Abstract
Under chronic conditions of neuropathic pain, nociceptive C terminals are lost from their target region in spinal lamina II, leading to reduced thermal hyperalgesia. This region of the spinal cord expresses high levels of polysialic acid (PSA), a cell surface carbohydrate known to weaken cell–cell interactions and promote plasticity. Experimental removal of PSA from the spinal cord exacerbates hyperalgesia and results in retention of C terminals, whereas it has no effect on plasticity of touch Aβ fibers and allodynia. We propose that expression of PSA at this stress pathway relay point could serve to protect central circuitry from chronic sensory overload.
Keywords: neuropathic pain, neuroplasticity, neural cell adhesion molecule
Conditions of chronic pain are associated with structural and functional changes in different parts of the nervous system. Among the structural changes, there appears to be a neuroplastic event involving reversible loss of the nociceptive C fibers from their terminal region in spinal lamina II (1–6). This region of the spinal dorsal horn exhibits an unusual molecular feature of neural plasticity, the expression of polysialic acid (PSA) (7, 8). In vertebrates, PSA is a specific glycosylation of the neural cell adhesion molecule (NCAM), consisting of a long linear homopolymer of α-2,8-linked sialic acid (9, 10). The large excluded volume of this polymer produces sufficient steric hindrance between apposing membranes (11) to interfere not only with NCAM-mediated adhesion but also with that mediated by other adhesion receptors such as L1, cadherins, and integrins (12).
PSA is known to modulate cell interactions and promote plasticity in the developing nervous system. In particular, it facilitates translocation of neural and nonneural precursor cells and shields them from inappropriate interactions (13–17) and optimizes neurite sprouting and branching during axon path-finding (13) and target innervation (18–20), as well as reducing the stabilization of inappropriate synapses (18, 20). Although in most cases PSA expression is down-regulated by the end of embryonic development, it is retained within certain regions of the adult brain believed to exhibit different forms and degrees of plasticity, for example, the hippocampus, hypothalamus, brainstem, and rostral migratory stream (7, 17, 21).
Given these molecular characteristics, biological activities, and adult expression patterns, it is reasonable to propose that PSA's ability to weaken cell interactions could contribute to the loss of C-fiber terminals associated with chronic pain. In the present study, we test this hypothesis in a well characterized chronic constriction injury (CCI) model for neuropathic pain (22), including hyperalgesia (increased sensitivity to external stimuli) and allodynia (transformation of touch stimuli to pain). The approach uses a loss-of-function perturbation based on the in vivo removal of PSA from mouse dorsal horn by intrathecal injection of the PSA-specific endoneuraminidase N (endo N) (20, 23, 24). The results indicate that removal of PSA increases hyperalgesia and inhibits the observed CCI-induced loss of C terminals in spinal lamina II.
Materials and Methods
Preparation of endo N. The enzyme endo N was prepared as described (20). In brief, a plasmid encoding endo N (23) was expressed in M15 cells. The enzyme was then purified and its specific activity determined. endo N specifically cleaves α-2,8-linked sialic acid chains with minimum chain length of 8 (23, 24). It diffuses rapidly in tissues and selectively removes PSA, sparing other sialic acid-containing moieties, which makes it suitable for in vivo application (13, 20, 25).
Sciatic Ligation and endo N Injection. Four groups of 10 adult male mice (CD-1, CRL locR/6) were used in this study. The first two groups were anesthetized (pentobarbital 50 mg/kg, Sigma), and their left sciatic nerve was exposed and lightly constricted at midthigh by placing two loose 7–0 braided silk (Ethicon, Somerville, NJ) ligatures ≈1.5 mm from one another (22). One of the two groups received a 1-μl intrathecal injection of endo N (200 units/μl) at the time of surgery. The second group received injection of a similar volume of vehicle solution. The limb was then closed and the animals returned to the husbandry after recovery from anesthesia. The third group received endo N injection and no surgery, whereas the remaining animals had no surgery and received no injection.
