Abstract
The technique of subcomponent self-assembly has been applied to the preparation of a set of copper(I) complexes from diamines and aldehydes in aqueous solution. These complexes may be synthesized alongside one another in solution despite the chemical nonorthogonality of their respective starting materials; thermodynamic equilibration eliminates all mixed products. The reactivity of these complexes has been studied, revealing that in certain cases, the substitution of both ligands and ligand subcomponents could be independently carried out. In one particular case, a complex was shown to be inert to ligand substitution but readily underwent ligand subcomponent substitution, creating the possibility of a previously undocumented kind of cascade reaction: Once ligand subcomponent substitution had occurred, ligand exchange could then happen, allowing both reactions to be triggered by a single chemical event.
Keywords: self-assembly, coordination chemistry, cascade reactions
The generation of complex metal-containing architectures from simple building blocks through self-assembly (1–8) is a compelling means by which to build up functional molecular machines (9–11). Such molecular devices are beginning to give substance to the promises of nanotechnology; recent examples include Mirkin and coworkers' (12) signal-amplifying allosteric catalyst, Nolte and colleagues' (13) “chain-walking” epoxidation catalyst, Leigh and coworkers' (14) reversible molecular motor, and the molecular elevator of Stoddart and colleagues (15).
A powerful technique in metallo-organic self-assembly consists of the simultaneous formation of covalent (carbon–heteroatom) and dative (heteroatom–metal) bonds, bringing both ligand and complex into being at the same time. This “subcomponent self-assembly” has its roots in the template synthesis of Busch and coworkers (16) and has recently been used in the synthesis of a wealth of structures, including rotaxanes (17), catenanes (18), helicates (19–22), grids (23–25), and a Borromean link (26). These structures belong to the domains of both dynamic covalent (27) and supramolecular (28) chemistry and offer a particularly rich set of possibilities for dynamic rearrangement at both covalent and dative linkages (29). (In ref. 29, Lehn refers to systems that are capable of rearrangement as “constitutionally dynamic.”)
Because subcomponent self-assembly reactions operate on two distinct levels simultaneously, one might expect that these reactions would give rise to mixtures of diverse products or large dynamic combinatorial libraries (30–35), as the multiple components combine in different ways. Although many structures might in theory be possible at both covalent and supramolecular levels, the metal and the subcomponents of the ligand may be chosen such that the thermodynamic preferences of both converge to give rise to a single product or a limited number of products (22–24, 36–38). It is important to note that it is the reversible formation of intraligand imine bonds (in addition to the metal–ligand bonds) that allows for this dynamic sorting/selection effect to occur.
Along similar lines, although the addition of further ligand components might be expected to increase the number of species present in the product mixture, it is nonetheless possible to choose the steric and electronic properties of the added ligand component such that clean substitution occurs (39).
In the present study, we detail how specific products may be obtained from aqueous mixtures of copper(I), certain diamines, and certain aldehydes under conditions of thermodynamic equilibration. It was demonstrated that selective exchange of both ligands and ligand subcomponents occurs, permitting the dynamic reassembly of these complexes on both supramolecular and molecular levels. A previously undocumented kind of cascade reaction (40, 41) was also introduced, whereby the addition of one ligand-component molecule cleanly induces two distinct rearrangements to occur.
Materials and Methods
All manipulations were carried out in degassed solvents, using reagents of the highest commercially available purity. Complexes 2a (42), 3 (43), and Cu(NCMe)4BF4 (44) were synthesized according to the literature. Complexes 1, 4, 5, and 6 decompose slowly in aqueous solution at room temperature (t1/2 ≈ 1 week at 25°C, giving 2-formylbenzenesulfonate and unidentified products) but are indefinitely stable in the solid state. Solutions of these complexes decompose within minutes in the presence of dioxygen.
