Abstract
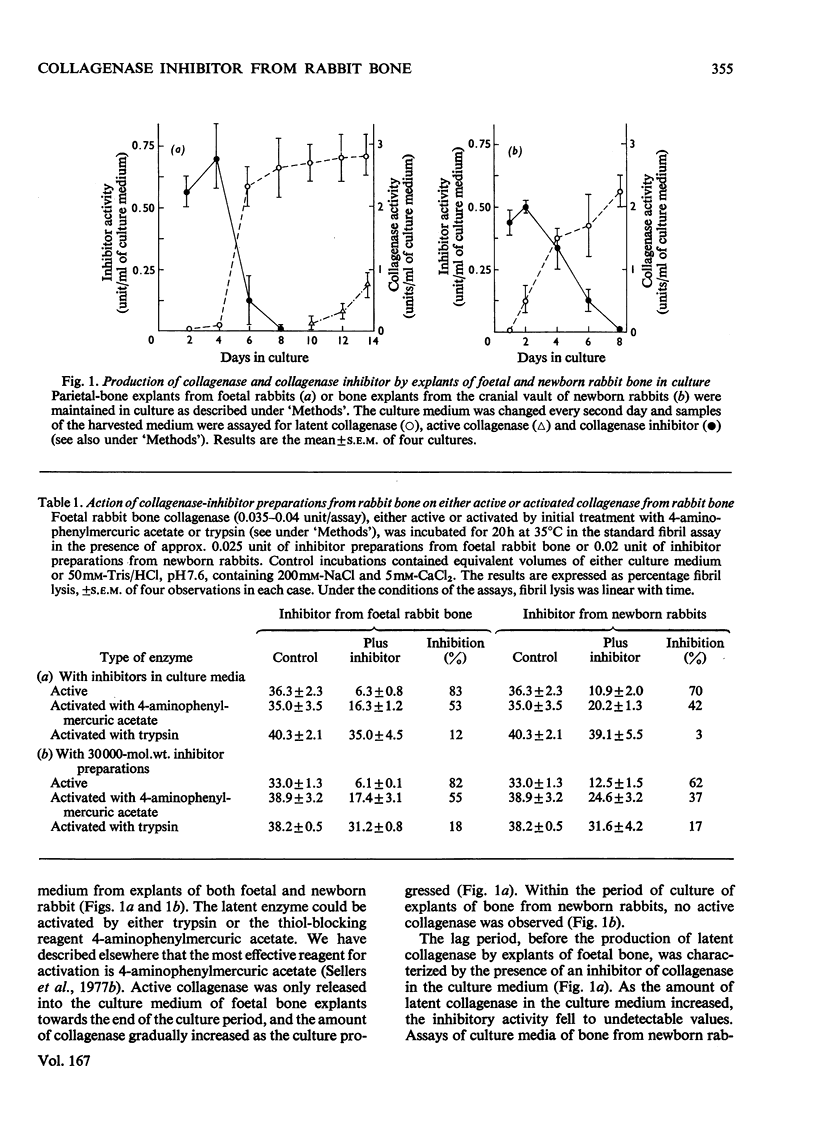
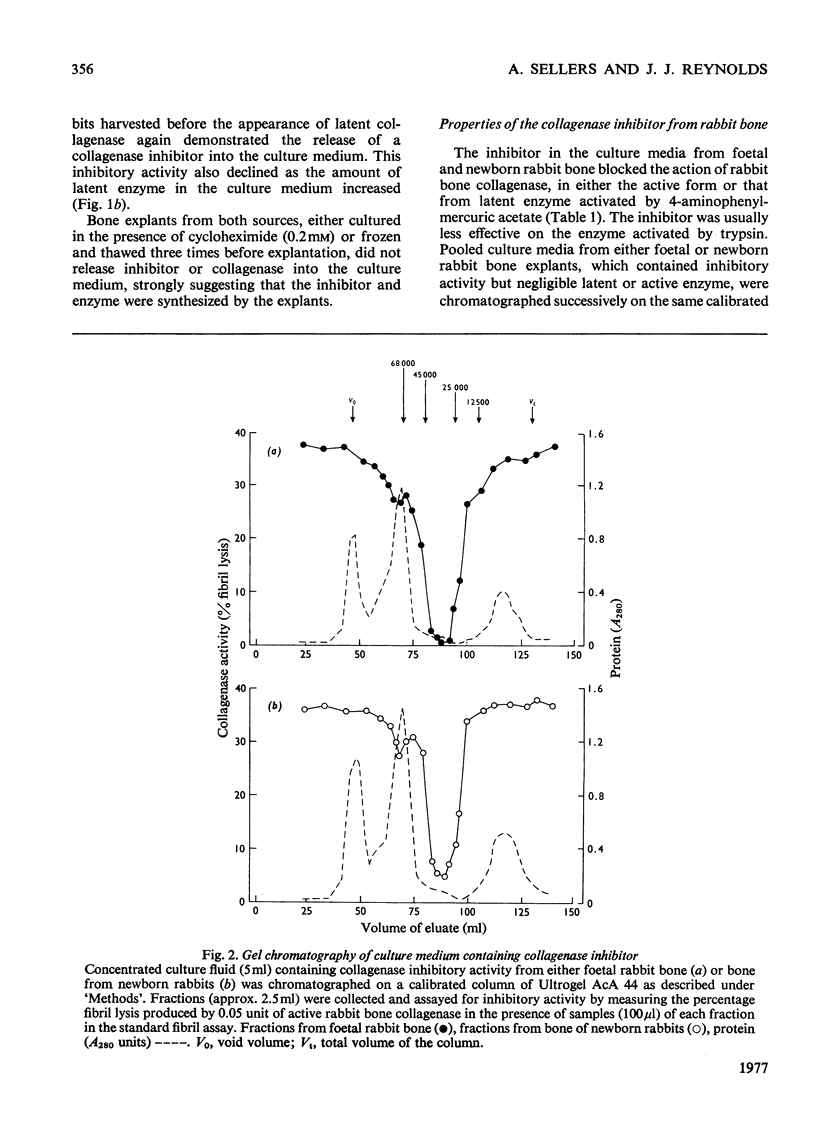
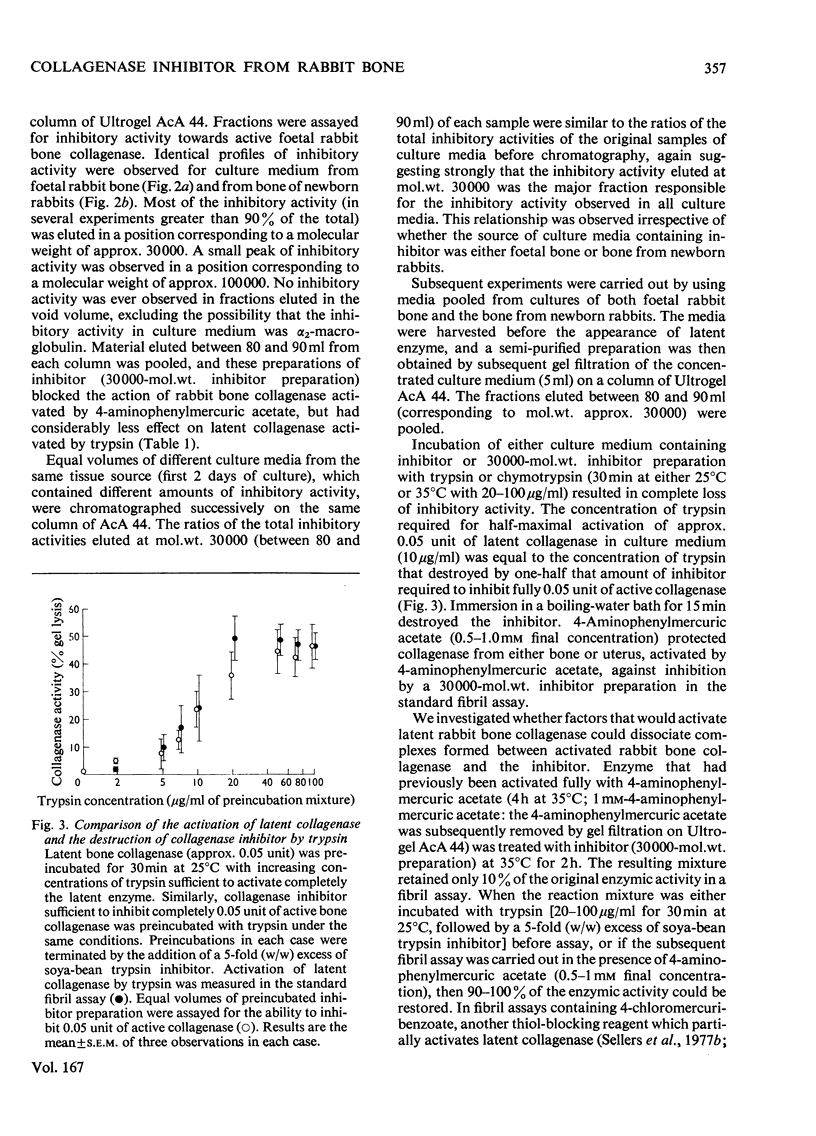
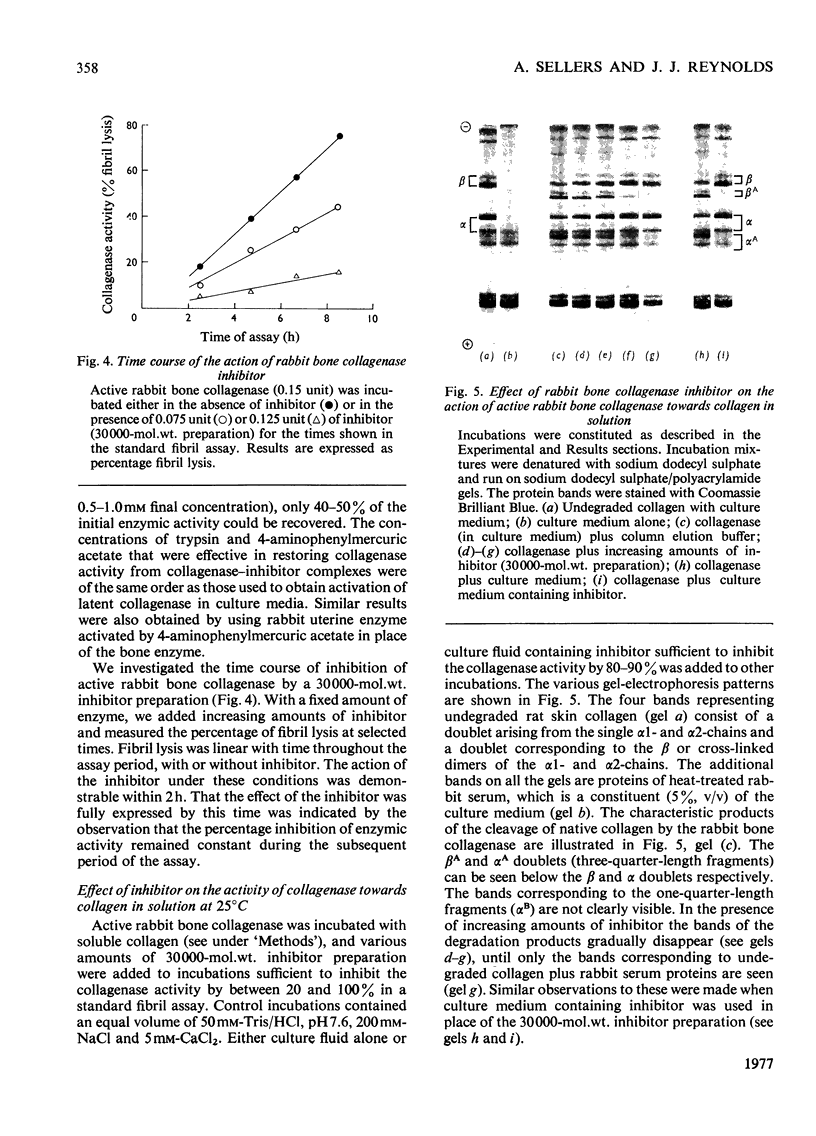
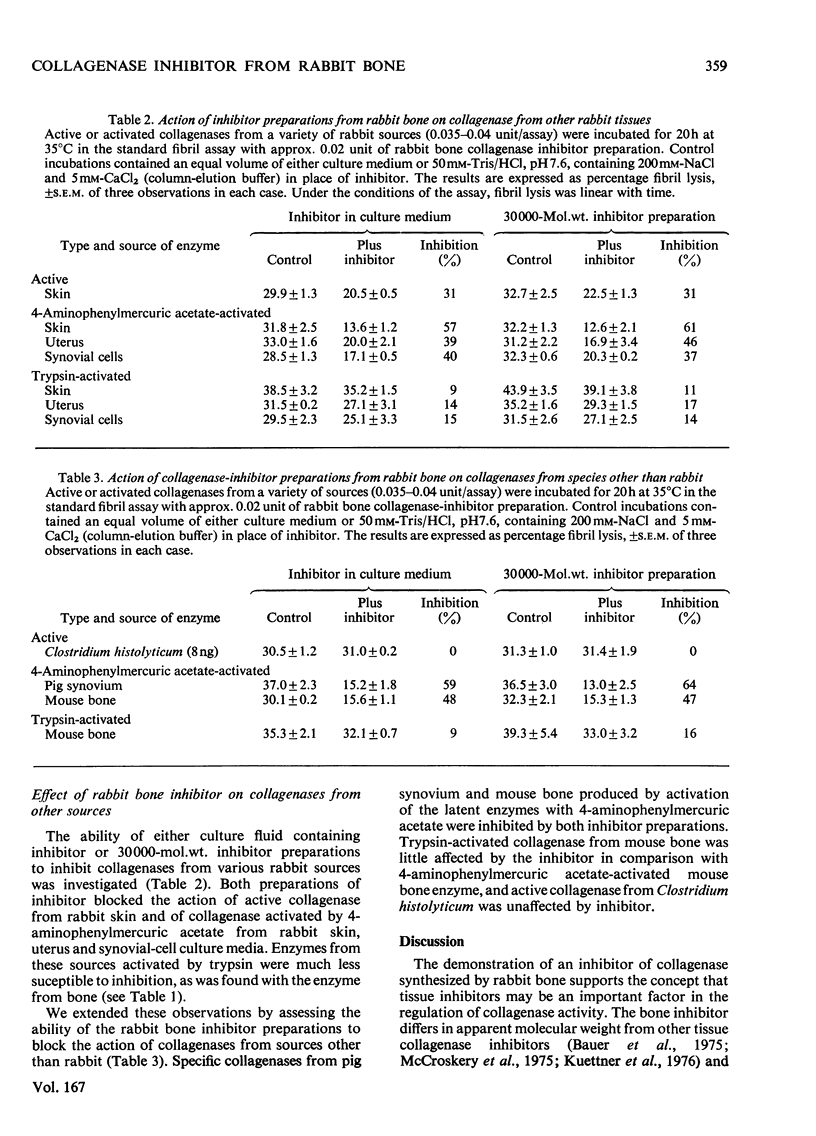
Bone explants from foetal and newborn rabbits synthesize and release a collagenase inhibitor into culture media. Inhibitor production in the early days of culture is followed first by latent collagenase and subsequently active collagenase in the culture media. A reciprocal relationship exists between the amounts of free inhibitor and latent collagenase in culture media, suggesting strongly that the inhibitor is a component of the latent form of the enzyme. Over 90% of the inhibitory activity of culture media is associated with a fraction of apparent mol.wt. 30000 when determined by gel filtration on Ultrogel AcA 44. The inhibitor blocks the action of rabbit collagenase on both reconstituted collagen fibrils and collagen in solution. It inhibits the action of either active collagenase or latent collagenase activated by 4-aminophenylmercuric acetate. Latent collagenase activated by trypsin is usually much less susceptible to inhibition. The activity of the inhibitor is destroyed by heat, by incubation with either trypsin or chymotrypsin and by 4-aminophenylmercuric acetate. Collagenase activity can be