Behavioral Testing
Thermal Algesia. The unilateral hot-plate test was used to test the sensitivity of the hindpaw to thermal stimuli (26). The animal was held by the operator, and one hindpaw at a time was allowed to touch a 55°C hot plate. The time of withdrawal of the limb was measured, and the mean of two readings was used as a tolerance threshold. The animals were habituated to the test before surgery, and presurgical values were used as a baseline reference for postsurgical measurements. Tests were repeated 1, 2, and 3 weeks after sciatic ligation.
Mechanical Algesia. The sensitivity to touch stimuli was assessed by using the dynamic plantar aesthesiometer (Ugo Basile, Varese, Italy), an automated apparatus based on the von Frey filament principle. The animals were housed over a grid, a stimulating probe was positioned under their hindpaw, and an increasing vertical force (continuous increase from 0 to 20 grams in 10 sec) was applied to the paw. The instrument registered the force intensity that triggered limb withdrawal, and the mean of three readings was used as the tolerance threshold. Before ligation, the animals were habituated to the apparatus, then tested to establish a baseline reference for postsurgical readings. They were retested under the same conditions 1, 2, and 3 weeks after surgery.
Transganglionic Labeling of Fibers. The animals were anesthetized 4–5 days before death, their left hindlimb opened, and 2 μl of fluorescent cholera toxin B (CTB)–Alexa Fluor 488 (Molecular Probes) was injected into the sciatic nerve. This nontoxic subunit labels Aβ fibers transganglionically (27). The specificity of this technique was confirmed by colabeling C fibers with wheat germ agglutinin (WGA)–Alexa Fluor 594; no colocalization of CTB and WGA could be seen in dorsal root fibers in control or CCI animals (see Supporting Text and Figs. 5 and 6, which are published as supporting information on the PNAS web site).
Histological Analysis. Four weeks after surgery, the animals were anesthetized and transcardially perfused with 25 ml of a 7.4 pH phosphate buffer saline solution followed by 25 ml of a 4% paraformaldehyde/0.1 M, 7.4 pH, phosphate buffer solution. The lumbar spinal cord was dissected and postfixed overnight at 4°C in the same fixative. Vibratome 50-μm transverse sections were then prepared. For fluorescent microscopy and confocal imaging of transganglionically labeled fibers, one set of sections was mounted directly on glass slides in mowiol (Calbiochem). A second set was processed for thiamine monophosphatase (TMP) staining to visualize C fiber terminal fields by light microscopy (27). The localization of this enzyme is restricted to lamina II C terminals in spinal dorsal horn, and changes in staining for its end product are a reliable marker for their plasticity (3, 28).
For TMP staining, sections were incubated for 90 min at 37°C in a solution of thiamine monophosphate chloride (0.25%) and lead nitrate (0.08%) in 0.04 M, 5.6 pH, Trismaleate buffer, then mounted on glass slides for microscopic examinations. Digital pictures were taken under a ×10 objective, and the density of TMP staining was measured in three sections from each sample by using the nih image 1.63 (http://rsb.info.nih.gov/nih-image) image analysis system. The ratio of measurements on the ligated side to those on the contralateral side was calculated, and the CCI-induced loss was expressed as percentage of the reduction obtained in vehicle-treated samples. The CTB-labeled Aβ fibers were examined by using a fluorescent microscope (Zeiss Axioplan 2), and ×400 confocal images were scanned by using a Zeiss LSM 510 imaging system. Three images from each sample were used for morphometric analysis. Lamina II of the spinal cord was delineated, and the density of labeled fibers inside its limits was determined by using nih image 1.63 software.
To analyze PSA expression in the spinal cord and to monitor its removal by intrathecal injection of endo N, the 5A5 antibody, a mouse monoclonal IgM specific for PSA, was applied to 50-μm floating vibratome sections at a 1:2,000 dilution. An appropriate Cy3 secondary antibody was used to visualize immunostaining by fluorescent microscopy.
The phosphorylated form of the microtubule-associated protein tau (P-tau) is expressed during terminal plasticity and degeneration (29). The mouse monoclonal anti-human P-tau antibody (clone AT8, Innogenetics, Gent, Belgium) was used (1:1,500) to analyze P-tau-associated terminal plasticity in spinal lamina II.
ANOVA tests were applied for statistical comparison of behavioral and morphometric data between endo N- and vehicle-treated samples. Animal care and experimentation were carried out according to institutional guidelines.