Preparative Synthesis of 1a. To a 100-ml Schlenk flask containing methanol (12 ml) and a stir bar were added ethylenediamine (0.125 g, 2.08 mmol), sodium 2-formylbenzenesulfonate (0.866 g, 4.16 mmol), and tetrakis(acetonitrile)copper(I) tetrafluoroborate (0.327 g, 1.04 mmol). All starting materials dissolved to give a red solution. The flask was sealed, and the atmosphere was purified of dioxygen by three evacuation/argon fill cycles. The reaction was allowed to stir for 2 h at room temperature. The solution was then cannula-filtered into a Schlenk flask containing diethyl ether (50 ml), and the yellow solid that precipitated was allowed to settle for 30 min. The supernatant was cannula-filtered off, and the product was dried under dynamic vacuum, giving an isolated yield of 95% (1.02 g, 0.989 mmol). 1H NMR (400 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 1.24 ppm as an internal standard): δ = 9.10 (s, 4 H, imine), 7.82 (br m, 4 H), 7.61 (br m, 4 H), 7.56 (br m, 8 H), 3.80 (s, 8 H); 13C NMR (100.62 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 30.29 ppm as an internal standard): δ = 163.4, 142.1, 133.1, 132.0, 131.9, 130.0, 127.1, 62.2; electrospray ionization MS (ESI-MS): m/z = –897.1 [1a + 2Na]–, –437.2 [1a + Na]2–, –197.2 [free ligand of 1a]2–; elemental analysis (percentage) calculated for (C32H28CuN4Na3O12S4·1.5H2O): C 40.53, H 3.29, N 5.91; found: C 40.79, H 3.65, N 6.07.
Synthesis of 1b. Into an NMR tube with a Teflon screw-cap was added 2,2′-(ethylenedioxy)bis(ethylamine) (0.0035 g, 0.024 mmol), sodium 2-formylbenzenesulfonate (0.0099 g, 0.048 mmol), tetrakis(acetonitrile)copper(I) tetraf luoroborate (0.0037 g, 0.012 mmol), and deuterium oxide (0.5 ml). All materials dissolved to give a yellow solution. The tube's atmosphere was purged of dioxygen with three evacuation/argon purge cycles. Only signals corresponding to the product were observed in the NMR spectra. 1H NMR (400 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 1.24 ppm as an internal standard): δ = 9.08 (s, 4 H, imine), 7.78 (d, J = 7.58 Hz, 4 H, 6-phenyl), 7.72 (d, J = 7.58 Hz, 4 H, 3-phenyl), 7.47 (t, J = 7.45 Hz, 4 H, 5-phenyl), 7.39 (t, J = 7.45 Hz, 4 H, 4-phenyl), 3.90 (br m, 16 H), 3.80 (br m, 8 H); 13C NMR (100.62 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 30.29 ppm as an internal standard): δ = 167.0, 142.3, 135.1, 132.3, 132.2, 128.0, 127.4, 70.6, 70.0, 60.9; ESI-MS: m/z = –1073.3 [1b + 2Na]–, –241.4 [free ligand of 1b]2–.
Synthesis of 1c. Into an NMR tube with a Teflon screw-cap was added 4,7,10-trioxa-1,13-tridecanediamine (0.0078 g, 0.035 mmol), sodium 2-formylbenzenesulfonate (0.0147 g, 0.0706 mmol), tetrakis(acetonitrile)copper(I) tetraf luoroborate (0.0055 g, 0.017 mmol), and deuterium oxide (0.5 ml). All materials dissolved to give a yellow solution. The tube's atmosphere was purged of dioxygen with three evacuation/argon purge cycles. Only signals corresponding to the product were observed in the NMR spectra. 1H NMR (400 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 1.24 ppm as an internal standard): δ = 9.07 (s, 4 H, imine), 7.90 (d, J = 8.09 Hz, 4 H, 6-phenyl), 7.79 (d, J = 8.09 Hz, 4 H, 3-phenyl), 7.58 (m, 8 H, 4,5-phenyl), 3.72 (br m, 8 H), 3.62 (br m, 16 H), 3.55 (br m, 8 H), 1.95 (br m, 8 H); 13C NMR (100.62 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 30.29 ppm as an internal standard): δ = 163.6, 142.4, 133.1, 132.4, 131.6, 128.7, 127.5, 70.2, 69.9, 69.1, 57.9, 30.2; ESI-MS: m/z = –1217.4 [1c + 2Na]–, –277.4 [free ligand of 1c]2–.
Synthesis of 2b. Into an NMR tube with a Teflon screw-cap was added 2,2′-(ethylenedioxy)bis(ethylamine) (0.0028 g, 0.019 mmol), pyridine-2-carboxaldehyde (0.0041 g, 0.038 mmol), tetrakis(acetonitrile)copper(I) tetrafluoroborate (0.0059 g, 0.019 mmol), and deuterium oxide (0.5 ml). All materials dissolved to give a dark red solution. The tube's atmosphere was purged of dioxygen with three evacuation/argon purge cycles. Only signals corresponding to the product were observed in the NMR and ESI-MS spectra. 1H NMR (400 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 1.24 ppm as an internal standard): δ = 8.69 (s, 2 H, imine), 8.50 (d, J = 4.55 Hz, 2 H, 6-pyridyl), 8.08 (t, J = 7.6 Hz, 2 H, 4-pyridyl), 7.88 (d, J = 7.6 Hz, 2 H, 3-pyridyl), 7.65 (m, 2 H, 5-pyridyl), 3.92 (br s, 4 H), 3.80 (br s, 4 H), 3.56 (br s, 4 H); 13C NMR (100.62 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 30.29 ppm as an internal standard): δ = 163.1, 151.2, 149.7, 138.9, 128.8, 127.5, 72.0, 70.9, 59.4; ESI-MS: m/z = 390.1 [2b+].
