Abstract
Fragile X syndrome, the most frequent form of hereditary mental retardation, is due to a mutation of the fragile X mental retardation 1 (FMR1) gene on the X chromosome. Like fragile X patients, FMR1-knockout (FMR1-KO) mice lack the normal fragile X mental retardation protein (FMRP) and show both cognitive alterations and an immature neuronal morphology. We reared FMR1-KO mice in a C57BL/6 background in enriched environmental conditions to examine the possibility that experience-dependent stimulation alleviates their behavioral and neuronal abnormalities. FMR1-KO mice kept in standard cages were hyperactive, displayed an altered pattern of open field exploration, and did not show habituation. Quantitative morphological analyses revealed a reduction in basal dendrite length and branching together with more immature-appearing spines along apical dendrites of layer five pyramidal neurons in the visual cortex. Enrichment largely rescued these behavioral and neuronal abnormalities while increasing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor subunit 1 (GluR1) levels in both genotypes. Enrichment did not, however, affect FMRP levels in the WT mice. These data suggest that FMRP-independent pathways activating glutamatergic signaling are preserved in FMR1-KO mice and that they can be elicited by environmental stimulation.
Keywords: fragile X mental retardation protein, mental retardation, FMR1 gene, AMPA receptor, dendritic spines
Several genes associated with mental retardation have been mapped on the X chromosome and, among them is the fragile X mental retardation 1 (FMR1) gene. The fragile X mental retardation protein (FMRP) absence or mutation is responsible for the fragile X syndrome (FXS), which is the most common form of inherited mental retardation. Most of the individuals affected carry a trinucleotide repeat that, after methylation, leads to transcriptional silencing of the FMR1 gene (1). Patients with the FXS do not express FMRP and exhibit phenotypic traits ranging from severe (IQ 20) to moderate (IQ 60) mental retardation, defective attention, autistic behavior, and physical features including an elongated face, large ears, joint laxity, and macroorchidism (2–5).
FMR1 is highly conserved between human and mouse, with a nucleotide and amino acid identity of 95% and 97%, respectively (6). The expression pattern of mouse FMR1 is similar to its human counterpart in both tissue specificity and timing (7). Interestingly, FMR1-knockout (FMR1-KO) mice, the mouse model for the FXS, lack the normal FMRP and show macroorchidism, hyperactivity, and mild learning deficits (8, 9) reminiscent of the human syndrome.
One common brain feature of fragile X patients and of the mouse model for the syndrome is the presence of long and thin immature dendritic spines indicative of defective pruning during development (10–14). At the molecular level, it has been shown that protein synthesis triggered by the type I metabotropic glutamate receptor (mGluR1) agonist dihydroxyphenylglycine is dramatically reduced in synaptoneurosomes of FMR1-KO mice (15). There is also evidence that FMRP is required for mGluR1-dependent translation of the postsynaptic density protein 95 (16), a scaffolding protein specifically involved in synaptic development and plasticity (17, 18). In addition to this observation, a reduction of cortical α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor subunit 1 (GluR1) expression and long-term potentiation (19) together with an enhancement of hippocampal long-term depression (20) have been reported in this genotype. Altogether, these findings support the view that the neuronal alterations associated with the FXS can be ascribed to a substantial impairment of mechanisms involved in neural plasticity.
Since the early observations of Hebb (21) and Krech and coworkers (22), environmental enrichment has been extensively used to demonstrate behavioral and brain plasticity in response to experience. Rearing animals in a complex environment reduces anxiety (23), accelerates habituation (24), enhances learning (24–26), and deeply affects brain morphology. In particular, an increase in dendrite length and branching (27), spine density and number of mature spines (29, 30, **), synaptogenesis (31), and neurogenesis (32) has been repeatedly found in cortical and subcortical areas of rodents experiencing enriched conditions during development. More recent evidence also indicates that environmental enrichment enhances the brain levels of several synaptic and structural proteins such as neurotrophins (33), cAMP response element-binding protein (34), synaptophysin (35, 36), and postsynaptic density protein 95 (36), thus playing a role in synapse formation and plasticity.
Here we examine the possibility of alleviating the behavioral and neuronal abnormalities of FMR1-KO mice by environmental stimulation. KO and their WT littermates reared in standard or enriched cages were first tested for motor activity then for their propensity to interact with external stimuli, i.e., two behavioral traits affected by FMRP deficiency (3, 37). Subsequently, the Golgi–Cox technique was used to examine the morphology of layer V neurons in the visual cortex. We observed an altered pattern of exploration and habituation in the open field, as well as abnormal dendrite and spine morphology in KO mice kept in standard cages. Interestingly, these behavioral and morphological alterations were largely rescued by environmental stimulation. Enrichment also increased the level of expression of the AMPA receptor subunit GluR1 in visual cortex of FMR1-KO mice but it did not affect expression of FMRP in the WT mice. These data suggest that neural plasticity mechanisms other than those modulated by the FMR1 gene are preserved in FMR1-KO mice and that they can be elicited by environmental stimulation.
Materials and Methods
Animals. Animal care was conducted according to the institutional guidelines that are in compliance with national†† and international laws and policies.‡‡ Mice used in this study were male C57BL/6WT (n = 18) and C57BL/6 FMR1-KO (n = 18). The KO mice were backcrossed at least eight times, and their genotype was determined according to Bakker et al. (8). Mice were 21 days old at the beginning of the experiment, and their weights ranged from 12 to 14 g.