Results
The neuropathic pain model using chronic constriction of the sciatic nerve (22) is amenable to both physiological analysis and histological evaluation of changes (1–6, 27, 30). After an initial validation of the experimental procedure to perturb PSA expression in the spinal cord, the first parameters examined here are the establishment of hyperalgesia for thermal stimuli and the remodeling of C-terminal fields in spinal lamina II. For comparison, the study then turns to the effects of PSA on another aspect of plasticity in this model, namely pathological pain sensitivity to mechanical stimuli (allodynia) associated with the sprouting of Aβ fibers into lamina II.
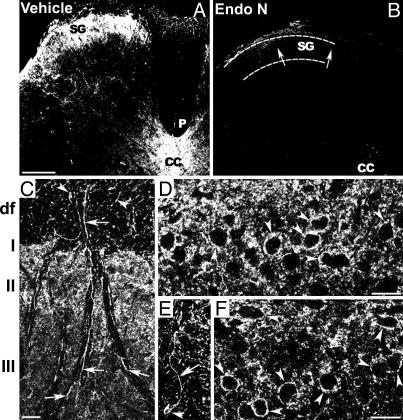
PSA Is Expressed in the Dorsal Spinal Cord and Can Be Suppressed by endo N. Immunostaining for PSA was carried out in lumbar sections of the spinal cord 4 weeks after intrathecal injections. The untreated and vehicle-treated controls showed high levels of PSA expression in the superficial layers of the dorsal horn (Fig. 1A). In contrast with a previous report using total dorsal rhizotomy (8), no detectable change in PSA expression was induced by more limited sciatic CCI lesion in vehicle-treated controls. PSA is expressed in membranes of lamina II interneurons as they were identified by their localization and size and by the shape and length of their immunopositive fibers. It is also expressed on projections to lamina III (Fig. 1 C–F), which presumably are intersegmental projections emanating from these interneurons. Interestingly, when projections of C fibers to lamina II were transganglionically labeled with fluorescent WGA, they appeared devoid of any PSA staining (data not shown). That is, PSA is expressed on the targets for C fibers but not on the C fiber presynaptic elements. PSA immunostaining was also observed in the epithelium of the central canal.
Fig. 1.
PSA is expressed in the spinal superficial laminae on interneurons and their projections and is completely removed by endo N. (A) A cross section of the spinal cord showing that PSA is expressed in superficial laminae (SG, substantia gelatinosa) and lamina X around the central canal (CC). (B) PSA is completely removed by intrathecal endo N injection in vivo; the SG is devoid of staining (arrows). (Bar, 200 μm.) (C) Dorsal portion of the spinal cord showing high levels of PSA in laminae I and II. (Bar, 20 μm.) Arrowheads point to PSA expression in a cross section of intersegmental projections in the dorsal funiculus (df). Some PSA-positive fibers (arrows) veer into the gray matter to branch and terminate in lamina III. (D–F) PSA expression on soma surfaces (arrowheads) and fibers (arrow) of lamina II interneurons. (Bar, 20 μm.)
A critical experimental issue in this investigation was the efficacy of PSA removal from the spinal tissue by intrathecal injection of endo N. Immunostaining for PSA in endo N-treated samples showed that treatment with an excess of enzyme (200 units) completely removes PSA from the spinal cord, and that the removal persists throughout the entire period of the study (Fig. 1B).
PSA Removal Exacerbates Thermal Hyperalgesia. Measurement of withdrawal time when holding the animal and allowing only one hindpaw at a time to touch the hot plate proved more feasible and reliable in assessing thermal sensitivity than when the animal moved freely on the hot plate. Preoperative testing established that the mean baseline latency for hindpaw withdrawal was 12.9 ± 0.1 s. After surgery, when the sensitivity to thermal stimuli of the hindpaw ipsilateral to the sciatic CCI was compared with baseline presurgical values, it appeared that sciatic nerve ligation alone caused a significant (P < 0.01) shortening in withdrawal latency, which decreased with time (Fig. 3A).
Fig. 3.
