Abstract
B cell homeostasis is maintained by a balance between the continual generation of new cells and their elimination. Here we show proapoptotic BCL-2 family members BAX and BAK are essential for regulating the number of B cells at both immature and mature developmental stages. BAX and BAK are critical mediators of B cell death induced by multiple stimuli. In addition, BAX- and BAK-deficient B cells display defective cell cycle progression to B cell receptor crosslinking and lipopolysaccharide, but not to CpG–DNA. Furthermore, inducible deletion of Bax and Bak in adult mice results in the development of severe autoimmune disease.
The development and maintenance of B lymphocytes is regulated at multiple checkpoints (1). Newly generated pro-B cells undergo B cell antigen-receptor (BCR) recombination in fetal liver and adult bone marrow (BM). B cells that fail to produce a functional BCR lack survival signals and thus undergo death by neglect (2). B cells that express surface BCRs that interact with self-antigens with too high affinity may be eliminated, at least in part, by apoptotic cell death, as well as anergy and receptor editing (3, 4). Immature B cells that successfully pass through developmental checkpoints in the BM migrate to the spleen as transitional B cells and undergo further maturation steps (5). The number of B lymphocytes in BM and secondary lymphoid organs is kept under tight control. Disruption of this homeostasis and accumulation of excess lymphocytes may give rise to the development of autoimmunity or malignancy.
BCL-2 family member proteins are requisite for the maintenance of lymphocyte numbers by regulating an intrinsic apoptosis pathway (2, 6–8). The antiapoptotic members, including BCL-2, BCL-xL, and MCL-1, display sequence conservation in four BCL-2 homology domains (BH1–BH4). Multidomain proapoptotic members, including BAX and BAK, possess the BH1, BH2, and BH3 domains. The “BH3-only” proteins, including BID, BAD, BIM, NOXA, and PUMA, show homology only within this single amphipathic α-helical segment. BH3-only molecules function as upstream sentinels that sense cellular abnormalities, such as lack of survival signals or DNA damage. Activated BH3-only molecules trigger the oligomerization of multidomain proapoptotic BCL-2 members by sequestering antiapoptotics and/or by direct activation of BAX or BAK (9–12). Oligomerization of BAX or BAK leads to the release of cytochrome c from mitochondria to the cytoplasm, causing the formation of the apoptosome and execution of cell death.
The role of the BCL-2 family in B cell homeostasis has been analyzed intensively by using mouse models that either have overexpression or deletion of BCL-2 family molecules (2, 13–15). Overexpression of BCL-2 protects B cells from death by neglect and by negative selection, and causes the accumulation of B cells (13, 16, 17). In addition, lymphocytes from Bcl-2 transgenic mice show defects in cell cycle progression (18, 19). In contrast, targeted deletion of the Bcl-2 gene in mice resulted in the massive loss of mature lymphocytes due to apoptosis, as well as increased turnover of thymocytes (15, 20). Deletion of another antiapoptotic, MCL-1, resulted in the loss of B cells throughout their development (21). Among BH3 only molecules, only BIM is implicated in the homeostatic maintenance of lymphocytes. Bim-deficient mice are reported to show abnormal development of T lymphocytes and accumulation of lymphocytes in secondary lymphoid organs (20, 22). Bim–/– B cells were resistant to apoptosis induced by the absence of IL-7 receptor signaling and by BCR ligation, and showed defective negative selection of B cells in vivo (23, 24). In addition, Bim–/– mice develop autoimmune kidney disease (22). However, it is still unclear whether this single BH3-only molecule functions as the sole regulator of lymphocyte number.
Mice deficient in either Bax or Bak showed minimal phenotype in lymphocytes except mild lymphoadenopathy reported in Bax-deficient mice (25). Deficiency in both Bax and Bak causes embryonic lethality in most cases. The surviving doubly deficient mice display lymphocyte abnormalities, including increased numbers of splenocytes and resistance of splenocytes and thymocytes to apoptotic stimuli (26). Mouse embryonic fibroblasts (MEFs) deficient for both Bax and Bak (Bax–/–Bak–/–) are highly resistant to induction of apoptosis by multiple intrinsic death stimuli and by the enforced expression of BH3-only molecules (9, 10, 26, 27). In addition, MEFs from Bax–/–Bak–/– mice have reduced endoplasmic reticulum (ER) Ca2+ stores, which results in impaired apoptotic responses to agents that induce Ca2+ release from ER (28). T cell development was further analyzed and shown to be perturbed by reconstituting immunodeficient mice with Bax–/–Bak–/– hematopoietic cells (29). However, analyses of other cell types is not amenable by this method. In this study, we show the establishment of conditional Bax and Bak doubly deficient mice, and investigate the effect of Bax and Bak deficiency specifically in the B cell lineage and compare this phenotype to that of Bim–/– mice. Deletion of Bax and Bak in B cells results in accumulation of immature and mature follicular B cells and in the abrogation of apoptosis. In contrast, Bim deficiency causes accumulation of only mature splenic B cells and partial resistance to apoptosis. In addition, Bax- and Bak-deficient B cells are defective in cell cycling in response to stimulation with BCR crosslinking and LPS. Furthermore, induced Bax and Bak deficiency in adulthood results in the development of severe autoimmune glomerular nephritis. These results demonstrate that BAX and BAK are essential gateways for both apoptosis and maintenance of B cell homeostasis.
Materials and Methods
Generation of Conditional Bax,Bak Double Knockout Mice. The targeting vector was constructed by inserting exons 2–4 of the Bax gene flanked by loxP sites, 6 kb of 5′ sequence, 1 kb of 3′ sequence, and a neomycin resistance (neo) gene flanked by loxP sites into pBluescript. The vector was electroporated into RW4 ES cells, and clones resistant to G418 were screened for homologous recombination by PCR and Southern blotting. The ES cells were transiently transfected with CMV–Cre to eliminate the neo gene. The successfully recombined ES clones were injected into C57BL/6 blastocysts. The chimeric mice were bred for germ-line transmission to obtain Baxfl/+ mice. The mice were further crossed with Bax+/–, Bak–/–, and CD19Cre or MxCre mice (Cre mice were kindly provided by K. Rajewsky, Harvard Medical School) (30, 31). Control mice carried at least one allele of either Bax or Bak.
