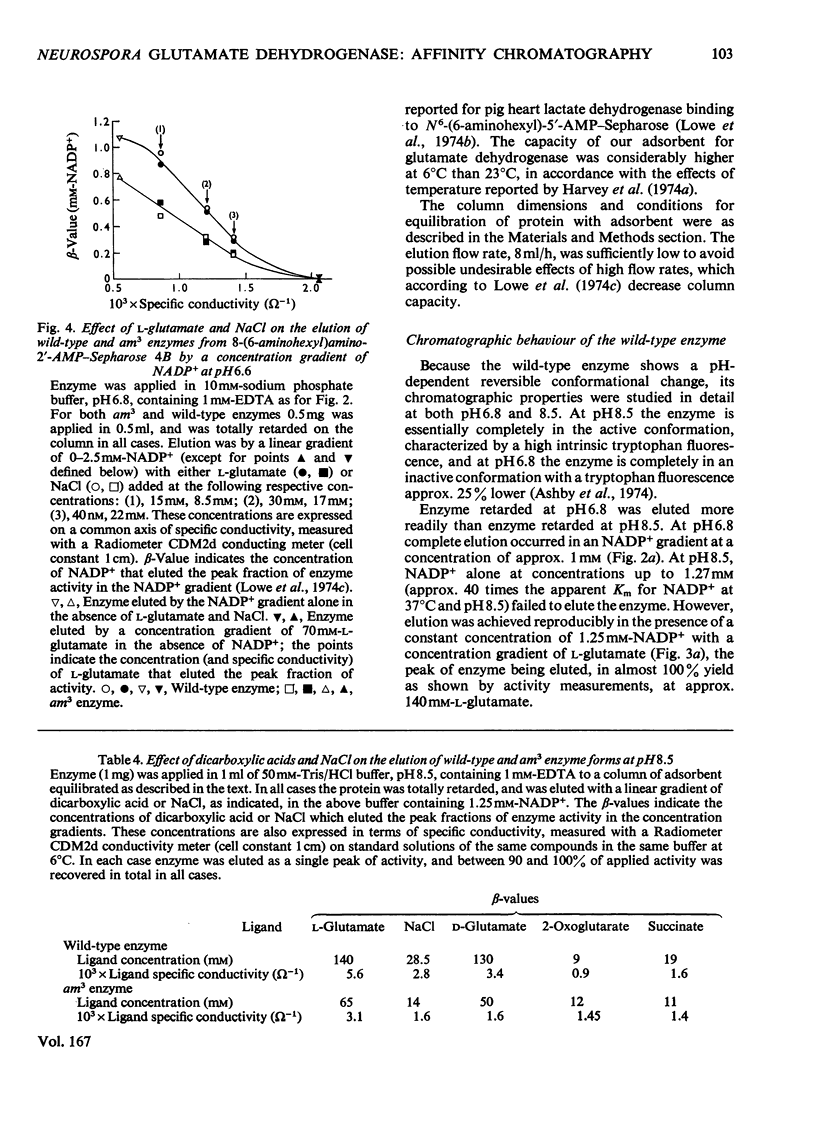
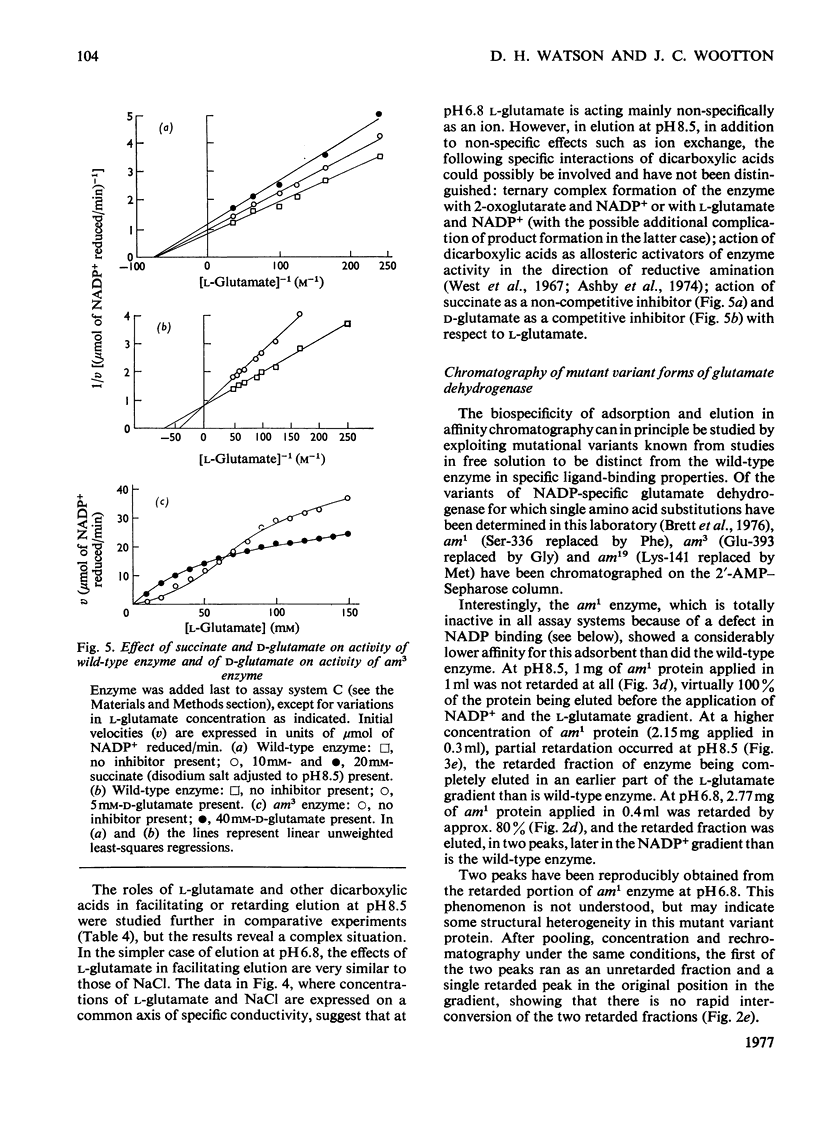
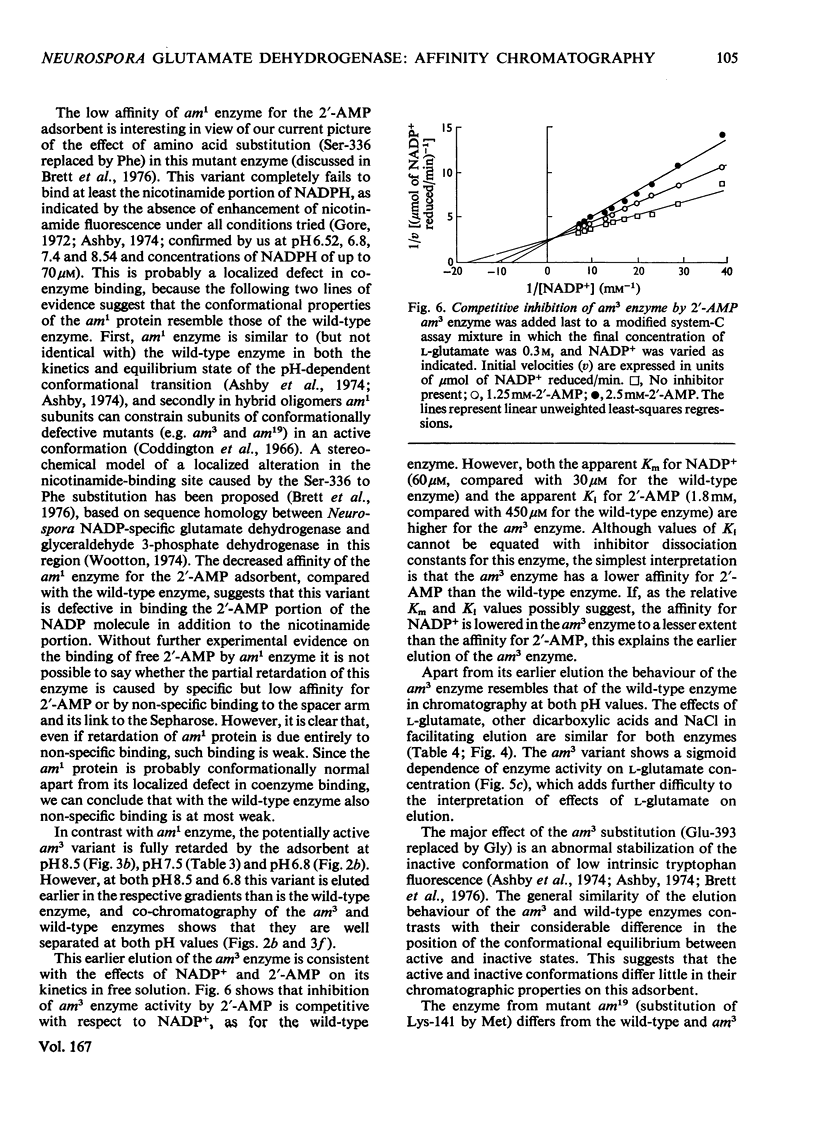
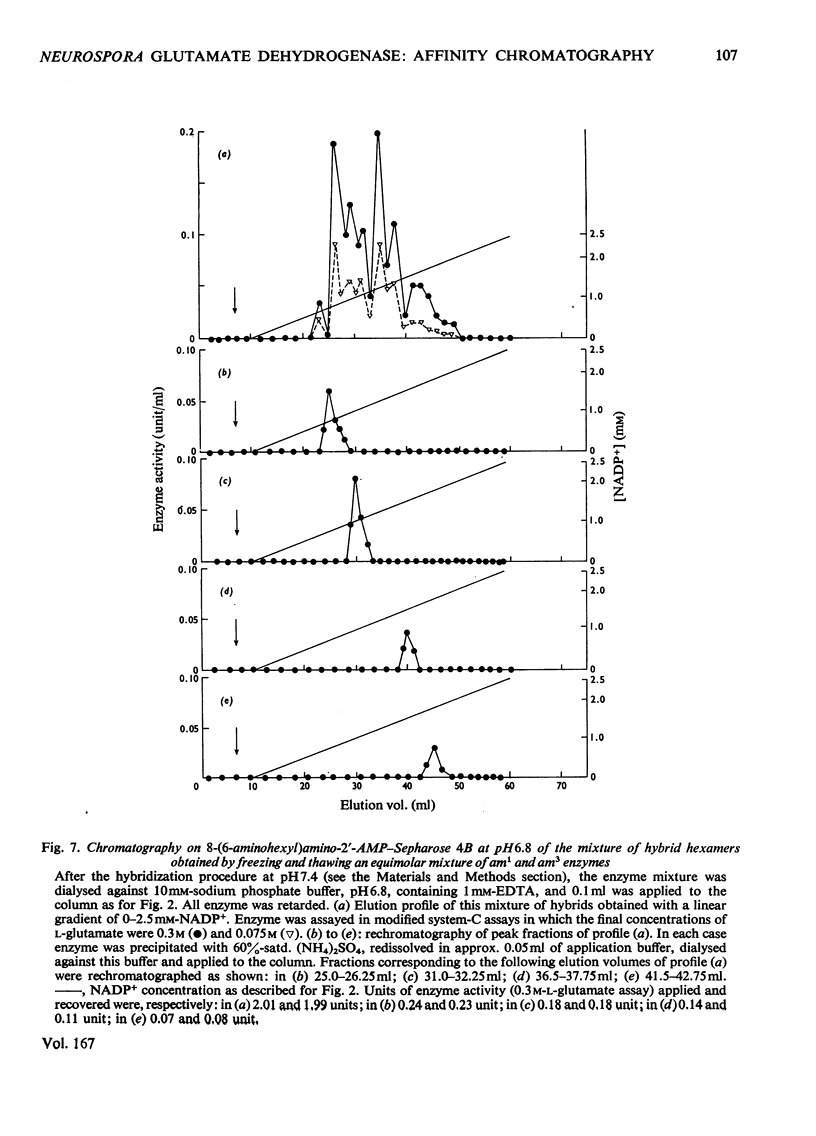
Abstract
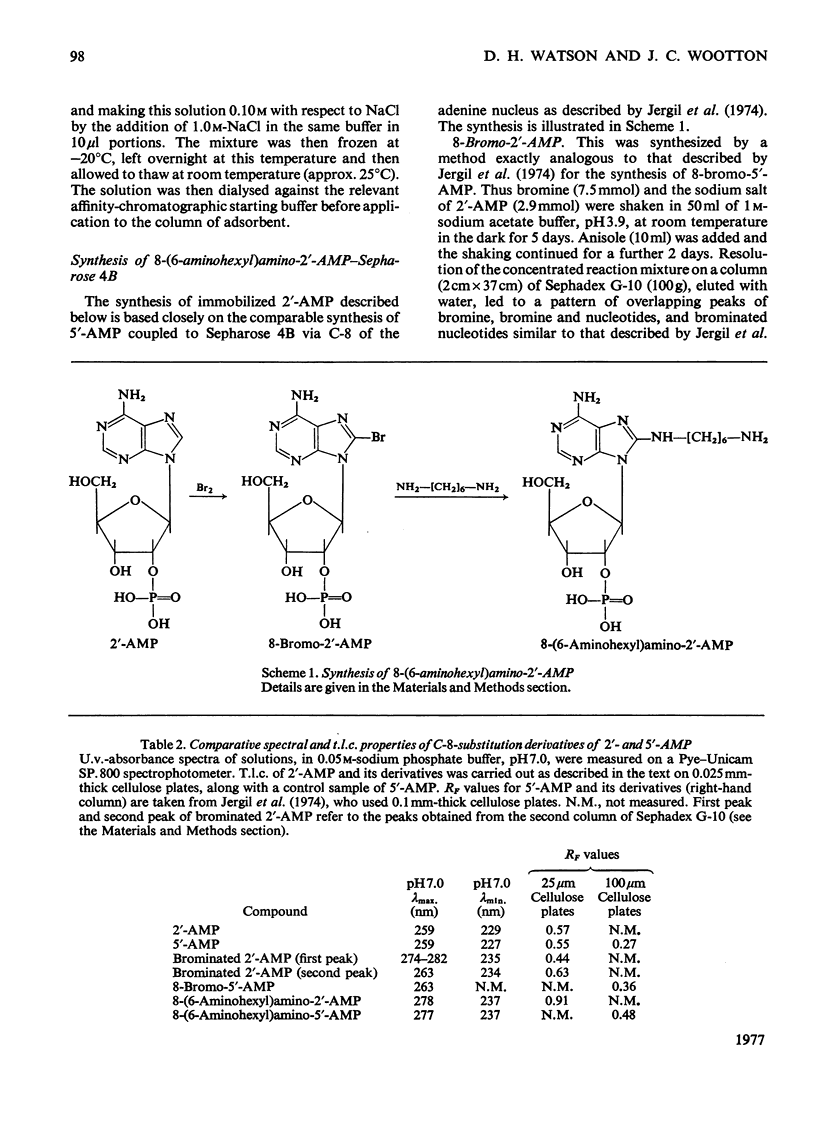
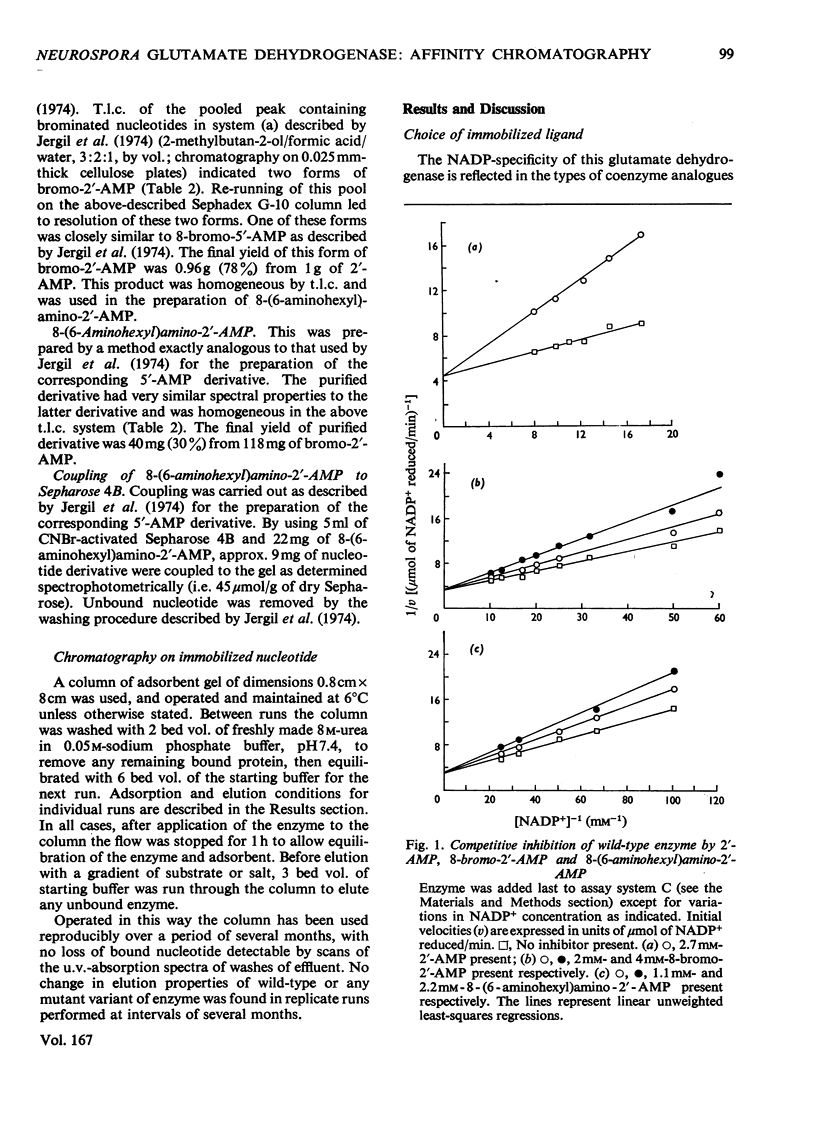
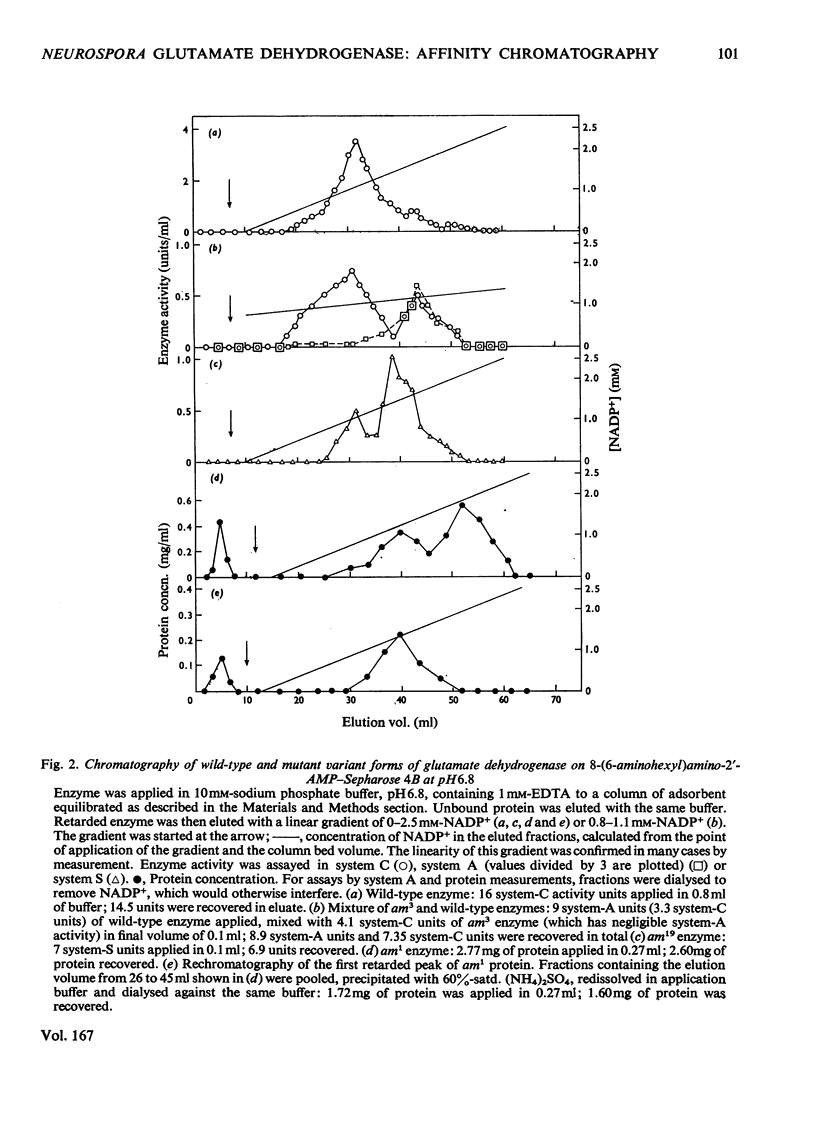
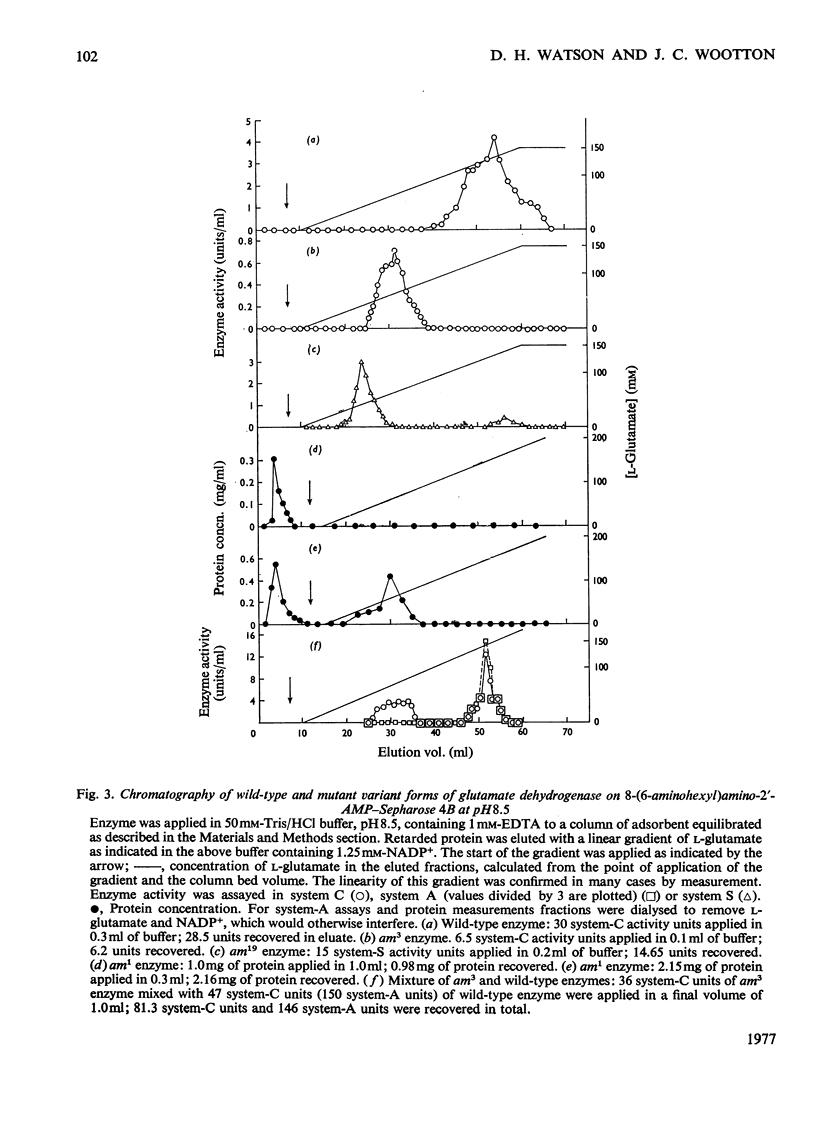
The synthesis of an affinity adsorbent, 8-(6-aminohexyl)aminoadenosine 2'-phosphate-Sepharose 4B, is described. The assembly of the 2'-AMP ligand and the hexanediamide spacer arm was synthesized in free solution before its attachment to the Sepharose matrix. This adsorbent retarded the hexameric NADP-specific glutamate dehydrogenase of Neurospora crassa, showing a capacity for this enzyme similar to that of comparable coenzyme-analogue adsorbents for other dehydrogenases. The enzyme was eluted either at pH 6.8 in a concentration gradient of NADP+, or at pH 8.5 in the presence of NADP+ in concentration gradients of either dicarboxylates or NaCl. Anomalous effects of dicarboxylates in facilitating elution are discussed. 2'-AMP and its derivatives, 8-bromoadenosine 2'-phosphate and 8-(l-aminohexyl)aminoadenosine 2'-phosphate, which were used in the synthesis of the adsorbent, all acted as enzyme inhibitors