Abstract
It is well known that mast cells are derived from hematopoietic stem cells. However, in adult hematopoiesis, a committed mast cell progenitor has not yet been identified in any species, nor is it clear at what point during adult hematopoiesis commitment to the mast cell lineage occurs. We identified a cell population in adult mouse bone marrow, characterized as Lin-c-Kit+Sca-1--Ly6c-FcεRIα-CD27-β7+T1/ST2+, that gives rise only to mast cells in culture and that can reconstitute the mast cell compartment when transferred into c-kit mutant mast cell-deficient mice. In addition, our experiments strongly suggest that these adult mast cell progenitors are derived directly from multipotential progenitors instead of, as previously proposed, common myeloid progenitors or granulocyte/macrophage progenitors.
Keywords: hematopoietic stem cell, multipotential progenitor
Mast cells are present in adult mammals in virtually all vascularized tissues (1, 2). Mast cells express on their surface the high-affinity receptor for IgE (i.e., FcεRI) and can be activated by IgE and specific antigen to release a diverse array of mediators, including histamine, leukotrienes, prostaglandins, serine proteases, and various cytokines, chemokines, and growth factors (2-4). Accordingly, mast cells are known primarily as critical effector cells of asthma and other IgE-associated allergic disorders (2-4). However, mast cells can be activated by many mechanisms in addition to IgE and specific antigen, and these cells have also been implicated in the pathogenesis of autoimmune disorders (5), in the expression of innate immunity to bacterial infection (6, 7), and in a wide variety of other biological processes (1-7).
It has long been known that mast cells arise from hematopoietic progenitors (8). Moreover, by using in vitro colony forming assays and in vivo bone marrow transplantation, it has been shown that cells with mast cell-generating activity are present in the bone marrow and certain peripheral tissues (1, 8, 9). These findings and other lines of evidence indicate that mast cells normally do not mature before leaving the bone marrow but circulate through the vascular system as immature progenitors that then complete their development peripherally within connective or mucosal tissues (1, 2, 4, 10, 11).
However, a definite mast cell progenitor (MCP) has been identified only in fetal blood (12). Such Thy-1loc-Kithi“promastocytes” lack expression of FcεRI but can generate FcεRI-expressing functional mast cells in vitro and in vivo (12). However, the counterpart of the promastocyte in adult-type hematopoiesis has remained elusive despite years of research. In part, the difficulty in isolating the MCP may reflect important differences between fetal and adult hematopoiesis. These processes have some broad similarities but also exhibit intrinsic differences in hematopoietic progenitor cell fate potentials, proliferation capacity, colony-forming activity, and differentiation fidelity (13). In this study, we identified and characterized a MCP in adult mouse bone marrow and demonstrated that this adult MCP can give rise to mast cells in the tissues of c-kit mutant mast cell-deficient mice in vivo. We also provide evidence that strongly supports the hypothesis that the adult MCP can be derived directly from multipotential progenitors (MPPs).
Materials and Methods
Animals. C57BL/Ka-Thy1.1 (CD45.2) mice (4-8 weeks old) were used for the isolation of MCPs, other myeloid progenitors, hematopoietic stem cells (HSCs), and MPPs. The congenic C57BL/Ka-Thy1.1-Ly5.2 (CD45.1) mice (4-8 weeks old) were used for the isolation of CD45.1+ MCPs for transferring into genetically mast cell-deficient C57BL/6-KitW-sh/KitW-sh (CD45.2) mice (6-8 weeks old). All animals were maintained in Stanford University Laboratory Animal Facility in accordance with Stanford Animal Care and Use Committee and National Institutes of Health guidelines.