Rearing Conditions. Mice were randomly assigned to the enriched or the standard environmental condition. Enriched environment cages consisted of clear Plexiglas cages (35 × 20 × 25 cm) with a horizontal platform (20 × 15 cm) dividing the cage into two floors. On the ground floor, there was a plastic running wheel, nesting material, and an assortment of differently colored and textured plastic toys (balls, tubes, boxes, and bells) that were changed every 3 days. A steel ladder allowed mice to reach the upper floor, where they had access to food and water. Mice were exposed, 2 h/day, to an additional enriched area situated in a different room. This area consisted of a Plexiglas cage (40 × 25 × 20 cm) containing polyurethane foam, cardboard boxes, and objects made of iron. Standard cages were clear Plexiglas laboratory cages (18 × 25 × 13 cm). Mice were caged in groups of three. Enriched and standard cages were placed in a temperature-controlled room (22°C) with a light–dark 12:12 cycle (light on 0700–1900 hours). Food and water were given ad libitum. Mice were housed in each experimental condition for 60 days. The behavioral experiments started when mice were 12 weeks old.
Open-Field Exploration and Habituation to the Objects. The apparatus was a circular arena (60 cm in diameter) with a white-painted floor divided into sectors by black lines. The arena was surrounded by a 20-cm-high wall. Testing consisted of four successive 5-min sessions separated by a 3-min delay during which mice were placed in an empty cage situated on the floor of the experimental room. One exploration session was first given in the empty arena (session 1). Subsequently, five differently colored, textured, and shaped objects (all ≈10 cm in height) were placed in the arena, and mice were allowed to explore freely these objects for three sessions (sessions 2–4). The number of central and peripheral sectors crossed during session 1 and the number of contacts with the five objects during sessions 2–4 were recorded. A contact was counted when the subject's snout was touching the object. In this experiment 18 WT and 12 FMR1-KO mice were used, with half of the mice in each group reared in standard cages and the other half in enriched cages.
Golgi–Cox Impregnation of Brain Tissue. At the completion of the behavioral experiments, mice were anesthetized with chloral hydrate (400 mg/kg) and perfused intracardially with 0.9% saline. The brains were dissected and impregnated by using a standard Golgi–Cox solution (1% potassium dichromate/1% mercuric chloride/0.8% potassium chromate) according to the method described by Glaser and Van der Loos (38). The brains immersed in the Golgi–Cox solution were stored at room temperature for 14 days, immersed in a sucrose solution (30%) for 5 days, and then sectioned coronally (200 μm) by using a Vibratome. Sections were mounted on gelatinized slides, stained according to the Gibb and Kolb (39) method, and covered with Permount.
Morphological Analysis. Four brains from mice reared in each experimental condition were processed for morphological analyses. Measurements were performed in fully impregnated pyramidal neurons displaying dendritic tree without obvious truncations (width of the first apical segment, 2.8–3.2 μm; length, >150 μm). Within each hemisphere, three visual cortex neurons with the soma in layer V and apical dendrites reaching layer III were selected. Because no interhemispheric difference was detected, the data were pooled so that six neurons per brain area were considered in each analysis. Measurements were carried out by using an Axio-scope microscope (Zeiss) equipped with a camera (Optronics, Chelmsford, MA) and the Neurolucida system (MicroBrightField, Williston, VT). Morphological measurements were made by an experimenter blind to the experimental condition of the animal.
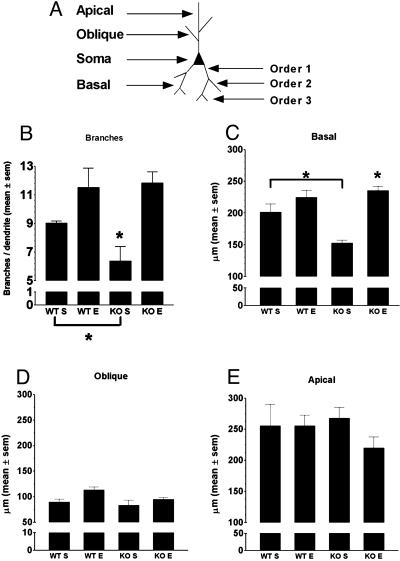
Dendritic Length and Branching. Dendrites from each category (apical, basal, and oblique) were traced along the entire length by means of the Neurolucida system connected to a stage controller, which allowed measurement of dendrites along the z axis. The primary branches of apical dendrites were considered as oblique dendrites. For branching analysis, basal dendrites were classified by using the centrifugal method (40): the branches arising from the soma were numbered as branch order 1 (see Fig. 2A). Bifurcations on order 1 branches were numbered as branch order 2 (Fig. 2A). In our Golgi–Cox-impregnated slices, the maximal branch order reached 5. The complexity of basal dendrite branching was estimated by counting the number of branches on each dendrite. Data were expressed as the mean of the number of branches (order ≥2) per dendrite.
Fig. 2.
Enrichment rescues basal dendrite structural abnormalities in FMR1-KO mice. (A) Schematic drawing of a typical layer V pyramidal neuron of the occipital cortex, showing the position of basal, apical, and oblique dendrites, and the different branch orders. (B) Basal dendrite branching in FMR1-KO (KO) and WT mice reared in standard (S) or enriched (E) conditions. The mean number of branches (±SEM) was significantly lower in KO mice reared in standard as opposed to enriched conditions. Enrichment significantly increased dendrite branching in KO mice but did not in WT mice. (C–E) Mean dendrite length (±SEM) of basal, oblique, and apical dendrites measured in KO and WT mice reared in standard or enriched conditions. (C) Basal dendrites were significantly shorter in KO mice reared in the standard condition than in the three other groups. Enrichment significantly increased dendrite length in KO mice. Oblique (D) and apical (E) dendrite length did not significantly vary among the four mouse groups. Dendrite length is expressed in micrometers. **, P < 0.01.
Spine Density. Neurons were first identified under low magnification (×200/0.5 numerical aperture). Subsequently, spines were analyzed under a higher magnification (×1,000/1.25 numerical aperture). All protrusions were counted as spines if they were in direct contact with the dendritic shaft. The average spine density (number of spines per 10 μm of dendritic length) was estimated on the focal plane along the entire apical dendrite and along three basal and oblique dendrites. Because this method has proven to produce reliable results (41), no attempt was made to introduce a correction factor for hidden spines.