competitive with NADP+. The chromatographic properties of the wild-type enzyme were compared with those of mutationally modified variants containing defined amino acid substitutions. This approach was used to assess the biospecificity of adsorption and elution and the contribution of non-specific binding. The adsorbent showed a low capacity for the enzyme from mutant am1 (Ser-336 replaced by Phe), a variant that has a localized defect in NADP binding, but an otherwise almost normal conformation, suggesting that non-specific interactions are at most weak. The enzyme from mutant am3, a variant modified in a conformational equilibrium, was fully retarded by the adsorbent, but showed a significantly earlier elution position than the wild-type enzyme. This is consistent with measurements in free solution that showed the am3 enzyme to have a higher Ki for 2'-AMP than the wild-type enzyme. The enzyme from mutant am19 was eluted as two distinct peaks at both pH 6.8 and 8.5. The adsorbent was used to separate hybrid hexamers constructed in vitro by a freeze-thaw procedure from pairs of purified variants. Several chromatographically distinct peaks of differing enzymological properties were purified from each hybridization mixture in quantities of up to a few milligrams, and represented distinct species of hybrid hexamers differing in subunit ratio.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L., Jörnvall H., Akeson A., Mosbach K. Separation of isozymes of horse liver alcohol dehydrogenase and purification of the enzyme by affinity chromatography on an immobilized AMP-analogue. Biochim Biophys Acta. 1974 Sep 11;364(1):1–8. doi: 10.1016/0005-2744(74)90126-0. [DOI] [PubMed] [Google Scholar]
- Ashby B., Wootton J. C., Fincham J. R. Slow conformational changes of a Neurospora glutamate dehydrogenase studied by protein fluorescence. Biochem J. 1974 Nov;143(2):317–329. doi: 10.1042/bj1430317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry S., O'Carra P. Affinity chromatography of nicotinamide-adenine dinucleotide-linked dehydrogenases on immobilized derivatives of the dinucleotide. Biochem J. 1973 Dec;135(4):595–607. doi: 10.1042/bj1350595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal K. M., Smith E. L. Nicotinamide adenine dinucleotide phosphate-specific glutamate dehydrogenase of Neurospora. I. Isolation, subunits, amino acid composition, sulfhydryl groups, and identification of a lysine residue reactive with pyridoxal phosphate and N-ethylmaleimide. J Biol Chem. 1973 Sep 10;248(17):6002–6008. [PubMed] [Google Scholar]