Synthesis of 2c. Into an NMR tube with a Teflon screw-cap was added 4,7,10-trioxa-1,13-tridecanediamine (0.0038 g, 0.017 mmol), pyridine-2-carboxaldehyde (0.0036 g, 0.034 mmol), tetrakis(acetonitrile)copper(I) tetrafluoroborate (0.0053 g, 0.017 mmol), and deuterium oxide (0.5 ml). All materials dissolved to give a dark red solution. The tube's atmosphere was purged of dioxygen with three evacuation/argon purge cycles. Only signals corresponding to the product were observed in the NMR and ESI-MS spectra. 1H NMR (400 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 1.24 ppm as an internal standard): δ = 8.62 (br s, 2 H, imine), 8.46 (br m, 2 H, 6-pyridyl), 8.03 (br m, 2 H, 4-pyridyl), 7.86 (br m, 2 H, 3-pyridyl), 7.62 (br m, 2 H, 5-pyridyl), 3.49–3.86 (br m, 16 H), 1.89 (br m, 4 H); ESI-MS: m/z = 461.1 [2c+].
Synthesis of 4. Into an NMR tube with a Teflon screw-cap was added 1a (0.0044 g, 0.0043 mmol), 3 (0.0043 g, 0.0043 mmol), and deuterium oxide (0.5 ml). All materials dissolved to give a dark orange-purple solution. The tube's atmosphere was purged of dioxygen with three evacuation/argon purge cycles. Only signals corresponding to the product were observed in the NMR spectra. 1H NMR (500 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 1.24 ppm as an internal standard): δ = 9.25 (s, 2 H, imine), 8.47 (s, 2 H, 3-quinoline), 8.05 (d, J = 8.19 Hz, 2 H, 5-quinoline), 7.98 (d, J = 8.19 Hz, 2 H, 8-quinoline), 7.70 (t, J = 7.25 Hz, 2 H, 7-quinoline), 7.62 (t, J = 7.33 Hz, 2 H, 6-quinoline), 7.25 (d, J = 7.57 Hz, 2 H, 3-phenyl), 7.13 (d, J = 7.72 Hz, 2 H, 6-phenyl), 6.81 (t, J = 7.33 Hz, 2 H, 5-phenyl), 6.34 (t, J = 7.33 Hz, 2 H, 4-phenyl), 4.44 (s, 4 H, N-CH2-CH2-N); 13C NMR (125.77 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 30.29 ppm as an internal standard): δ = 175.1, 163.3, 152.2, 147.9, 145.5, 141.6, 132.0, 131.9, 130.9, 129.5, 129.4, 129.3, 128.3, 126.1, 125.8, 125.7, 117.3, 62.6; ESI-MS: m/z = –266.7 [4]3–.
Synthesis of 5. Into an NMR tube with a Teflon screw-cap was added 1a (0.0296 g, 0.0287 mmol), 1,2-phenylenediamine dihydrochloride (0.0104 g, 0.0574 mmol), and deuterium oxide (0.5 ml). The tube's atmosphere was purged of dioxygen with three evacuation/argon purge cycles. Only signals corresponding to the product were observed in the NMR spectra. Complex 5 may also be prepared directly by mixing 1,2-phenylenediamine, sodium 2-formylbenzenesulfonate, and tetrakis(acetonitrile)copper(I) tetrafluoroborate, according to the procedure described for the synthesis of complex 1a. Proton spectra of the complex prepared by both methods were identical. 1H NMR (400 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 1.24 ppm as an internal standard): δ = 9.37 (s, 4 H, imine), 7.69 (d, J = 6.32 Hz, 4 H, 6-phenyl), 7.58 (d, J = 5.55 Hz, 4 H, 3-phenyl), 7.55 (br m, 4 H, 4-phenylene), 7.31 (br m, 4 H, 4-phenyl), 7.24 (br m, 4H, 3-phenylene), 6.71 (br m, 4 H, 5-phenyl); 13C NMR (100.62 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 30.29 ppm as an internal standard): δ = 160.9, 144.3, 143.1, 133.0, 131.5, 131.4, 130.4, 127.8, 127.2, 120.3; ESI-MS: m/z =–993.2 [5 + 2Na]–, –485.1 [5 + Na]2–, –221.4 [free ligand of 5]2–.