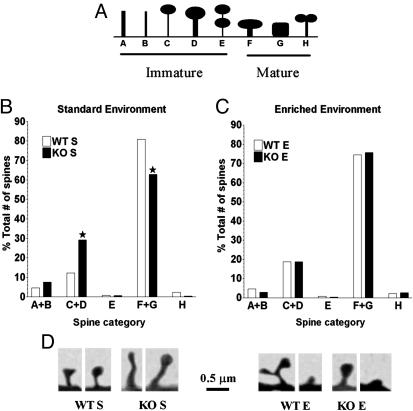
Spine Morphology. The morphology of up to 35 spines on apical dendrite segments starting 50 μm out from the soma was acquired under high magnification (×1,000/1.25 numerical aperture). The spines were categorized according to ref. 28, along an immature- vs. mature-appearing spine continuum (see Fig. 4A)
Fig. 4.
Enrichment enhances the proportion of mature-appearing spines in FMR1-KO mice. (A) The schema shows a standard categorization of spines along an immature–mature continuum (28) in FMR1-KO (KO) and WT mice reared in standard (S) or enriched (E) conditions. [Reproduced with permission from ref. 28 (Copyright 2000, Oxford University Press).] (B) KO mice reared in the enriched condition exhibited significantly more immature- and fewer immature-appearing spines than KO mice reared in the standard condition. (C) KO and WT mice reared in the enriched condition displayed a similar proportion of immature- and mature-appearing spines. (D) Computer-based reconstruction of the categories of spines most frequently observed in KO and WT mice reared in the standard (Left) and the enriched (Right) condition.
Western Blotting. Five brains from mice reared in each experimental condition were processed for Western blotting. Visual cortex from standard and enriched WT and FMR1-KO mice were dissected and homogenized in lysis buffer (50 mM NaCl/50 mM Tris, pH 7.5/1% Triton X-100/10% glycerol/320 mM sucrose containing 10 μl/ml Sigma protease inhibitor). For each sample, two different amounts of protein extract were separated by SDS/PAGE, blotted, and probed with antibodies against GluR1 (Upstate Biotechnology, Lake Placid, NY), FMRP (42), and β-actin (Sigma), followed by secondary antibodies conjugated to horseradish peroxidase (Promega) and developed with the chemiluminescence reaction (ECL-plus, Amersham Pharmacia). Images were acquired by using a Storm 840 by Amersham Pharmacia, and quantification was performed by using imagequant (version 5.0 TL v2003.02). GluR1 and FMRP levels were then normalized to β-actin levels and expressed as arbitrary units. For each animal three independent gels were loaded to reduce experimental variability.
Results
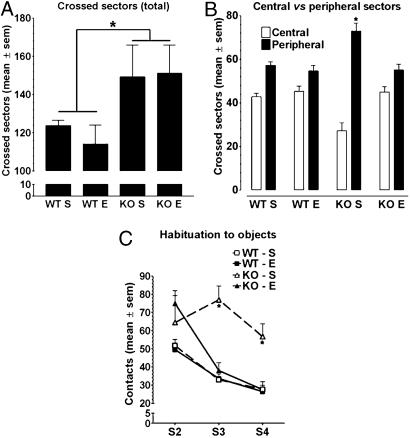
Enrichment Does Not Affect Hyperactivity of FMR1-KO Mice. Hyperactivity is the most robust and reproducible behavioral phenotypic trait of C57BL/6 FMR1-KO mice (8, 9). Therefore, we reexamined motor activity by counting the number of sectors crossed by mice of each group in an empty open field to assess the effect of enrichment on this behavioral parameter. A two-way ANOVA with genotype and rearing condition as main factors revealed only a significant effect of genotype (F1,24 = 6.69, P < 0.025). This result indicates that, irrespective of the environment experienced during development, FMR1-KO mice crossed significantly more sectors than did the WT mice (Fig. 1A).
Fig. 1.
Motor activity and object exploration in FMR1-KO (KO) and WT mice reared in standard (S) or enriched (E) conditions. (A) Mean (±SEM) number of sectors crossed during session 1 in the empty open field. KO mice were more active than WT mice irrespective of the rearing conditions experienced during development. (B) Mean (±SEM) peripheral and central sectors crossed during session 1. KO mice reared in standard conditions explored significantly less the central than the peripheral sectors in comparison with the three other mouse groups that similarly explored each type of sector. Enrichment restored the pattern of open-field exploration in KO mice. (C) Mean number of contacts (±SEM) with the five objects during sessions 2–4. KO mice reared in standard conditions did not show habituation of object exploration. Enrichment restored habituation in KO mice. Each session lasted 5 min. *, P < 0. 05.
Enrichment Rescues the Inner Exploration Deficit Shown by FMR1-KO Mice. Examination of the motor activity in the center and at the periphery of the empty open field revealed, however, a different pattern of exploration between FMR1-KO and WT mice reared in standard cages (Fig. 1B). In particular, the KO mice showed an anxiety-like behavioral trait because they crossed the peripheral sectors more than the central ones in contrast with the WT mice, which similarly explored all of the sectors of the apparatus. Interestingly, enrichment restored a normal pattern of exploration in KO mice without affecting exploration in WT mice. These findings are based on a three-way ANOVA indicating a significant “genotype × rearing condition × sectors” interaction (F1,24 = 7.29, P < 0.025) with simple effects showing a significant effect of sectors only for the group of FMR1-KO mice reared in standard cages (P < 0.01).