Removal of PSA from the spinal cord exacerbates thermal hyperalgesia and reduces C-fiber atrophy. (A) Graph representing tolerance to thermal stimuli as assessed by hindpaw withdrawal in the hot-plate test. Sciatic ligation alone shortens the latency of withdrawal. Removal of PSA by endo N significantly (P < 0.01) and persistently intensifies this hyperalgesia. W, week; B, graph showing the CCI-induced loss of lamina II C terminals. The density of TMP staining in lamina II was quantified (see Materials and Methods), and the ratio of the density on the ligated side to that on the unligated side was calculated. The CCI-induced loss of TMP staining was then expressed as percentage of that obtained in vehicle-treated samples. The loss of C terminals is maximal in the presence of PSA (Vehicle). The absence of this carbohydrate from the tissue significantly reduces (47.5% ± 9.7; P < 0.01) the C-terminal loss in lamina II (endo N). (C) Spinal cross sections processed for TMP histoenzymology, a specific staining for C terminals. Note the normal staining pattern in lamina II of the control unligated side. A marked reduction in lamina II TMP staining is induced by ligation in the presence of PSA in the vehicle-treated group (arrows). Removal of PSA by endo N results in the preservation of more terminals. (Bar, 150 μm.)
Comparison of the performances of vehicle- and endo N-treated animals established that the enzymatic removal of PSA exacerbated the CCI-induced thermal hyperalgesia 1 week after surgery by ≈36% (Fig. 3A, P < 0.01), and assessments in the following 2 weeks revealed that these effects persist. endo N injection did not alter the withdrawal sensitivity of unoperated animals (12.7 ± 0.2 s 1 week after injection). These results suggest that the presence of PSA in the spinal cord serves to alleviate the thermal hyperalgesia associated with neuropathic pain.
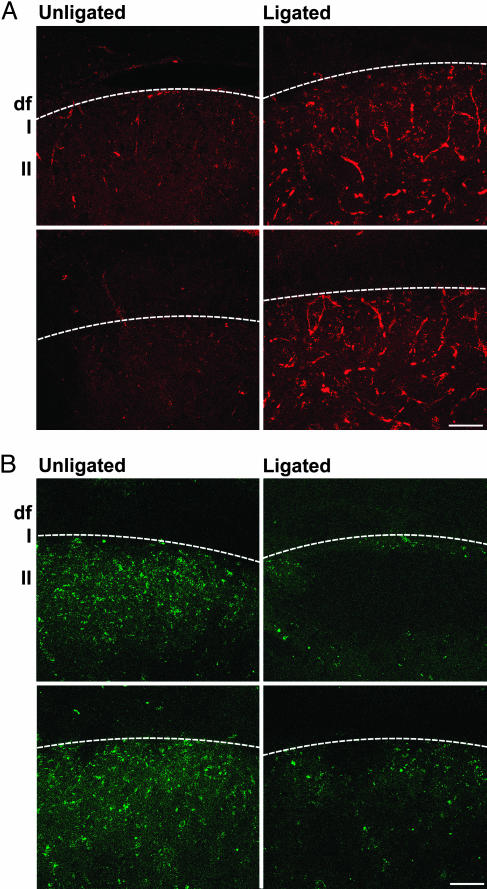
CCI Results in Loss of C Terminals from Lamina II. With these suggestive behavioral results in hand, the study turned to a histological evaluation of the effect of PSA removal on C-terminal loss. It was, however, necessary to first establish that the loss of terminals reported for other models (1–6) also occurs in the CCI model. Four weeks after CCI surgery, the animals were killed and processed for histological examination by using three independent marker systems: fluorescent WGA imaging to visualize C fibers; TMP histoenzymology (27) to detect lamina II C terminals (3, 28); and immunostaining for P-tau, which is expressed during axonal plasticity and terminal regression (29).
In the substantia gelatinosa, numerous fibers became immunopositive for P-tau after ligation (Fig. 2A). In addition, the WGA labeling of C terminals normally found in lamina II was reduced after CCI (Fig. 2B). For TMP staining, the dark/brown reaction product on the unligated side extended from the lateral extremity of lamina II to its medial border with the ascending pathways, and CCI ligation produced a marked disappearance of TMP-labeled terminals (Fig. 3C).
