases NO concentration and coronary blood velocity. However, there are discrepancies between them in dose–response relation and time profile, indicating that ACh-induced change in coronary blood flow is not a precise measure of the bioavailability of NO. The catheter-type NO sensor for coronary sinus is useful to evaluate the endogenously and exogenously derived NO in the coronary circulation.
Acknowledgments
We thank Dr. Ziad Taha of Innovative Instruments, Mr. Akio Nakano of Nakano Mercantile, and Mr. Kouichi Muraki and Mr. Masanori Kimura of Hirakawa Hewtech Corp. for their valuable comments and assistance in developing the catheter-type sensors. This work was supported by Grant-in-Aid for Scientific Research (C) (2) 14570703 (to K.Y.) and Grant-in-Aid for Scientific Research Exploratory Research 14658297 (to S.M.).
Author contributions: M.G. designed research; Y.N., S.M., T. Kawamoto, T. Kume, R.S., and M.T. performed research; S.M., T.M., Y.O., and F.K. contributed new reagents/analytic tools; Y.N., S.M., Y.O., T.A., K.Y., and M.G. analyzed data; and Y.N., S.M., and M.G. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACh, acetylcholine; APV, average peak velocity; ISDN, isosorbide dinitrate; l-NAME, NG-methyl-l-arginine; NOS, nitric oxide synthase.
References
- 1.Moncada, S., Palmer, R. M. & Higgs, E. A. (1991) Pharmacol. Rev. 43, 109-142. [PubMed] [Google Scholar]
- 2.Kuo, L., Davis, M. J. & Chilian, W. M. (1990) Am. J. Physiol. 259, H1063-H1070. [DOI] [PubMed] [Google Scholar]
- 3.Buga, G. M., Gold, M. E., Fukuto, J. M. & Ignarro, L. J. (1991) Hypertension 17, 187-193. [DOI] [PubMed] [Google Scholar]
- 4.Lamontagne, D., Pohl, U. & Busse, R. (1992) Circ. Res. 70, 123-130. [DOI] [PubMed] [Google Scholar]
- 5.Koller, A., Sun, D. & Kaley, G. (1993) Circ. Res. 72, 1276-1284. [DOI] [PubMed] [Google Scholar]
- 6.Stepp, D. W., Nishikawa, Y. & Chilian, W. M. (1999) Circulation 100, 1555-1561. [DOI] [PubMed] [Google Scholar]
- 7.Hutcheson, I. R. & Griffith, T. M. (1991) Am. J. Physiol. 261, H257-H262. [DOI] [PubMed] [Google Scholar]
- 8.Canty, J. M., Jr., & Schwartz, J. S. (1994) Circulation 89, 375-384. [DOI] [PubMed] [Google Scholar]
- 9.Awolesi, M. A., Sessa, W. C. & Sumpio, B. E. (1995) J. Clin. Invest. 96, 1449-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman, J. I. & Spaan, J. A. (1990) Physiol. Rev. 70, 331-390. [DOI] [PubMed] [Google Scholar]
- 11.Yada, T., Hiramatsu, O., Kimura, A., Goto, M., Ogasawara, Y., Tsujioka, K., Yamamori, S., Ohno, K., Hosaka, H. & Kajiya, F. (1993) Circ. Res. 72, 939-946. [DOI] [PubMed] [Google Scholar]
- 12.Kajiya, F. & Goto, M. (1999) Jpn. J. Physiol. 49, 229-241. [DOI] [PubMed] [Google Scholar]
- 13.Pinsky, D. J., Patton, S., Mesaros, S., Brovkovych, V., Kubaszewski, E., Grunfeld, S. & Malinski, T. (1997) Circ. Res. 81, 372-379. [DOI] [PubMed] [Google Scholar]
- 14.Tune, J. D., Gorman, M. W. & Feigl, E. O. (2004) J. Appl. Physiol. 97, 404-415. [DOI] [PubMed] [Google Scholar]
- 15.Prasad, A., Zhu, J., Halcox, J. P., Waclawiw, M. A., Epstein, S. E. & Quyyumi, A. A. (2002) Circulation 106, 184-190. [DOI] [PubMed] [Google Scholar]
- 16.Mohri, M., Egashira, K., Tagawa, T., Kuga, T., Tagawa, H., Harasawa, Y., Shimokawa, H. & Takeshita, A. (1997) Hypertension 30, 50-56. [DOI] [PubMed] [Google Scholar]
