Abstract
Human serotonin [5-hydroxytryptamine (5-HT)] transporters (hSERT, 5HTT, and SLC6A4) inactivate 5-HT after release and are prominent targets for therapeutic intervention in mood, anxiety, and obsessive-compulsive disorders. Multiple hSERT coding variants have been identified, although to date no comprehensive functional analysis of these variants has been reported. We transfected hSERT or 10 hSERT coding variants and examined total and surface protein expression, antagonist recognition, and transporter modulation by posttranslational, regulatory pathways. Two variants, Pro339Leu and Ile425Val, demonstrated significant changes in surface expression supporting alterations in 5-HT transport capacity (Vmax). Regardless of basal transport activity, all SERT variants displayed a capacity for rapid, phorbol ester-triggered down-regulation. Remarkably, five variants (Thr4Ala, Gly56Ala, Glu215Lys, Lys605Asn, and Pro612Ser) demonstrated no capacity for 5-HT uptake stimulation after acute protein kinase G (PKG)/p38 mitogen-activated protein kinase (MAPK) activation. Epstein–Barr virus (EBV)-transformed lymphocytes natively expressing the most common of these variants (Gly56Ala) exhibited a similar loss of 5-HT uptake stimulation by PKG/p38 MAPK activators. HeLa cells transfected with the Gly56Ala variant demonstrated elevated basal phosphorylation and, unlike hSERT, could not be further phosphorylated after 8-bromo cGMP (8BrcGMP) treatments. These studies reveal cellular phenotypes associated with naturally occurring human SERT coding variants and suggest that altered transporter regulation by means of PKG/p38 MAPK-linked pathways may influence risk for disorders attributed to compromised 5-HT signaling.
Keywords: transport, antidepressant, polymorphism, regulation, autism
Serotonin [5-hydroxytryptamine (5-HT)] is a neurotransmitter in the brain and periphery, and modulates a wide variety of physiological processes, including vasoconstriction, gastrointestinal motility and secretion, respiration, sleep, appetite, aggression, and mood (1, 2). Disrupted 5-HT signaling has been implicated in a similarly wide spectrum of disorders, including primary pulmonary hypertension, irritable bowel syndrome, sudden infant death syndrome (SIDS), anorexia, obsessive-compulsive disorder (OCD), autism, depression, and suicide (3–6). A major determinant of 5-HT signaling is the antidepressant-sensitive 5-HT transporter (SERT, 5HTT). Human SERT (hSERT) protein is encoded by a single locus mapping to chromosome 17q11.2 (7). Although evidence of alternative splicing of 5′ noncoding exons exists (8, 9), the same ORF is translated in brain, platelets, lymphocytes, and placenta, producing a protein of 630 aa with closest identify to norepinephrine and dopamine transporters (NET and DAT respectively). Initial hydropathy-based predictions of SERT secondary structure proposed 12 transmembrane domains (TMs) with intracellular NH2 and COOH termini (10), a model supported by biochemical and immunocytochemical studies (11, 12). SERT proteins can be rapidly regulated by multiple G protein-coupled receptors and protein kinase-linked pathways, including those triggered by activation of PKC, protein kinase G (PKG), and p38 mitogen-activated protein kinase (MAPK) (13–17). Phosphorylation and down-regulation of SERT through the PKC-linked pathway is sensitive to extracellular 5-HT (14), revealing an intrinsic capacity for temporal integration of ongoing 5-HT clearance demand with modulatory inputs.
The importance of SERT in presynaptic 5-HT homeostasis, synaptic 5-HT clearance, and psychoactive drug action has raised questions as to whether the hSERT gene exhibits functional polymorphisms that impact expression and activity in vivo (18). A common promoter variant (5HTTLPR) was found to support altered hSERT mRNA and protein expression (19) and has been associated with anxiety traits as well as multiple psychiatric syndromes, including autism, OCD, and depression (18). A variable nucleotide tandem repeat sequence (VNTR) in the intron following the first coding exon has also been described and seems to have enhancer-like properties (20). Ten nonsynonymous SNPs have been identified in hSERT (21, 22), although few have been explored for their functional impact. Recently, Kilic et al. (23) established a gain-of-function phenotype associated with the hSERT Ile425Val variant, attributing alterations to constitutive elaboration of regulation normally supported by PKG stimulation. Ozaki et al. (24) found the variant in two families, tracking the allele (as well as the 5HTTLPR “L” allele) with subjects exhibiting a complex psychiatric phenotype, including, among other things, OCD and Asperger's syndrome. The increasing awareness that rare, functional alleles can define disrupted pathways bearing other disease susceptibility genes (25, 26) encouraged us to achieve a comprehensive, functional evaluation of known hSERT coding variants. Among the changes observed is a striking pattern of regulatory disruption, wherein half of the hSERT variants, including all four that are present on cytoplasmic domains, seem specifically refractory to PKG and p38 MAPK-linked signaling pathways. We discuss our findings with respect to a possible role for compromised hSERT regulation in disorders linked to 5-HT dysfunction.
Materials and Methods
DNA Constructs. The full-length cDNA encoding hSERT in the mammalian expression vector pcDNA3.1(Invitrogen) has been described (27). Mutations in hSERT were produced by using the QuikChange mutagenesis kit (Stratagene). All mutations were confirmed by fluorescent dideoxynucleotide sequencing (Center for Molecular Neuroscience Neurogenomics Core).
Transfection and Transport Studies. HeLa cells, maintained at 37°C in a 5% CO2 humidified incubator, were grown in complete medium [DMEM, (Invitrogen)/10% FBS/2 mM l-glutamine/100 units/ml penicillin/100 μg/ml streptomycin]. Transfections (1 μgof DNA per 500,000 cells per 6-well-plate or 0.05 μg per 10,000 cells per 24-well-plate) were performed by using FuGENE 6 reagent (Roche, Indianapolis) in Opti-MEM I (Invitrogen) as suggested by the manufacturer. Transfected cells were cultured as above for 36 h before 5-HT transport and biochemical assays.
