Abstract
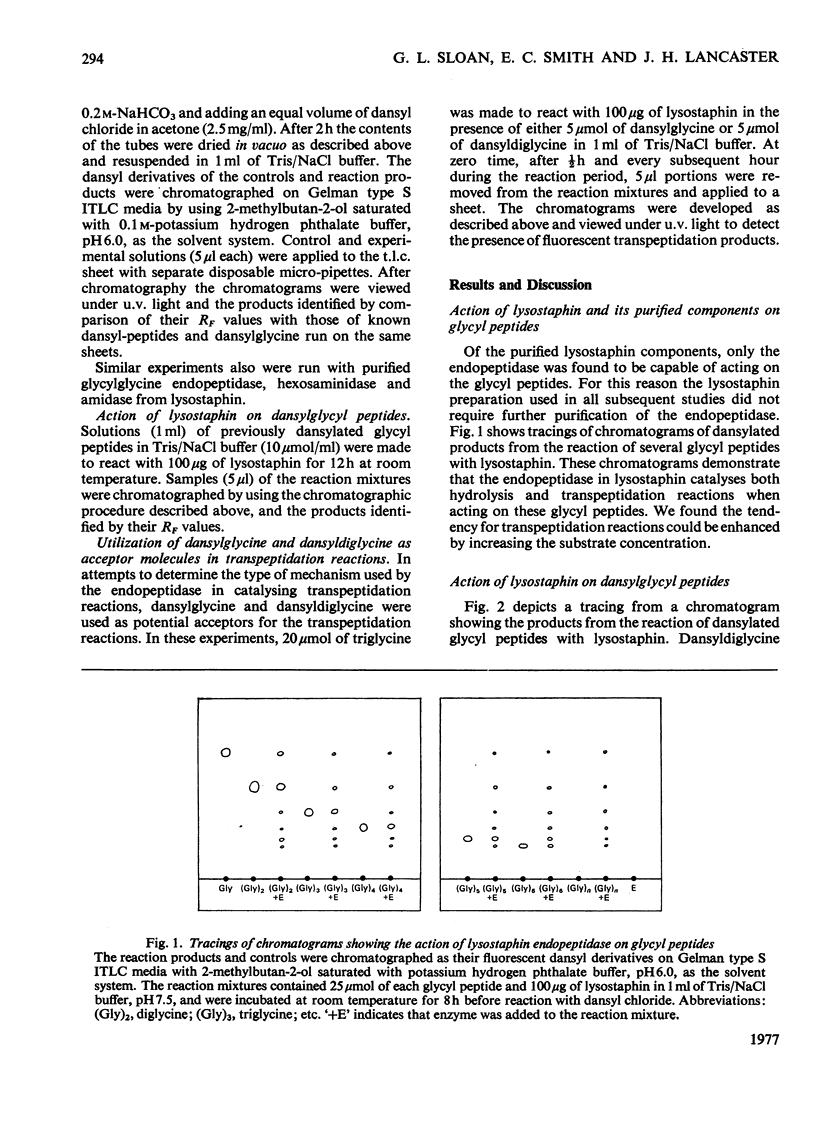
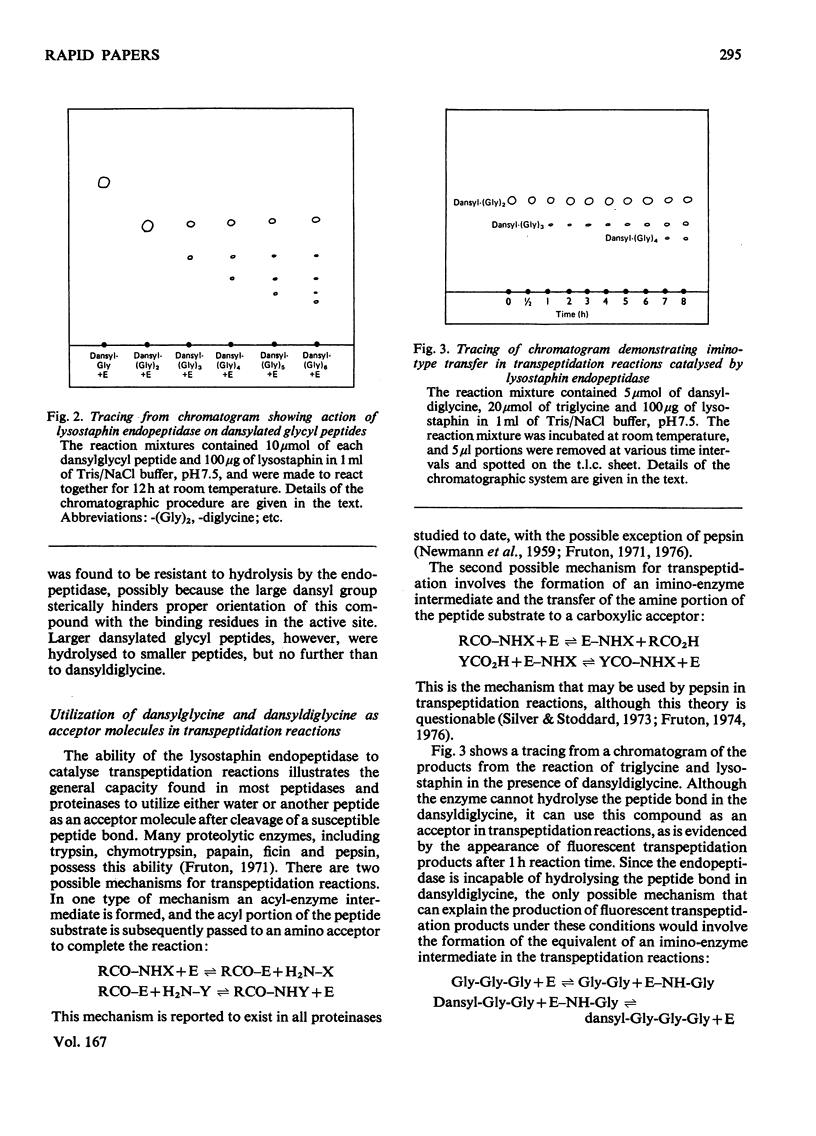
The glycylglycine endopeptidase in lysostaphin has been found capable of catalysing both hydrolysis and transpeptidation reactions when acting on glycyl peptides. The ability of the enzyme to utilize dansyldiglycine (5-dimethylaminoaphthalene-1-sulphonylglycylglycine) as an acceptor molecule in transpeptidation reactions, although it is incapable of hydrolysing the peptide bond in this compound, indicates the enzyme must be capable of forming the equivalent of an imino-enzyme intermediate during the catalytic process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWDER H. P., ZYGMUNT W. A., YOUNG J. R., TAVORMINA P. A. LYSOSTAPHIN: ENZYMATIC MODE OF ACTION. Biochem Biophys Res Commun. 1965 Apr 23;19:383–389. doi: 10.1016/0006-291x(65)90473-0. [DOI] [PubMed] [Google Scholar]
- CROPP C. B., HARRISON E. F. THE IN VITRO EFFECT OF LYSOSTAPHIN ON CLINICAL ISOLATES OF STAPHYLOCOCCUS AUREUS. Can J Microbiol. 1964 Dec;10:823–828. doi: 10.1139/m64-107. [DOI] [PubMed] [Google Scholar]
- Fruton J. S. The mechanism of the catalytic action of pepsin and related acid proteinases. Adv Enzymol Relat Areas Mol Biol. 1976;44:1–36. doi: 10.1002/9780470122891.ch1. [DOI] [PubMed] [Google Scholar]