Flow Cytometric Analysis. Single cell suspensions were incubated with anti-CD16/32 to block nonspecific binding, and subsequently stained with cocktails of various mAbs conjugated with FITC, phycoerythrin (PE), allophycocyanin (APC), or biotin for 30 min at 4°C. AA4.1-PE was purchased from eBioscience. The other antibodies for FACS analysis were purchased from PharMingen unless otherwise indicated. Streptavidin–APC was used to develop biotinylated mAbs. Flow cytometry was performed by using FACSCalibur (Becton Dickinson) and analyzed with flowjo software (Treestar, Ashland, OR).
Immunohistochemistry. Frozen 6-μm sections were fixed in acetone and stained with rat anti-MOMA-1 (Cedarlane Laboratories), followed by alkaline phosphatase-conjugated streptavidin. The sections were subsequently stained with biotinylated rat anti-CD19 (Pharmingen) followed with horseradish peroxidase-conjugated goat anti-rat IgG.
Cell Death Assays. Sorted or bead-purified cells were cultured in RPMI medium 1640 supplemented with 10% FCS with cytotoxic stimuli including 100 nM dexamethasone (Sigma), 250-rad γ-irradiation, 20 μg/ml VP-16 (Sigma), and indicated concentrations of anti-IgM F(ab′)2 fragment (Jackson Immuno-Research). The cells were stained with Annexin V-Cy3 (Bio-Vision) and analyzed by flow cytometry.
Carboxyfluorescein Succinimidyl Ester (CFSE) Labeling. Purified splenic B cells were incubated with 5 μM CFSE (Molecular Probes) for 7 min in PBS, then further incubated for 3 min in the presence of 10% FCS, followed by three washes with RPMI medium 1640 containing 10% FCS. The labeled cells were stimulated with 5 μg/ml anti-IgM F(ab′)2 fragment, 5 μg/ml LPS (O55:B5, Sigma) or 50 μM CpG–DNA (synthesized by IDT) for 3 days. Fluorescence intensity was evaluated by flow cytometry.
For further details, see Supporting Text and Fig. 6, which are published as supporting information on the PNAS web site.
Results
Generation of Conditional Bax-Mutant Mice. A conditional Bax allele was generated by targeting loxP sites upstream of exon 2 and downstream of exon 4 to generate the Baxflox allele (Baxfl) (Fig. 6 A and B). Baxfl/+ mice were bred to the previously described null Bax mice (Bax–/–) to generate Baxfl/– mice (25). Baxfl/– mice were crossed with mice expressing Cre recombinase under the control of the CD19 promoter to restrict the deletion of the remaining Bax allele in B lineage cells. The mice were further crossed with Bak–/– mice to generate mice in which both BAX and BAK protein is specifically deleted in B cells. To determine the efficiency of Bax deletion in B cells, splenic B cells were purified from CD19Cre+Baxfl/–Bak–/– mice and analyzed by Southern blotting. Highly efficient deletion of Bax in B cells was observed (Fig. 6 C and D). The expression of BAX in thymocytes was not altered in CD19Cre+Baxfl/–Bak–/– mice, demonstrating the B cell specificity of deletion.
Generation of Bim Null Mice. To generate mice completely null for Bim, we adopted a targeting strategy different from that reported by Bouillet et al. (22). Newly targeted mice in which the first 4 exons of the Bim gene are deleted by homologous recombination of ES cells were generated to ensure the lack of any aberrant BIM protein (Fig. 7, which is published as supporting information on the PNAS web site); this was especially important because the Bim-targeted mice generated by Bouillet et al. were reported to still express an aberrant BIM protein without a BH3 domain, raising the possibility that this partial protein may have affected the phenotype of these mice.
Increased Abundance of B Cells in CD19Cre+Baxfl/–Bak–/– Mice. First, we examined the numbers and developmental subsets of B cells in BM. As shown in Table 1, the total cellularity of CD19Cre+Baxfl/–-Bak–/– BM cells was increased ≈2-fold compared to that of control mice that carried one allele of either BAX or BAK. Flow cytometric analysis revealed that the increase is due to an accumulation of developing B cells. The number of CD43+IgM– Pro-B cells and IgM+ immature B cells was increased by 4-fold compared to those of control mice (Fig. 1A and Table 1). In contrast, the number of BM B cells from Bim–/– mice is equivalent to that from control cells at all differentiation stages.
Table 1. B cell subpopulations in CD19Cre+BaxFl/-Bak-/- mice compared to control and Bim–/– mice.