Cells Staining and Sorting. For stem cells and progenitor isolation, bone marrow cells were stained with unconjugated antibodies specific for the following lineage makers: CD3 (KT31.1), CD4 (GK1.5), CD5 (53-7.3/7.8), CD8 (53-6.7), CD11b (M1/70), B220 (6B2), Gr-1 (8C5), and TER119. Cells were then stained with goat anti-rat IgG microbeads (Miltenyi Biotec, Auburn, CA) and passed through an LD column (Miltenyi Biotec) to remove the Lin+ cells. For isolation of MCPs, the remaining lineage-depleted cells were blocked with unconjugated anti-FcγRII/III (2.4G2, Pharmingen). The cells were then stained with FITC-conjugated anti-T1/ST2 (DJ8, MD Biosciences, St. Paul); phycoerythrin (PE)-conjugated anti-FcεRIα (MAR-1, eBioscience, San Diego) and anti-Ly6C (HK1.4, Southern Biotech, Birmingham, AL); PE/Cy5-conjugated lineage-specific antibodies (eBioscience) CD3 (145-2C11), CD4, CD8, CD11b, B220, Gr-1, and TER119; Texas red-conjugated anti-Sca-1 (E13-161-7); allophycocyanin (APC)-conjugated anti-CD27 (LG.7F9, eBioscience); biotin-conjugated anti-β7 integrin (M293, Pharmingen) [revealed by PE/Cy7-conjugated strepavidin (Caltag, Burlingame, CA)]; and APC/Cy7-conjugated anti-c-Kit (2B8, eBioscience). For assessing CD9 and β1 integrin expression levels on β7+ progenitors, the lineage-depleted cells were stained with FITC-conjugated anti-FcεRIα (eBioscience) and Ly6C (AL-21, Pharmingen), PE-conjugated anti-β7 integrin (Pharmingen), PE/Cy5-conjugated lineage-specific antibodies, Texas red-conjugated anti-Sca-1, APC-conjugated anti-CD27, APC/Cy7-conjugated anti-c-Kit, and biotin-conjugated anti-CD9 (KMC8, Pharmingen) or anti-β1 integrin (Ha2/5, Pharmingen) revealed by PE/Cy7-conjugated strepavidin. For isolation of β7+ progenitors and other myeloid progenitors, the remaining cells were stained with FITC-conjugated anti-CD34 (RAM34, Pharmingen), PE-conjugated anti-FcεRIα, PE/Cy5-conjugated lineage-specific antibodies, Texas red-conjugated anti-Sca-1, APC-conjugated anti-FcγRII/III (93, eBioscience), biotin-conjugated anti-β7 integrin (revealed by PE/Cy7-conjugated strepavidin), and APC/Cy7-conjugated anti-c-Kit. For isolation of long-term (LT)-HSCs, short-term (ST)-HSCs, and MPPs, the cells were blocked with unconjugated anti-FcγRII/III, then stained with FITC-conjugated anti-FcεRIα and Ly6C, PE-conjugated anti-Flk2 (A2F10.1, Pharmingen), PE/Cy5-conjugated lineage-specific antibodies, Texas red-conjugated anti-Sca-1, APC-conjugated anti-Thy1.1 (HIS51, eBioscience), and APC/Cy7-conjugated anti-c-Kit. Cells were sorted or analyzed by using a triple-laser (407-nm krypton laser, 488-nm argon laser, and 598-nm dye laser) FACS Vantage SE/DiVa (Becton Dickinson) with facsdiva software. Fluorescence spill-over compensations were generated with the autocompensation feature of the facsdiva software (14). For cultured progenitors, cells were blocked with unconjugated anti-FcγRII/III, then stained with PE-conjugated anti-FcεRIα and APC-conjugated anti-c-Kit (2B8, Pharmingen). Stained cells were analyzed on a FACSCalibur (Becton Dickinson) machine. The resulting data were analyzed with flowjo (Treestar, Ashland, OR).
In Vitro Differentiation Assay. For liquid culture, sorted cells were cultured in Iscove's modified Dulbecco's medium (Invitrogen) supplemented with 10% FCS (Sigma), 5 × 10-5 M 2-mercaptoethanol, l-glutamine, sodium pyruvate, nonessential amino acid, and antibiotics (Mediatech, Arlington, VA). Two kinds of cytokine mixtures were added to the culture. All cytokines were used at a concentration of 10 ng/ml. The mast cell-specific mixture consisted of stem cell factor (SCF; Amgen Biologicals), IL-3, IL-6, and IL-9 (PeproTech, Rocky Hill, NJ). The multipotential cytokine mixture consisted of SCF, erythropoietin (EPO; R & D Systems), thrombopoietin (TPO), Flt-3 ligand (Flt-3L), granulocyte/macrophage colony-stimulating factor (GM-CSF; Peprotech), and IL-1 (R & D Systems), -3, -6, -7, -9, and -11 (PeproTech). For colony-forming assays, sorted cells were added into methycellulose medium (Mehocult M3231, StemCell Technologies, Vancouver) supplemented with the multipotential cytokine mixture. After 10 days, colonies were picked up, cytospun, and stained with May Grunwald-Giemsa stain.
RT-PCR Analysis. RNA (50 ng) was isolated from cells with an RNeasy mini kit (Qiagen, Valencia, CA) and converted to first-strand cDNA with oligo(dT)12-18 primers (Promega) and Sensiscript reverse transcriptase (Qiagen) before amplification with specific primers using AccuPrime polymerase (Invitrogen). The resulting PCR products were resolved on 2% agarose gels. The primer pairs used for amplification were the following: mouse mast cell carboxypeptidase A, GCAGGCAGGCACAGTTATG (forward) and TGTTGGTGTTTGGAGAAGAGTC (reverse); mast cell protease-2, TTGTTCACCCAAAGTTTCAG (forward) and CCTGGAGTTGATAATCGTA-ATC (reverse); mast cell protease-4, GACAGAATCCACACAGCAGAAG (forward) and CCTCCAGA-GTCTCCCTTGTATG (reverse); mast cell protease-6, GGGAGGACATGAGGCTTC (forward) and AGTATAGATACTGCTCACGAAG (reverse).