- Blumenthal K. M., Smith E. L. Nicotinamide adenine dinucleotide phosphate-specific glutamate dehydrogenase of Neurospora. III. Inactivation by nitration of a tyrosine residue involved in coenzyme binding. J Biol Chem. 1975 Aug 25;250(16):6560–6563. [PubMed] [Google Scholar]
- Brett M., Chambers G. K., Holder A. A., Fincham J. R., Wootton J. C. Mutational amino acid replacements in Neurospora crassa NADP-specific glutamate dehydrogenase. J Mol Biol. 1976 Sep 5;106(1):1–22. doi: 10.1016/0022-2836(76)90297-7. [DOI] [PubMed] [Google Scholar]
- Brodelius P., Larsson P. O., Mosbach K. The synthesis of three AMP-analogues: N6-(6-aminohexyl)-adenosine 5'-monophosphate, N6-(6-aminohexyl)-adenosine 2',5'-bisphosphate, and N6-(6-aminohexyl)-adenosine 3',5'-bisphosphate and their application as general ligands in biospecific affinity chromatography. Eur J Biochem. 1974 Aug 15;47(1):81–89. doi: 10.1111/j.1432-1033.1974.tb03670.x. [DOI] [PubMed] [Google Scholar]
- Brodelius P., Mosbach K. Separation of the isoenzymes of lactate dehydrogenase by affinity chromatography using an immobilized AMP-analogue. FEBS Lett. 1973 Sep 15;35(2):223–226. doi: 10.1016/0014-5793(73)80290-x. [DOI] [PubMed] [Google Scholar]
