Abstract
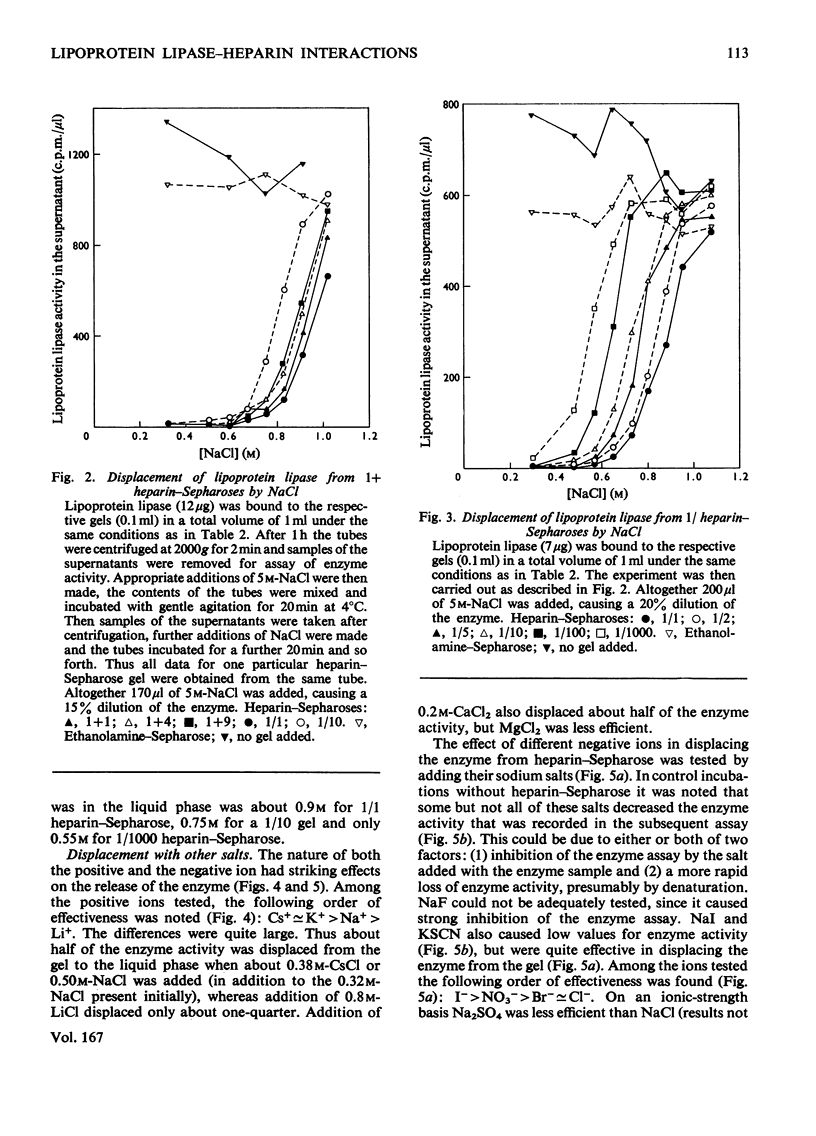
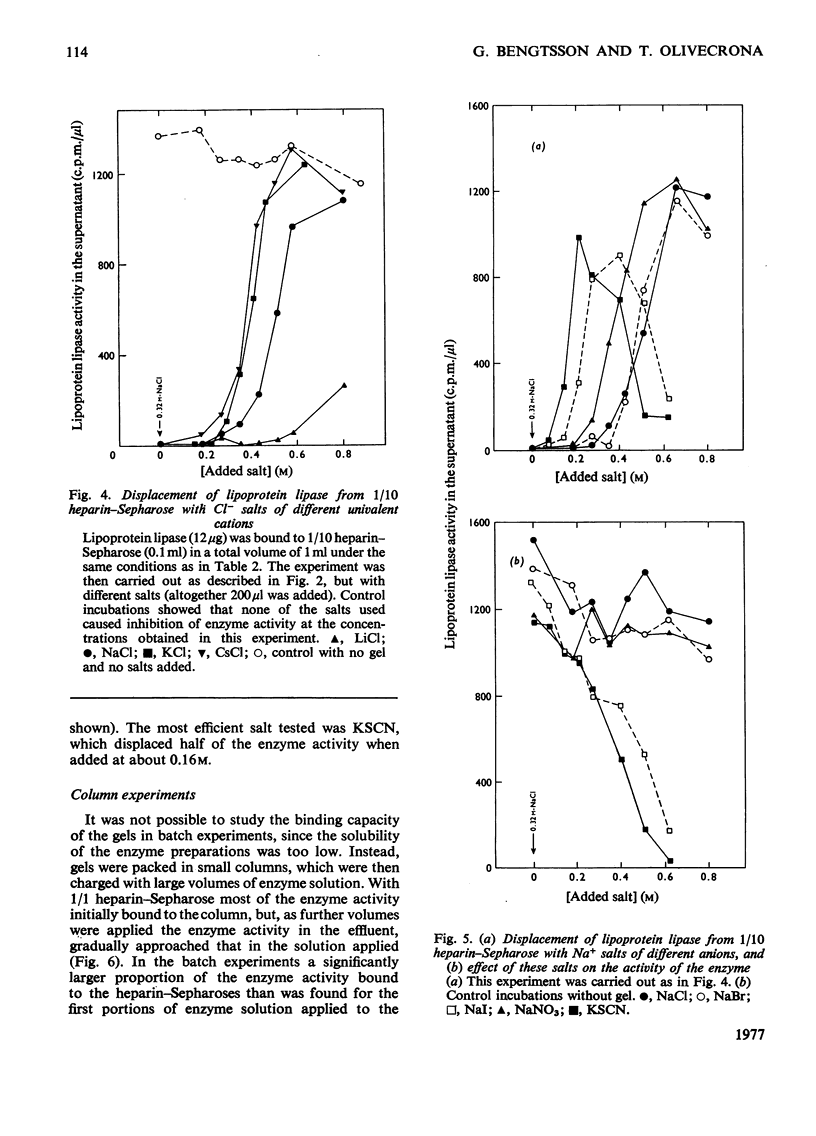
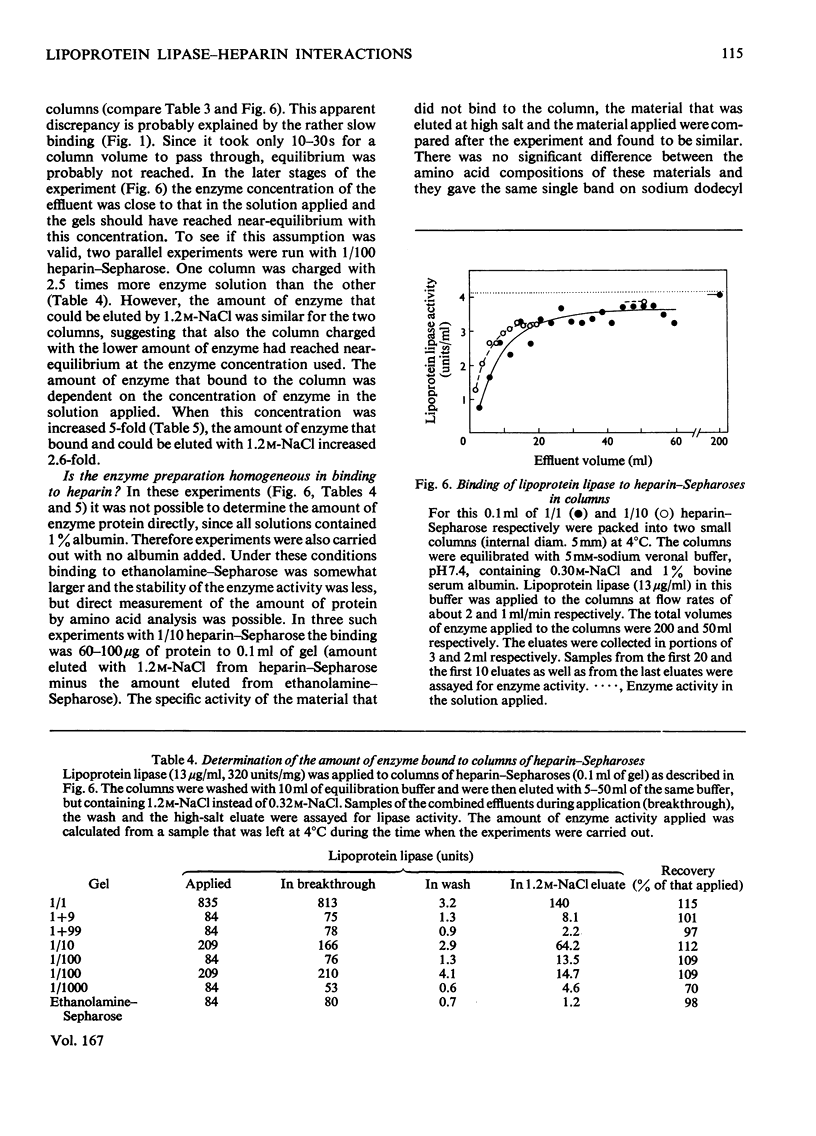
Lipoprotein lipases from a variety of sources have been shown previously to bind to heparin and some related polysaccharides. For the present studies lipoprotein lipase purified from bovine milk was used. 1. In batch experiments binding of the enzyme activity to heparin–Sepharose occurred relatively slowly, so that 30min was required for the system to come to near-equilibrium. In contrast, release of the enzyme activity from heparin–Sepharose by addition of salt to the liquid phase occurred rapidly. 2. Some binding was observed also with unsubstituted Sepharose, but this binding had a low capacity compared with that observed with heparin–Sepharose. High salt concentrations, heparin or deoxycholate decreased the binding to unsubstituted Sepharose. These factors also increase the solubility of the enzyme, which is low. 3. Addition of heparin to the liquid phase caused a concentration-dependent release of enzyme activity from the gel. These results suggested that the binding of the enzyme to heparin–Sepharose was mainly through interaction with heparin. 4. The enzyme activity was also quantitatively displaced to the liquid phase at increased concentrations of salt. Among the positive ions tested the following order of effectiveness was noted: Cs+≃K+>Na+>Li+; and among the negative the following: SCN−>I−> NO3−>Br−≃Cl−. The differences were quite large. Thus addition of 0.16m-KSCN (in addition to the 0.32m-NaCl originally present) displaced one-half of the enzyme activity to the supernatant, whereas 0.8m-LiCl only displaced one-quarter. 