Fig. 2.
CCI causes increase in P-tau immunostaining and depletion of WGA-labeled terminals in lamina II. (A) Spinal cord cross sections showing that P-tau is expressed in fibers of the substantia gelatinosa after CCI. (B) Confocal images of WGA-labeled C terminals in lamina II. Note the abundance of labeling in normal spinal cord and its reduction on the ligated side. The white line separates the white (Upper) and gray (Lower) matters. Df, dorsal funiculus; I and II, spinal laminae. (Bars, 50 μm.)
PSA Removal Inhibits CCI-Induced Loss of C Terminals. There is an intrinsic variability in all CCI studies because of differences in the efficacy of the ligatures applied to the sciatic nerve. The TMP technique, which was found to have the least amount of additional experimental variability and could be most easily quantitated by morphometry, was therefore chosen as the most reliable readout for the PSA perturbation studies. CCI by itself resulted in a substantial (≈50%) loss of TMP staining from large segments of the lamina as well as a diminution of terminal density in some remaining areas (Fig. 3 B and C, P < 0.01). By contrast, in endo N-treated animals, the loss in TMP staining was significantly attenuated (Fig. 3 B and C, P < 0.01). These results indicate that PSA has a facilitating role in C-terminal loss.
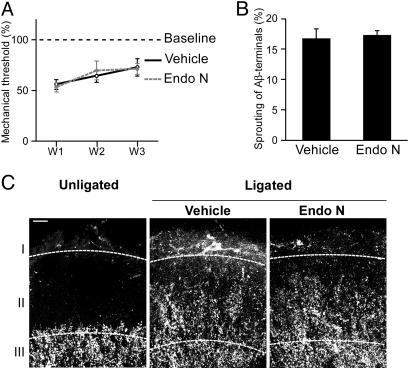
PSA Removal Does Not Affect Mechanical Hyperalgesia. Nerve injury-induced neuropathic pain also involves a deleterious manifestation of Aβ-fiber plasticity, namely the transformation of touch to painful sensations, called allodynia. For instance, sensitivity to mechanical stimuli as assessed by a dynamic plantar aesthesiometer revealed a significant (P < 0.01) and persistent increase in touch sensitivity after ligation (Fig. 4A). However, the removal of PSA from the spinal tissue did not alter ligation-induced mechanical hyperalgesia (Fig. 4A). Therefore, although PSA reduces the severity of thermal hyperalgesia, it does not appear to alter the intensity of allodynia.
Fig. 4.
Removal of PSA from the spinal cord has no effect on ligation-induced allodynia and Aβ-fiber plasticity. (A) This graph shows thresholds of sensitivity to touch as measured by the plantar aesthesiometer. Postsurgical values are significantly lower than baseline levels (allodynia). Note that the presence or absence of PSA does not influence this parameter. Error bars, SEM; W, week. (B) Graph showing the density of CTB-labeled Aβ fibers in lamina II. The amount of sprouting into lamina II is the same in the presence and absence of PSA. Error bars, SEM. (C) Confocal images of CTB-labeled Aβ fibers invading lamina II. This lamina is normally devoid of Aβ terminals, as shown in the control. The sciatic ligation causes these touch fibers to sprout into lamina II, an area containing nociceptive target neurons. endo N- and vehicle-treated animals show similar patterns of sprouting into lamina II. Layers I, II, and III, three superficial laminae of the spinal dorsal horn. (Bar, 20 μm.)
PSA Does Not Promote Sprouting of Aβ Fibers into Lamina II. The absence of effect on allodynia was somewhat surprising, in that sprouting of Aβ fibers into lamina II has been proposed as an anatomical correlate of allodynia (30–32), and PSA is known to promote branching and sprouting during development (13, 19, 20). To assess the effect of PSA on Aβ-fiber sprouting directly, we transganglionically labeled Aβ fibers by preinjection of fluorescent CTB in the sciatic nerve. This labeling is specific for Aβ fibers, in that it does not colocalize with the C-fiber marker WGA when both markers are coinjected in the ligated sciatic nerve (see Supporting Text). In the unligated animals, the Aβ-terminal fields were seen as large fluorescent areas within and below lamina III, whereas lamina II was free of staining. As expected, there was a robust invasion of lamina II by Aβ terminals after ligation (Fig. 4 B and C). However, in agreement with measurements of the ligation-induced allodynia (Fig. 4A), the presence or absence of PSA did not detectably alter the amount of this sprouting (Fig. 4 B and C).