Immunofluorescence Microscopy. Stomach and ear pinna specimens were carefully dissected and embedded in OCT compound (Tissue Tek, Torrance, CA) for cryopresevation. For each tissue, two consecutive sections (10 μm each) were cut and stained with 0.1% toluidine blue (pH 1.0) or FITC-conjugated anti-CD45.1 (A20, Phramingen) followed by Rhodamine-conjugated streptavidin (Vector Laboratories). The samples were mounted by using fluorescent mounting medium (DakoCytomation, Carpinteria, CA). Images were captured with an Olympus BX60 fluorescence microscope and bioquant image analysis software (R & M Biometrics, Nashville).
Supporting Information. For more information, see Figs. 5-9, which are published as supporting information on the PNAS web site.
Results and Discussion
We began our search for adult MCPs in mouse bone marrow because this compartment contains most of the hematopoietic progenitors (Lin-c-Kit+Sca-1-), which possess mast cell-generating activity. However, the relatively long (4- to 8-week) culture time required for such bone marrow cells to generate essentially homogenous populations of bone marrow-derived cultured mast cells (BMCMCs) through standard culture methods represented a major limitation in the search for the MCP (15). In our in vitro liquid culture system, we cultured sorted candidate progenitors in Iscove's modified Dulbecco's medium containing 10% FCS and cytokine mixtures. With this approach, we were able to generate mast cells within 11-14 days after culture (Fig. 5).
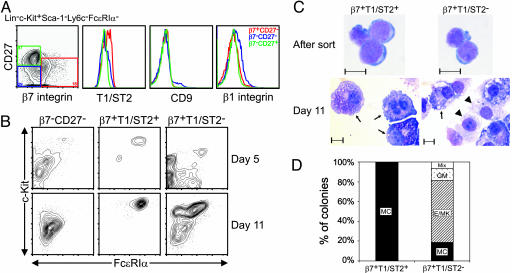
To remove any mature mast cells that might contaminate candidate progenitor populations, FcεRIα+ cells were excluded. We then divided the progenitor compartment into three fractions based on β7 integrin (β7) [an integrin expressed on BMCMCs (11, 16)] and CD27 [a marker for hematopoietic stem cells and progenitors (17)]. By using our liquid culture assay, we found that the majority of the mast cell-generating activity is in the β7+CD27- fraction (Fig. 6). We then analyzed this fraction for surface expression of T1/ST2, an orphan receptor in the IL-1 receptor family that is expressed on T helper type 2 cells and mast cells (18-20); CD9, a marker for megakaryocyte progenitors (21); and β1 integrin, another integrin expressed on BMCMCs (16) and HSCs (22). We found that the β7+CD27- progenitors can be separated into two populations based on T1/ST2 expression (Fig. 1A).
Fig. 1.
Identification of MCPs in adult mouse bone marrow. (A) Lin-c-Kit+Sca-1-Ly6c-FcεRIα- cells were subdivided into β7+CD27-, β7-CD27-, and β7-CD27+ populations (red, blue, and green, respectively) and analyzed for their surface expression of T1/ST2, CD9, and β1 integrin. (B) Double-sorted β7-CD27-, β7+T1/ST2+ (MCPs), and β7+T1/ST2- cells were cultured with SCF, EPO, TPO, Flt-3L, GM-CSF, and IL-1, -3, -6, -7, -9, and -11 and harvested at days 5 and 11 for flow cytometry analysis for mast cells to be detected by expression of c-Kit and FcεRIα.(C) Cytospin of double-sorted β7+T1/ST2+ (MCPs) and β7+T1/ST2- cells at isolation (Upper) and after 11 days in culture (Lower) and May Grunwald-Giemsa staining of mast cells (arrows) and macrophages (arrowheads). (Scale bars, 10 μm.) (D) Day 10 results of colony-forming assay in methylcellulose containing SCF, EPO, TPO, Flt-3L, GM-CSF, and IL-1, -3, -6, -7, -9, and -11. Data shown are representative of those obtained in three (A, C, and D) or four (B) experiments. MC, mast cells; Mix, mixed; GM, granulocyte/macrophage; E/MK, erythrocyte/megakaryocyte.