5. The distribution of heparin in the gel also profoundly influenced the binding. Two series of gels were studied. One series was made by mixing heparin–Sepharose with unsubstituted Sepharose. Results obtained with these gels were those expected from a series of decreasing volumes of heparin–Sepharose. In contrast, a series of heparin–Sepharoses made with different degrees of substitution gave quite different results. With these gels the amount of enzyme activity bound per amount of heparin increased markedly, whereas the salt concentration needed to displace the enzyme activity from the gel decreased markedly with decreased concentration of heparin in the gel. 6. On stepwise elution of small columns of heparin–Sepharose the enzyme activity was eluted over a remarkably wide range of salt concentrations. When enzyme eluted at one salt concentration was re-applied, it gave the same elution profile as enzyme previously eluted at other salt concentrations or the entire enzyme preparation. These and other results suggested that, whereas the enzyme preparation was rather homogeneous in its binding to heparin, the heparin preparation was polydisperse in binding of lipoprotein lipase.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNFELD P., KELLEY T. F. Inhibitory and activating effects of polyanions on lipoprotein lipase. J Biol Chem. 1963 Apr;238:1236–1241. [PubMed] [Google Scholar]
- Bengtsson G., Olivecrona T. Interaction of heparin with proteins. Demonstration of different binding sites for antithrombin and lipoprotein lipase. FEBS Lett. 1977 Jul 1;79(1):59–63. doi: 10.1016/0014-5793(77)80350-5. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Ehnholm C., Steinberg D., Brown W. V. Purification and characterization of lipoprotein lipase from pig adipose tissue. J Biol Chem. 1974 Apr 10;249(7):2220–2227. [PubMed] [Google Scholar]
- Dolphin P. J., Rubinstein D. The metabolism of very low density lipoprotein and chylomicrons by purified lipoprotein lipase from rat heart and adipose tissue. Biochem Biophys Res Commun. 1974 Apr 8;57(3):808–814. doi: 10.1016/0006-291x(74)90618-4. [DOI] [PubMed] [Google Scholar]