- CODDINGTON A., FINCHAM J. R. PROOF OF HYBRID ENZYME FORMATION IN A CASE OF INTER-ALLELIC COMPLEMENTATION IN NEUROSPORA CRASSA. J Mol Biol. 1965 May;12:152–161. doi: 10.1016/s0022-2836(65)80289-3. [DOI] [PubMed] [Google Scholar]
- Coddington A., Fincham J. R., Sundaram T. K. Multiple active varieties of Neurospora glutamate dehydrogenase formed by hybridization between two inactive mutant proteins in vivo and in vitro. J Mol Biol. 1966 Jun;17(2):503–512. doi: 10.1016/s0022-2836(66)80160-2. [DOI] [PubMed] [Google Scholar]
- Craven D. B., Harvey M. J., Lowe C. R., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 1. A new synthesis and some properties of an N6-immobilised 5'-AMP. Eur J Biochem. 1974 Jan 16;41(2):329–333. doi: 10.1111/j.1432-1033.1974.tb03273.x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINCHAM J. R., CODDINGTON A. Complementation at the am locus of Neurospora crassa: a reaction between different mutant forms of glutamate dehydrogenase. J Mol Biol. 1963 May;6:361–373. doi: 10.1016/s0022-2836(63)80049-2. [DOI] [PubMed] [Google Scholar]
- Godinot C., Julliard J. H., Gautheron D. C. A rapid and efficient new method of purification of glutamate dehydrogenase by affinity chromatography on GTP-sepharose. Anal Biochem. 1974 Sep;61(1):264–270. doi: 10.1016/0003-2697(74)90353-4. [DOI] [PubMed] [Google Scholar]
