Abstract
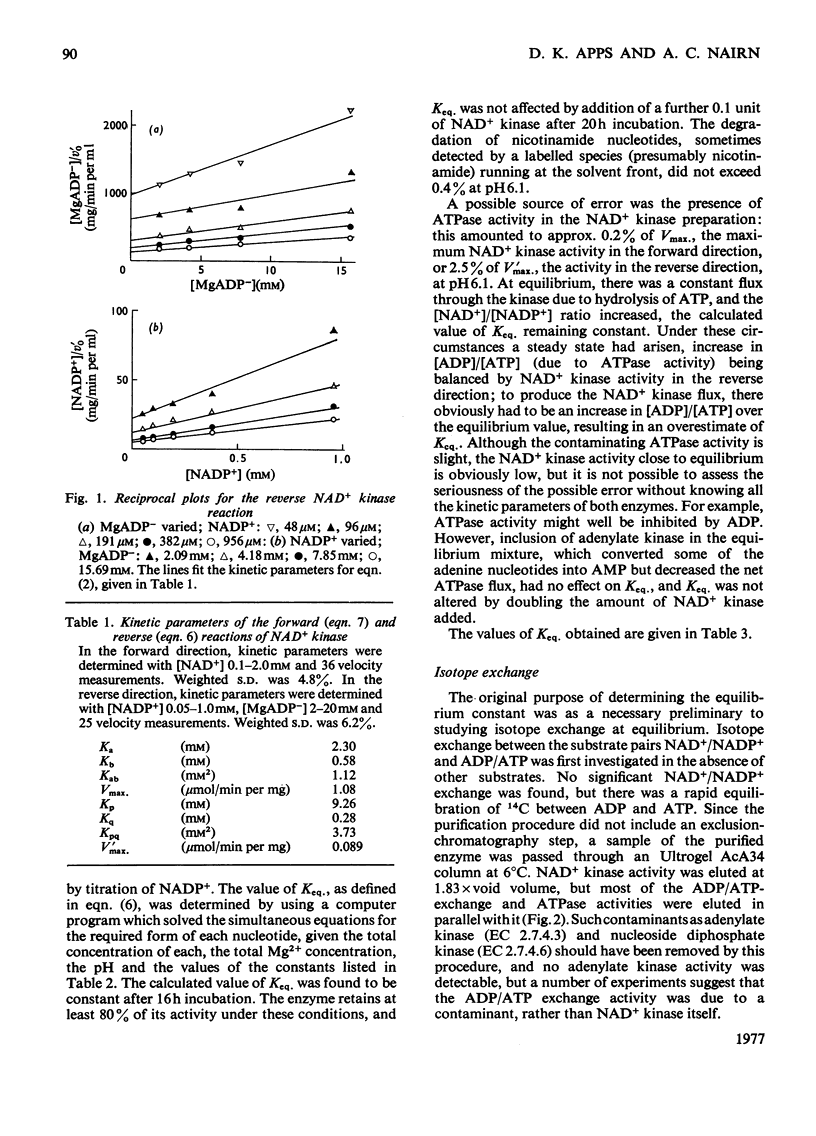
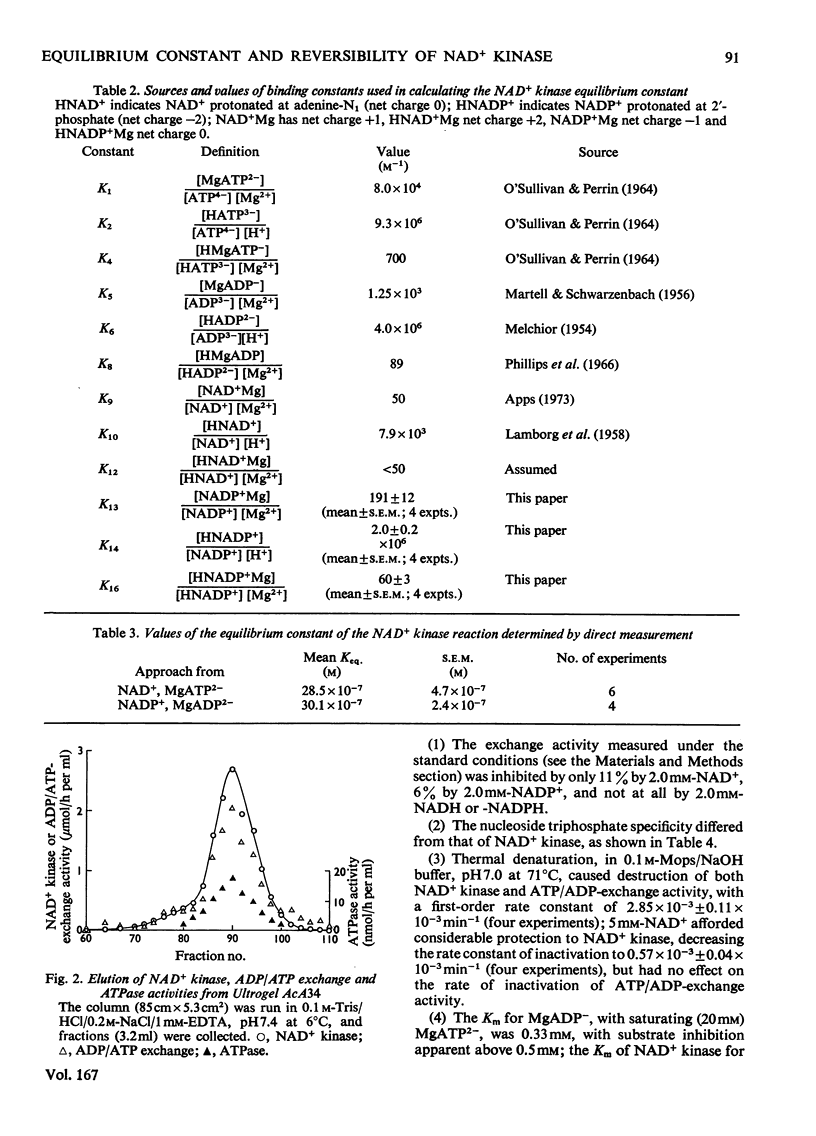
The reversibility of the NAD+ kinase reaction was established, and the kinetic parameters of the rate equation in the reverse direction were determined. The equilibrium constant of the reaction was determined by using the purified pigeon liver enzyme and radioactively labelled nicotinamide nucleotides. The relationship between kinetic parameters of the forward and reverse reactions is in reasonable agreement with the measured equilibrium constant. As expected from the proposed mechanism of action, the enzyme does not catalyse isotope exchange between NAD+ and NADP+ in the absence of ADP and ATP. Although homogeneous as judged by polyacrylamide-gel electrophoresis, the enzyme preparation exhibits ADP/ATP isotope-exchange activity which could not be separated from NAD+ kinase activity, but kinetic evidence suggests that this is probably due to a contaminant.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apps D. K. Complex formation between magnesium ions and pyridine nucleotide coenzymes. Biochim Biophys Acta. 1973 Sep 14;320(2):379–387. doi: 10.1016/0304-4165(73)90319-x. [DOI] [PubMed] [Google Scholar]
- Apps D. K., Gleed C. D. Interaction of pigeon-liver nicotinamide-adenine dinucleotide kinase with cibacron blue F3GA. Biochem J. 1976 Nov;159(2):441–443. doi: 10.1042/bj1590441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps D. K. Kinetic studies of pigeon liver NAD kinase. Eur J Biochem. 1968 Aug;5(3):444–450. doi: 10.1111/j.1432-1033.1968.tb00390.x. [DOI] [PubMed] [Google Scholar]
- Apps D. K., Marsh A. Kinetic studies of the interaction of pigeon-liver NAD kinase with adenine nucleotides and divalent cations. Eur J Biochem. 1972 Jun 23;28(1):12–19. doi: 10.1111/j.1432-1033.1972.tb01878.x. [DOI] [PubMed] [Google Scholar]