| Number of cells per mouse
|
|||
|---|---|---|---|
| Cell populations | Control | CD19Cre+-BaxFl/-Bak-/- | Bim-/- |
| BM | |||
| Bone marrow (×106) | 30.5 ± 5.7 | 47.5 ± 7.4 | 28.2 ± 5.2 |
| B220+ cells (×106) | 7.84 ± 2.38 | 21.1 ± 5.09 | 8.67 ± 2.57 |
| Pro-B cells (×106) | 1.57 ± 0.51 | 4.77 ± 0.52 | 1.25 ± 0.46 |
| Pre-B cells (×106) | 3.29 ± 1.35 | 3.88 ± 1.46 | 2.99 ± 2.25 |
| Immature B cells (×106) | 1.54 ± 0.75 | 6.39 ± 3.94 | 2.22 ± 1.54 |
| Mature B cells (×106) | 1.22 ± 0.61 | 5.10 ± 1.43 | 1.62 ± 0.53 |
| Spleen | |||
| Total splenocytes (×107) | 11.5 ± 3.1 | 44.1 ± 8.8 | 31.8 ± 4.03 |
| B220+ cells (×107) | 5.77 ± 1.67 | 33.1 ± 6.85 | 17.4 ± 4.24 |
| CD3+ cells (×107) | 3.73 ± 0.73 | 5.25 ± 1.74 | 10.7 ± 4.56 |
| B220+AA4.1+ (×106) | 7.39 ± 2.57 | 33.9 ± 10.4 | 22.4 ± 9.53 |
| T1 (×106) | 1.31 ± 0.45 | 4.09 ± 2.05 | 2.85 ± 1.67 |
| T2 (×106) | 2.39 ± 1.71 | 8.48 ± 4.29 | 7.97 ± 2.98 |
| T3 (×106) | 1.92 ± 0.71 | 8.60 ± 2.47 | 5.03 ± 1.81 |
| Follicular (×107) | 4.20 ± 1.50 | 27.7 ± 6.58 | 15.1 ± 3.18 |
| MZ (×106) | 5.14 ± 1.20 | 1.97 ± 1.70 | 7.54 ± 3.21 |
| PC | |||
| B-1 (×105) | 3.0 ± 2.3 | 2.7 ± 1.6 | 3.2 ± 2.2 |
| B-2 (×105) | 2.2 ± 1.1 | 24.8 ± 17.1 | 5.8 ± 0.6 |
Number of cells per mouse. Numbers represent the mean (+/- SD) of 12 control, 12 CD 19Cre+BaxFl/-Bak-/-, and 9 Bim-/- mice. Results were obtained by flow cytometric analysis of BM cells obtained from two femurs based on B220, CD43, and IgM expression, splenocytes based on the expression of B220, AA4.1, IgM, CD21, and CD23, and peritoneal cells (PC) based on the expression of CD5 and B220.
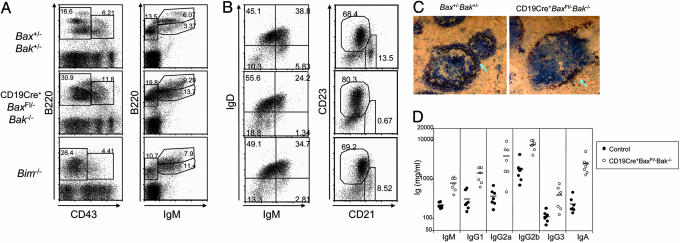
Fig. 1.
Increased number of B cells and augmented Ig production in CD19Cre+Baxfl/–Bak–/– and Bim–/– mice. (A) Development of B cells in BM. Surface B220, CD43, and IgM expression on BM cells obtained from Bax+/–Bak+/–, CD19Cre+Baxfl/–Bak–/–, and Bim–/– mice. (B) Analysis of B cell markers on B cells from spleen. Surface IgM and IgD expression, and CD21 and CD23 expression on B220-gated splenic B cells, is shown. (C) Immunohistochemical staining of spleen sections for MOMA-1+ (red) sinusoidal macrophages and CD19+ (blue) B cells. The arrow indicates MZ B cells surrounding the sinus. (D) Serum Ig levels in CD19Cre+Baxfl/–Bak–/– mice. Serum was collected from 8-week-old control and CD19Cre+Baxfl/–Bak–/– mice, and Ig levels were determined by ELISA. Mean values are indicated by a bar.
Immature B cells that have passed checkpoints in the BM emigrate to the spleen where they undergo further transitional stages to become mature follicular or marginal zone (MZ) B cells. These transitional B cells express the surface marker AA4.1. The proportion of B220+ splenic cells that are AA4.1+ transitional B cells is comparable in control, CD19Cre+Baxfl/–Bak–/–, and Bim–/– mice (Fig. 8A, which is published as supporting information on the PNAS web site). However, the absolute number of AA4.1+ transitional B cells was increased by 4 times in CD19Cre+Baxfl/–-Bak–/– mice compared to control mice (Table 1). The transitional B cells can be further divided into three different stages based on the expression of IgM and CD23 and designated transitional (T) 1 (IgMhighCD23–), T2 (IgMhighCD23+), and T3 (IgMlowCD23+) (32). The numbers of transitional B cells at all three stages were increased by 3–5 times in CD19Cre+Baxfl/–Bak–/– mice than control (Fig. 8B and Table 1). The number of transitional and follicular B cells was also markedly increased in the spleens of Bim–/– mice. Interestingly, although CD21intCD23high follicular B cells are strikingly increased in CD19Cre+Baxfl/–Bak–/– mice, the relative population of CD21highCD23low MZ B cells was remarkably lower than control mice (Fig. 1B). We confirmed this result by immunohistological analysis of spleen sections with the B cell marker CD19 and the metallophilic macrophage marker MOMA-1. Few B cells existed in the MZ area of spleen sections of CD19Cre+Baxfl/–-Bak–/– mice, in contrast to control mice (Fig. 1C). Although the absolute number of MZ B cells in Bim–/– mice was approximately equal to control, the proportion of MZ relative to transitional and follicular B cells is reduced. Furthermore, B-2 cells, but not B-1 cells obtained from the peritoneal cavity, were increased in CD19Cre+Baxfl/–Bak–/– mice and to a lesser extent in Bim–/– mice (Fig. 8B and Table 1). These results demonstrate that BAX and BAK deficiency causes accumulation of transitional and follicular, but not MZ, B cells.
To study the functional capacity of aberrantly increased B cells from CD19Cre+Baxfl/–Bak–/– mice, we measured the serum Ig concentrations in nonimmunized mice by ELISA. All isotypes tested were 5–10 times higher in CD19Cre+Baxfl/–Bak–/– mice compared to control mice (Fig. 1D). The Ig concentrations of Bim–/– mice were also increased, in agreement with previous reports (data not shown) (22). These results indicate that, in the absence of BAX and BAK, the elevated number of B cells correlates with abnormal production of Ig.
