Abstract
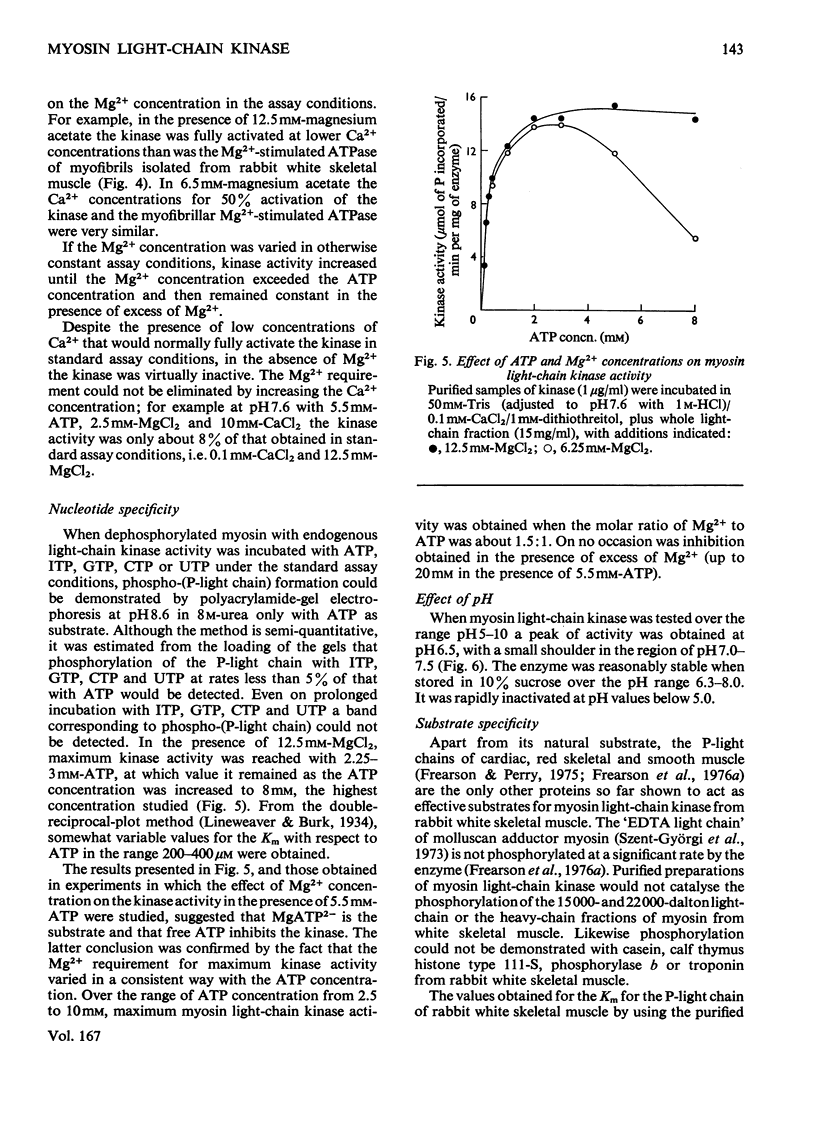
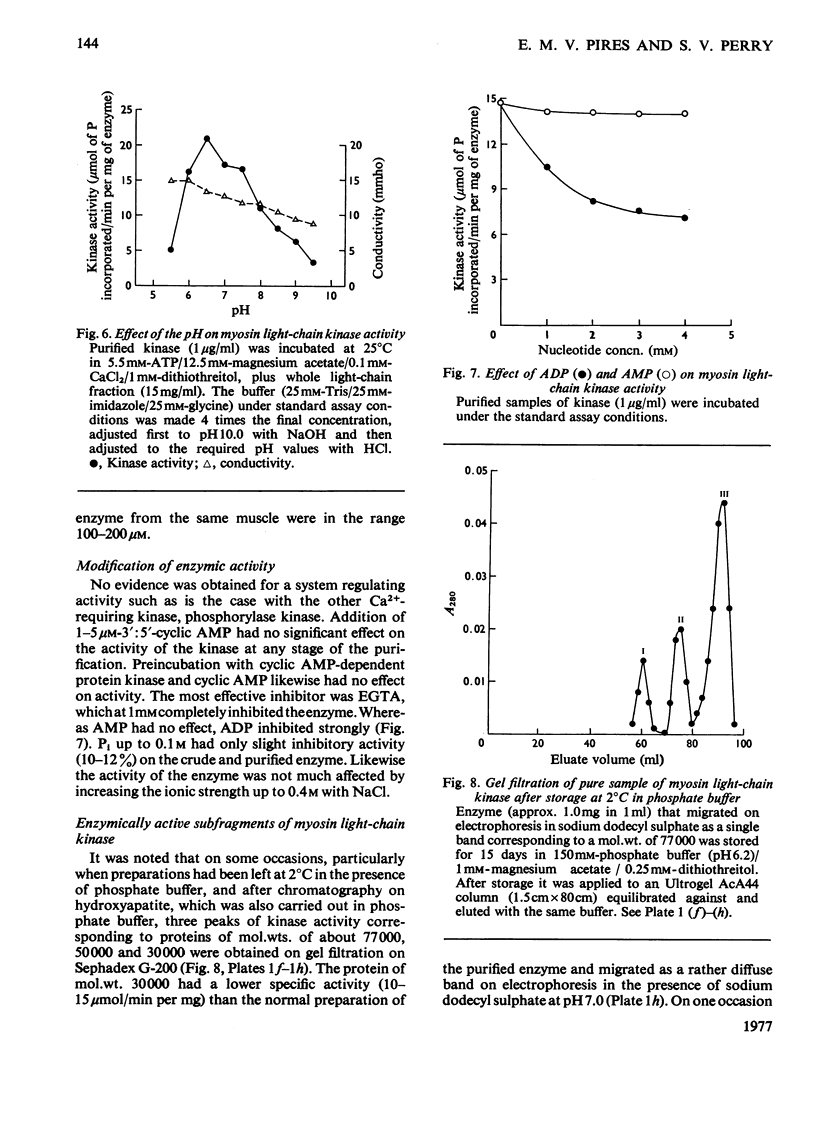
1. A procedure is described for the isolation of myosin light-chain kinase from rabbit fast skeletal muscle as a homogeneous protein. 2. Myosin light-chain kinase is a monomeric enzyme of mol.wt. 77000. Under some conditions of storage it is converted into components of mol.wts. about 50000 and 30000 that possess enzymic activity. 3. The enzyme is clearly different in structure and properties from any other protein kinase so far isolated from muscle. 4. The enzyme is highly specific for the P-light chain (18000-20000-dalton light chain) of myosin and requires Ca2+ for activity. 5. The P-light chain is phosphorylated at a similar rate whether isolated or associated with the rest of the myosin molecule. 6. The effects of pH, bivalent cation and other nucleotides on the enzymic activity are described. 7. The role of the phosphorylation of the P-light chain of myosin in muscle function is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J. L., Adelstein R. S. Isolation and properties of platelet myosin light chain kinase. Biochemistry. 1976 Jun 1;15(11):2370–2377. doi: 10.1021/bi00656a019. [DOI] [PubMed] [Google Scholar]
- Frearson N., Focant B. W., Perry S. V. Phosphorylation of a light chain component of myosin from smooth muscle. FEBS Lett. 1976 Mar 15;63(1):27–32. doi: 10.1016/0014-5793(76)80187-1. [DOI] [PubMed] [Google Scholar]
- Frearson N., Perry S. V. Phosphorylation of the light-chain components of myosin from cardiac and red skeletal muscles. Biochem J. 1975 Oct;151(1):99–107. doi: 10.1042/bj1510099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frearson N., Solaro R. J., Perry S. V. Changes in phosphorylation of P light chain of myosin in perfused rabbit heart. Nature. 1976 Dec 23;264(5588):801–802. doi: 10.1038/264801a0. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Cather R., Winget G. D. Advantages of the use of Cerenkov vounting for determination of P 32 in photophosphorylation research. Anal Biochem. 1972 Dec;50(2):540–548. doi: 10.1016/0003-2697(72)90064-4. [DOI] [PubMed] [Google Scholar]