We double-sorted the β7+CD27- progenitors into T1/ST2+ and T1/ST2- fractions and cultured them in the presence of SCF, EPO, TPO, Flt-3L, GM-CSF, and IL-1, -3, -6, -7, -9 and -11, a cytokine mixture that allows the development of most lineages except T cells. β7+T1/ST2+ progenitors represented the only fraction that generated mast cells exclusively after 5 or 11 days in culture (Fig. 1B). Cytospins of β7+T1/ST2+ and β7+T1/ST2- cells prepared immediately after sorting showed that they looked very similar and, in contrast to promastocytes (12), did not contain prominent cytoplasmic granules (Fig. 1C, After sort). But after 11 days in liquid culture, β7+T1/ST2+ progenitors give rise to pure populations of mast cells, whereas the β7+T1/ST2- fraction gave rise to mixed populations of mast cells (Fig. 1C, day 11, arrows) and macrophages (Fig. 1C, day 11, arrowheads). In methylcellulose assays, β7+T1/ST2+ progenitors again generated only mast cell colonies, whereas β7+T1/ST2- cells formed mainly erythrocyte/megakaryocyte colonies (Fig. 1D). Notably, flow cytometry analysis of the β7+CD27- fraction indicated that it contained mainly megakaryocyte/erythrocyte progenitors (MEPs) and common myeloid progenitors (CMPs) (Fig. 7). Based on these in vitro data, we provisionally named the β7+T1/ST2+ population MCPs.
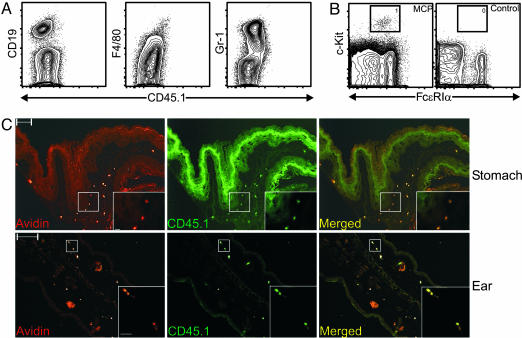
To verify the in vitro observations, we transplanted 10,000 CD45.1+ MCPs into lethally irradiated congenic KitW-sh/KitW-sh mice (23, 24). Although KitW-sh/KitW-sh mice are profoundly mast cell-deficient, they have a normal hematocrit and their other hematopoietic lineages are nearly normal (23, 24). After 4 weeks, no donor-derived myeloid cells or lymphoid cells were detected in the blood, spleen, peritoneal lavage fluid, or bone marrow of the MCP-transplanted animals (Fig. 2A and data not shown). Donor-derived peritoneal mast cells were present in MCP-transplanted animals but not in control mice that received only rescuing KitW-sh/KitW-sh bone marrow cells (Fig. 2B). We also performed immunofluorescence microscope analysis on sections from stomach and ear pinna specimens of MCP-transplanted animals. By using rhodamine-conjugated avidin to identify mast cells and FITC-labeled anti-CD45.1 antibody to identify donor-derived cells, we found only donor-derived mast cells in these tissues (i.e., the only rhodamine-conjugated avidin+ mast cells present were FITC-labeled anti-CD45.1 antibody+) (e.g., Fig. 2C and data not shown). Based on the intense staining of their cytoplasmic granules in toluidine blue-stained sections of forestomach and ear pinna from the MCP-transplanted KitW-sh/KitW-sh mice, such donor-derived mast cells appeared to be rather mature by morphology (Fig. 8). However, we found no evidence of any other donor-derived cell lineages in sections from such mice (i.e., there were no identifiable rhodamine-conjugated avidin-, FITC-labeled anti-CD45.1 antibody+ cells) (e.g., Fig. 2C and data not shown). Thus, MCPs can give rise to tissue mast cells in vivo. Moreover, MCPs do not appear to be able to generate any lineage other than mast cells in vivo.
Fig. 2.
MCPs reconstitute mast cell compartments in mast cell-deficient KitW-sh/KitW-sh mice. Lethally irradiated KitW-sh/KitW-sh mice were injected i.v. with double-sorted CD45.1+ MCPs (104 cells per mouse) mixed with rescuing KitW-sh/KitW-sh bone marrow cells (3 × 105 cells per mouse) or with rescuing KitW-sh/KitW-sh bone marrow cells only (negative control). Mice were killed 4 weeks after transplantation. (A) Flow cytometry analysis of the bone marrow from MCP-transplanted KitW-sh/KitW-sh mice. No donor-derived B cells (CD19), macrophages (F4/80), or granulocytes (Gr-1) were found. (B) Peritoneal lavage cells from MCP-transplanted or control KitW-sh/KitW-sh mice were analyzed by flow cytometry. Mast cells (gated population) were identified only in MCP-transplanted animals. (C) Immunofluorescence microscopy analysis of forestomach (Upper) and ear pinna (Lower) of MCP-transplanted KitW-sh/KitW-sh mice. Sections were stained with avidin for mast cells (red) and CD45.1 for donor-derived cells (green). Acquired images were then merged with photoshop (Adobe Systems, San Jose, CA). (Scale bars, 100 μm.) (Insets) Magnified views of the white boxed areas. Data shown are representative of those obtained in three experiments. (Scale bars, 10 μm.)