BAX and BAK Are Essential Mediators of B Cell Apoptosis. In BM, B cell genesis is regulated by a delicate balance between proliferation and death. Developing B cells cultured in the absence of cytokines and B cells expressing BCR that can react with autoantigens are eliminated by apoptosis. Because CD19Cre+Baxf/–Bak–/– mice showed increased abundance of BM B cells, we examined whether loss of Bax and Bak or Bim affect the survival of developing BM B cells cultured in vitro. B220+IgM– and B220+IgM+ immature B cells were purified by flow cytometry, and cultured in the absence of cytokines for 2 and 4 days. Whereas control B cells died in the course of culture so that there were almost no viable cells after 4 days, CD19Cre+Baxfl/–Bak–/– cells were highly resistant to cell death (Fig. 2A). Although the number of B cells in the bone marrow was not increased in Bim–/– mice, the survival of Bim–/– IgM– and IgM+ B cells in the absence of cytokines was intermediate between that of control and CD19Cre+Baxfl/–Bak–/– cells (Fig. 2A).
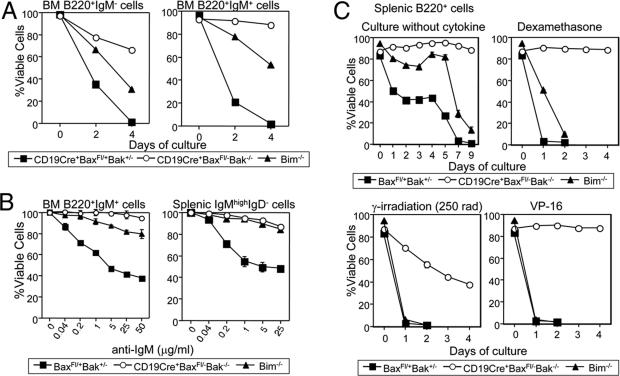
Fig. 2.
Defective cell death CD19Cre+Baxfl/–Bak–/– and Bim–/– B cells. (A) Cell death by neglect in BM B cells. Sorted B220+IgM– and B220+IgM+ BM B cells were cultured without cytokine stimulation. Cell survival after days 2 and 4 was quantified by annexin V staining and flow cytometric analysis. (B) BCR ligation-induced cell death. Immature BM B cells sorted as B220+IgM+ and immature splenic B cells sorted as B220+IgMhighIgD– were stimulated with increasing amounts of anti-IgM F(ab′)2 antibody for 18 h. The number of viable cells was determined by flow cytometry. Cell survival of anti-IgM F(ab′)2 antibody-stimulated B cells was calculated as a percentage of the number of B cells surviving in unstimulated cultures. (C) Purified splenic B cells were cultured in medium without cytokines, with 10 nM dexamethasone after 250 rad γ-irradiation, or with 20 μg/ml VP-16 for the indicated time period. Cell viability was determined.
Next, we examined whether BAX and BAK is involved in the negative selection of B cells. Immature B cells are known to undergo apoptosis after BCR crosslinking, and this system has been used as an in vitro model for negative selection. Immature BM (B220+IgM+) B cells and immature splenic (IgMhighIgDlow) B cells were sorted from control and mutant mice and stimulated with increasing concentrations of anti-IgM F(ab′)2 fragment. Control B cells died after 18 h of culture in response to BCR ligation in a dose-dependent manner (Fig. 2B). In contrast, B cells from CD19Cre+Baxfl/–Bak–/– mice were viable after stimulation with up to 50 μg/ml anti-IgM F(ab′)2 fragment. Immature B cells from Bim–/– mice were also highly resistant to induction of cell death in response to BCR crosslinking, consistent with previous reports.
We next examined the role of BAX, BAK, and BIM in the cell death pathway of various apoptosis stimuli in mature splenic B cells. Purified B cells from spleens were cultured either without cytokines, in the presence of dexamethasone or VP-16, or after γ-irradiation. Bax- and Bak-deficient splenic B cells were dramatically resistant to all of these death stimuli, consistent with enhanced accumulation of B cells in these mice (Fig. 2C). Bim–/– mature splenic B cells showed only partial viability relative to control and CD19Cre+Baxfl/–Bak–/– cells. Bim deficiency afforded enhanced survival for only the short term when cells were cultured in the absence of cytokines or in the presence of dexamethasone; it was not sufficient to block death after 7–9 days of culture without cytokines or 2 days after dexamethasone. Furthermore, Bim–/– cells were as sensitive to VP16 and γ-irradiation as control cells. CD19Cre+Baxfl/–Bak–/– cells were significantly more resistant to all of the death stimuli. These results indicate that Bim ablation does not recapitulate the phenotype of BAX/BAK doubly deficient cells, suggesting the existence of alternative apoptotic signaling pathway via BAX/BAK distinct from BIM.
Impaired Cell Cycle Progression in CD19Cre+Baxfl/–Bak–/– but Not in Bim–/– Splenic Cells After Stimulation with Mitogens. The importance of BCL-2 in controlling cell cycle entry in T cells was established from studies using Bcl-2 transgenic mice (18, 33). Mature B cells are activated and proliferate upon encountering cognate antigens as well as pathogen-specific components, such as LPS and bacterial DNA with a CpG motif (34). To investigate the impact of Bax and Bak deficiency in cell cycle in B cells, we stimulated carboxyfluorescein succinimidyl ester-labeled splenic B cells with various B cell mitogens, such as anti-IgM Ab and Toll-like receptor (TLR) ligands. More than 90% of control cells underwent cell divisions in 3 days of culture in the presence of 5 μg/ml LPS or 50 nM CpG–DNA. Bim–/– cells divided similarly to control cells upon mitogen stimulation. In contrast, cells from CD19Cre+ Baxfl/–Bak–/– mice showed impaired proliferation in response to LPS and anti-IgM stimulation, but surprisingly not to CpG–DNA (Fig. 3A).