Synthesis of 6. A solution of 5 in deuterium oxide (0.25 ml, 2.4 × 10–2 M) was added to a solution of 3 in deuterium oxide (0.4 ml, 1.5 × 10–2 M) in an NMR tube with a Teflon screw-cap. The tube's atmosphere was purged of dioxygen with three evacuation/argon purge cycles. Only signals corresponding to the product were observed in the NMR spectra. Complex 6 may also be obtained through the addition of 2 eq of o-phenylenediamine dihydrochloride to a mixture of 1 eq of 1b and 1 eq. of 3 in deuterium oxide. Proton spectra of the complex prepared by both methods were identical. 1H NMR (500 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 1.24 ppm as an internal standard): δ = 9.54 (s, 2 H, imine), 8.47 (s, 2 H, 3-quinoline), 8.10 (d, J = 8.35 Hz, 2 H, 5-quinoline), 7.79 (d, J = 8.51 Hz, 2 H, 8-quinoline), 7.65 (br s, 4 H, 3,4-phenylene), 7.58 (t, J = 7.49 Hz, 2 H, 6-quinoline), 7.50 (t, J = 7.64 Hz, 2 H, 7-quinoline), 7.39 (d, J = 7.57 Hz, 2 H, 3-phenyl), 7.21 (d, J = 7.88 Hz, 2 H, 6-phenyl), 6.86 (t, J = 7.56 Hz, 2 H, 5-phenyl), 6.35 (t, J = 7.49 Hz, 2 H, 4-phenyl); 13C NMR (125.77 MHz, 300 K, D2O, referenced to 2-methyl-2-propanol at 30.29 ppm as an internal standard): δ = 174.9, 160.8, 151.9, 148.5, 145.7, 144.4, 142.6, 132.7, 132.1, 131.7, 130.5, 130.0, 129.6, 129.0, 128.1, 126.2, 125.8, 119.5, 116.9; ESI-MS: m/z = –282.7 [6]3–.
Results and Discussion
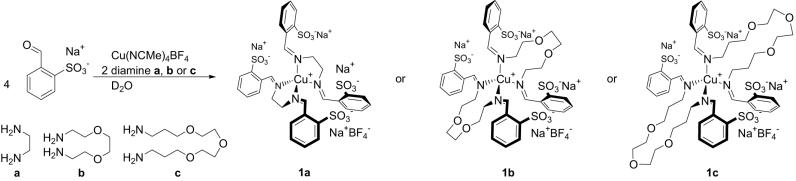
The aqueous reaction of 4 eq of sodium benzaldehyde-2-sulfonate with 1 eq of copper(I) tetrakis(acetonitrile) tetrafluoroborate and 2 eq of diamine a, b, or c gave product 1a, 1b, or 1c, respectively (Scheme 1). The spectroscopic data of these products were consistent with their formulation as the pseudotetrahedral copper(I) structures shown in Scheme 1; only one product was observed in each case. Compounds similar to 1a have been structurally characterized (45–47). The simultaneous template synthesis (16) of two macrocycles that are 11 (1b) or 16 (1c) atoms in circumference is noteworthy; such macrocycles can be difficult to generate in synthetically useful yields (48).
Scheme 1.
Syntheses of CuI complexes 1a, 1b, and 1c.
Complexes 1a, 1b, and 1c represent a potentially useful motif in ligand-component self-assembly around CuI templates. Such moieties could in principle be used in conjunction with others (22, 38) to build up larger structures. To employ these complexes as subcomponents of larger self-assembled structures, it is necessary to know whether their self-assembly occurs robustly in the presence of “differently programmed” aldehydes and amines or whether “crosstalk”† gives mixtures of compounds.
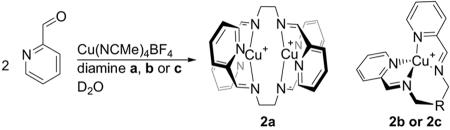
To investigate the question of crosstalk, we undertook the syntheses of a series of copper(I) complexes incorporating diamines a, b, and c and 2-pyridinecarboxaldehyde (Scheme 2). In the case of diamine a, the product was the known dinuclear double helicate 2a (42, 49), identical by NMR to the previously prepared material. Diamines b and c gave mononuclear products 2b and 2c that were analogous to those that had been synthesized from monoamines (38).
Scheme 2.
Syntheses of 2a, 2b, and 2c.