Transport, Binding, Biotinylation, and Phosphorylation Studies. Transport of [3H]5-HT {5-hydroxy[3H]tryptamine trifluoroacetate, Amersham Pharmacia Biosciences, 20 nM final concentration} was conducted in a total assay volume of 500 μl of Krebs–Ringer–Hepes (KRH) assay buffer containing 130 mM NaCl, 1.3 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1.8 g/liter glucose, and 10 mM Hepes (pH 7.4), as described (15), defining specific 5-HT uptake by using 10 μM paroxetine. For quantitative assessment of SERT total and surface density, we measured [125I]RTI-55 (3β-(4-iodophenyl)tropan-2β-carboxylic acid methyl ester) binding (5 nM) to intact cells on ice, using either paroxetine (10 μM) or 5-HT (100 μM) as displacer (15). To establish levels and biosynthetic progression of hSERT protein produced from mutant cDNAs, HeLa cells were plated in six-well dishes at 500,000 cells per well and transfected 12 h later. Twenty four hours after transfection, whole-cell detergent extracts were blotted for hSERT (mAb Technologies, Stone Mountain, GA, 1:1000) by using enhanced chemiluminescence (ECL, Amersham Pharmacia) detection. Altered density of SERT surface proteins was validated by using immunoblotting of biotinylated whole-cell extracts produced by using the lysine-directed, membrane-impermeant biotinylating reagent sulfo-NHS-SS-biotin (Pierce) (27). Phosphorylation of hSERT and Gly56Ala variants in transfected HeLa cells was examined as described (13) by using 100 μM 8-bromo cGMP (8BrcGMP) (60 min) as stimulus. Specificity of labeling was verified by using parallel cultures transfected with pcDNA3.
Genotyping and Lymphocyte Studies. Lymphocyte lines were derived from autistic pedigrees recruited by S. E. Folstein (The Johns Hopkins University, Baltimore), from the Autism Genetics Resource Exchange (AGRE) collection (www.agre.org), and from the National Institute of Mental Health Human Genetics Initiative Repository (www.nimhgenetics.org) at Rutgers University. DNA from lymphoblastoid cell lines was genotyped by means of TaqMan-based allelic discrimination, by using an Applied Biosystems Assay-by-Design and independently confirmed by PCR and direct sequence analysis. Genotyped lymphocytes were cultured in suspension in RPMI medium 1640, supplemented with 15% FBS, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified incubator at 5% CO2 and maintained in uniform growth conditions. Lymphocyte were pelleted at 200 × g for 5 min and washed with Krebs–Ringer–Hepes (KRH) assay buffer. A total of 1 × 106 cells in triplicate were prewarmed (37°C) in a shaking water bath (10 min) in 12 × 75 polypropylene tubes in KRH buffer containing 100 μM pargyline and 100 μM ascorbic acid ± modifiers. After a 5-min incubation with [3H]5-HT (20 nM) ± 10 μM paroxetine at 37°C, uptake assays were terminated by immersion on ice, and uptake in pelleted, SDS (1%)-extracted cells was quantitated by scintillation spectrometry.
Results
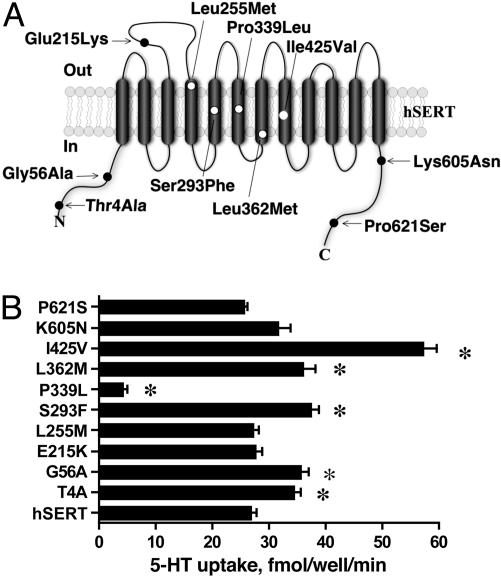
The location of 10 identified hSERT coding variants is described in Fig. 1A (see also Tables 1 and 2, which are published as supporting information on the PNAS web site). The reference hSERT cDNA and each hSERT variant were separately transfected into HeLa cells, and 5-HT transport activity was assessed as described in Materials and Methods. As shown in Fig. 1B, five variants (Thr4Ala, Gly56Ala, Ser293Phe, Leu362Met, and Ile425Val) displayed enhanced 5-HT transport activity relative to hSERT and one variant (Pro339Leu) displayed markedly reduced uptake activity. These variations persisted across multiple plasmid preparations and thus seem to arise from intrinsic differences in protein abundance, transport rates, or both. More detailed kinetic studies were pursued for the two variants displaying the largest shifts in transport activity, Ile425Val and Pro339Leu. Kinetic analysis of Ile425Val revealed significant changes in 5-HT Vmax (190 ± 28% of hSERT, P < 0.05) and Km (0.56 ± 0.20 μM vs. hSERT 1.00 ± 0.47 μM, P < 0.05) (see also Fig. 6, which is published as supporting information on the PNAS web site). With Pro339Leu, 5-HT Vmax was significantly reduced (3.0 ± 1.2% of hSERT, P < 0.05). The activity of Pro339Leu was too low to allow the 5-HT Km to be reliably determined.
Fig. 1.
Location and 5-HT transport activity of human SERT coding variants. (A) Variants are overlayed on a 12 TM model of a single SERT subunit, with NH2 and COOH termini oriented inside the cell. Variants in extramembrane domains are shaded black whereas those in membrane domains are shaded white. (B) 5-HT transport activity of SERT-coding variants in transfected HeLa cells. Data reflect mean values ± SEM of three separate experiments. Means were compared with hSERT cDNA by using a one-way ANOVA followed by Dunnett's test of individual means against hSERT values (*, P < 0.05 taken as significant).