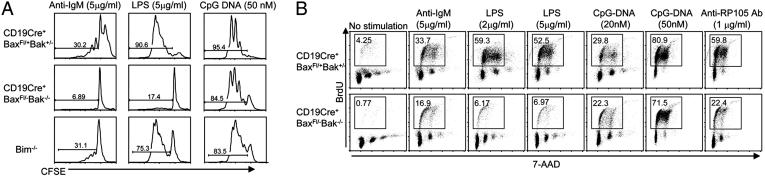
Fig. 3.
Impaired B cell proliferation in Bax- and Bak-deficient B cells. (A) Impaired cell division in response to anti-IgM and LPS in B cells from CD19Cre+ Baxfl/–Bak–/– mice. Carboxyfluorescein succinimidyl ester-labeled splenic B cells were stimulated with the indicated concentrations of anti-IgM, LPS, and CpG–DNA. Fluorescence was measured 3 days after stimulation. (B) Impaired DNA synthesis in response to anti-IgM and LPS in CD19Cre+Baxfl/–Bak–/– B cells. Splenic B cells were cultured in the presence or absence of anti-IgM, LPS, CpG–DNA, and anti-RP105 Ab for 24 h and pulsed with BrdUrd for 16 h. The cells were stained with anti-BrdUrd antibody and 7-AAD and analyzed by flow cytometry. Percentage of cells in S phase (box) are indicated.
DNA synthesis after mitogen stimulation was also analyzed by pulsing the cells with BrdUrd and intracellular staining with anti-BrdUrd Ab. Cells from CD19Cre+Baxfl/–Bak–/– mice showed defective incorporation of BrdUrd in response to LPS and anti-IgM Ab, but not to CpG–DNA (Fig. 3B). In contrast, BrdUrd incorporation upon mitogen stimulation was not impaired in B cells from control mice deficient in either BAX or BAK alone (data not shown). These results indicate that BAX and BAK deficiency, but not BIM deficiency, strongly effects cell cycle regulation by inhibiting S phase entry.
To further dissect the defect in cell cycle progression, we measured the expression of the cyclin-dependent kinase (cdk) inhibitor p27 protein with time after stimulation with either CpG–DNA or LPS. p27 protein was down-regulated 12 h after CpG–DNA stimulation in control and CD19Cre+Baxfl/–Bak–/– B cells (Fig. 4C). In response to LPS, however, p27 expression was reduced in control but not CD19Cre+Baxfl/–Bak–/– B cells. The normal response of CD19Cre+Baxfl/–Bak–/– B cells to CpG–DNA indicates that BAX and BAK do not control p27 expression directly, suggesting an upstream signaling defect in response to LPS.
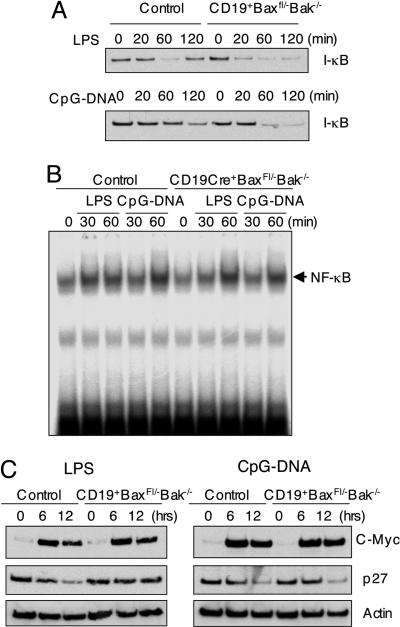
Fig. 4.
Analysis of signaling pathway activated by LPS and CpG–DNA. (A) Comparable degradation of IκBα in control and CD19Cre+Baxfl/–Bak–/– B cells. Splenic B cells were stimulated with LPS and CpG–DNA for the indicated times, and protein cell lysates were prepared and immunoblotting was performed by using anti-IκBα antibody. (B) Comparable activation of NF-κB in control and CD19Cre+Baxfl/–Bak–/– B cells. Splenic B cells were stimulated with LPS and CpG–DNA for the indicated time. Nuclear extracts were prepared and electrophoretic mobility-shift assays were performed by using an NF-κB probe. (C) Expression of c-Myc and degradation of p27 in CD19Cre+Baxfl/–Bak–/– B cells. Protein lysates from splenic B cells stimulated with LPS and CpG–DNA for the indicated times were analyzed for the level of c-Myc and p27. Actin serves as a control for protein loading.
Differential Activation of Signaling Pathways in Bax and Bak-Deficient B Cells in Response to LPS and CpG–DNA. The differential response of Bax- and Bak-deficient B cells in response to TLR agonists prompted us to explore the signaling pathways triggered by them. LPS and CpG–DNA are recognized by TLR4 and TLR9, respectively (34). Signaling pathways emanating from both TLR4 and TLR9 are thought to share most signaling molecules resulting in the activation of the transcription factor NF-κB and mitogen-activated protein kinases (34). However, the response of B cells to LPS has been shown to require another member of the TLR family, RP105 (CD180), in addition to TLR4 (35). To analyze the mechanism for the differential cell cycling response observed in CD19Cre+-Baxfl/–Bak–/– B cells, the signaling pathways that participate in LPS and CpG–DNA were examined. Several parameters indicated that the canonical signaling pathway leading to NFκB-induced gene expression is not affected by Bax and Bak-deficiency. These include comparable activation of IκB degradation (Fig. 4A), NFκB DNA-binding (Fig. 4B), and normal c-myc induction (Fig. 4C).
Because LPS utilizes RP105 as a receptor in addition to TLR4, we tested whether B cell proliferation induced by an anti-RP105 stimulating Ab was affected. The cell cycle progression in response to anti-RP105 Ab was also impaired (Fig. 3B), suggesting that the impaired RP105 signaling is partly involved in the impaired responsiveness against LPS in Bax and Bak deficiency.