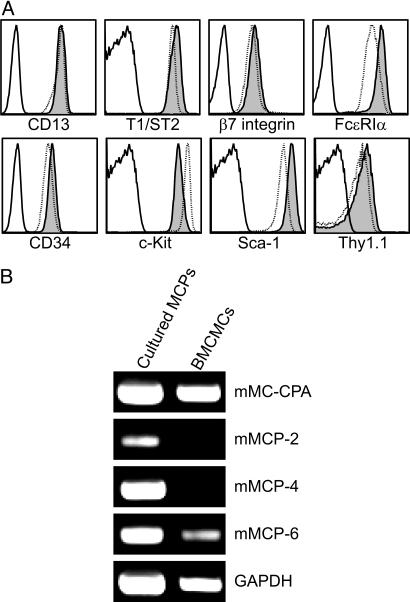
After 14 days in vitro, MCP-derived cells, compared with ex vivo freshly sorted MCPs, maintained similar levels of expression of c-Kit, β7, and T1/ST2 but exhibited increased expression of FcεRIα, CD13, CD34, Sca-1, and Thy-1.1 to levels comparable with or higher than those in 6-week-old IL-3-derived BMCMCs (Fig. 3A). We also evaluated by RT-PCR the expression of mast cell-associated proteases in cultured MCPs. Whereas cultured MCPs expressed levels of mRNA for mouse mast cell carboxypeptidase A, which were similar to those in standard IL-3-derived BMCMCs, the levels of expression of mRNA for mouse mast cell protease-2, -4, and, -6 were higher in cultured MCPs than in BMCMCs. This result may reflect differences in the cytokine mixtures in the two culture conditions: That for MCPs contains IL-9, which can up-regulate mouse mast cell protease-2 and -4 (25); SCF, which can up-regulate mouse mast cell protease-4 and -6 (26); and IL-3, which is used to generate BMCMCs (15, 27).
Fig. 3.
MCPs give rise to mast cells in vitro. Double-sorted MCPs were cultured with SCF and IL-3, -6, and -9 for 14 days. (A) Flow cytometry analysis of surface antigen expression of cultured MCPs (shaded), BMCMCs (dotted line), and isotype control (solid line). (B) RT-PCR analysis of mast cell-associated protease expression in cultured MCPs and BMCMCs. Data shown are representative of those obtained in four experiments.
The developmental relationship of mast cells to other myeloid cells has yet to be fully resolved. It had been proposed that CMPs can give rise to all myeloid cells, including mast cells (28). Although we are not aware of actual data to support this claim, mast cells do express transcription factors required for granulocyte/macrophage lineage development, such as PU.1 (29), and share the expression of GATA-1 and GATA-2 with erythrocyte/megakaryocyte lineage cells (30). Indeed, PU.1 and GATA-2 are required for normal mast cell development (31). These findings suggest a close developmental relationship between mast cells and these two other lineages.
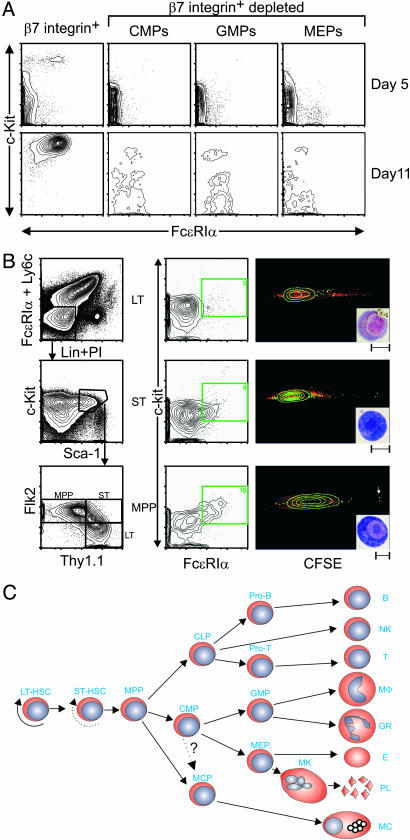
To investigate the developmental relationship of MCPs to other myeloid progenitors, we separated the Lin-c-Kit+Sca-+-FcεRIα- hematopoietic progenitors based on their β7 expression. β7- cells were further sorted based on their FcγRII/III and CD34 expression into CMPs, granulocyte/macrophage progenitor (GMPs), and MEPs (32). These sorted populations were cultured with SCF, EPO, TPO, Flt-3L, GM-CSF, and IL-1, -3, -6, -7, -9, and -11. We found that the β7+ progenitors were the only population that could give rise to mast cells. Under the same culture conditions, β7-depleted CMPs, GMPs, and MEPs did not generate mast cells, even after 11 days in culture (Fig. 4A).