- NEUMANN H., LEVIN Y., BERGER A., KATCHALSKI E. Pepsincatalysed transpeptidation of the amino-transfer type. Biochem J. 1959 Sep;73:33–41. doi: 10.1042/bj0730033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. LYSOSTAPHIN: A NEW BACTERIOLYTIC AGENT FOR THE STAPHYLOCOCCUS. Proc Natl Acad Sci U S A. 1964 Mar;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C. A. Staphylococcal strains with relation to lysostaphin sensistivity. Nature. 1966 Mar 26;209(5030):1368–1369. doi: 10.1038/2091368a0. [DOI] [PubMed] [Google Scholar]
- Silver M. S., Stoddard M. Amino-enzyme intermediates in pepsin-catalyzed reactions. Biochemistry. 1972 Jan 18;11(2):191–200. doi: 10.1021/bi00752a008. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Isolation of 4-O-beta-N-acetylmuramyl-N-acetylglucosamine and 4-O-beta-N, 6-O-diacetylmuramyl-N-acetylglucosamine and the structure of the cell wall polysaccharide of Staphylococcus aureus. Biochem Biophys Res Commun. 1966 Jan 4;22(1):48–56. doi: 10.1016/0006-291x(66)90601-2. [DOI] [PubMed] [Google Scholar]
- Trayer H. R., Buckley C. E., 3rd Molecular properties of lysostaphin, a bacteriolytic agent specific for Staphylococcus aureus. J Biol Chem. 1970 Sep 25;245(18):4842–4846. [PubMed] [Google Scholar]
- Wadstrom T., Vesterberg O. Studies on endo-beta-acetylglucosaminidase, staphylolytic peptidase, and N-acetylmuramyl-L-alanine amidase in lysostaphin and from Staphylococcus aureus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):248–264. doi: 10.1111/j.1699-0463.1971.tb02152.x. [DOI] [PubMed] [Google Scholar]