Enrichment Restores Habituation to Objects in FMR1-KO Mice. A decreased reactivity to external stimuli has been reported for FMR1-KO mice (37). Therefore, in this experiment, we examined their propensity to interact with five differently colored, textured, and shaped objects placed in an open field. Mice were given three sessions of exploration (sessions 2–4) separated by 5-min intervals. The data are shown in Fig. 1C. A three-way ANOVA with genotype and rearing condition as main factors, and sessions as a within-factor, revealed a significant genotype × rearing condition × sessions interaction (F2,48 = 12.09, P < 0.001). Subsequent simple effect analyses indicated that, on the first session of exploration, the number of contacts with the five objects was significantly higher in KO than in WT mice (significant effect of genotype on session 2, P < 0.01). The standard-caged FMR1-KO mice, however, did not show habituation to the objects across sessions 2–4 differently from their littermates reared in complex cages and from the WT mice reared in standard or complex cages. In fact, a significant effect of sessions (P < 0.01) was found in all groups except in standard-caged FMR1-KO mice.
Enrichment Increases Basal Dendrite Branching in FMR1-KO Mice. The statistical comparison of order 2 to order 5 branches counted on basal dendrites in the four groups was performed by means of a two-way ANOVA with genotype and rearing conditions as main factors. The results showed a main effect of the rearing condition (F1,12 = 18.21; P < 0.01), indicating higher-order branching in mice experiencing enrichment (Fig. 2B). Pair-wise comparisons, however, revealed that FMR1-KO mice reared in standard cages exhibited fewer branches than their WT counterpart [Fisher's least significant difference (LSD), P < 0.05] and that the effect of enrichment on branching was significant only for the FMR1-KO mice (Fisher's LSD, P < 0.01) In fact, no difference in basal dendrite branching was found between FMR1-KO mice reared in complex cages and WT mice reared in either environmental conditions (P > 0.1 for all pair-wise comparisons).
Enrichment Increases Basal Dendrite Length in FMR1-KO Mice. The two-way ANOVA showed a significant main effect of environment (F1,12 = 30.52; P < 0.001), indicating that, in general, mice reared in standard cages exhibited shorter basal dendrites than their counterpart reared in enriched cages. However, a significant genotype × rearing condition interaction (F1,12 = 9,55; P < 0.01) revealed that enrichment promoted basal dendrite lengthening in FMR1-KO mice (enriched vs. standard FMR1-KO mice, LSD test, P < 0.05) without affecting significantly the length of dendrites in WT mice (Fig. 2C). For the oblique and the apical dendrites, no statistically significant variation in length was detected among the four groups (Fig. 2 D and E).
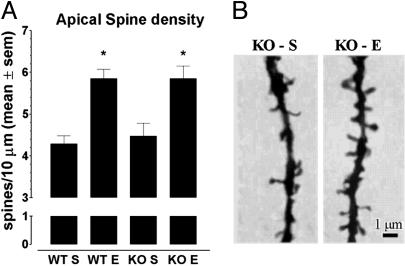
Enrichment Increases Spine Density Along Apical Dendrites in both Genotypes. The two-way ANOVA revealed a significant main effect of enrichment (F1,12 = 31.41; P < 0.001). In fact, both FMR1-KO (Fisher's LSD test, P < 0.05) and WT mice (Fisher's LSD test, P < 0.01) reared in enriched conditions showed a higher density of spines along apical dendrites than did their counterparts reared in standard cages (Fig. 3). Spine density along basal or oblique dendrites did not vary significantly according to the genotype or the rearing condition (data not shown).
Fig. 3.
Spine density (mean ± SEM) along apical dendrites in FMR1-KO (KO) and WT mice reared in standard (S) or enriched (E) conditions. (A) Spine density on apical dendrites was significantly higher in both genotypes reared in the enriched as opposed to the standard condition. (B) Representative Golgi–Cox-stained apical dendrites showing increased spine density in FMR1-KO mice reared in the enriched condition. Spine density along basal and oblique dendrites did not differ significantly according to the genotype or the rearing condition (data not shown). Spine density is expressed as the number of spines per 10 μm of dendrite segments. *, P < 0.05.
Enrichment Rescues the Immature-Appearing Spine Morphology in FMR1-KO Mice. The observation that enrichment enhances spine density prompted us to assess whether the dendritic spines could have also undergone a morphological change. As shown in Fig. 4B, FMR1-KO mice reared in standard cages showed more immature- and fewer mature-appearing spines than their WT counterpart (χ2 = 85.32, 4 df, P < 0.0001; 1.478 spines). Enrichment significantly decreased immature-appearing spines while promoting mature-appearing ones in KO mice (enriched vs. standard FMR1-KO mice, χ2 = 58.55, 4 df P < 0.001; 1.664 spines). In fact, there was no difference in the level of maturation of spines between KO and WT mice reared in enriched environmental conditions (Fig. 4C).
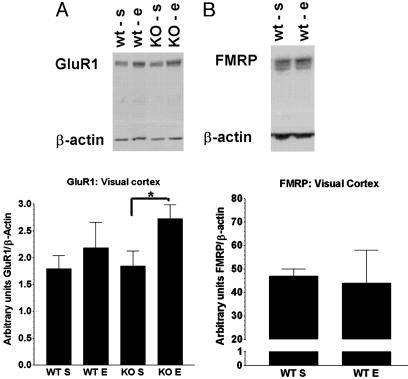
The Rescue of the Behavioral and Neuronal Alterations Observed in FMR1-KO Mice Is GluR1-Dependent. To evaluate whether the partial rescue of immature morphology by enrichment was actually due to an increase in mRNA translation and/or stability of key molecules involved in synaptic events, we analyzed the GluR1 levels in the visual cortex of KO mice reared in standard or enriched conditions in parallel to their WT counterpart reared in the same conditions. A two-way ANOVA with genotype and enrichment as main factors showed a significant effect only for the rearing condition (F1,16 = 5.62, P < 0.05). This result indicates that GluR1 levels were significantly increased in mice exposed to enrichment. Subsequent pair-wise comparisons revealed, however, that this increase was significant (Fisher's LSD test, P < 0.05) only for the KO mice (Fig. 5A).