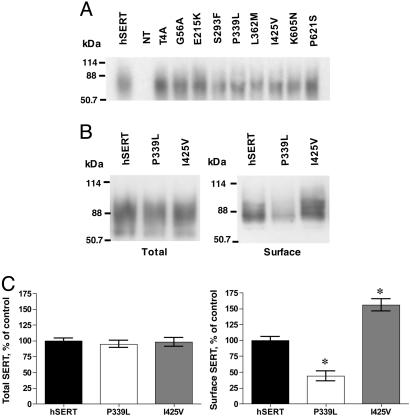
To establish a physical basis for the altered transport activities, immunoblots of transfected HeLa cell extracts were obtained. As shown in Fig. 2A, hSERT and almost all variants produced comparable levels of the 80-kDa protein that is characteristic of mature N-glycosylated hSERT protein (27). Biotinylation studies of the two variants bearing the largest changes in 5HT uptake, Pro339Val and Ile425Val, revealed a significant reduction and elevation, respectively, of surface protein, paralleling shifts in 5-HT transport activity (Fig. 2 B and C). Total [125I]RTI-55 binding, defined with the hydrophobic displacer paroxetine, did not demonstrate differences between hSERT and hSERT variants (Fig. 7A, which is published as supporting information on the PNAS web site). Surface SERT density, as defined by 5-HT displacement, was significantly depressed for Pro339Val and elevated for Ile425Val (Fig. 7B), but unchanged for the other variants. In contrast, Thr4Ala, Gly56Ala, Ser293Phe, and Leu362Met variants also display enhanced basal transport activity (30–50%) that could not be explained by enhanced surface density. We also evaluated whether variant SERTs retained normal antagonist sensitivities. Several variants demonstrated altered sensitivity to either r/s-fluoxetine, r/s-citalopram, or cocaine (Table 3, which is published as supporting information on the PNAS web site). Most prominently, we observed a 10-fold shift in cocaine potency with Pro339Leu, accompanied by a significant although less substantial loss of potency for citalopram and fluoxetine.
Fig. 2.
Analysis of protein expression of hSERT and coding variants. (A) Immunoblots of total cell extracts prepared from HeLa cells transfected with hSERT or one of the variants described in the study. (B) Cell surface expression alterations in hSERT Pro339Leu and Ile425Val. Variants were transfected in parallel with hSERT into HeLa cells, and cell surface transporters were identified by immunoblotting of biotinylated samples, captured as described in Materials and Methods.(C) Quantitative estimations of relative surface density of hSERT, Pro339Leu, and Ile425Val based on densitometry of biotinylation immunoblots. Data reflect mean values of three separate experiments ± SEM. Means were compared with a one-way ANOVA followed by Dunnett's test to compare variant surface expression to that achieved with hSERT (*, P < 0.05 taken as significant).
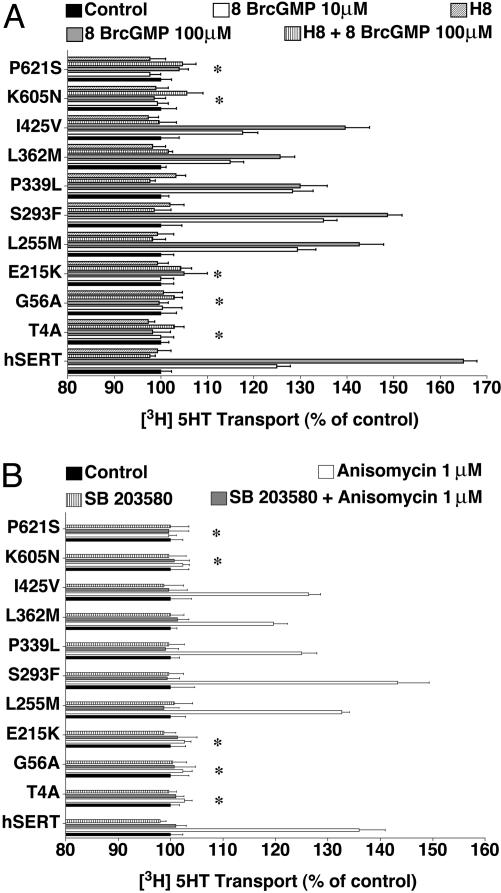
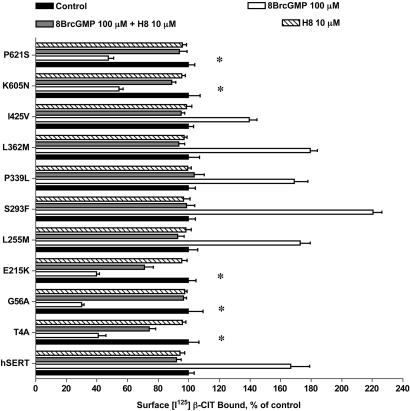
cGMP-linked pathways enhance SERT activity in native (15, 28, 29) and transfected (15, 23, 30) cells. Similarly, when we treated hSERT-expressing HeLa cells with 8BrcGMP (10–100 μM, 10 min), we achieved a dose-dependent stimulation of 5-HT transport activity that peaked at a 50–70% increase at 100 μM 8BrcGMP, and which could be completely antagonized by coincubation with the PKG antagonist H8 (10 μM) (Fig. 3A). Parallel changes in [125I]RTI-55 surface-binding support increased surface trafficking triggered by the PKG pathway (Fig. 4). For variants Leu255Met, Ser293Phe, Pro339Leu, Leu362Met, and Ile425Met, 8BrcGMP triggered a dose-dependent, H8-sensitive stimulation of SERT activity comparable with hSERT. In contrast, Thr4Ala, Gly56Ala, Glu215Lys, Lys605Asn, and Pro621Ser were completely insensitive to 8BrcGMP application. The hSERT variants that responded with uptake increases also demonstrated elevated [125I]RTI-55 surface binding. Remarkably, the five hSERT variants that failed to elicit uptake increases after 8BrcGMP treatments actually demonstrated a reduction in [125I]RTI-55 surface binding (Fig. 4). These reductions in surface density were still specific because they could be completely blocked by H8. 8BrcGMP and H8 had no effects on total binding as assessed in parallel assays using paroxetine as the displacer (data not shown).
Fig. 3.