Discussion
Although PSA is used during development to prevent premature or ectopic formation of synapses by preventing the establishment of strong cell contacts (18, 20), the present results suggest that in the context of adult pain pathways this carbohydrate appears to facilitate the loss of C terminals from lamina II in the CCI model of chronic pain.
In our studies, the experimental removal of PSA diminished the reduction of TMP-positive C-terminal density in spinal cord lamina II and exacerbated the intensity of hyperalgesia to thermal stimuli. These findings strengthen the connection between the anatomical and physiological changes observed in response to stressful pain and demonstrate that the PSA-dependent changes in these terminals serve to limit the overflow of painful peripheral insults from reaching central pain pathways.
Although PSA has also been shown to promote branching and sprouting during development (13, 19, 20), no change in the amount of sprouting of Aβ-touch fibers into lamina II could be detected after endo N treatment. This anatomical finding is in agreement with the absence of a role for PSA in ligation-induced allodynia, which is believed to result from Aβ-fiber sprouting and synapse formation with lamina II pain neurons (30, 31). That removal of PSA affects changes in C terminals without influencing the amount of Aβ-fiber sprouting into the same spinal region probably reflects the fact that the underlying cellular mechanisms are likely to be quite different for these two phenomena and therefore could differ in their susceptibility to PSA removal. However, the possibility remains that PSA might have an effect on the rate of Aβ-fiber sprouting and not its amount. If this is the case, a difference might be visible only during early stages of sprouting. In any case, it appears that use of PSA-dependent plasticity in spinal lamina II is precisely orchestrated to alleviate the severity of chronic pain without increasing deleterious secondary effects.
The exact nature of the cellular mechanism underlying the functional uncoupling and terminal loss promoted by PSA in lamina II remains to be determined. In addition to our observations with WGA, P-tau, and TMP stainings in the CCI model, studies using a combination of TMP staining and electron microscopy showed that the loss of TMP produced by peripheral nerve transection, nerve crush (2, 3), or blockade of axonal transport (33) correlates with a reversible degeneration of the C terminals in lamina II. If this degeneration process includes a disassembly of the synaptic complex, which comprises several modes of cell–cell interaction, then the unique ability of PSA to simultaneously affect a wide variety of adhesive mechanisms (12, 34) makes it a particularly potent means for achieving this type of plasticity. However, other mechanisms such as a direct effect of PSA on neurotransmission could contribute as well (for review, see ref. 35).
It is interesting that afferents to the ventral auditory nucleus also express high levels of PSA (our unpublished studies), and that noxious acoustic insults to the auditory system are associated with a reversible atrophy of nerve terminals in the ventral auditory nucleus (36–38). Similarly, mossy fibers and cornu ammonis (CA) neurons of the hippocampus express PSA (18, 21, 39), and CA3 apical dendrites undergo a transient stress-induced withdrawal (40–45) that is believed to help reduce detrimental effects of stress (46). On this basis, the possibility is raised that the PSA-dependent alteration of neuronal connections observed in the chronic pain system may be applicable to all three sensory systems. We propose that the presence of PSA at a particular site along an input pathway allows for a local and reversible break in that pathway in response to excessive stimulation and thereby could protect the brain from overload and consequent deleterious alterations.
Supplementary Material
Acknowledgments
We thank Dr. McEwen's laboratory (The Rockefeller University, New York) for providing antibodies.
Author contributions: A.E.M. designed research; A.E.M. and Y.K. performed research; G.P., Y.K., and U.R. contributed new reagents/analytic tools; A.E.M. analyzed data; A.E.M. and U.R. wrote the paper; and G.P. and U.R. supervised the project.
Abbreviations: PSA, polysialic acid; TMP, thiamine monophosphatase; CCI, chronic constriction injury; endo N, endoneuraminidase N; WGA, wheat germ agglutinin; CTB, cholera toxin B; P-tau, protein tau.