Fig. 5.
GluR1 and FMRP levels in visual cortex. (A Upper) Western blot analysis of GluR1 and β-actin from FMR1-KO (KO) and WT mice reared in standard (S) or enriched (E) conditions. Five animals and two different protein concentrations were analyzed (only one shown), and the intensities of the bands were quantified by using imagequant (Lower). *, P < 0. 05. Enrichment enhanced GluR1 levels in both KO and WT mice, but this effect was more marked in KO mice. Error bars indicate SEM. (B Upper) Western blot analysis of FMRP and β-actin from WT mice reared in standard or enriched conditions. Five animals and two different protein concentrations were analyzed (only one shown), and the intensities of the bands were analyzed by using imagequant (Lower). FMRP level was unaffected by enrichment in WT mice. Error bars indicate SEM.
Enrichment Does Not Affect FMRP Levels in WT Mice. In addition, we analyzed the effect of enrichment on the level of FMRP expression in the visual cortex of the WT mice. As shown in Fig. 5B, FMRP levels did not differ between mice reared in standard and enriched cages (t = 0.024, Fig. 5B).
Discussion
Hyperactivity is a constant feature of the FXS (8, 9). Here we show that independently from the rearing condition experienced during development, FMR1-KO mice crossed more sectors in the empty open field than did WT mice and that enrichment failed to reverse this KO-specific behavioral trait.
Another main feature of human patients is anxiety (2). Interestingly, we observed that FMR1-KO mice reared in standard cages consistently avoided the central sectors of the open field, unlike their counterparts reared in complex cages and unlike WT mice reared in either environmental condition. Thus, enrichment seems to decrease anxiety in FMR1-KO mice. It is worth remembering that an opposite tendency, i.e., a higher rate of permanence in the center of the field, has been reported in FMR1-KO mice in a FVB strain background (43). However, the retinal degeneration identified in the FVB strain as well as differences in testing procedures, namely in the size of the field and the duration of testing (10), may account for this discrepancy. Of importance, our findings show that FMR1-KO mice in a C57BL/6 background exhibit an anxiety-like phenotype, which is consistent with the human syndrome (2) and is fully rescued by enrichment.
Most studies dealing with the characterization of the cognitive profile of the FMR1-KO mice have assessed superior functions (8, 9, 37). How these mice simply explore a set of objects and whether they show habituation when repeatedly exposed to the same situation has, to our knowledge, never been examined. Because autistic behavior is also a feature of the fragile X patients (44) and because FMR1-KO mice show a decreased reactivity to external stimuli (37), we therefore expected abnormal interactions of KO mice with any element situated in their proximal environment. In fact, we observed that standard-reared FMR1-KO mice intensively explored the objects on the first session and failed to show habituation on repeated exposure. Interestingly, enrichment did not attenuate the initial intense object exploration of the objects but fully restored habituation.
In any species, exploration is the result of a complex balance between attention, curiosity, and neophobia, whereas habituation, i.e., a decreased reaction to external stimuli, presupposes a comparison between a current perception and previously stored representations of those stimuli. Exploration and habituation therefore assess emotional and cognitive functions known to be altered in fragile X patients (2, 44). Thus, our finding that enrichment rescued inner open-field exploration and habituation in FMR1-KO mice indicates that environment stimulation may exert beneficial effects on anxiety and memory disturbances of fragile X patients.
Finally, based on the previously reported modest influence of enrichment on exploratory activity (45) and anxiety (46) in the C57BL/6 inbred strain, we did not expect large behavioral differences between WT mice reared in standard or in complex cages. In fact, none of the behaviors examined in this study were found to be affected by enrichment in the WT mice. Thus, we suggest that silencing the FMR1 gene in a C57BL/6 background may be particularly suitable for detecting KO-specific effects of environmental stimulation.
Early investigations on neuronal abnormalities related to the mutation or the induction of FMR1 deficiency established that one common morphological feature of fragile X patients and FMR1-KO mice was the presence of thin, long, and abundant dendritic spines along pyramidal neurons in layer V of the visual cortex (10). However, as mentioned above, the FVB strain used to generate these KO mice was undergoing a mutation-induced retinal degeneration with possible effects on cell morphology in the visual cortex. This degeneration made it necessary to confirm the presence of abnormalities in spine morphology and number in FMR1-KO mice generated in a different background. Using KO mice derived from FVB × 129J crossings, Irwin et al. (12) observed more immature spines but did not find an increase in spine density. Moreover, these authors reported that the amount of dendrite arbor and dendrite branching complexity did not vary between the KO mice and their WT controls. Given that the morphology of pyramidal neurons in the visual cortex of FMR1-KO mice in C57BL/6 background has not yet been examined, we analyzed dendrites and dendritic spines in KO and WT mice reared in standard cages or in an enriched environment.
One consequence of the FMRP absence on the neuronal morphology of C57BL/6 mice housed in standard cages was the reduced length and branching of basal dendrites. Thus, contrary to the findings obtained in KO mice derived from a FVB × 129J background (12), our data show that the absence of the FMRP protein can modify the extent and the complexity of the dendritic tree in layer V pyramidal neurons of the visual cortex. Consistent with this possibility, dendrite abnormalities in somatosensory cortical barrels have been reported in the same C57BL/6 FMR1-KO mice (14).