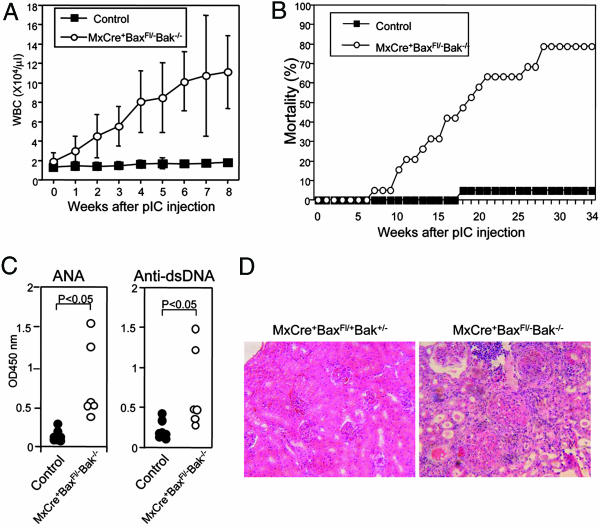
Deficiency in Bax and Bak Leads to the Development of Autoimmune Disease. Because most Bax–/–Bak–/– mice are embryonic lethal as a result of developmental defects, and those that survive have limited lifespan, the effects of BAX and BAK deficiency in mature animals has not been characterized. In addition, enhanced resistance of BAX/BAK doubly-deficient B cells to apoptotic stimuli warranted examination of pathologic consequences in mature animals. To achieve inducible deletion of Bax and Bak in adulthood, we crossed Baxfl/– mice with Mx-Cre mice that express CRE protein in response to type I IFN (31). Injection of poly(I:C), an IFN-inducer, induced accumulation of WBC in MxCre+Baxfl/–-Bak–/– mice in a time-dependent fashion (Fig. 5A). The WBC increased in these mice were mainly lymphocytes (data not shown). Almost no BAX protein was detected in thymocytes and splenocytes 4 weeks after poly(I:C) injection in MxCre+Baxfl/–Bak–/– mice. In these mice, thymic T cell development was perturbed as reported for Bax–/–Bak–/–-deficient T cells in chimeric mice (data not shown) (29). Six weeks after poly(I:C) injection, MxCre+-Baxfl/–Bak–/– mice showed accumulation of B cells in the spleen and BM to a similar extent as observed in CD19Cre+Baxfl/–Bak–/– mice (data not shown). By 35 weeks after induction of Bax deletion, 78% of MxCre+Baxfl/–Bak–/– mice died, compared to 5% of control (Fig. 5B). All MxCre+Baxfl/–Bak–/– mice showed elevated serum antinuclear antibodies (ANA) and anti-dsDNA Ab after 30 weeks of Bax deletion (Fig. 5C). Histological analysis of sick mice showed that the MxCre+Baxfl/–Bak–/– mice had severe glomeluronephritis and arthritis (Fig. 5D). These results indicate that BAX and BAK play a critical role in preventing the development of autoimmune disease.
Fig. 5.
Induced deletion of the Bax and Bak genes resulted in the development of autoimmune disease. (A) Inducible accumulation of peripheral white blood cells in MxCre+Baxfl/–Bak–/– mice after poly(I:C) injection. Four-week-old control and MxCre+Baxfl/–Bak–/– mice were injected i.p. three times with 400 μg of poly(I:C) every other day. Blood was collected, and the number of WBC was counted weekly. (B) Mortality in MxCre+Baxfl/–Bak–/– mice (n = 19) and control littermates (n = 20) in mixed genotypes after poly IC injection. (C) Production of autoantibodies. Sera were collected from control and MxCre+Baxfl/–Bak–/– mice at the age of 30 weeks. The levels of anti-nuclear Abs (ANA) and anti-dsDNA Abs were determined by ELISA. (D) Histological analysis of renal cortex of control and MxCre+Baxfl/–Bak–/– mice. Kidneys from MxCre+Baxfl/+Bak+/– and MxCre+Baxfl/–Bak–/– mice were sectioned and stained with hematoxylin and eosin.
Discussion
In this study, we used a conditional BAX/BAK-deficient mouse model as a valuable tool to demonstrate that the proapoptotic BCL-2 family members BAX and BAK are essential for controlling the numbers of both BM and splenic B cells. B cells prepared from B cell-specific Bax- and Bak-deficient mice are highly resistant to multiple cell death stimuli, including cytokine withdrawal, BCR crosslinking, steroid treatment, and DNA damage. This finding is consistent with previous observations showing extensive resistance of Bax–/–Bak–/– mouse embryonic fibroblasts to intrinsic apoptotic stimuli. Although a previous report showed that Bim–/– B cells have partial resistance to BCR crosslinking, B cells from CD19Cre+-Baxfl/–Bak–/– mice showed almost complete survival, clarifying that BCR-induced cell death is mediated fully by the intrinsic cell death pathway. Our results clearly show that BAX and BAK is the exclusive gateway for intrinsic cell death in B cells. Here we show that Bax and Bak deficiency resulted in a dramatic increase in the number of B cells at all stages of development in the BM and spleen. This phenotype is consistent with previous studies showing increased abundance of lymphocytes and the resistance of cells to apoptotic stimuli in the few mice that survive Bax and Bak deficiency to adulthood (26).
The increase of splenic B cell was reflected in transitional and mature follicular B cells. Interestingly, however, the number of MZ B cells and B-1 cells was not increased. Both follicular and MZ B cells are differentiated from immature B cells supplied from BM; however, the precise mechanism of their differentiation from transitional B cell populations is still controversial (36). Our study implies that apoptosis may play an important role in controlling the abundance of follicular, but not MZ, B cells. It is suggested that follicular B cells turnover more rapidly than MZ B cells (37). Homeostatic proliferation is implicated in the maintenance of MZ B cell pool. Therefore, the regulation of cell cycle progression by BAX and BAK may affect the generation of MZ B cells. The role of BAX and BAK in controlling any of these processes may be differentially involved at particular stages of B cell development. Stage-specific deletion studies will clarify the mechanism how these different B cell subsets are maintained.