Impact of 8BrcGMP and p38 MAPK on hSERT activity. (A) Activity modulation. HeLa cells transfected with hSERT- or hSERT-coding variants were examined for 5-HT transport activities as described in Materials and Methods after pretreatments of cells with either 100 μM 8BrcGMP ± H8 or vehicle for 1 h. (B) Altered p38 MAPK-dependent regulation of hSERT in transfected HeLa cells. Cells transfected with hSERT or hSERT-coding variants were examined after pretreatments of cells with either 1 μM anisomycin ± SB203580 or vehicle for 10 min. Results reflect mean values ± SEM of three separate experiments normalized to each mutant's control measured under vehicletreated conditions (100%). Results in A and B reflect mean values ± SEM of three separate experiments normalized to each mutant's level under vehicletreated conditions (100%). Data were analyzed by a one-way ANOVA with post hoc Bonferonni tests comparing variant to hSERT 8BrcGMP/anisomycin responses with P < 0.05 taken as significant.
Fig. 4.
Impact of 8BrcGMP on hSERT surface binding. HeLa cells transfected with hSERT- or hSERT-coding variants were treated with either 100 μM 8BrcGMP ± H8 or vehicle for 1 h. Cells were subjected to cell surface [125I]RTI-55 (5 nM) binding with 5-HT (100 μM) as displacer. Data were analyzed by a one-way ANOVA with post hoc Bonferonni tests comparing variant to hSERT anisomycin responses, with P < 0.05 taken as significant.
SERTs are known to be rapidly internalized by phorbol ester treatments, effects that are blocked by PKC antagonists (14). Thus, we treated transfected cells with the phorbol ester β-phorbol 12-myristate 13-acetate (β-PMA) and monitored changes in 5-HT transport activity. As expected, hSERT expressed transiently in HeLa cells displays an ≈40% down-regulation after a 15-min treatment with 10 μM β-PMA, down-regulation blocked by the PKC antagonist bisindolylmaleimide (BIM, 1 μM) (Fig. 8, which is published as supporting information on the PNAS web site). In contrast to findings with hSERT variants for 8BrcGMP treatments, each of the hSERT variants (Thr4Ala, Gly56Ala, Lys605Asn, and Pro621Ser) displayed down-regulation after β-PMA treatments equal to or slightly greater than that seen for hSERT.
In studies of adenosine receptor and PKG-linked up-regulation of SERT, we discovered that enhanced SERT activity requires activated p38 MAPK (15). More recent studies reveal that direct p38 MAPK activators such as anisomycin trigger a rapid, trafficking-independent up-regulation of hSERT (17). We treated hSERT-transfected cells with 1 μM anisomycin for 10 min before 5-HT transport assays and, as previously found, achieved a 40–50% stimulation of uptake activity (Fig. 3B). Just as with 8BrcGMP treatments, variants Leu255Met, Ser293Phe, Pro339Leu, Leu362Met, and Ile425Val each responded to anisomycin treatment comparable with hSERT, with increased activity blocked by cotreatments with SB203580. Neither the uptake stimulation of hSERT nor the up-regulation achieved with these five variants was accompanied by changes in total or surface [125I]RTI-55 binding (data not shown), consistent with a trafficking-independent mode of action of the p38 MAPK pathway (15, 17). Remarkably, when the five hSERT variants lacking 8BrcGMP sensitivity (Thr4Ala, Gly56Ala, Glu215Lys, Lys605Asn, and Pro621Ser) were tested with anisomycin, no uptake stimulation was observed.
Of the variants studied, only one, Gly56Ala, is found at frequencies sufficient to permit identification of subjects carrying modified alleles. We genotyped a large collection of 340 autism families possessing, in many cases, banked, Epstein–Barr virus (EBV)-transformed lymphocytes, because SERT is natively expressed in lymphocytes (19) and because the 17q11.2 region harboring the SERT gene demonstrated linkage in autism families (31). We found the 56Ala allele at a frequency of 1.1% in all families, but this frequency increased to 2.3% in 120 families most contributing to linkage. This frequency represents a significant difference (χ2 = 9.94, df = 1, P = 0.0016) between our sample and a separately collected, nonclinical sample (21). Importantly, we identified two probands bearing a homozygous Ala-56 genotype as well as multiple subjects carrying heterozygous genotypes.
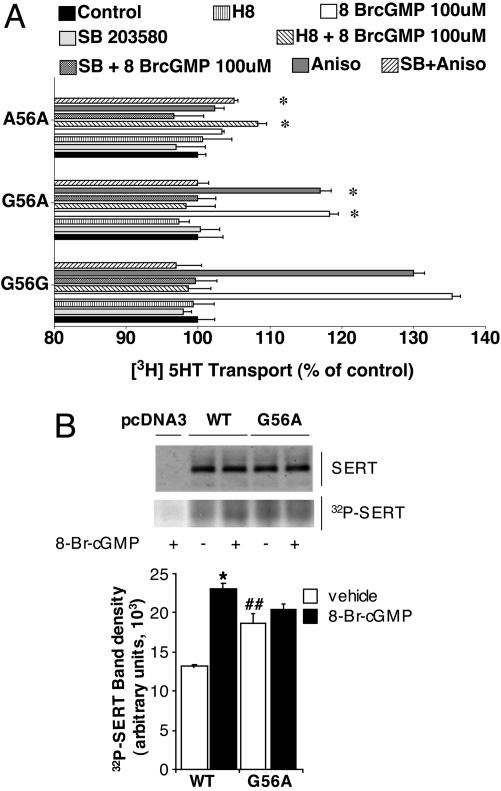
As seen with transfected cells, lymphocytes homozygous for the Gly variant (identical to reference hSERT) provided robust 8BrcGMP stimulation of 5-HT transport, stimulation that is sensitive to H8 (Fig. 5A). Additionally, anisomycin stimulated uptake activity, and this stimulation was sensitive to SB203580. In contrast, the Ala-56 homozygous lines lacked sensitivity to either 8BrcGMP or anisomycin. The Gly56Ala heterozygous cells displayed intermediate sensitivity to these agents, consistent with a gene dosage-dependent impact on regulation. As detected in transfected cells, we found that all three genotypes displayed a similar degree of down-regulation after β-PMA treatments (data not shown). SERT is phosphorylated under basal conditions, and phosphorylation can be significantly elevated after PKG activation (13). To examine whether the loss of regulation exhibited by the Gly56Ala variant might derive from changes in its ability to receive regulatory phosphorylation, we performed in situ phosphorylation studies, immunoprecipitating SERT proteins after stimulation of 32P-prelabeled cells with 8BrcGMP. In hSERT-transfected HeLa cells, 8BrcGMP (100 μM, 60 min) triggered an ≈80% elevation of basal phosphorylation (Fig. 5B). In contrast, Gly56Ala-transfected cells exhibited significantly elevated basal phosphorylation levels and could not be further phosphorylated by 8BrcGMP treatments. Western blotting of cell extracts revealed no differences in total hSERT protein levels.
