Abstract
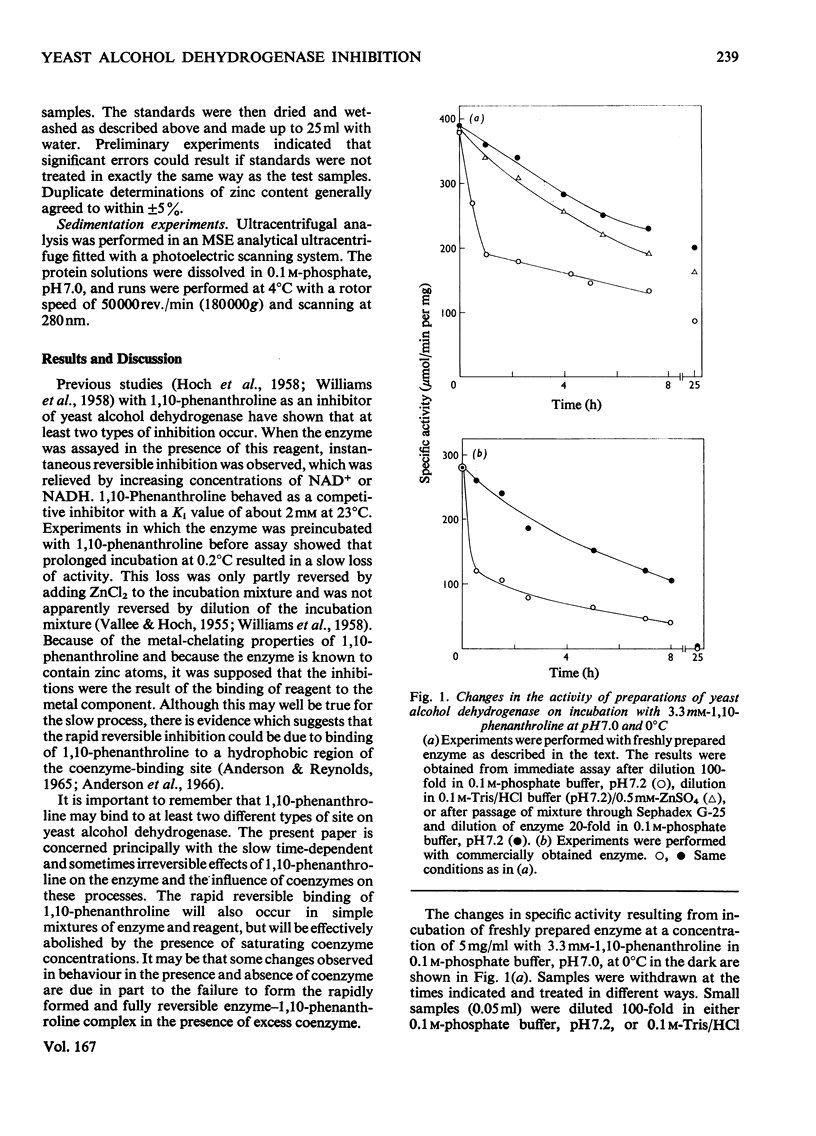
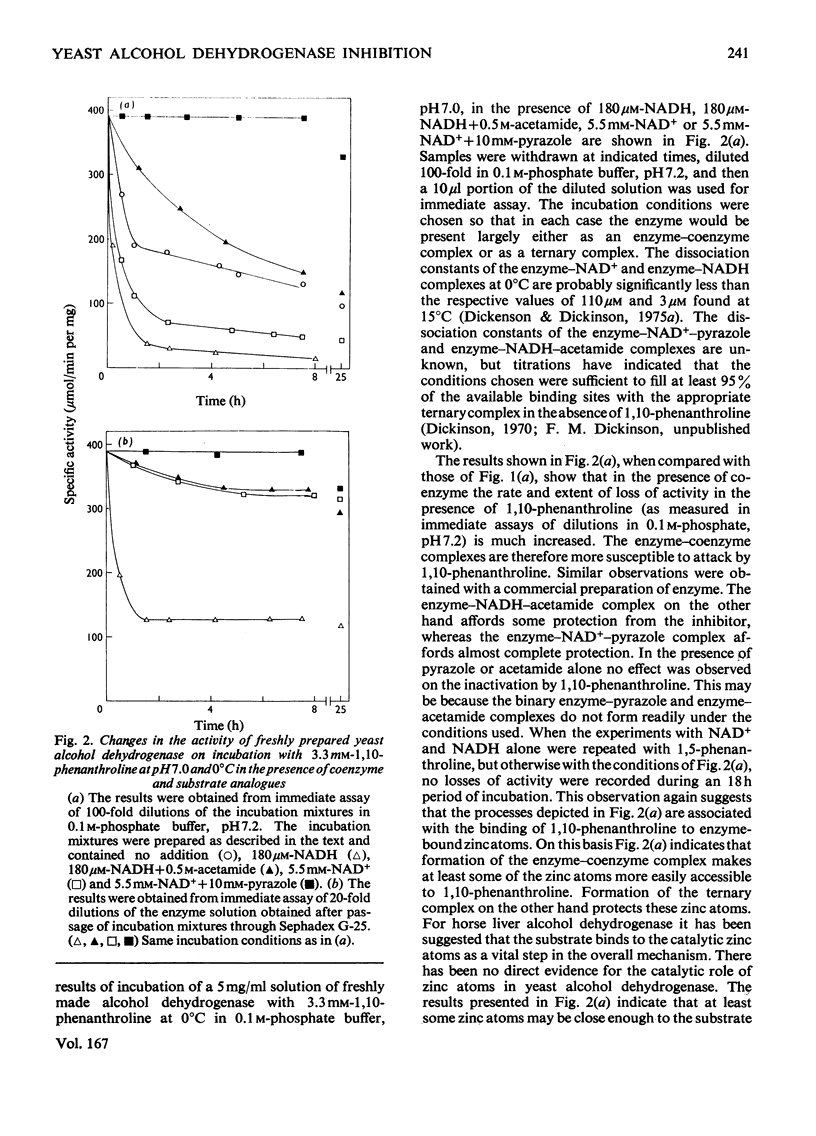
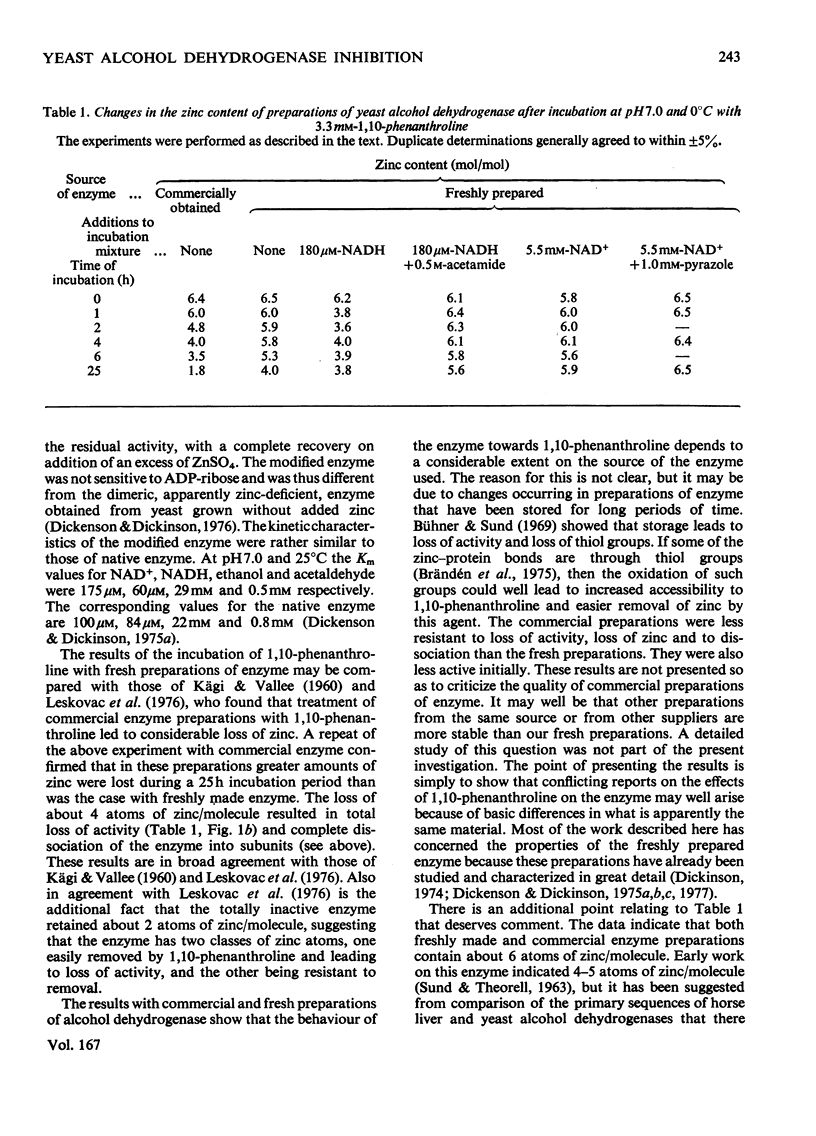
Freshly prepared samples of yeast alcohol dehydrogenase (EC 1.1.1.1) were inhibited by 1,10-phenanthroline at pH 7.0 and 0 degrees C in a two-stage process. The first step appeared to be slowly established, but was rendered reversible by removal of reagent or by addition of excess Zn2+ ions. The second step was irreversible and was associated with the dissociation of the tetrameric enzyme. The presence of saturating concentrations of NAD+ or NADH promoted and enhanced inhibition by the slowly established reversible process, but prevented dissociation of the enzyme. For the incubation mixtures containing NAD+, removal of the 1,10-phenanthroline resulted in virtually complete recovery of activity, whereas, for the incubation mixtures containing NADH, removal of the reagent gave only partial re-activation. The presence of NAD+ and pyrazole, or NADH and acetamide, in incubation mixtures with the enzyme gave rise to ternary complexes that gave protection against both forms of inactivation by 1,10-phenanthroline. The results support the view that at least some of the Zn2+ ions associated with yeast alcohol dehydrogenase have a catalytic, as opposed to a purely structural, role.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. M., Reynolds M. L., Anderson C. D. Nitrogen base inhibition of yeast alcohol dehydrogenase. Biochim Biophys Acta. 1966 Feb 14;113(2):235–243. doi: 10.1016/s0926-6593(66)80064-4. [DOI] [PubMed] [Google Scholar]