The newly generated Bim-deficient mice without any detectable BIM protein expression displayed a similar phenotype to the mice reported previously (20, 22). A direct comparison of the phenotype of Bim–/– B cells to that of CD19Cre+Baxfl/–Bak–/– cells reveals that Bim–/– B cells show only a partial resistance to apoptotic stimuli, indicating that BIM is not a sole player in the regulation of lymphocyte number. Comparison of Bax- and Bak-deficient cells and cells deficient in combinations of multideficient BH3-only mice will be highly informative in evaluating the precise role of upstream apoptosis regulators in different tissues.
The defect in cell cycle progression of Bax and Bak deficient B cells in response to LPS and anti-IgM stimulation (Fig. 3) is consistent with that observed in Bcl-2 transgenic mice (18). Previous reports showed that Bcl-2 overexpressing cells had elevated expression of the cdk inhibitors, p27 and p107, which would account for the inhibition of cell cycle progression (38). However, our observation that p27 is down-regulated normally after CpG–DNA stimulation indicates that BAX or BAK are not direct regulators of cdk inhibitors, but rather that regulation of p27 is a consequence of impaired upstream signaling pathway(s) in the response to LPS.
Bax- and Bak-deficient B cells displayed normal response to a TLR9 ligand, CpG–DNA, despite the severe defect in cell cycle progression to LPS, a TLR4 ligand. This finding is surprising given the overlapping signaling pathways of these two receptors. The analysis of LPS signaling pathway revealed that the NF-κB signaling pathway is intact in B cells from CD19Cre+Baxfl/–Bak–/– mice. The defective response to RP105 stimulation, a coreceptor required in response to LPS, may contribute to the abnormal LPS response. These pathways should be further dissected in future studies.
Interestingly, the inducible deletion of Bax and Bak in adulthood in MxCre+Baxfl/–Bak–/– mice resulted in the development of a severe autoimmune disease. Because ANA and anti-dsDNA Ab are produced in the serum of these mice, Bax and Bak deficiency is responsible not only for the increased production of natural Ig leading to the accumulation of immune complexes, but also the production of autoantibodies. In contrast, neither mortality nor accumulation of autoantibodies was observed in CD19Cre+ Baxfl/–Bak–/– mice by 35 weeks of age, suggesting that deficiency of Bax and Bak in cells other than B cells is also required to cause a complex immune disease. This cumulative mortality owing to the development of autoimmune disease is also observed in Bim–/– mice and in mice overexpressing Bcl-2 in B cells (13, 14, 22). The overlapping phenotype of these mutant mice fits with the current model of the function of BCL-2 family members in vivo in immune cells, where BH3-only proteins act as upstream sentinels competing for downstream pro- and antiapoptotic BCL-2 members in response to apoptotic or survival signals, suggesting that stapled BH3 peptides may be a promising therapeutic for autoimmune diseases (11, 39).
Supplementary Material
Acknowledgments
We thank N. Danial for helpful discussions, R. Bronson for histological analysis, Y. Sasaki and K. Rajewsky for assistance with immunohistochemistry protocols, S. Wade for animal husbandry, and E. Smith for editorial assistance. O.T. is the recipient of the Human Frontier Science Program Long–Term Fellowship. This work is supported in part by a grant from the National Institutes of Health.
Author contributions: O.T. and S.J.K. designed research; O.T., J.F., H.S., and H.H. performed research; O.T. and B.A.M. analyzed data; and O.T. and B.A.M. wrote the paper.
Abbreviations: BCR, B cell receptor; BM, bone marrow; MZ, marginal zone; TLR, Toll-like receptor.
References
- 1.Hardy, R. R. & Hayakawa, K. (2001) Annu. Rev. Immunol. 19, 595–621. [DOI] [PubMed] [Google Scholar]
- 2.Marsden, V. S. & Strasser, A. (2003) Annu. Rev. Immunol. 21, 71–105. [DOI] [PubMed] [Google Scholar]
- 3.Rolink, A. G., Schaniel, C., Andersson, J. & Melchers, F. (2001) Curr. Opin. Immunol. 13, 202–207. [DOI] [PubMed] [Google Scholar]
- 4.Strasser, A. & Bouillet, P. (2003) Immunol. Rev. 193, 82–92. [DOI] [PubMed] [Google Scholar]
- 5.Allman, D., Srivastava, B. & Lindsley, R. C. (2004) Immunol. Rev. 197, 147–160. [DOI] [PubMed] [Google Scholar]
- 6.Rathmell, J. C. & Thompson, C. B. (2002) Cell 109, Suppl., S97–S107. [DOI] [PubMed] [Google Scholar]
- 7.Opferman, J. T. & Korsmeyer, S. J. (2003) Nat. Immunol. 4, 410–415. [DOI] [PubMed] [Google Scholar]
- 8.Danial, N. N. & Korsmeyer, S. J. (200) Cell 116, 205–219. [DOI] [PubMed] [Google Scholar]