Fig. 5.
Altered PKG/p38 MAPK sensitivity of 56Ala is evident in native lymphocytes and may involve altered transporter phosphorylation. (A) Lymphocytes were genotyped and cultured as described in Materials and Methods and assessed for 5-HT uptake regulation as described for transfected HeLa cells. Data presented derive from individual lymphocyte lines of determined genotype. Findings were replicated in a separate set of genotyped lines with equivalent results. Uptake levels for each genotype with vehicle-treated conditions were taken as 100%. Transport activities were analyzed by a two-way ANOVA with post hoc Bonferroni tests, with P < 0.05 taken as significant. (B) hSERT Gly56Ala variant displays altered basal phosphorylation and sensitivity to 8BrcGMP. SERTs expressed in transfected HeLa cells were examined 36 h after transfection. (Upper) Representative total extract immunoblot and autoradiogram from SERT immunoprecipitations. (Lower) Quantitation of SERT labeling from phosphorylation studies (n = 3). Values are expressed as mean ± SEM. *, P < 0.01 versus WT-vehicle; ##, P < 0.05 versus WT-vehicle by one-way ANOVA with Bonferroni post hoc analysis.
Discussion
A number of disorders including anxiety, major depression, OCD, autism, and irritable bowel syndrome have been associated with the a common, functional variant in the hSERT promoter termed the 5HTTLPR (18, 32, 33). Less attention has been given to the functional status of hSERT-coding variants. In an early report, Lesch and coworkers (22) identified a single Leu255Met allele. Glatt et al. (21) greatly expanded the list of known variants in a study of 450 nonclinical subjects, revealing nine new coding variants, but only one, Gly56Ala, was found more than once and still at a frequency of <0.5% (4/900 chromosomes; Table 1). Gly56Ala was also identified at low frequency by Cargill et al. (34), along with Lys605Asn, also found by Glatt et al. (21). None of these initial studies characterized the function of hSERT-coding variants. Possibly, they could impact hSERT function and provide clues to pathways contributing risk for 5-HT-linked clinical phenotypes. By analogy, we identified Ala457Pro in human norepinephrine (hNET) in a single family with orthostatic intolerance (OI) (35). Despite the rarity of this variant, its segregation with tachycardia and plasma catecholamines provides important evidence that idiopathic OI likely involves a hypernoradrenergic state. Each of the major SERT alleles studied is highly conserved across currently sequenced mammalian SERTs (Table 2). Conservation has been demonstrated as one predictor of functional perturbations (36). Overall, we found that 7 of the 10 variants bore functional perturbations, including altered protein expression and basal 5-HT uptake, cocaine and antidepressant recognition, or loss of regulation.
We found two variants whose changes in 5-HT uptake capacity were accompanied by parallel changes in total and/or cell surface protein expression. Pro339Leu exhibits a major loss of mature, N-glycosylated protein consistent with improper folding leading to inefficient biosynthetic progression or rerouting to degradative pathways. Relative to the amount that reaches the surface, a greater functional loss is observed, suggesting further disruption of the 5-HT translocation mechanism. Pro339Leu lies in TM6, a domain suggested to participate in transporter oligomerization (37). Whereas Pro-339 is conserved down to Caenorhabditis elegans, the residue is not conserved in norepinephrine and dopamine, consistent with a more specific role in 5-HT translocation or unique aspects of the transporter's biosynthesis not tested in our studies. Ile425Val presented the opposite phenotype, with greatly enhanced transport activity coupled to increased cell surface density. Recently, Ozaki et al. (24) identified this variant in two families with a complex psychiatric phenotype including OCD and Asperger's syndrome. Of the nine affected subjects in these two families, seven carried a single copy of the allele in the background of a homozygous 5HTTLPR L/L genotype. We were unable to reproduce the loss of regulatory sensitivity for Ile425Val reported by Kilic et al. (23), although we did observe the reported increase in basal 5-HT transport capacity. We have found, using hSERT-inducible cell lines (C.-B.Z. and R.D.B., unpublished findings), that regulation by the PKG/p38 MAPK pathways become less evident or is nondetectible with higher level expression of hSERT, and, thus, a variant such as Ile425Val, bearing constitutively elevated surface density and 5-HT uptake, may more readily saturate the regulatory machinery upon heterologous expression. Regardless, both studies agree that Ile425Val represents a hypermorphic mutation whose altered activity, presuming similar effects in vivo, may constitutively inappropriately elevate synaptic 5-HT clearance.
The most striking finding in the current article is the complete lack of sensitivity of 5 of the 10 hSERT variants to acute actrivators of PKG or p38 MAPK. This loss of sensitivity does not correlate with changes in basal 5-HT transport activity: both Pro339Leu and Ile425Val, which show hypomorphic and hypermorphic phenotypes, respectively, demonstrated stimulation by 8BrcGMP and anisomycin. Additionally, all of the variants displayed a relatively robust sensitivity to phorbol ester-triggered down-regulation. Finally, although there were changes noted for cocaine and antidepressant recognition, these changes were not highly correlated with loss of transporter stimulation. In fact, the greatest number of changes in antagonist recognition occurred within TMs 4–8 whereas four of the five PKG/p38 MAPK-insensitive variants lie in either the cytoplasmic NH2 or COOH termini. At these latter sites, it is reasonable to speculate that variants may disrupt interactions with accessory proteins that, in past years, have grown to include syntaxin 1A (38), protein phosphatase 2A catalytic subunit (PP2Ac) (39), Hic-5 (40), and MacMARCKS (41). The loss of regulation by Glu215Lys is more difficult to explain but could suggest a conformational linkage of EL2 to regions of TM3 linked to 5-HT recognition (42). EL2 movement, when limited by zinc coordination, blocks substrate transport in homologous dopamine proteins (43), and thus sequence variation in this loop may perturb regulatory conformational changes propagated from intracellular domains to the substrate binding site. In this regard, the p38 MAPK pathway triggers an increase in 5-HT affinity, as assessed in antagonist binding assays (17).