- Apps D. K. Pigeon-liver NAD kinase. The structural and kinetic basis of regulation of NADPH. Eur J Biochem. 1975 Jul 1;55(2):475–483. doi: 10.1111/j.1432-1033.1975.tb02184.x. [DOI] [PubMed] [Google Scholar]
- Apps D. K. The substrate specificity of pigeon liver NAD kinase. Eur J Biochem. 1969 Jan;7(2):260–266. doi: 10.1111/j.1432-1033.1969.tb19601.x. [DOI] [PubMed] [Google Scholar]
- Apps David K. Inhibition of pigeon liver NAD kinase by a non-phosphorylatible analogue of NAD. FEBS Lett. 1971 Jul 1;15(4):277–280. doi: 10.1016/0014-5793(71)80637-3. [DOI] [PubMed] [Google Scholar]
- Bernofsky C., Swan M. An improved cycling assay for nicotinamide adenine dinucleotide. Anal Biochem. 1973 Jun;53(2):452–458. doi: 10.1016/0003-2697(73)90094-8. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMBORG M., STOLZENBACH F. E., KAPLAN N. O. The nicotinic acid analogue of diphosphopyridine nucleotide. J Biol Chem. 1958 Apr;231(2):685–694. [PubMed] [Google Scholar]
- MELCHIOR N. C. Sodium and potassium complexes of adenosinetriphosphate: equilibrium studies. J Biol Chem. 1954 Jun;208(2):615–627. [PubMed] [Google Scholar]
- O'SULLIVAN W. J., PERRIN D. D. THE STABILITY CONSTANTS OF METAL-ADENINE NUCLEOTIDE COMPLEXES. Biochemistry. 1964 Jan;3:18–26. doi: 10.1021/bi00889a005. [DOI] [PubMed] [Google Scholar]
- Orringer B. P., Chung A. E. Nicotinamide adenine dinucleotide kinase from Azotobacter vinelandii cells. A possible mechanism for the enzyme reaction. Biochim Biophys Acta. 1971 Oct;250(1):86–91. doi: 10.1016/0005-2744(71)90122-7. [DOI] [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic data for the hydrolysis of adenosine triphosphate as a function of pH, Mg2+ ion concentration, and ionic strength. J Biol Chem. 1969 Jun 25;244(12):3330–3342. [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic studies of the formation and ionization of the magnesium(II) complexes of ADP and ATP over the pH range 5 to 9. J Am Chem Soc. 1966 Jun 20;88(12):2631–2640. doi: 10.1021/ja00964a002. [DOI] [PubMed] [Google Scholar]