Fig. 4.
Evidence that MCPs are derived from MPPs but not from CMPs or GMPs. (A) Lin-c-Kit+Sca-1- cells were sorted into β7+ and β7- fractions. β7- cells were sorted into CMP (FcγRII/IIIloCD34+), GMP (FcγRII/IIIhiCD34+), and MEP (FcγRII/IIIloCD34-) populations. Double-sorted cells were cultured with SCF, EPO, TPO, Flt-3L, GM-CSF, and IL-1, -3, -6, -7, -9, and -11 and harvested at days 5 and 11 for flow cytometry analysis for mast cells detected by c-Kit and FcεRIα. (B) Lin-c-Kit+Sca-1+Ly6c-FcεRIα- cells were double-sorted into LT-HSCs (Thy1.1+Flk2-), ST-HSCs (Thy1.1+Flk2+), and MPPs (Thy1.1-Flk2+). Sorted cells were labeled with CFSE and then cultured with SCF and IL-3, -6, and -9 for 5 days. Harvested cells were stained for c-Kit and FcεRIα to identify mast cells. c-Kit+FcεRIα+ cells (green) were gated, and their CFSE level was analyzed. (Insets) May Grunwald-Giemsa staining of sorted c-Kit+FcεRIα+ cells (Scale bars, 10 μm). (A and B) Data shown are representative of those obtained in three (A) or two (B) experiments. (C) Proposed lineage relationship model of MCPs to other hematopoietic progenitors.
In addition, we performed two experiments in which Lin-c-Kit+Sca-1-FcεRIα-β7+T1/ST2- hematopoietic progenitors were further sorted based on their FcγRII/III and CD34 expression into CMPs, GMPs and MEPs, and then cultured with SCF, EPO, TPO, Flt-3L, GM-CSF and IL-1, -3, -6, -7, -9, and -11 for 5 or 11 days. Little or no mast cell-generating potential was detected in any of the three populations (Fig. 9). In each experiment, a few mast cells arose from the population of β7+T1/ST2- GMPs. However, the mast cell-generating potential of this population was very modest compared with that of MCPs (Fig. 1 A). Accordingly, we think that the results shown in Fig. 9 more likely reflect the contamination of this β7+T1/ST2- GMP population with a few β7+T1/ST2+ MCPs, rather than the true mast cell-generating potential of β7+T1/ST2- GMPs.
We then examined the lineage relationship of MCPs to HSCs and MPPs. The lin-c-Kit+ Sca-1+Ly6c-FcεRIα- cells were separated into LT-HSCs, ST-HSCs, and MPPs based on their Thy1.1 and Flk2 expression (33). The cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) so that cell division could be traced (34), and the cells were cultured with SCF and IL-3, -6, and -9, a cytokine mixture that can more specifically stimulate the growth of mast cells. After 5 days in culture, a c-Kit+FcεRIα+ immature mast cell population was present in cultures derived from all three fractions. The percentage of the immature mast cell population and their c-Kit and FcεRIα expression increased as HSCs lost self-renewal capacity (Fig. 4B Center). CFSE analysis demonstrated that LT-HSCs and ST-HSCs require more cell divisions than do these MPPs to generate this immature mast cell population. Indeed, some cells derived from MPPs do not even require a single cell division before they express the FcεRIα (Fig. 4B Right, white arrow). Cytospins of the sorted c-Kit+FcεRIα+ cells indicated that this population had the morphology of mast cells, but without as fully developed a complement of cytoplasmic granules as is present in more mature mast cells (Fig. 4B, Right Inset). These data strongly suggest that a common progenitor for mast cells and other myeloid cells exists in the MPP compartment (Fig. 4C). It should be noted that this compartment is heterogeneous and contains cells that have different efficiencies at producing platelets and erythrocytes (33, 35, 36).
Isolation of the MCP resolves the long-standing debate about whether there is a true mast cell-committed progenitor in adult hematopoiesis. Taken together, our findings also strongly support the hypothesis that mast cells are derived from a branch in the pathway of adult hematopoiesis that is distinct from those giving rise to the CMP or the common lymphoid progenitor (CLP) (arrow from MPP to MCP in Fig. 4C). Indeed, we consistently failed to detect mast cell generating potential in either β7- CMPs (Fig. 4A) or β7+ CMPs (Fig. 9). The observation that the development of some mast cells from MPPs does not require even a single cell division represents additional evidence for the direct generation of mast cells from MPPs.