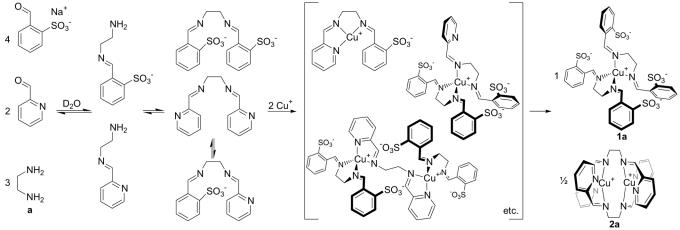
The mixture of diamine a with both pyridinecarboxaldehyde and benzaldehydesulfonate in water gave a mixture of products, the NMR spectra of which were attributed to bis(pyridyl)imine, bis(benzaldehydesulfonate)imine, and the mixed (pyridyl)(benzaldehydesulfonate)diimine, in addition to monoimines and unreacted amine and aldehyde starting materials (Scheme 3). The addition of Cu(MeCN)4BF4 to this library of compounds resulted in the exclusive formation of complexes 1a and 2a, eliminating the mixed ligand and the free-ligand components from the equilibrium.
Scheme 3.
The simultaneous formation of 1a and 2a from a dynamic library of ligands and ligand components.
The mixed ligand, bearing three nitrogen donor atoms, appeared thus to be a poor fit for the coordination preferences of the CuI ion. Copper(I) complexes of this ligand would have to either be coordinatively unsaturated (50) or assemble into an entropically disfavored Cu3L4 structure that saturates all ligand and metal-binding sites by means of a vernier-type mechanism (51). The formation of complexes incorporating both ligands would likewise involve either coordinative unsaturation or the formation of entropically disfavored structures. Such complexes would thus be thermodynamically unstable with respect to a mixture of 1a and 2a and would be transformed into these products under equilibrium conditions, as shown in Scheme 3.
When diamine b was added to an aqueous mixture of the two aldehydes, the formation of a mixture of homoligands and heteroligands was likewise observed by NMR. The addition of CuI similarly collapsed the dynamic library of compounds present, eliminating all but 1b and 2b from solution. When CuI was added to the mixture of the two aldehydes and amine c, a broad spectrum was obtained, within which it was not possible to clearly distinguish the spectra of 1c and 2c; multiple products may have been obtained.
The addition of copper(I) to a mixture of the two aldehydes and amines a and b did not induce any additional selectivity. The four distinct products 1a, 1b, 2a, and 2b were noted in the NMR spectrum, with neither amine pairing preferentially with either of the aldehydes.
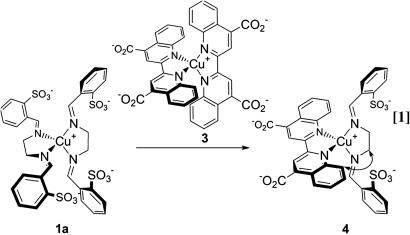
Following the work of Schmittel and colleagues (52, 53), we reasoned that the bulky ligands of 1a might favor exchange with those of a less hindered copper(I) complex to give a mixed-ligand species as the thermodynamic product. Indeed, this substitution occurred; the aqueous reaction of the two homoleptic complexes 1a and 3 (43) resulted in ligand exchange, giving heteroleptic complex 4 (Eq. 1). NMR and ESI-MS results were consistent with a quantitative reaction, and a nuclear Overhauser effect (54), indicating spatial proximity, was observed between the 8-quinoline and ethylene protons, as noted by the double-headed arrow in Eq. 1. Contrary to expectation, complexes 1b and 1c provided no evidence of reacting with 3 by NMR or ESI-MS. In the absence of steric programming, copper(I) bis-imine complexes generally give statistical mixtures of homoleptic and heteroleptic complexes (52); the rationale behind this lack of reactivity is currently unknown.
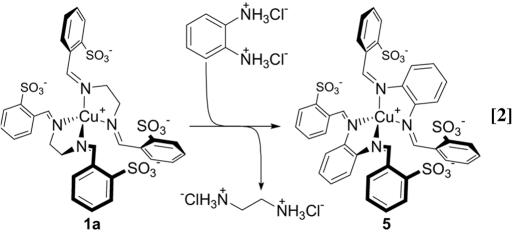
In addition to selective ligand exchange, complex 1a underwent quantitative ligand-component exchange (38). When treated with o-phenylenediamine dihydrochloride in aqueous solution, NMR spectra indicated that 1a reacted to give 5 (Eq. 2); similar copper(I) bis(phenylenediimine) complexes have been structurally characterized (47). The driving force for this substitution may be understood in terms of the difference in pKas between o-phenylenediamine (pKa1 = 1.86 and pKa2 = 4.65) and ethylenediamine (pKa1 = 6.85 and pKa2 = 9.93) (55), which favors the displacement of the protonated form of the weaker acid and the incorporation of the deprotonated form of the stronger acid (38).