Measurement of Gly-56 and Ala-56 hSERT lymphocyte mRNA by real-time PCR revealed no differences in SERT mRNA levels (data not shown), consistent with a posttranscriptional origin for the defects in transporter regulation observed. hSERT proteins exhibit basal phosphorylation and become further phosphorylated in response to activators of PKA, PKC, and PKG (13). To consider the integrity of hSERT PKG phosphorylation, we explored the extent of phosphorylation of the Gly56Ala variant in transiently transfected HeLa cells and obtained evidence that the variant exhibits elevated basal phosphorylation and cannot be further phosphorylated in response to 8BrcGMP treatments. The Gly56Ala variant, possibly through disrupted phosphatase interactions (39), may lack normal inhibitory mechanisms restricting basal phosphorylation. Alternatively, the Gly56Ala variant may impart a gain-of-function phenotype that leads to elevated basal phosphorylation. For example, 5-HT-gated channel activity is unmasked in SERT proteins by elimination of regulatory syntaxin 1A interactions (38), and, possibly, changes in basal phosphorylation are indicative of novel states that directly or indirectly enhance basal phosphorylation of SERT. Additional studies are needed to expand this effort to p38 MAPK stimulation, to clarify which of the several kinases targeting SERT supports enhanced basal phosphorylation, and to extend phosphorylation studies to the other affected variants. Loss of regulation through the PKG pathway may leave the endocytic mechanisms supported by PKC-linked pathways (13, 27) unopposed. Four of the five PKG/p38 MAPK nonresponsive alleles (Thr4Ala, Gly56Ala, Lys605Asn, and Pro621Ser) actually demonstrated significantly enhanced phorbol ester-mediated down-regulation, further enhancing this possibility. Enhanced sensitivity to phorbol ester-mediated down-regulation may also be a clue as to why the variants that fail to show enhanced 5-HT uptake after 8BrcGMP treatments actually show decreased [125I]RTI-55 surface binding. Signals that trigger the PKG-dependent shuttling of new transporters to the surface may also enhance endocytic recycling rates (44) possibly through crosstalk with PKC-linked pathways. As such, diminished [125I]RTI-55 binding to the variants may report a stabilized, partial conformational transition on the endocytic limb. We have recently reported (45) that platelet SERT exhibits surface-resident inactive states and that it is conceivable that [125I]RTI-55 binding might report a state occupied before uptake inactivation. Although these ideas remain speculative at best, the five variants lacking uptake stimulation by PKG/p38 MAPK activators would seem to be useful in probing different steps in the complex regulatory pathways that ultimately establish 5-HT uptake capacity.
Because the changes we report are genetically encoded and because SERT expression occurs early in development, the nonresponsive alleles could compromise the ability of SERT to modulate in response to environmental demands and elevate risk for developmental disorders linked to altered 5-HT signaling (46–48). Modulation of SERT activity in neonatal animals has lasting effects on emotional behavior in adults, and genetic variation at the hSERT promoter has been reported to interact with early childhood stressors to influence risk for depression and suicide in later life (33). Possibly, carriers of regulatory nonresponsive hSERT alleles may be at greater risk for adult onset disorders arising from inappropriate hSERT activity at critical periods in development. The Gly56Ala allele, although uncommon, is still carried by ≈1:200 Caucasian subjects (21), representing more than a million Americans. Disrupted 5-HT signaling has long been discussed as a potential underlying determinant of altered development and behavior in autism (6, 49–51). Because of our prior study noting linkage of autism to the SERT locus at 17q11.2 (31) and our access to a large collection of autism family DNA samples with matching lymphocyte lines, we genotyped subjects for the Gly56Ala allele and accessed banked cell lymphocyte lines to determine the functional impact of the variant allele within native hSERT expressing cells. Although details of allelic segregation with the autism phenotype must be revealed through dedicated studies, we found the Gly56Ala allele at a frequency of 2.3% in 120 families selected on the basis of linkage to autism at 17q11.2, a >5-fold increase in allele frequency over the Gly56Ala frequency published by Glatt et al. (21) in a study of 450 nonclinical subjects. The homozygous Gly56Ala lines we identified derive from two male subjects with autism. Recent evidence for linkage of markers at 17q11.2 to autism (31, 52, 53) and male-specific autism risk in particular (54) argue that further evaluation of the phenotype of hSERT Gly56Ala carriers, as well as a directed search for additional hSERT alleles that can similarly impact transporter regulation through PKG/p38 MAPK pathways, is warranted.
Supplementary Material
Acknowledgments
We gratefully acknowledge technical assistance from Qiao Han in cell culture support, Dr. Keith Henry for guidance on transport and biotinylation studies, and Dr. Louis DeFelice for critical review of the manuscript. This work was supported by funds from National Institutes of Health (NIH) Grants DA07390 (to R.D.B.), MH62612 (to S.R.), MH61009 (to J.S.S.), and MH55135 (to Dr. Susan E. Folstein), and funds from Vanderbilt Kennedy Center Hobbs Research Awards (to R.D.B. and J.S.S.). H.C.P. and R.C.S. were supported in part by a Vanderbilt Discovery Grant. J.L.M. is supported by a predoctoral training grant from the National Alliance for Autism Research. We gratefully acknowledge assistance from the Vanderbilt Neurogenomics Core, the Center for Human Genetics Research DNA Resources, and the General Clinical Research Center (M01 RR-00095, NIH National Center for Research Resources), and the resources provided by the Autism Genetic Resource Exchange Consortium, a program of Cure Autism Now.