- Anderson B. M., Reynolds M. L. Multiple inhibition of yeast alcohol dehydrogenase. Arch Biochem Biophys. 1965 Jul;111(1):1–7. doi: 10.1016/0003-9861(65)90315-2. [DOI] [PubMed] [Google Scholar]
- Bühner M., Sund H. Yeast alcohol dehydrogenase: SH groups, disulfide groups, quaternary structure, and reactivation by reductive cleavage of disulfide groups. Eur J Biochem. 1969 Nov;11(1):73–79. doi: 10.1111/j.1432-1033.1969.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. A study of the ionic properties of the essential histidine residue of yeast alcohol dehydrogenase in complexes of the enzyme with its coenzymes and substrates. Biochem J. 1977 Jan 1;161(1):73–82. doi: 10.1042/bj1610073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. A study of the oxidation of butan-1-ol and propan-2-ol by nicotinamide-adenine dinucleotide catalysed by yeast alcohol dehydrogenase. Biochem J. 1975 Jun;147(3):541–547. doi: 10.1042/bj1470541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. A study of the pH- and temperature-dependence of the reactions of yeast alcohol dehydrogenase with ethanol, acetaldehyde and butyraldehyde as substrates. Biochem J. 1975 May;147(2):303–311. doi: 10.1042/bj1470303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. Some properties of an alcohol dehydrogenase partially purified from baker's yeast grown without added zinc. Biochem J. 1976 Feb 1;153(2):309–319. doi: 10.1042/bj1530309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. The role of an essential histidine residue of yeast alcohol dehydrogenase. Eur J Biochem. 1975 Apr 1;52(3):595–603. doi: 10.1111/j.1432-1033.1975.tb04031.x. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M. Role of the essential thiol groups of yeast alcohol dehydrogenase. Biochem J. 1972 Jan;126(1):133–138. doi: 10.1042/bj1260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. The binding of dihydronicotinamide--adenine dinucleotide and pyridine-3-aldehyde--adenine dinucleotide by yeast alcohol dehydrogenase. Biochem J. 1970 Dec;120(4):821–830. doi: 10.1042/bj1200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. The interaction of 1-anilino-8-naphthalene sulphonate with yeast alcohol dehydrogenase. FEBS Lett. 1971 Jun 2;15(1):17–20. doi: 10.1016/0014-5793(71)80068-6. [DOI] [PubMed] [Google Scholar]
- Dickinson M. Measurements of the concentration of active sites in preparations of yeast alcohol dehydrogenase. Eur J Biochem. 1974 Jan 3;41(1):31–36. doi: 10.1111/j.1432-1033.1974.tb03240.x. [DOI] [PubMed] [Google Scholar]
- HAYES J. E., Jr, VELICK S. F. Yeast alcohol dehydrogenase: molecular weight, coenzyme binding, and reaction equilibria. J Biol Chem. 1954 Mar;207(1):225–244. [PubMed] [Google Scholar]
- HOCH F. L., WILLIAMS R. J., VALLEE B. L. The role of zinc in alcohol dehydrogenases. II. The kinetics of the instantaneous reversible inhibition of yeast alcohol dehydrogenase by 1,10-phenanthroline. J Biol Chem. 1958 May;232(1):453–464. [PubMed] [Google Scholar]
- HVIDT A., KAEGI J. H., OTTESEN M. EFFECT OF OXIDIZED NICOTINAMIDE-ADENINE DINUCLEOTIDE ON HYDROGEN-DEUTERIUM EXCHANGE OF YEAST ALCOHOL DEHYDROGENASE AS MEASURED BY INFRARED SPECTROPHOTOMETRY. Biochim Biophys Acta. 1963 Sep 24;75:290–292. doi: 