- 9.Wei, M. C., Zong, W. X., Cheng, E. H., Lindsten, T., Panoutsakopoulou, V., Ross, A. J., Roth, K. A., MacGregor, G. R., Thompson, C. B. & Korsmeyer, S. J. (2001) Science 292, 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng, E. H., Wei, M. C., Weiler, S., Flavell, R. A., Mak, T. W., Lindsten, T. & Korsmeyer, S. J. (2001) Mol. Cell 8, 705–711. [DOI] [PubMed] [Google Scholar]
- 11.Letai, A., Bassik, M. C., Walensky, L. D., Sorcinelli, M. D., Weiler, S. & Korsmeyer, S. J. (2002) Cancer Cell 2, 183–192. [DOI] [PubMed] [Google Scholar]
- 12.Kuwana, T., Bouchier-Hayes, L., Chipuk, J. E., Bonzon, C., Sullivan, B. A., Green, D. R. & Newmeyer, D. D. (2005) Mol. Cell 17, 525–535. [DOI] [PubMed] [Google Scholar]
- 13.McDonnell, T. J., Deane, N., Platt, F. M., Nunez, G., Jaeger, U., McKearn, J. P. & Korsmeyer, S. J. (1989) Cell 57, 79–88. [DOI] [PubMed] [Google Scholar]
- 14.Strasser, A., Harris, A. W., Vaux, D. L., Webb, E., Bath, M. L., Adams, J. M. & Cory, S. (1990) Curr. Top. Microbiol. Immunol. 166, 175–181. [DOI] [PubMed] [Google Scholar]
- 15.Veis, D. J., Sorenson, C. M., Shutter, J. R. & Korsmeyer, S. J. (1993) Cell 75, 229–240. [DOI] [PubMed] [Google Scholar]
- 16.Strasser, A., Whittingham, S., Vaux, D. L., Bath, M. L., Adams, J. M., Cory, S. & Harris, A. W. (1991) Proc. Natl. Acad. Sci. USA 88, 8661–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartley, S. B., Cooke, M. P., Fulcher, D. A., Harris, A. W., Cory, S., Basten, A. & Goodnow, C. C. (1993) Cell 72, 325–335. [DOI] [PubMed] [Google Scholar]
- 18.O'Reilly, L. A., Harris, A. W., Tarlinton, D. M., Corcoran, L. M. & Strasser, A. (1997) J. Immunol. 159, 2301–2311. [PubMed] [Google Scholar]
- 19.Linette, G. P., Li, Y., Roth, K. & Korsmeyer, S. J. (1996) Proc. Natl. Acad. Sci. USA 93, 9545–9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouillet, P., Purton, J. F., Godfrey, D. I., Zhang, L. C., Coultas, L., Puthalakath, H., Pellegrini, M., Cory, S., Adams, J. M. & Strasser, A. (2002) Nature 415, 922–926. [DOI] [PubMed] [Google Scholar]
- 21.Opferman, J. T., Letai, A., Beard, C., Sorcinelli, M. D., Ong, C. C. & Korsmeyer, S. J. (2003) Nature 426, 671–676. [DOI] [PubMed] [Google Scholar]
- 22.Bouillet, P., Metcalf, D., Huang, D. C., Tarlinton, D. M., Kay, T. W., Kontgen, F., Adams, J. M. & Strasser, A. (1999) Science 286, 1735–1738. [DOI] [PubMed] [Google Scholar]
- 23.Enders, A., Bouillet, P., Puthalakath, H., Xu, Y., Tarlinton, D. M. & Strasser, A. (2003) J. Exp. Med. 198, 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver, P. M., Wang, M., Zhu, Y., White, J., Kappler, J. & Marrack, P. (2004) J. Exp. Med. 200, 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knudson, C. M., Tung, K. S., Tourtellotte, W. G., Brown, G. A. & Korsmeyer, S. J. (1995) Science 270, 96–99. [DOI] [PubMed] [Google Scholar]
- 26.Lindsten, T., Ross, A. J., King, A., Zong, W. X., Rathmell, J. C., Shiels, H. A., Ulrich, E., Waymire, K. G., Mahar, P., Frauwirth, K., et al. (2000) Mol. Cell 6, 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zong, W. X., Lindsten, T., Ross, A. J., MacGregor, G. R. & Thompson, C. B. (2001) Genes Dev. 15, 1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scorrano, L., Oakes, S. A., Opferman, J. T., Cheng, E. H., Sorcinelli, M. D., Pozzan, T. & Korsmeyer, S. J. (2003) Science 300, 135–139. [DOI] [PubMed] [Google Scholar]
- 29.Rathmell, J. C., Lindsten, T., Zong, W. X., Cinalli, R. M. & Thompson, C. B. (2002) Nat. Immunol. 3, 932–939. [DOI] [PubMed] [Google Scholar]
- 30.Rickert, R. C., Roes, J. & Rajewsky, K. (1997) Nucleic Acids Res. 25, 1317–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn, R., Schwenk, F., Aguet, M. & Rajewsky, K. (1995) Science 269, 1427–1429. [DOI] [PubMed] [Google Scholar]
- 32.Allman, D., Lindsley, R. C., DeMuth, W., Rudd, K., Shinton, S. A. & Hardy, R. R. (2001) J. Immunol. 167, 6834–6840. [DOI] [PubMed] [Google Scholar]
- 33.O'Reilly, L. A., Huang, D. C. & Strasser, A. (1996) EMBO J. 15, 6979–6990. [PMC free article] [PubMed] [Google Scholar]
- 34.Akira, S. & Takeda, K. (2004) Nat. Rev. Immunol. 4, 499–511. [DOI] [PubMed] [Google Scholar]
- 35.Ogata, H., Su, I., Miyake, K., Nagai, Y., Akashi, S., Mecklenbrauker, I., Rajewsky, K., Kimoto, M. & Tarakhovsky, A. (2000) J. Exp. Med. 192, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su, T. T., Guo, B., Wei, B., Braun, J. & Rawlings, D. J. (2004) Immunol. Rev. 197, 161–178. [DOI] [PubMed] [Google Scholar]
- 37.Hao, Z. & Rajewsky, K. (2001) J. Exp. Med. 194, 1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vairo, G., Soos, T. J., Upton, T. M., Zalvide, J., DeCaprio, J. A., Ewen, M. E., Koff, A. & Adams, J. M. (2000) Mol. Cell. Biol. 20, 4745–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walensky, L. D., Kung, A. L., Escher, I., Malia, T. J., Barbuto, S., Wright, R. D., Wagner, G., Verdine, G. L. & Korsmeyer, S. J. (2004) Science 305, 1466–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.