As for the morphology and the number of spines, we observed a pattern of alterations basically similar to that previously reported in FVB × 129J FMR1-KO mice (12). That is, gene silencing was not found to affect spine density in any part of the dendritic tree but strongly modified the proportion of mature- vs. immature-appearing spines counted on apical dendrite segments. In particular, using a standard categorization (12), we found fewer multiple head, stubby, and mushroom-structured spines and more thin and elongated ones in KO mice than in their WT littermates. The main finding of our study, however, is that the abnormal morphology of dendrites and dendritic spines observed in the KO mice was fully rescued by enrichment. These data therefore indicate that some aspects of the molecular machinery responsible for experience-dependent neuronal plasticity are preserved in FMR1-KO mice.
The fact that, despite the rescue of FMRP deficiency-related neuronal abnormalities, other morphological indexes such as the length of oblique dendrites and the density of spines on apical dendrites were stimulated by enrichment in both genotypes raises the question of the mechanisms whereby plasticity is induced when the FMR1 gene product is absent or present at lower levels. Hence, we investigated the role of FMRP in promoting enrichment-induced plastic changes in the WT group and found that FMRP levels were unaffected by the rearing condition. This finding may seem in contrast with data showing enhanced expression of FMRP in rats exposed to a complex environment (47). However, species and timing differences in enrichment-induced FMRP expression, as well as the method used for protein quantification, may account for this discrepancy. In fact, our mice were kept in complex cages from weaning until 3 months of age, whereas in the previous report, rats, already adult, were exposed to an enriched environment for a 20-day period. It is possible that FMRP is highly responsive to initial environmental stimulation but that its expression returns to basal levels if the housing is maintained for a longer period. In agreement with this hypothesis, it has been shown that visual experience induces a rapid but transient expression of FMRP in the visual cortex of rats reared in a dark environment (48).
We can therefore assume that, in the C57BL/6 mouse strain, FMRP is important for normal development of dendrite length and complexity but not for experience-dependent enhancement of these parameters. Thus, possibly, some of the mechanisms whereby enrichment stimulates neuronal plasticity in both the KO mice and their WT controls are FMRP-independent.
In view of their role in the maturation of dendritic spines (49, 50) and the regulation of dendritic architecture (51), glutamatergic receptor expression is likely to be affected by both FMRP deficiency and enrichment. There is evidence that protein synthesis triggered by the type I metabotropic glutamate receptor (mGluR1) in brain synaptosomes (15) and expression of the AMPA receptor subunit 1 in the somatosensory cortex (19) are dramatically reduced in FMR1-KO mice. Enrichment, however, selectively increases AMPA activity (52) and gene expression of GluR1 (53) in the hippocampus. Based on these findings, we assessed GluR1 expression in the visual cortex of FMR1-KO and WT mice reared in standard and enriched conditions. Surprisingly, we did not detect differences in GluR1 levels between KO and WT mice reared in a standard environment.
Rather, these levels were significantly higher in both genotypes exposed to enrichment, with a major increase in the KO mice. Our data appear in contrast with previous findings showing lower levels of GluR1 in FMR1-KO mice relative to their WT controls (19). In these experiments, however, GluR1 levels were measured in a different brain region (somatosensory cortex), by using a different technique, and, most importantly, in younger animals (8 weeks).
Of interest, a phase II clinical trial for FXS involves a class of drugs enhancing AMPA receptor activity called ampakines or AMPA receptor modulators (ref. 54; E. Berry-Kravis, www.traxa.org). Although the trafficking of FMRP is regulated by glutamatergic signals (55, 56) and that FMRP is required for group I GluR1-dependent translation of scaffolding proteins (16), FMRP-independent pathways such as the calcium/calmodulin-dependent protein kinase II have been shown to increase AMPA signaling into synapses (57) and to play a role in stabilization of newly formed NMDA-dependent synapses (48). Such pathways may therefore be involved in promoting enrichment-induced behavioral and brain plasticity.
In summary, we show that enrichment can reverse several structural and behavioral abnormalities resulting from the silencing of the FMR1 gene. Our behavioral data are thus consistent with the observations by Reiss and coworkers (58, 59), indicating that environmental factors positively influence the behavioral outcome in children with FXS. Although further investigations are needed to elucidate the molecular mechanisms involved in the enrichment effects, our findings demonstrate that some mechanisms of neuronal plasticity are preserved in FMR1-KO mice and can be triggered by environmental stimulation.
Acknowledgments
We are grateful to M. Segal and K. Braun for their help with the morphological experiments. The work in the laboratory of C.B. was supported by Telethon-Italy (GGP02357) and Progetto Genomica Funzionale (Consiglio Nazionale delle Ricerche-Ministero dell'Istruzione dell'Università e della Ricerca). B.A.O. was supported by National Institutes of Health Grant 5R01 HD38038.
Author contributions: C.B. and M.A.-T. designed research; L.R., F.F., E.P., and C.S. performed research; E.P., B.A.O., C.B., and M.A.-T. contributed new reagents/analytic tools; L.R., F.F., C.S., J.B., C.B., and M.A.-T. analyzed data; and C.B. and M.A.-T. wrote the paper.
Abbreviations: FMRP, fragile X mental retardation protein; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GluR1, subunit 1 of AMPA ionotropic glutamate receptor; KO, knockout; FXS, fragile X syndrome; LSD, least significant difference.
Footnotes
Diamond, J., Gray, E. G. & Yasargil, G. M. (1969) J. Physiol. 202, 116P (abstr.).
Decreto Legislativo N116, Gazzetta Ufficiale, Suppl. 40, 18-2-1992.
European Community Council Directive 86/609, Official Journal L358, Dec. 18, 1986, and the U.S. National Research Council (1996) National Institutes of Health Guide for the Care and Use of Laboratory Animals.