However, we cannot formally rule out the possibility that MCPs (which are β7+T1/ST2+) are derived from a small subpopulation of β7+T1/ST2- cells that are found within the major β7+ CMP population that gives rise to few or no mast cells when cultured en masse for 5 or 11 days in vitro (Fig. 9). This possibility is indicated by the dotted arrow from CMP to MCP in Fig. 4C. Alternatively, a common progenitor derived from the MPP might give rise to MCPs and CMPs. Either of these possibilities could account for the ability of a population of β7+CD27-T1/ST2- progenitors to generate a few mast cell colonies in addition to other lineages of hematopoietic cells (e.g., Fig. 1 C and D). However, we think that it is more likely that the existence of mast cell-generating capacity within the β7+CD27-T1/ST2- progenitor population simply reflects contamination of this population with a few β7+CD27-T1/ST2+ MCPs.
Establishing the precise relationship among MPPs, CMPs, and MCPs may require the identification of additional surface markers to better define these three populations. However, our identification of the adult MCP will facilitate the investigation of many other important questions. These questions include the identification of candidate genes involved in mast cell commitment and the investigation of their functions by manipulating such genes in MCPs for functional analysis; a more precise determination of the lineage relationship of mast cells to other hematopoietic cells types, including basophils and eosinophils; and the tracking of MCPs during their migration, tissue invasion, and maturation, both physiologically and in settings such as parasite infections, allergic diseases, and other pathological processes. Finally, the finding that HSCs can quickly develop into mast cells raises the possibility that circulating HSCs may also serve as a source of recruited mast cell precursors during infectious challenges or in other settings.
Supplementary Material
Acknowledgments
We thank members of the I.L.W. and S.J.G. laboratories for helpful discussions, Peter Besmer (Memorial Sloan-Kettering Cancer Center, New York) for the generous gift of C57BL/6-KitW-sh/KitW-sh mice, Zhen-Sheng Wang for help with the histology, and Libuse Jerabek for excellent laboratory management. This work was supported by National Institutes of Health Grants AI23990, CA72074, HL67674, Project 1 (to S.J.G.), and CA086017 (to I.L.W.) and a National Health and Medical Research Council of Australia C. J. Martin Fellowship (to M.A.G.).
Abbreviations: HSC, hematopoietic stem cell; MCP, mast cell progenitor; MPP, multipotential progenitor; CMP, common myeloid progenitors; GMP, granulocyte/macrophage progenitor; BMCMC, bone marrow-derived cultured mast cell; MEP, megakaryocyte/erythrocyte progenitor; PE, phycoerythrin; APC, allophycocyanin; SCF, stem cell factor; TPO, thrombopoietin; EPO, erythropoietin; GM-CSF, granulocyte/macrophage colony-stimulating factor; Flt-3L, Flt-3 ligand; CFSE, carboxyfluorescein diacetate succinimidyl ester.
See Commentary on page 11129.
References
- 1.Kitamura, Y. (1989) Annu. Rev. Immunol. 7, 59-76. [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe, D. D., Baram, D. & Mekori, Y. A. (1997) Physiol. Rev. 77, 1033-1079. [DOI] [PubMed] [Google Scholar]
- 3.Kinet, J. P. (1999) Annu. Rev. Immunol. 17, 931-972. [DOI] [PubMed] [Google Scholar]
- 4.Galli, S. J., Kalesnikoff, J., Grimbaldeston, M. A., Piliponsky, A. M., Williams, C. M. M. & Tsai, M. (2005) Annu. Rev. Immunol. 23, 749-786. [DOI] [PubMed] [Google Scholar]
- 5.Benoist, C. & Mathis, D. (2002) Nature 420, 875-878. [DOI] [PubMed] [Google Scholar]
- 6.Marshall, J. S. (2004) Nat. Rev. Immunol. 4, 787-799. [DOI] [PubMed] [Google Scholar]
- 7.Maurer, M., Wedemeyer, J., Metz, M., Piliponsky, A. M., Weller, K., Chatterjea, D., Clouthier, D. E., Yanagisawa, M. M., Tsai, M. & Galli, S. J. (2004) Nature 432, 512-516. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura, Y., Shimada, M., Hatanaka, K. & Miyano, Y. (1977) Nature 268, 442-443. [DOI] [PubMed] [Google Scholar]