References
- 1.Knyihar-Csillik, E., Csillik, B. & Rakic, P. (1982) J. Comp. Neurol. 210, 357-375. [DOI] [PubMed] [Google Scholar]
- 2.Knyihar-Csillik, E., Kreutzberg, G. W. & Csillik, B. (1989) J. Neurosci. Res. 22, 74-82. [DOI] [PubMed] [Google Scholar]
- 3.Knyihar-Csillik, E. & Torok, A. (1989) Neuroscience 33, 75-91. [DOI] [PubMed] [Google Scholar]
- 4.Knyihar-Csillik, E., Torok, A. & Csillik, B. (1990) J. Comp. Neurol. 297, 594-612. [DOI] [PubMed] [Google Scholar]
- 5.Por, I. (1985) Acta Physiol. Hung. 65, 255-262. [PubMed] [Google Scholar]
- 6.Doubell, T. P. & Woolf, C. J. (1997) J. Comp. Neurol. 386, 111-118. [DOI] [PubMed] [Google Scholar]
- 7.Bonfanti, L., Olive, S., Poulain, D. A. & Theodosis, D. T. (1992) Neuroscience 49, 419-436. [DOI] [PubMed] [Google Scholar]
- 8.Bonfanti, L., Merighi, A. & Theodosis D. T. (1996) Neuroscience 74, 619-623. [DOI] [PubMed] [Google Scholar]
- 9.Rutishauser, U. (1996) Curr. Opin. Cell Biol. 8, 679-684. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama, J., Angata, K., Ong, E., Katsuyama, T. & Fukuda, M. (1998) Pathol. Int. 48, 665-677. [DOI] [PubMed] [Google Scholar]
- 11.Yang, P., Yin, X. & Rutishauser, U. (1992) J. Cell. Biol. 116, 1487-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto, I., Bruses, J. L. & Rutishauser, U. (2001) J. Biol. Chem. 276, 31745-31751. [DOI] [PubMed] [Google Scholar]
- 13.Rutishauser, U. & Landmesser, L. (1996) Trends Neurosci. 19, 422-427. [DOI] [PubMed] [Google Scholar]
- 14.Hu, H., Tomasiewicz, H., Magnuson, T. & Rutishauser, U. (1996) Neuron 16, 735-743. [DOI] [PubMed] [Google Scholar]
- 15.Wang, C., Rougon, G. & Kiss, J. Z. (1994) J. Neurosci. 14, 4446-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida, K., Rutishauser, U., Crandall, J. E. & Schwarting, G. A. (1999) J. Neurosci. 19, 794-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petridis, A. K., El Maarouf, A. & Rutishauser, U. (2004) Dev. Dyn. 230, 675-684. [DOI] [PubMed] [Google Scholar]
- 18.Seki, T. & Rutishauser, U. (1998) J. Neurosci. 18, 3757-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto, N., Inui, K., Matsuyama, Y., Harada, A., Hanamura, K., Murakami, F., Ruthazer, E. S., Rutishauser, U. & Seki, T. (2000) J. Neurosci. 20, 9145-9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Maarouf, A. & Rutishauser, U. (2003) J. Comp. Neurol. 460, 203-211. [DOI] [PubMed] [Google Scholar]
- 21.Seki, T. & Arai, Y. (1993) Brain Res. Dev. Brain Res. 73, 141-145. [DOI] [PubMed] [Google Scholar]
- 22.Seltzer, Z., Dubner, R. & Shir, Y. (1990) Pain 43, 205-218. [DOI] [PubMed] [Google Scholar]
- 23.Vimr, E. R., McCoy, R. D., Vollger, H. F., Wilkison, N. C. & Troy, F. A. (1984) Proc. Natl. Acad. Sci. USA 81, 1971-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallenbeck, P. C., Vimr, E. R., Yu, F., Bassler, B. & Troy, F. A. (1987) J. Biol. Chem. 262, 3553-3556. [PubMed] [Google Scholar]