References
- 1.Hagerman, R. J., Staley, L. W., O'Conner, R., Lugenbeel, K., Nelson, D., McLean, S. D. & Taylor, A. (1996) Pediatrics 97, 122-126. [PubMed] [Google Scholar]
- 2.Hagerman, R. J. & Hagerman, P. J. (2002) Curr. Opin. Genet. Dev. 12, 278-283. [DOI] [PubMed] [Google Scholar]
- 3.O'Donnell, W. T. & Warren, S. T. (2002) Annu. Rev. Neurosci. 25, 315-338. [DOI] [PubMed] [Google Scholar]
- 4.Chiurazzi, P., Neri, G. & Oostra, B. A. (2003) Curr. Opin. Pediatr. 15, 559-566. [DOI] [PubMed] [Google Scholar]
- 5.Zalfa, F. & Bagni, C. (2004) Curr. Issues Mol. Biol. 6, 73-88. [PubMed] [Google Scholar]
- 6.Ashley, C. T., Jr., Wilkinson, K. D., Reines, D. & Warren, S. T. (1993) Science 262, 563-566. [DOI] [PubMed] [Google Scholar]
- 7.Hinds, H. L., Ashley, C. T., Sutcliffe, J. S., Nelson, D. L., Warren, S. T., Housman, D. E. & Schalling, M. (1993) Nat. Genet. 3, 36-43. [DOI] [PubMed] [Google Scholar]
- 8.Bakker, C. E., Verheij, C., Willemsen, R., Vanderhelm, R., Oerlemans, F., Vermey, M., Bygrace, A., Hoogeveen, A. T., Oostra, B. A., Reyniers, E., et al. (1994) Cell 78, 23-33.8033209 [Google Scholar]
- 9.Mineur, Y. S., Sluyter, F., de Wit, S., Oostra, B. A. & Crusio, W. E. (2002) Hippocampus 12, 39-46. [DOI] [PubMed] [Google Scholar]
- 10.Comery, T. A., Harris, J. B., Willems, P. J., Oostra, B. A., Irwin, S. A., Weiler, I. J. & Greenough, W. T. (1997) Proc. Natl. Acad. Sci. USA 94, 5401-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irwin, S. A., Patel, B., Idupulapati, M., Harris, J. B., Crisostomo, R. A., Larsen, B. P., Kooy, F., Willems, P. J., Cras, P., Kozlowski, P. B., et al. (2001) Am. J. Med. Genet. 98, 161-167. [DOI] [PubMed] [Google Scholar]
- 12.Irwin, S. A., Idupulapati, M., Gilbert, M. E., Harris, J. B., Chakravarti, A. B., Rogers, E. J., Crisostomo, R. A., Larsen, B. P., Mehta, A., Alcantara, C. J., et al. (2002) Am. J. Med. Genet. 111, 140-146. [DOI] [PubMed] [Google Scholar]
- 13.Nimchinsky, E. A., Oberlander, A. M. & Svoboda, K. (2001) J. Neurosci. 21, 5139-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galvez, R., Gopal, A. R. & Greenough, W. T. (2003) Brain Res. 971, 83-89. [DOI] [PubMed] [Google Scholar]
- 15.Greenough, W. T., Klintsova, A. Y., Irwin, S. A., Galvez, R., Bates, K. E. & Weiler, I. J. (2001) Proc. Natl. Acad. Sci. USA 98, 7101-7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todd, P. K., Mack, K. J. & Malter, J. S. (2003) Proc. Natl. Acad. Sci. USA 100, 14374-81437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Husseini, A. E., Schnell, E., Chetkovich, D. M., Nicoll, R. A. & Bredt, D. S. (2000) Science 290, 1364-1368. [PubMed] [Google Scholar]
- 18.Beique, J. C. & Andrade, R. (2003) J. Physiol. (London) 546, 859-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, J., Pelletier, M. R., Perez Velazquez, J. L. & Carlen, P. L. (2002) Mol. Cell. Neurosci. 19, 138-151. [DOI] [PubMed] [Google Scholar]
- 20.Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. (2002) Proc. Natl. Acad. Sci. USA 99, 7746-7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hebb, D. O. (1947) Am. Psychol. 2, 306-307. [Google Scholar]
- 22.Krech, D., Rosenzweig, M. R. & Bennett, E. L. (1960) J. Comp. Physiol. Psychol. 53, 509-519. [DOI] [PubMed] [Google Scholar]
- 23.Benaroya-Milshtein, N., Hollander, N., Apter, A., Kukulansky, T., Raz, N., Wilf, A., Yaniv, I. & Pick, C. G. (2004) Eur. J. Neurosci. 20, 1341-1347. [DOI] [PubMed] [Google Scholar]
- 24.Schrijver, N. C., Bahr, N. I., Weiss, I. C. & Wurbel, H. (2002) Pharmacol. Biochem. Behav. 73, 209-224. [DOI] [PubMed] [Google Scholar]
- 25.Lee, E. H., Hsu, W. L., Ma, Y. L., Lee, P. J. & Chao, C. C. (2003) Eur. J. Neurosci. 18, 2842-2852. [DOI] [PubMed] [Google Scholar]
- 26.Moser, M. B., Trommald, M., Egeland, T. & Andersen, P. (1997) J. Comp. Neurol. 380, 373-381. [DOI] [PubMed] [Google Scholar]
- 27.Greenough, W. T., Volkmar, F. R. & Juraska, J. M. (1973) Exp. Neurol. 41, 371-378. [DOI] [PubMed] [Google Scholar]
- 28.Irwin, S. A., Galvez, R. & Greenough, W. T. (2000) Cereb. Cortex 10, 1038-1044. [DOI] [PubMed] [Google Scholar]
- 29.Diamond, M. C. & Connor, J. R., Jr. (1982) Exp. Brain Res. 5, 36-44. [DOI] [PubMed] [Google Scholar]