recovered from complexes of enzyme (activated with 4-aminophenylmercuric acetate) with free inhibitor by incubation with either trypsin or 4-aminophenylmercuric acetate, at concentrations similar to those that activate latent collagenase from culture media. The rabbit bone inhibitor does not affect the activity of bacterial collagenase, but blocks the action of collagenases not only from a variety of rabbit tissues but also from other mammalian species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer E. A., Eisen A. Z., Jeffrey J. J. Regulation of vertebrate collagenase activity in vivo and in vitro. J Invest Dermatol. 1972 Jul;59(1):50–55. doi: 10.1111/1523-1747.ep12625767. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Stricklin G. P., Jeffrey J. J., Eisen A. Z. Collagenase production by human skin fibroblasts. Biochem Biophys Res Commun. 1975 May 5;64(1):232–240. doi: 10.1016/0006-291x(75)90243-0. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Cobb C. M., Taylor R. E., Fullmer H. M. Procollagenase from bovine gingiva. Biochim Biophys Acta. 1976 Mar 11;429(1):229–238. doi: 10.1016/0005-2744(76)90046-2. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper E., Gross J. Collagenase, procollagenase and activator relationships in tadpole tissue cultures. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1147–1152. doi: 10.1016/0006-291x(72)90830-3. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (first of three parts). N Engl J Med. 1974 Sep 12;291(11):557–563. doi: 10.1056/NEJM197409122911105. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (second of three parts). N Engl J Med. 1974 Sep 19;291(12):605–609. doi: 10.1056/NEJM197409192911205. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (third of three parts). N Engl J Med. 1974 Sep 26;291(13):652–661. doi: 10.1056/NEJM197409262911305. [DOI] [PubMed] [Google Scholar]
- Hook R. M., Hook C. W., Brown S. I. Fibroblast collagenase partial purification and characterization. Invest Ophthalmol. 1973 Oct;12(10):771–776. [PubMed] [Google Scholar]
- Horwitz A. L., Crystal R. G. Collagenase from rabbit pulmonary alveolar macrophages. Biochem Biophys Res Commun. 1976 Mar 22;69(2):296–303. doi: 10.1016/0006-291x(76)90521-0. [DOI] [PubMed] [Google Scholar]
- Kruze D., Wojtecka E. Activation of leucocyte collagenase proenzyme by rheumatoid synovial fluid. Biochim Biophys Acta. 1972 Dec 28;285(2):436–446. doi: 10.1016/0005-2795(72)90330-3. [DOI] [PubMed] [Google Scholar]
- Kuettner K. E., Hiti J., Eisenstein R., Harper E. Collagenase inhibition by cationic proteins derived from cartilage and aorta. Biochem Biophys Res Commun. 1976 Sep 7;72(1):40–46. doi: 10.1016/0006-291x(76)90957-8. [DOI] [PubMed] [Google Scholar]