- 17.Quyyumi, A. A., Mulcahy, D., Andrews, N. P., Husain, S., Panza, J. A. & Cannon, R. O., 3rd. (1997) Circulation 95, 104-110. [DOI] [PubMed] [Google Scholar]
- 18.Cai, H. & Harrison, D. G. (2000) Circ. Res. 87, 840-844. [DOI] [PubMed] [Google Scholar]
- 19.Ji, Y., Han, Y., Diao, J., Huang, Y., Chen, Q. & Ferro, A. (2003) Br. J. Pharmacol. 140, 1272-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luscher, T. F., Tanner, F. C., Tschudi, M. R. & Noll, G. (1993) Annu. Rev. Med. 44, 395-418. [DOI] [PubMed] [Google Scholar]
- 21.Perticone, F., Ceravolo, R., Pujia, A., Ventura, G., Iacopino, S., Scozzafava, A., Ferraro, A., Chello, M., Mastroroberto, P., Verdecchia, P., et al. (2001) Circulation 104, 191-196. [DOI] [PubMed] [Google Scholar]
- 22.Celermajer, D. S., Adams, M. R., Clarkson, P., Robinson, J., McCredie, R., Donald, A. & Deanfield, J. E. (1996) N. Engl. J. Med. 334, 150-154. [DOI] [PubMed] [Google Scholar]
- 23.Cardillo, C., Kilcoyne, C. M., Quyyumi, A. A., Cannon, R. O., 3rd, & Panza, J. A. (1998) Circulation 97, 851-856. [DOI] [PubMed] [Google Scholar]
- 24.Suwaidi, J. A., Hamasaki, S., Higano, S. T., Nishimura, R. A., Holmes, D. R., Jr., & Lerman, A. (2000) Circulation 101, 948-954. [DOI] [PubMed] [Google Scholar]
- 25.Schachinger, V., Britten, M. B. & Zeiher, A. M. (2000) Circulation 101, 1899-1906. [DOI] [PubMed] [Google Scholar]
- 26.Halcox, J. P., Schenke, W. H., Zalos, G., Mincemoyer, R., Prasad, A., Waclawiw, M. A., Nour, K. R. & Quyyumi, A. A. (2002) Circulation 106, 653-658. [DOI] [PubMed] [Google Scholar]
- 27.Targonski, P. V., Bonetti, P. O., Pumper, G. M., Higano, S. T., Holmes, D. R., Jr., & Lerman, A. (2003) Circulation 107, 2805-2809. [DOI] [PubMed] [Google Scholar]
- 28.Kugiyama, K., Yasue, H., Okumura, K., Ogawa, H., Fujimoto, K., Nakao, K., Yoshimura, M., Motoyama, T., Inobe, Y. & Kawano, H. (1996) Circulation 94, 266-271. [DOI] [PubMed] [Google Scholar]
- 29.Kuvin, J. T. & Karas, R. H. (2003) Circulation 107, 3243-3247. [DOI] [PubMed] [Google Scholar]
- 30.Ludmer, P. L., Selwyn, A. P., Shook, T. L., Wayne, R. R., Mudge, G. H., Alexander, R. W. & Ganz, P. (1986) N. Engl. J. Med. 315, 1046-1051. [DOI] [PubMed] [Google Scholar]
- 31.Vita, J. A., Treasure, C. B., Nabel, E. G., McLenachan, J. M., Fish, R. D., Yeung, A. C., Vekshtein, V. I., Selwyn, A. P. & Ganz, P. (1990) Circulation 81, 491-497. [DOI] [PubMed] [Google Scholar]
- 32.Bache, R. J. (1998) Circulation 98, 1257-1260. [DOI] [PubMed] [Google Scholar]
- 33.Kinlay, S., Libby, P. & Ganz, P. (2001) Curr. Opin. Lipidol. 12, 383-389. [DOI] [PubMed] [Google Scholar]
- 34.Neri Serneri, G. G., Boddi, M., Modesti, P. A., Cecioni, I., Coppo, M., Papa, M. L., Toscano, T., Marullo, A. & Chiavarelli, M. (2003) Circ. Res. 92, 1359-1366. [DOI] [PubMed] [Google Scholar]
- 35.Quyyumi, A. A., Dakak, N., Andrews, N. P., Gilligan, D. M., Panza, J. A. & Cannon, R. O., 3rd. (1995) Circulation 92, 320-326. [DOI] [PubMed] [Google Scholar]
- 36.Dinerman, J. L., Lowenstein, C. J. & Snyder, S. H. (1993) Circ. Res. 73, 217-222. [DOI] [PubMed] [Google Scholar]
- 37.Wennmalm, A., Benthin, G. & Petersson, A. S. (1992) Br. J. Pharmacol. 106, 507-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schechter, A. N. & Gladwin, M. T. (2003) N. Engl. J. Med. 348, 1483-1485. [DOI] [PubMed] [Google Scholar]