In contrast to their lack of reactivity toward ligand exchange, complexes 1b and 1c readily underwent ligand-component exchange. Complex 5 was likewise identified as the product in the reaction of o-phenylenediamine dihydrochloride with either 1b or 1c.
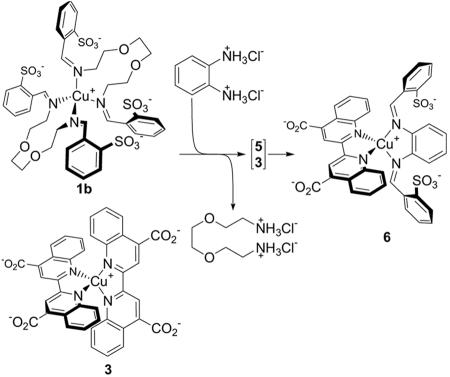
Complex 5 also underwent selective ligand exchange with 3, which created the possibility of a previously undocumented kind of cascade reaction. When a mixture of 1b and 3, which do not react together, is treated with o-phenylenediamine dihydrochloride, two reactions occur. The first is the ligand-component substitution of the aromatic diamine for the aliphatic diamine b, yielding 5. The second is ligand exchange between 3 and 5, giving 6 as the uniquely observed product (Scheme 4).
Scheme 4.
The cascade reaction of 1b, 3, and o-phenylenediamine dihydrochloride, yielding 6.
Cascade (or domino) reactions are common in natural (enzyme-catalyzed) systems (40) and are of increasing importance in organic synthesis (41). To the best of our knowledge, the reaction of Scheme 4 represents the first example of a cascade reaction in which (molecular) chemistry that was performed on a ligand triggered (supramolecular) ligand exchange.
Conclusion
In summary, investigations of the self-assembly reactions leading to the series of CuI complexes 1 and 2 demonstrated that these complexes may self-assemble quantitatively in each other's presence, despite the observation of mixed ligand in the absence of copper. Both ligand and ligand-component substitution occur cleanly in the case of complex 1a, allowing one to effect the reassembly of this species on either of two orthogonal levels. In contrast, complex 1b was susceptible to ligand-component substitution but not ligand substitution. Once ligand-component substitution had occurred, however, ligand exchange became possible, which opened the door to a previously undocumented kind of cascade rearrangement operating at both covalent and supramolecular levels. This cascade reaction could allow larger assemblies that incorporate moieties similar to 1 and 2 to selectively rearrange in different ways upon the application of different chemical signals.
The concepts behind the syntheses of complexes 4 and 6 are also of interest from a materials perspective. The use of an aromatic dialdehyde in place of the benzaldehydesulfonate ligand component could lead to the generation of a conjugated, water-soluble, metal-containing polymer that is capable of dynamic rearrangement (self-healing) (56). The degree of crosslinking could be controlled by varying the proportion of 3 added during the preparative self-assembly process or at some later moment.
Figure 4.
Figure 5.
Acknowledgments
We thank P. Perrottet for mass spectrometric analyses and D. Jeannerat for help with NMR spectra and gratefully acknowledge the support of the Swiss National Science Foundation.
Author contributions: J.R.N. designed research; D.S. performed research; D.S. and J.R.N. analyzed data; and J.R.N. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: ESI-MS, electrospray ionization MS.
Footnotes
Crosstalk is the intermingling of molecules bearing different chemical information, in analogy with the electrical engineering phenomenon whereby signals are intermingled between closely spaced wires.