Author contributions: H.C.P., J.L.M., D.J.S., and S.R. performed research; H.C.P., J.L.M., D.J.S., S.R., J.S.S., and R.D.B. analyzed data; H.C.P., C.-B.Z., D.J.S., S.R., and R.D.B. designed research; J.L.M., D.J.S., S.R., and J.S.S. contributed new reagents/analytic tools; and H.C.P., C.-B.Z., J.L.M., D.J.S., S.R., R.C.S., W.A.H., J.S.S., and R.D.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: 5-HT, 5-hydroxytryptamine; SERT, serotonin transporter; hSERT, human SERT; PKG, protein kinase G; MAPK, mitogen-activated protein kinase; OCD, obsessive-compulsive disorder; TM, transmembrane domain; 8BrcGMP, 8-bromo cGMP; β-PMA, β-phorbol 12-myristate 13-acetate; RTI-55, (3β-(4-iodophenyl)tropan-2β-carboxylic acid methyl ester.
References
- 1.Jacobs, B. & Azmitia, E. C. (1992) Physiol. Rev. 72, 165-229. [DOI] [PubMed] [Google Scholar]
- 2.Fozzard, J. E. (1989) Peripheral Actions of 5-Hydroxytryptamine (Oxford Univ. Press, New York).
- 3.Insel, T. R., Zohar, J., Benkelfat, C. & Murphy, D. L. (1990) Ann. N.Y. Acad. Sci., 574-586. [DOI] [PubMed]
- 4.Meltzer, H. Y. (1990) Ann. N.Y. Acad. Sci., 486-499. [DOI] [PubMed]
- 5.Gershon, M. D. (1999) Aliment. Pharmacol. Ther. 13, Suppl. 2, 15-30. [PubMed] [Google Scholar]
- 6.Cook, E. H., Jr., & Leventhal, B. L. (1996) Curr. Opin. Pediatr. 8, 348-354. [DOI] [PubMed] [Google Scholar]
- 7.Ramamoorthy, S., Bauman, A. L., Moore, K. R., Han, H., Yang-Feng, T., Chang, A. S., Ganapathy, V. & Blakely, R. D. (1993) Proc. Natl. Acad Sci. USA 90, 2542-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley, C. C. & Blakely, R. D. (1997) J. Neurochem. 69, 1356-1367. [DOI] [PubMed] [Google Scholar]
- 9.Ozsarac, N., Santha, E. & Hoffman, B. J. (2002) J. Neurochem. 82, 336-344. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman, B. J., Mezey, E. & Brownstein, M. J. (1991) Science 254, 579-580. [DOI] [PubMed] [Google Scholar]
- 11.Chen, J. G., Liu-Chen, S. & Rudnick, G. (1998) J. Biol. Chem. 273, 12675-12681. [DOI] [PubMed] [Google Scholar]
- 12.Miner, L. H., Schroeter, S., Blakely, R. D. & Sesack, S. R. (2000) J. Comp. Neurol. 427, 220-234. [DOI] [PubMed] [Google Scholar]
- 13.Ramamoorthy, S., Giovanetti, E., Qian, Y. & Blakely, R. D. (1998) J. Biol. Chem. 273, 2458-2466. [DOI] [PubMed] [Google Scholar]
- 14.Ramamoorthy, S. & Blakely, R. D. (1999) Science 285, 763-766. [DOI] [PubMed] [Google Scholar]
- 15.Zhu, C. B., Hewlett, W. A., Feoktistov, I., Biaggioni, I. & Blakely, R. D. (2004) Mol. Pharmacol. 65, 1462-1474. [DOI] [PubMed] [Google Scholar]
- 16.Samuvel, D. J., Jayanthi, L. D., Bhat, N. R. & Ramamoorthy, S. (2005) J. Neurosci. 5, 29-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu, C.-B., Carneiro, A. M., Dostmann, W. R., Hewlett, W. A. & Blakely, R. D. (2005) J. Biol. Chem. 280, 15649-15658. [DOI] [PubMed] [Google Scholar]
- 18.Murphy, D. L., Lerner, A., Rudnick, G. & Lesch, K. P. (2004) Mol. Interv. 4, 109-123. [DOI] [PubMed] [Google Scholar]
- 19.Lesch, K.-P., Bengel, D., Heils, A., Sabol, S. Z., Greenberg, B. D., Petri, S., Benjamin, J., Müller, C. R., Hamer, D. H. & Murphy, D. L. (1996) Science 274, 1527-1531. [DOI] [PubMed] [Google Scholar]
- 20.MacKenzie, A. & Quinn, J. (1999) Proc. Natl. Acad. Sci. USA 96, 15251-15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glatt, C. E., DeYoung, J. A., Delgado, S., Service, S. K., Giacomini, K. M., Edwards, R. H., Risch, N. & Freimer, N. B. (2001) Nat. Genet. 27, 435-438. [DOI] [PubMed] [Google Scholar]
- 22.Di Bella, D., Catalano, M., Balling, U., Smeraldi, E. & Lesch, K. P. (1996) Am. J. Med. Genet. 67, 541-545. [DOI] [PubMed] [Google Scholar]
- 23.Kilic, F., Murphy, D. L. & Rudnick, G. (2003) Mol. Pharmacol. 64, 440-446. [DOI] [PubMed] [Google Scholar]
- 24.Ozaki, N., Goldman, D., Kaye, W. H., Plotnicov, K., Greenberg, B. D., Lappalainen, J., Rudnick, G. & Murphy, D. L. (2003) Mol. Psychiatry 8, 895, 933-936. [DOI] [PubMed] [Google Scholar]
- 25.Pritchard, J. K. (2001) Am. J. Hum. Genet. 69, 124-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen, J. C., Kiss, R. S., Pertsemlidis, A., Marcel, Y. L., McPherson, R. & Hobbs, H. H. (2004) Science 305, 869-872. [DOI] [PubMed] [Google Scholar]
- 27.Qian, Y., Galli, A., Ramamoorthy, S., Risso, S., DeFelice, L. J. & Blakely, R. D. (1997) J. Neurosci. 17, 45-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, K. J. & Hoffman, B. J. (1994) J. Biol. Chem. 269, 27351-27356. [PubMed] [Google Scholar]