- Harvey M. J., Lowe C. R., Craven D. B., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 2. Some parameters relating to the selection and concentration of the immobilised ligand. Eur J Biochem. 1974 Jan 16;41(2):335–340. doi: 10.1111/j.1432-1033.1974.tb03274.x. [DOI] [PubMed] [Google Scholar]
- Harvey M. J., Lowe C. R., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 5. Some applications of the influence of temperature on the binding of dehydrogenases and kinases. Eur J Biochem. 1974 Jan 16;41(2):353–357. doi: 10.1111/j.1432-1033.1974.tb03277.x. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Wootton J. C., Baron A. J., Chambers G. K., Fincham J. R. The amino acid sequence of Neurospora NADP-specific glutamate dehydrogenase. Peptic and chymotryptic peptides and the complete sequence. Biochem J. 1975 Sep;149(3):757–773. doi: 10.1042/bj1490757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergil B., Guilford H., Mosbach K. Biospecific affinity chromatography of an adenosine 3':5'-cyclic monophosphate-stimulated protein kinase (protamine kinase from trout testis) by using immobilized adenine nucleotides. Biochem J. 1974 May;139(2):441–448. doi: 10.1042/bj1390441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. R., Harvey M. J., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 3. The binding of glycerokinase and lactate dehydrogenase in relation to column geometry and dynamics. Eur J Biochem. 1974 Jan 16;41(2):341–345. doi: 10.1111/j.1432-1033.1974.tb03275.x. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Harvey M. J., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 4. Variation of the binding of dehydrogenases and kinases with pH. Eur J Biochem. 1974 Jan 16;41(2):347–351. doi: 10.1111/j.1432-1033.1974.tb03276.x. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Harvey M. J., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. Some kinetic parameters involved in the binding of group-specific enzymes. Eur J Biochem. 1974 Feb 15;42(1):1–6. doi: 10.1111/j.1432-1033.1974.tb03306.x. [DOI] [PubMed] [Google Scholar]
- Mosbach K. AMP and NAD as "General Ligands". Methods Enzymol. 1974;34:229–242. doi: 10.1016/s0076-6879(74)34019-0. [DOI] [PubMed] [Google Scholar]