- Egelrud T., Olivecrona T. The purification of a lipoprotein lipase from bovine skim milk. J Biol Chem. 1972 Oct 10;247(19):6212–6217. [PubMed] [Google Scholar]
- Ehnholm C., Kinnunen P. K., Huttunen J. K., Nikkilä E. A., Ota M. Purification and characterization of lipoprotein lipase from pig myocardium. Biochem J. 1975 Sep;149(3):649–655. doi: 10.1042/bj1490649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten H., Walter B. Purification of rat adipose tissue lipoprotein lipase. FEBS Lett. 1973 Sep 1;35(1):36–40. doi: 10.1016/0014-5793(73)80571-x. [DOI] [PubMed] [Google Scholar]
- Harvey M. J., Lowe C. R., Craven D. B., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 2. Some parameters relating to the selection and concentration of the immobilised ligand. Eur J Biochem. 1974 Jan 16;41(2):335–340. doi: 10.1111/j.1432-1033.1974.tb03274.x. [DOI] [PubMed] [Google Scholar]
- Hernell O., Egelrud T., Olivecrona T. Serum-stimulated lipases (lipoprotein lipases). Immunological crossreaction between the bovine and the human enzymes. Biochim Biophys Acta. 1975 Feb 13;381(2):233–241. [PubMed] [Google Scholar]
- Hernell O., Olivecrona T. Human milk lipases. I. Serum-stimulated lipase. J Lipid Res. 1974 Jul;15(4):367–374. [PubMed] [Google Scholar]
- Hofstee B. H. Non-specific binding of proteins by substituted agaroses. Adv Exp Med Biol. 1974;42(0):43–59. doi: 10.1007/978-1-4684-6982-0_4. [DOI] [PubMed] [Google Scholar]
- Hök M., Björk I., Hopwood J., Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett. 1976 Jul 1;66(1):90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- Iverius P. H. Coupling of glycosaminoglycans to agarose beads (sepharose 4B). Biochem J. 1971 Oct;124(4):677–683. doi: 10.1042/bj1240677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverius P. H., Lindahl U., Egelrud T., Olivecrona T. Effects of heparin on lipoprotein lipase from bovine milk. J Biol Chem. 1972 Oct 25;247(20):6610–6616. [PubMed] [Google Scholar]