- 29.Launay, J., Bondoux, D., Oset-Gasque, M., Emami, S., Mutel, V., Haimart, M. & Gespach, C. (1994) Am. J. Physiol. 266, 526-536. [DOI] [PubMed] [Google Scholar]
- 30.Zhu, C. B., Hewlett, W. A., Francis, S. H., Corbin, J. D. & Blakely, R. D. (2004) Eur. J. Pharmacol. 504, 1-6. [DOI] [PubMed] [Google Scholar]
- 31.McCauley, J. L., Olson, L. M., Dowd, M., Amin, T., Steele, A., Blakely, R. D., Folstein, S. E., Haines, J. L. & Sutcliffe, J. S. (2004) Am. J. Med. Genet. B Neuropsychiatr. Genet. 127, 104-112. [DOI] [PubMed] [Google Scholar]
- 32.Hahn, M. K. & Blakely, R. D. (2002) Pharmacogenomics J. 2, 217-235. [DOI] [PubMed] [Google Scholar]
- 33.Caspi, A., Sugden, K., Moffitt, T. E., Taylor, A., Craig, I. W., Harrington, H., McClay, J., Mill, J., Martin, J., Braithwaite, A. & Poulton, R. (2003) Science 301, 386-389. [DOI] [PubMed] [Google Scholar]
- 34.Cargill, M., Altshuler, D., Ireland, J., Sklar, P., Ardlie, K., Patil, N., Lane, C. R., Lim, E. P., Kalayanaraman, N., Nemesh, J., et al. (1999) Nat. Genet. 22, 231-238. [DOI] [PubMed] [Google Scholar]
- 35.Shannon, J. R., Flattem, N. L., Jordan, J., Jacob, G., Black, B. K., Biaggioni, I., Blakely, R. D. & Robertson, D. (2000) N. Engl. J. Med. 342, 541-549. [DOI] [PubMed] [Google Scholar]
- 36.Shu, Y., Leabman, M. K., Feng, B., Mangravite, L. M., Huang, C. C., Stryke, D., Kawamoto, M., Johns, S. J., DeYoung, J., Carlson, E., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 5902-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hastrup, H., Karlin, A. & Javitch, J. A. (2001) Proc. Natl. Acad. Sci. USA 98, 10055-10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haase, J., Killian, A. M., Magnani, F. & Williams, C. (2001) Biochem. Soc. Trans. 29, 722-728. [DOI] [PubMed] [Google Scholar]
- 39.Bauman, A. L., Apparsundaram, S., Ramamoorthy, S., Wadzinski, B. E., Vaughan, R. A. & Blakely, R. D. (2000) J. Neurosci. 20, 7571-7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carneiro, A., Ingram, S. L., Beaulieu, J.-M., Sweeney, A., Amara, S. G., Thomas, S. M., Caron, M. G. & Torres, G. E. (2002) J. Neurosci. 22, 7045-7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jess, U., El Far, O., Kirsch, J. & Betz, H. (2002) Biochem. Biophys. Res. Commun. 294, 272-279. [DOI] [PubMed] [Google Scholar]
- 42.Chen, J. G., Sachpatzidis, A. & Rudnick, G. (1997) J. Biol. Chem. 272, 28321-28327. [DOI] [PubMed] [Google Scholar]
- 43.Norregaard, L., Frederiksen, D., Nielsen, E. O. & Gether, U. (1998) EMBO J. 17, 4266-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melikian, H. E. (2004) Pharmacol. Ther. 104, 17-27. [DOI] [PubMed] [Google Scholar]
- 45.Jayanthi, L. D., Samuvel, D. J., Blakely, R. D. & Ramamoorthy, S. (2005) Mol. Pharmacol. 67, 2077-2087. [DOI] [PubMed] [Google Scholar]
- 46.Lebrand, C., Cases, O., Wehrle, R., Blakely, R. D., Edwards, R. H. & Gaspar, P. (1998) J. Comp. Neurol. 401, 506-524. [PubMed] [Google Scholar]
- 47.Persico, A. M., Mengual, E., Moessner, R., Hall, F. S., Revay, R. S., Sora, I., Arellano, J., DeFelipe, J., Gimenez-Amaya, J. M., Conciatori, M., et al. (2001) J. Neurosci. 21, 6862-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ansorge, M. S., Zhou, M., Lira, A., Hen, R. & Gingrich, J. A. (2004) Science 306, 879-881. [DOI] [PubMed] [Google Scholar]
- 49.Ciaranello, R. D. (1982) N. Engl. J. Med. 307, 181-183. [DOI] [PubMed] [Google Scholar]
- 50.Piven, J., Tsai, G. C., Nehme, E., Coyle, J. T., Chase, G. A. & Folstein, S. E. (1991) J. Autism Dev. Disord. 21, 51-59. [DOI] [PubMed] [Google Scholar]
- 51.Chugani, D. C., Muzik, O., Behen, M., Rothermel, R., Janisse, J. J., Lee, J. & Chugani, H. T. (1999) Ann. Neurol. 45, 287-295. [DOI] [PubMed] [Google Scholar]
- 52.Yonan, A. L., Alarcon, M., Cheng, R., Magnusson, P. K., Spence, S. J., Palmer, A. A., Grunn, A., Hank Juo, S. H., Terwilliger, J. D., Liu, J., et al. (2003) Am. J. Hum. Genet. 73, 886-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.International Molecular Genetic Study of Autism Consortium (2001) Am. J. Hum. Genet. 69, 570-581.11481586 [Google Scholar]
- 54.Stone, J. L., Merriman, B., Cantor, R. M., Yonan, A. L., Gilliam, T. C., Geschwind, D. H. & Nelson, S. F. (2004) Am. J. Hum. Genet. 75, 1117-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.