- Mosbach K., Guilford H., Ohlsson R., Scott M. General ligands in affinity chromatography. Cofactor-substrate elution of enzymes bound to the immobilized nucleotides adenosine 5'-monophosphate and nicotinamide-adenine dinucleotide. Biochem J. 1972 May;127(4):625–631. doi: 10.1042/bj1270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayer I. P., Trayer H. R. Affinity chromatography of nicotinamide nucleotide-dependent dehydrogenases on immobilized nucleotide derivatives. Biochem J. 1974 Sep;141(3):775–787. doi: 10.1042/bj1410775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayer I. P., Trayer H. R., Small D. P., Bottomley R. C. Preparation of adenosine nucleotide derivatives suitable for affinity chromatography. Biochem J. 1974 Jun;139(3):609–623. doi: 10.1042/bj1390609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West D. J., Tuveson R. W., Barratt R. W., Fincham J. R. Allosteric effects in nicotinamide adenine dinucleotide phosphate-specific glutamate dehydrogenase from Neurospora. J Biol Chem. 1967 May 10;242(9):2134–2138. [PubMed] [Google Scholar]
- Wootton J. C. The coenzyme-binding domains of glutamate dehydrogenases. Nature. 1974 Dec 13;252(5484):542–546. doi: 10.1038/252542a0. [DOI] [PubMed] [Google Scholar]
- Zabin I., Villarejo M. R. Protein complementation. Annu Rev Biochem. 1975;44:295–313. doi: 10.1146/annurev.bi.44.070175.001455. [DOI] [PubMed] [Google Scholar]