- 39.Stamler, J. S., Jia, L., Eu, J. P., McMahon, T. J., Demchenko, I. T., Bonaventura, J., Gernert, K. & Piantadosi, C. A. (1997) Science 276, 2034-2037. [DOI] [PubMed] [Google Scholar]
- 40.Mochizuki, S., Himi, N., Miyasaka, T., Nakamoto, H., Takemoto, M., Hirano, K., Tsujioka, K., Ogasawara, Y. & Kajiya, F. (2002) Physiol. Meas. 23, 261-268. [DOI] [PubMed] [Google Scholar]
- 41.Mochizuki, S., Miyasaka, T., Goto, M., Ogasawara, Y., Yada, T., Akiyama, M., Neishi, Y., Toyoda, T., Tomita, J., Koyama, Y., et al. (2003) Biochem. Biophys. Res. Commun. 306, 505-508. [DOI] [PubMed] [Google Scholar]
- 42.Kanai, A. J., Strauss, H. C., Truskey, G. A., Crews, A. L., Grunfeld, S. & Malinski, T. (1995) Circ. Res. 77, 284-293. [DOI] [PubMed] [Google Scholar]
- 43.Blatter, L. A., Taha, Z., Mesaros, S., Shacklock, P. S., Wier, W. G. & Malinski, T. (1995) Circ. Res. 76, 922-924. [DOI] [PubMed] [Google Scholar]
- 44.Vallance, P., Patton, S., Bhagat, K., MacAllister, R., Radomski, M., Moncada, S., Malinski, T. (1995) Lancet 346, 153-154. [DOI] [PubMed] [Google Scholar]
- 45.Malinski, T., Taha, Z. (1992) Nature 358, 676-678. [DOI] [PubMed] [Google Scholar]
- 46.Rassaf, T., Kleinbongard, P., Preik, M., Dejam, A., Gharini, P., Lauer, T., Erckenbrecht, J., Duschin, A., Schulz, R., Heusch, G., et al. (2002) Circ. Res. 91, 470-477. [DOI] [PubMed] [Google Scholar]
- 47.Rassaf, T., Preik, M., Kleinbongard, P., Lauer, T., Heiss, C., Strauer, B. E., Feelisch, M. & Kelm, M. (2002) J. Clin. Invest. 109, 1241-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ford, P. C., Wink, D. A. & Stanbury, D. M. (1993) FEBS Lett. 326, 1-3. [DOI] [PubMed] [Google Scholar]
- 49.Allard, M. F., Kamimura, C. T., English, D. R., Henning, S. L. & Wiggs, B. R. (1993) Circ. Res. 72, 187-193. [DOI] [PubMed] [Google Scholar]
- 50.Goddard, C. M., Allard, M. F., Hogg, J. C., Herbertson, M. J. & Walley, K. R. (1995) Am. J. Physiol. 269, H1389-H1397. [DOI] [PubMed] [Google Scholar]
- 51.Broere, A., Van Den Meiracker, A. H., Boomsma, F., Derkx, F. H., Veld, A. J. & Schalekamp, M. A. (1998) Am. J. Physiol. 275, F870-F877. [DOI] [PubMed] [Google Scholar]
- 52.Gerova, M., Mesaros, S., Kristek, F., Kittova, M. & Malinski, T. (1998) Physiol. Res. 47, 169-175. [PubMed] [Google Scholar]
- 53.Stoen, R., Lossius, K. & Karlsson, J. O. (2003) Br. J. Pharmacol. 138, 39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin, C. M., Beltran-Del-Rio, A., Albrecht, A., Lorenz, R. R. & Joyner, M. J. (1996) Am. J. Physiol. 270, H442-H446. [DOI] [PubMed] [Google Scholar]
- 55.Woo, K. S., Chook, P., Lolin, Y. I., Cheung, A. S., Chan, L. T., Sun, Y. Y., Sanderson, J. E., Metreweli, C. & Celermajer, D. S. (1997) Circulation 96, 2542-2544. [DOI] [PubMed] [Google Scholar]
- 56.Traverse, J. H., Wang, Y. L., Du, R., Nelson, D., Lindstrom, P., Archer, S. L., Gong, G. & Bache, R. J. (2000) Circulation 101, 2526-2531. [DOI] [PubMed] [Google Scholar]
- 57.Cosby, K., Partovi, K. S., Crawford, J. H., Patel, R. P., Reiter, C. D., Martyr, S., Yang, B. K., Waclawiw, M. A., Zalos, G., Xu, X. L., et al. (2003) Nat. Med. 9, 1498-1505. [DOI] [PubMed] [Google Scholar]
- 58.Kleschyov, A. L., Oelze, M., Daiber, A., Huang, Y., Mollnau, H., Schulz, E., Sydow, K., Fichtlscherer, B., Mulsch, A. & Munzel, T. (2003) Circ. Res. 93, e104-e112. [DOI] [PubMed] [Google Scholar]
- 59.Antony, I., Lerebours, G. & Nitenberg, A. (1995) Circulation 91, 1624-1628. [DOI] [PubMed] [Google Scholar]