10.1016/0006-3002(63)90614-0. [DOI] [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. The role of zinc in alcohol dehydrogenase. V. The effect of metal-binding agents on thestructure of the yeast alcohol dehydrogenase molecule. J Biol Chem. 1960 Nov;235:3188–3192. [PubMed] [Google Scholar]
- Klinman J. P., Welsh K. The zinc content of yeast alcohol dehydrogenase. Biochem Biophys Res Commun. 1976 Jun 7;70(3):878–884. doi: 10.1016/0006-291x(76)90673-2. [DOI] [PubMed] [Google Scholar]
- Leskovac V., Trivić S., Latkovska M. State and accessibility of zinic in yeast alcohol dehydrogenase. Biochem J. 1976 Apr 1;155(1):155–161. doi: 10.1042/bj1550155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Hoch F. L. ZINC, A COMPONENT OF YEAST ALCOHOL DEHYDROGENASE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):327–338. doi: 10.1073/pnas.41.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillon C., Sytkowski A. J. The intrinsic zinc atoms of yeast alcohol dehydrogenase. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1494–1500. doi: 10.1016/0006-291x(75)90195-3. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. J., HOCH F. L., VALLEE B. L. The role of zinc in alcohol dehydrogenases. III. The kinetics of a time-dependent inhibition of yeast alcohol dehydrogenase by 1,10-phenanthroline. J Biol Chem. 1958 May;232(1):465–474. [PubMed] [Google Scholar]
- YONETANI T., THEORELL H. STUDIES ON LIVER ALCOHOL HYDROGENASE COMPLEXES. 3. MULTIPLE INHIBITION KINETICS IN THE PRESENCE OF TWO COMPETITIVE INHIBITORS. Arch Biochem Biophys. 1964 Jul 20;106:243–251. doi: 10.1016/0003-9861(64)90184-5. [DOI] [PubMed] [Google Scholar]