- 30.Turner, A. M. & Greenough, W. T. (1985) Brain Res. 329, 195-203. [DOI] [PubMed] [Google Scholar]
- 31.Rampon, C., Jiang, C. H., Dong, H., Tang, Y. P., Lockhart, D. J., Schultz, P. G., Tsien, J. Z. & Hu, Y. (2000) Proc. Natl. Acad. Sci. USA 97, 12880-12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kempermann, G. & Gage, F. H. (1999) Hippocampus 9, 321-332. [DOI] [PubMed] [Google Scholar]
- 33.Ickes, B. R., Pham, T. M., Sanders, L. A., Albeck, D. S., Mohammed, A. H. & Granholm, A. C. (2000) Exp. Neurol. 164, 45-52. [DOI] [PubMed] [Google Scholar]
- 34.Williams, B. M., Luo, Y., Ward, C., Redd, K., Gibson, R., Kuczaj, S. A. & McCoy, J. G. (2001) Physiol. Behav. 73, 649-658. [DOI] [PubMed] [Google Scholar]
- 35.Frick, K. M. & Fernandez, S. M. (2003) Neurobiol. Aging 24, 615-626. [DOI] [PubMed] [Google Scholar]
- 36.Nithianantharajah, J., Levis, H. & Murphy, M. (2004) Neurobiol. Learn. Mem. 81, 200-210. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen, D. M., Derber, W. J., McClellan, D. A. & Crnic, L. S. (2002) Brain. Res. 927, 8-17. [DOI] [PubMed] [Google Scholar]
- 38.Glaser, E. M. & Van der Loos, H. (1981) J. Neurosci. Methods 4, 117-125. [DOI] [PubMed] [Google Scholar]
- 39.Gibb, R. & Kolb, B. (1998) J. Neurosci. Methods 79, 1-4. [DOI] [PubMed] [Google Scholar]
- 40.Uylings, H. B., Ruiz-Marcos, A. & van Pelt, J. (1986) J. Neurosci. Methods 18, 127-151. [DOI] [PubMed] [Google Scholar]
- 41.Horner, C. H. & Arbuthnott, E. (1991) J. Anat. 177, 179-184. [PMC free article] [PubMed] [Google Scholar]
- 42.Zalfa, F., Giorgi, M., Primerano, B., Moro, A., Di Penta, A., Reis, S., Oostra, B. & Bagni, C. (2003) Cell 112, 317-327. [DOI] [PubMed] [Google Scholar]
- 43.Qin, M., Kang, J. & Smith, C. B. (2002) Proc. Natl. Acad. Sci. USA 99, 15758-15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin, P. & Warren, S. T. (2000) Hum. Mol. Genet. 9, 901-908. [DOI] [PubMed] [Google Scholar]
- 45.Faherty, C. J., Kerley, D. & Smeyne, R. J. (2003) Brain Res. Dev. Brain Res. 141, 55-61. [DOI] [PubMed] [Google Scholar]
- 46.Griebel, G., Belzung, C., Perrault, G. & Sanger, D. J. (2000) Psychopharmacology 148, 164-170. [DOI] [PubMed] [Google Scholar]
- 47.Irwin, S. A., Swain, R. A., Christmon, C. A., Chakravarti, A., Weiler, I. J. & Greenough, W. T. (2000) Neurobiol. Learn. Mem. 73, 87-93. [DOI] [PubMed] [Google Scholar]
- 48.Gabel, L. A., Won, S., Mc Kinney, M., Tartakoff, A. M. & Fallon, J. R. (2004) J. Neurosci. 24, 10579-10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer, M., Kaech, S., Wagner, U., Brinkhaus, H. & Matus, A. (2000) Nat. Neurosci. 3, 887-894. [DOI] [PubMed] [Google Scholar]
- 50.Passafaro, M., Nakagawa, T., Sala, C. & Sheng, M. (2003) Nature 424, 677-681. [DOI] [PubMed] [Google Scholar]
- 51.Inglis, F. M., Crockett, R., Korada, S., Abraham, W. C., Hollmann, M. & Kalb, R. G. (2002) J. Neurosci. 22, 8042-8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gagne, J., Gelinas, S., Martinoli, M. G., Foster, T. C., Ohayon, M., Thompson, R. F., Baudry, M. & Massicotte, G. (1998) Brain Res. 799, 16-25. [DOI] [PubMed] [Google Scholar]
- 53.Mlynarik, M., Johansson, B. B. & Jezova, D. (2004) Ann. N.Y. Acad. Sci. 1018, 273-280. [DOI] [PubMed] [Google Scholar]
- 54.Danysz, W. (2002) Curr. Opin. Investig. Drugs 3, 1081-1088. [PubMed] [Google Scholar]
- 55.Weiler, I. J. & Greenough, W. T. (1999) Am. J. Med. Genet. 83, 248-252. [DOI] [PubMed] [Google Scholar]
- 56.Antar, L. N., Afroz, R., Dictenberg, J. B., Carroll, R. C. & Bassell, G. J. (2004) J. Neurosci. 24, 2648-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi, Y., Shi, S. H., Esteban, J. A., Piccini, A., Poncer, J. C. & Malinow, R. (2000) Science 287, 2262-2267. [DOI] [PubMed] [Google Scholar]
- 58.Hessl, D., Dyer-Friedman, J., Glaser B., Wisbek, J., Barajas, R. G., Taylor, A. & Reiss, A. (2001) Pediatrics 108, 88-96. [DOI] [PubMed] [Google Scholar]
- 59.Dyer-Friedman, J., Glaser, B., Hessl, D., Johnston, C., Huffman, L.C., Taylor, A., Wisbeck, J. & Reiss, A. L. (2002) J. Am. Acad. Child Adolesc. Psychiatry 41, 237-244. [DOI] [PubMed] [Google Scholar]