- 9.Nabel, G., Galli, S. J., Dvorak, A. M., Dvorak, H. F. & Cantor, H. (1981) Nature 291, 332-334. [DOI] [PubMed] [Google Scholar]
- 10.Gurish, M. F. & Austen, K. F. (2001) J. Exp. Med. 194, F1-F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, J. K., Knight, P. A., Pemberton, A. D., Wright, S. H., Pate, J. A., Thornton, E. M. & Miller, H. R. (2004) Am. J. Pathol. 165, 95-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodewald, H. R., Dessing, M., Dvorak, A. M. & Galli, S. J. (1996) Science 271, 818-822. [DOI] [PubMed] [Google Scholar]
- 13.Traver, D., Miyamoto, T., Christensen, J., Iwasaki-Arai, J., Akashi, K. & Weissman, I. L. (2001) Blood 98, 627-635. [DOI] [PubMed] [Google Scholar]
- 14.Tung, J. W., Parks, D. R., Moore, W. A., Herzenberg, L. A. & Herzenberg, L. A. (2004) Clin. Immunol. 110, 277-283. [DOI] [PubMed] [Google Scholar]
- 15.Galli, S. J. (2000) Curr. Opin. Hematol. 7, 32-39. [DOI] [PubMed] [Google Scholar]
- 16.Gurish, M. F., Bell, A. F., Smith, T. J., Ducharme, L. A., Wang, R. K. & Weis, J. H. (1992) J. Immunol. 149, 1964-1972. [PubMed] [Google Scholar]
- 17.Wiesmann, A., Phillips, R. L., Mojica, M., Pierce, L. J., Searles, A. E., Spangrude, G. J. & Lemischka, I. (2000) Immunity 12, 193-199. [DOI] [PubMed] [Google Scholar]
- 18.Klemenz, R., Hoffmann, S. & Werenskiold, A. K. (1989) Proc. Natl. Acad. Sci. USA 86, 5708-5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino, K., Kashiwamura, S.-I., Kuribayashi, K., Kodama, T., Tsujimura, T., Nakanishi, K., Matsuyama, T., Takeda, K. & Akira, S. (1999) J. Exp. Med. 190, 1541-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moritz, D. R., Rodewald, H. R., Gheyselinck, J. & Klemenz, R. (1998) J. Immunol. 161, 4866-4874. [PubMed] [Google Scholar]
- 21.Nakorn, T. N., Miyamoto, T. & Weissman, I. L. (2003) Proc. Natl. Acad. Sci. USA 100, 205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voura, E. B., Billia, F., Iscove, N. N. & Hawley, R. G. (1997) Exp. Hematol. 25, 1172-1179. [PubMed] [Google Scholar]
- 23.Stevens, J. & Loutit, J. F. (1982) Proc. R. Soc. London B 215, 405-409. [DOI] [PubMed] [Google Scholar]
- 24.Grimbaldeston, M. A., Chen, C.-C., Piliponsky, A. M., Tsai, M., Tam, S.-Y. & Galli, S. J. (2005) Am. J. Pathol., in press. [DOI] [PMC free article] [PubMed]
- 25.Rupp, B., Lohning, M. & Werenskiold, A. K. (2000) Eur. J. Immunol. 30, 2954-2961. [DOI] [PubMed] [Google Scholar]
- 26.Gurish, M. F., Ghildyal, N., McNeil, H. P., Austen, K. F., Gillis, S. & Stevens, R. L. (1992) J. Exp. Med. 175, 1003-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghildyal, N., Friend, D. S., Nicodemus, C. F., Austen, K. F. & Stevens, R. L. (1993) J. Immunol. 151, 3206-3214. [PubMed] [Google Scholar]
- 28.Janeway, C., Travers, P., Walport, M. & Shlomchik, M. (2001) Immunobiology (Garland, New York).
- 29.Scott, E. W., Simon, M. C., Anastasi, J. & Singh, H. (1994) Science 265, 1573-1577. [DOI] [PubMed] [Google Scholar]
- 30.Martin, D. I., Zon, L. I., Mutter, G. & Orkin, S. H. (1990) Nature 344, 444-447. [DOI] [PubMed] [Google Scholar]
- 31.Walsh, J. C., DeKoter, R. P., Lee, H. J., Smith, E. D., Lancki, D. W., Gurish, M. F., Friend, D. S., Stevens, R. L., Anastasi, J. & Singh, H. (2002) Immunity 17, 665-676. [DOI] [PubMed] [Google Scholar]
- 32.Akashi, K., Traver, D., Miyamoto, T. & Weissman, I. L. (2000) Nature 404, 193-197. [DOI] [PubMed] [Google Scholar]
- 33.Christensen, J. L. & Weissman, I. L. (2001) Proc. Natl. Acad. Sci. USA 98, 14541-14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyons, A. B. & Parish, C. R. (1994) J. Immunol. Methods 171, 131-137. [DOI] [PubMed] [Google Scholar]
- 35.Morrison, S. J., Wandycz, A. M., Hemmati, H. D., Wright, D. E. & Weissman, I. L. (1997) Development (Cambridge, U.K.) 124, 1929-1939. [DOI] [PubMed] [Google Scholar]
- 36.Adolfsson, J., Mansson, R., Buza-Vidas, N., Hultquist, A., Liuba, K., Jensen, C. T., Bryder, D., Yang, L., Borge, O. J., Thoren, L. A., et al. (2005) Cell 121, 295-306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.