References
- 1.Holliday, B. J. & Mirkin, C. A. (2001) Angew. Chem. Int. Ed. 40, 2022–2043. [PubMed] [Google Scholar]
- 2.Piguet, C., Bernardinelli, G. & Hopfgartner, G. (1997) Chem. Rev. 97, 2005–2062. [DOI] [PubMed] [Google Scholar]
- 3.Sun, W.-Y., Yoshizawa, M., Kusukawa, T. & Fujita, M. (2002) Curr. Opin. Chem. Biol. 6, 757–764. [DOI] [PubMed] [Google Scholar]
- 4.Caulder, D. L. & Raymond, K. N. (1999) Acc. Chem. Res. 32, 975–982. [Google Scholar]
- 5.Cotton, F. A., Lin, C. & Murillo, C. A. (2001) Acc. Chem. Res. 34, 759–771. [DOI] [PubMed] [Google Scholar]
- 6.Albrecht, M. (2000) J. Inclusion Phenom. Macrocyclic Chem. 36, 127–151. [Google Scholar]
- 7.Seidel, S. R. & Stang, P. J. (2002) Acc. Chem. Res. 35, 972–983. [DOI] [PubMed] [Google Scholar]
- 8.Linton, B. & Hamilton, A. D. (1997) Chem. Rev. 97, 1669–1680. [DOI] [PubMed] [Google Scholar]
- 9.Balzani, V., Credi, A., Raymo, F. M. & Stoddart, J. F. (2000) Angew. Chem. Int. Ed. 39, 3348. [DOI] [PubMed] [Google Scholar]
- 10.Stoddart, J. F. (2001) Acc. Chem. Res. 34, 410–411. [DOI] [PubMed] [Google Scholar]
- 11.Collin, J.-P., Dietrich-Buchecker, C., Gaviña, P., Jimenez-Molero, M. C. & Sauvage, J.-P. (2001) Acc. Chem. Res. 34, 477–487. [DOI] [PubMed] [Google Scholar]
- 12.Gianneschi, N. C., Nguyen, S. T. & Mirkin, C. A. (2005) J. Am. Chem. Soc. 127, 1644–1645. [DOI] [PubMed] [Google Scholar]
- 13.Thordarson, P., Bijsterveld, E. J. A., Rowan, A. E. & Nolte, R. J. M. (2003) Nature 424, 915–918. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez, J. V., Kay, E. R. & Leigh, D. A. (2004) Science 306, 1532–1537. [DOI] [PubMed] [Google Scholar]
- 15.Badjic, J. D., Balzani, V., Credi, A., Silvi, S. & Stoddart, J. F. (2004) Science 303, 1845–1849. [DOI] [PubMed] [Google Scholar]
- 16.Hubin, T. J. & Busch, D. H. (2000) Coord. Chem. Rev. 200, 5–52. [Google Scholar]
- 17.Hogg, L., Leigh, D. A., Lusby, P. J., Morelli, A., Parsons, S. & Wong, J. K. Y. (2004) Angew. Chem. Int. Ed. 43, 1218–1221. [DOI] [PubMed] [Google Scholar]
- 18.Leigh, D. A., Lusby, P. J., Teat, S. J., Wilson, A. J. & Wong, J. K. Y. (2001) Angew. Chem. Int. Ed. 40, 1538–1543. [PubMed] [Google Scholar]
- 19.Houjou, H., Iwasaki, A., Ogihara, T., Kanesato, M., Akabori, S. & Hiratani, K. (2003) New J. Chem. 27, 886–889. [Google Scholar]
- 20.Childs, L. J., Alcock, N. W. & Hannon, M. J. (2002) Angew. Chem. Int. Ed. 41, 4244–4247. [DOI] [PubMed] [Google Scholar]
- 21.Hamblin, J., Childs, L. J., Alcock, N. W. & Hannon, M. J. (2002) Dalton 2, 164–169. [Google Scholar]
- 22.Nitschke, J. R., Schultz, D., Bernardinelli, G. & Gérard, D. (2004) J. Am. Chem. Soc. 126, 16538–16543. [DOI] [PubMed] [Google Scholar]
- 23.Nitschke, J. R. & Lehn, J.-M. (2003) Proc. Natl. Acad. Sci. USA 100, 11970–11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitschke, J. R., Hutin, M. & Bernardinelli, G. (2004) Angew. Chem. Int. Ed. 43, 6724–6727. [DOI] [PubMed] [Google Scholar]
- 25.Brooker, S., Hay, S. J. & Plieger, P. G. (2000) Angew. Chem. Int. Ed. 39, 1968–1970. [PubMed] [Google Scholar]
- 26.Chichak, K. S., Cantrill, S. J., Pease, A. R., Chiu, S.-H., Cave, G. W. V., Atwood, J. L. & Stoddart, J. F. (2004) Science 304, 1308–1312. [DOI] [PubMed] [Google Scholar]
- 27.Rowan, S. J., Cantrill, S. J., Cousins, G. R. L., Sanders, J. K. M. & Stoddart, J. F. (2002) Angew. Chem. Int. Ed. 41, 898–952. [DOI] [PubMed] [Google Scholar]
- 28.Lehn, J.-M. (1995) Supramolecular Chemistry: Concepts and Perspectives (Wiley–VCH, Weinheim, Germany).