- 25.Glass, J. D., Shen, H., Fedorkova, L., Chen, L., Tomasiewicz, H. & Watanabe, M. (2000) Neurosci. Lett. 280, 207-210. [DOI] [PubMed] [Google Scholar]
- 26.Menéndez, L., Lastra, A., Hidalgo A. & Baamonde, A. (2002) J. Neurosci. Methods 113, 91-97. [DOI] [PubMed] [Google Scholar]
- 27.Mannion, R. J., Doubell, T. P., Coggeshall, R. E. & Woolf, C. J. (1996) J. Neurosci. 16, 5189-5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knyihar-Csillik, E., Bezzegh, A., Boti, S. & Csillik B. (1986) J. Histochem. Cytochem. 34, 363-371. [DOI] [PubMed] [Google Scholar]
- 29.Arendt, T., Stieler, J., Strijkstra, A. M., Hut, R. A., Rudiger, J., Van der Zee, E. A., Harkany, T., Holzer, M. & Hartig, W. (2003) J. Neurosci. 23, 6972-6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cousins, M. J. (1999) Anesthesiology 91, 538-551. [DOI] [PubMed] [Google Scholar]
- 31.Woolf, C. J., Shortland, P. & Coggeshall, R. E. (1992) Nature 355, 75-78. [DOI] [PubMed] [Google Scholar]
- 32.Soares, S., von Boxberg, Y., Lombard, M. C., Ravaille-Veron, M., Fischer, I., Eyer, J. & Nothias, F. (2002) Eur. J. Neurosci. 16, 593-606. [DOI] [PubMed] [Google Scholar]
- 33.Csillik, B., Knyihar, E., Jojart, I., Elshiekh, A. A. & Por, I. (1978) Res. Commun. Chem. Pathol. Pharmacol. 21, 467-484. [PubMed] [Google Scholar]
- 34.Johnson, C. P., Fujimoto, I., Rutishauser, U. & Leckband, D. E. (2005) J. Biol. Chem. 280, 137-145. [DOI] [PubMed] [Google Scholar]
- 35.Sandi, C. (2004) Nat. Rev. Neurosci. 5, 917-930. [DOI] [PubMed] [Google Scholar]
- 36.Bilak, M., Kim, J., Potashner, S. J., Bohne, B. A. & Morest, D. K. (1997) Exp. Neurol. 147, 256-268. [DOI] [PubMed] [Google Scholar]
- 37.Kim, J., Morest, D. K. & Bohne, B. A. (1997) Hear. Res. 103, 169-191. [DOI] [PubMed] [Google Scholar]
- 38.Morest, D. K., Kim, J., Potashner, S. J. & Bohne, B. A. (1998) Microsc. Res. Technol. 41, 205-216. [DOI] [PubMed] [Google Scholar]
- 39.Nacher, J., Blasco-Ibanez, J. M. & McEwen, B. S. (2002) Brain Res. 930, 1-11. [DOI] [PubMed] [Google Scholar]
- 40.Sousa, N., Lukoyanov, N. V., Madeira, M. D., Almeida, O. F. & Paula-Barbosa, M. M. (2000) Neuroscience 97, 253-266. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe, Y., Gould, E. & McEwen, B. S. (1992) Brain Res. 588, 341-345. [DOI] [PubMed] [Google Scholar]
- 42.Magarinos, A. M. & McEwen, B. S. (1995) Neuroscience 69, 83-88. [DOI] [PubMed] [Google Scholar]
- 43.Magarinos, A. M., McEwen, B. S., Flugge, G. & Fuchs, E. (1996) J. Neurosci. 16, 3534-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conrad, C. D., LeDoux, J. E., Magarinos, A. M. & McEwen, B. S. (1999) Behav. Neurosci. 113, 902-913. [DOI] [PubMed] [Google Scholar]
- 45.McKittrick, C. R., Magarinos, A. M., Blanchard, D. C., Blanchard, R. J., McEwen, B. S. & Sakai, R. R. (2000) Synapse 36, 85-94. [DOI] [PubMed] [Google Scholar]
- 46.Magarinos, A. M., Verdugo, J. M. & McEwen, B. S. (1997) Proc. Natl. Acad. Sci. USA 94, 14002-14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.