- Iverius P. H., Ostlund-Lindqvist A. M. Lipoprotein lipase from bovine milk. Isolation procedure, chemical characterization, and molecular weight analysis. J Biol Chem. 1976 Dec 25;251(24):7791–7795. [PubMed] [Google Scholar]
- Iverius P. H. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem. 1972 Apr 25;247(8):2607–2613. [PubMed] [Google Scholar]
- KORN E. D. The kinetics of the inhibition of lipoprotein lipase by polyanions and polycations. J Biol Chem. 1962 Nov;237:3423–3429. [PubMed] [Google Scholar]
- Kinnunen P. K., Huttunen J. K., Ehnholm C. Properties of purified bovine milk lipoprotein lipase. Biochim Biophys Acta. 1976 Dec 20;450(3):342–351. doi: 10.1016/0005-2760(76)90007-2. [DOI] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. Affinity chromatography--old problems and new approaches. Adv Exp Med Biol. 1974;42(0):3–14. doi: 10.1007/978-1-4684-6982-0_1. [DOI] [PubMed] [Google Scholar]
- O'carra P., Barry S., Griffin T. Interfering and complicating adsorption effects in bioaffinity chromatography. Methods Enzymol. 1974;34:108–126. doi: 10.1016/s0076-6879(74)34011-6. [DOI] [PubMed] [Google Scholar]
- Olivecrona T., Bengtsson G., Marklund S. E., Lindahl U., Hök M. Heparin-lipoprotein lipase interactions. Fed Proc. 1977 Jan;36(1):60–65. [PubMed] [Google Scholar]
- Olivecrona T., Egelrud T., Iverius P. H., Lindahl U. Evidence for an ionic binding of lipoprotein lipase to heparin. Biochem Biophys Res Commun. 1971 May 7;43(3):524–529. doi: 10.1016/0006-291x(71)90645-0. [DOI] [PubMed] [Google Scholar]
- Patten R. L., Hollenberg C. H. The mechanism of heparin stimulation of rat adipocyte lipoprotein lipase. J Lipid Res. 1969 Jul;10(4):374–382. [PubMed] [Google Scholar]
- Twu J. S., Garfinkel A. S., Schotz M. C. Rat heart lipoprotein lipase. Atherosclerosis. 1975 Nov-Dec;22(3):463–472. doi: 10.1016/0021-9150(75)90025-8. [DOI] [PubMed] [Google Scholar]