- 29.Lehn, J.-M. (2002) Proc. Natl. Acad. Sci. USA 99, 4763–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Telfer, S. G., Yang, X. J. & Williams, A. F. (2004) Dalton Trans. 5, 699–705. [DOI] [PubMed] [Google Scholar]
- 31.Severin, K. (2004) Chem. Eur. J. 10, 2565–2580. [DOI] [PubMed] [Google Scholar]
- 32.Otto, S., Furlan, R. L. E. & Sanders, J. K. M. (2002) Science 297, 590–593. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen, R. & Huc, I. (2003) Chem. Commun., 942–943. [DOI] [PubMed]
- 34.Lehn, J.-M. & Eliseev, A. V. (2001) Science 291, 2331–2332. [DOI] [PubMed] [Google Scholar]
- 35.Hof, F., Nuckolls, C. & Rebek, J., Jr. (2000) J. Am. Chem. Soc. 122, 4251–4252. [Google Scholar]
- 36.Albrecht, M., Janser, I., Runsink, J., Raabe, G., Weis, P. & Froehlich, R. (2004) Angew. Chem. Int. Ed. 43, 6662–6666. [DOI] [PubMed] [Google Scholar]
- 37.Calama, M. C., Timmerman, P. & Reinhoudt, D. N. (2000) Angew. Chem. Int. Ed. 39, 755–758. [DOI] [PubMed] [Google Scholar]
- 38.Nitschke, J. R. (2004) Angew. Chem. Int. Ed. 43, 3073–3075. [DOI] [PubMed] [Google Scholar]
- 39.Constable, E. C. (1995) Metals and Ligand Reactivity: An Introduction to the Organic Chemistry of Metal Complexes (VCH, Weinheim, Germany).
- 40.Mayer, S. F., Kroutil, W. & Faber, K. (2001) Chem. Soc. Rev. 30, 332–339. [Google Scholar]
- 41.Tietze, L. F. (1996) Chem. Rev. 96, 115–136. [DOI] [PubMed] [Google Scholar]
- 42.Amendola, V., Fabbrizzi, L., Gianelli, L., Maggi, C., Mangano, C., Pallavicini, P. & Zema, M. (2001) Inorg. Chem. 40, 3579–3587. [DOI] [PubMed] [Google Scholar]
- 43.Braun, R. D., Wiechelman, K. J. & Gallo, A. A. (1989) Anal. Chim. Acta 221, 223–238. [Google Scholar]
- 44.Ogura, T. (1976) Transition Met. Chem. (Dordrecht, The Netherlands) 1, 179–182. [Google Scholar]
- 45.Chowdhury, S., Patra, G. K., Drew, M. G. B., Chattopadhyay, N. & Datta, D. (2000) Dalton 2000, 235–237. [Google Scholar]
- 46.Patra, G. K. & Goldberg, I. (2003) Eur. J. Inorg. Chem. 2003, 969–977. [Google Scholar]
- 47.Li, P., Scowen, I. J., Davies, J. E. & Halcrow, M. A. (1998) Dalton 1998, 3791–3799. [Google Scholar]
- 48.Parker, D, ed. (1996) Macrocycle Synthesis: A Practical Approach (Oxford Univ. Press, Oxford).
- 49.Van Stein, G. C., Van Koten, G., Vrieze, K. & Brevard, C. (1984) Inorg. Chem. 23, 4269–4278. [Google Scholar]
- 50.Drew, M. G. B., Lavery, A., McKee, V. & Nelson, S. M. (1985) Dalton 1985, 1771–1774. [Google Scholar]
- 51.Kelly, T. R., Xie, R. L., Weinreb, C. K. & Bregant, T. (1998) Tetrahedron Lett. 39, 3675–3678. [Google Scholar]
- 52.Schmittel, M. & Ganz, A. (1997) Chem. Commun., 999–1000.
- 53.Kalsani, V., Ammon, H., Jäckel, F., Rabe, J. P. & Schmittel, M. (2004) Chem. Eur. J. 2004, 5481–5492. [DOI] [PubMed] [Google Scholar]
- 54.Braun, S., Kalinowski, H.-O. & Berger, S. (1998) 150 and More Basic NMR Experiments (Wiley–VCH, Weinheim, Germany).
- 55.Buckingham, J. (1982) Dictionary of Organic Compounds (Chapman & Hall, New York).
- 56.Skene, W. G. & Lehn, J.-M. (2004) Proc. Natl. Acad. Sci. USA 101, 8270–8275. [DOI] [PMC free article] [PubMed] [Google Scholar]