- McCroskery P. A., Richards J. F., Harris E. D., Jr Purification and characterization of a collagenase extracted from rabbit tumours. Biochem J. 1975 Oct;152(1):131–142. doi: 10.1042/bj1520131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cartwright E. C., Sellers A., Reynolds J. J. The detection and characterisation of collagenase inhibitors from rabbit tissues in culture. Biochim Biophys Acta. 1977 Aug 11;483(2):493–498. doi: 10.1016/0005-2744(77)90080-8. [DOI] [PubMed] [Google Scholar]
- Nagai Y. Vertebrate collagenase: further characterization and the significance of its latent form in vivo. Mol Cell Biochem. 1973 Jun 27;1(2):137–145. doi: 10.1007/BF01659325. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Sellers A., Cartwright E., Murphy G., Reynolds J. J. An inhibitor of mammalian collagenase from foetal rabbit bone in culture. Biochem Soc Trans. 1977;5(1):227–229. doi: 10.1042/bst0050227. [DOI] [PubMed] [Google Scholar]
- Sellers A., Cartwright E., Murphy G., Reynolds J. J. Evidence that latent collagenases are enzyme-inhibitor complexes. Biochem J. 1977 May 1;163(2):303–307. doi: 10.1042/bj1630303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terato K., Nagai Y., Kawanishi K., Yamamoto S. A rapid assay method of collagenase activity using 14C-labeled soluble collagen as substrate. Biochim Biophys Acta. 1976 Oct 11;445(3):753–762. doi: 10.1016/0005-2744(76)90125-x. [DOI] [PubMed] [Google Scholar]
- Vaes G. The release of collagenase as an inactive proenzyme by bone explants in culture. Biochem J. 1972 Jan;126(2):275–289. doi: 10.1042/bj1260275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Burleigh M. C. A specific collagenase from rabbit fibroblasts in monolayer culture. Biochem J. 1974 Feb;137(2):373–385. doi: 10.1042/bj1370373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Burleigh M. C., Barrett A. J., Starkey P. M. The interaction of alpha2-macroglobulin with proteinases. Binding and inhibition of mammalian collagenases and other metal proteinases. Biochem J. 1974 May;139(2):359–368. doi: 10.1042/bj1390359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Werb Z., Reynolds J. J. Stimulation by endocytosis of the secretion of collagenase and neutral proteinase from rabbit synovial fibroblasts. J Exp Med. 1974 Dec 1;140(6):1482–1497. doi: 10.1084/jem.140.6.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. E., Roberts D. R., Evanson J. M. Small molecular weight beta 1 serum protein which specifically inhibits human collagenases. Nature. 1976 May 27;261(5558):325–327. doi: 10.1038/261325a0. [DOI] [PubMed] [Google Scholar]