- Górecka A., Aksoy M. O., Hartshorne D. J. The effect of phosphorylation of gizzard myosin on actin activation. Biochem Biophys Res Commun. 1976 Jul 12;71(1):325–331. doi: 10.1016/0006-291x(76)90286-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morgan M., Perry S. V., Ottaway J. Myosin light-chain phosphatase. Biochem J. 1976 Sep 1;157(3):687–697. doi: 10.1042/bj1570687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V., ZYDOWO M. A ribonucleoprotein of skeletal muscle and its relation to the myofibril. Biochem J. 1959 Aug;72:682–690. doi: 10.1042/bj0720682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires E., Perry S. V., Thomas M. A. Myosin light-chain kinase, a new enzyme from striated muscle. FEBS Lett. 1974 May 1;41(2):292–296. doi: 10.1016/0014-5793(74)81232-9. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Ray K. P., England P. J. Phosphorylation of the inhibitory subunit of troponin and its effect on the calcium dependence of cardiac myofibril adenosine triphosphatase. FEBS Lett. 1976 Nov;70(1):11–16. doi: 10.1016/0014-5793(76)80716-8. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Righetti P., Drysdale J. W. Isoelectric focusing in polyacrylamide gels. Biochim Biophys Acta. 1971 Apr 27;236(1):17–28. doi: 10.1016/0005-2795(71)90144-9. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Molecular forms and subunit composition of a cyclic adenosine 3',5'-monophosphate-dependent protein kinase purified from bovine heart muscle. J Biol Chem. 1972 Jan 10;247(1):36–44. [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V. The relaxing protein system of striated muscle. Resolution of the troponin complex into inhibitory and calcium ion-sensitizing factors and their relationship to tropomyosin. Biochem J. 1969 Dec;115(5):993–1004. doi: 10.1042/bj1150993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Myosin-linked calcium regulation in vertebrate smooth muscle. J Mol Biol. 1976 Mar 25;102(1):75–92. doi: 10.1016/0022-2836(76)90074-7. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Moir A. J., Perry S. V. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976 Aug 12;262(5569):615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. G., Szentkiralyi E. M., Kendrick-Jonas J. The light chains of scallop myosin as regulatory subunits. J Mol Biol. 1973 Feb 25;74(2):179–203. doi: 10.1016/0022-2836(73)90106-x. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]