Abstract
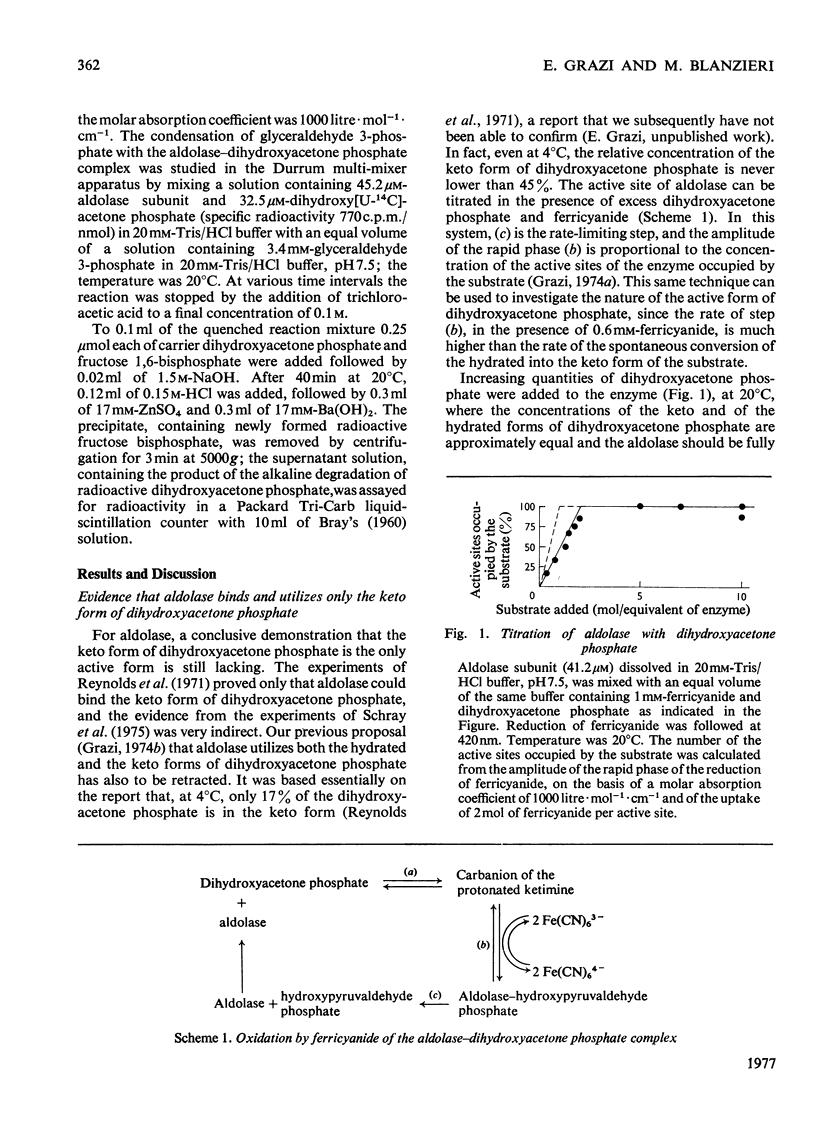
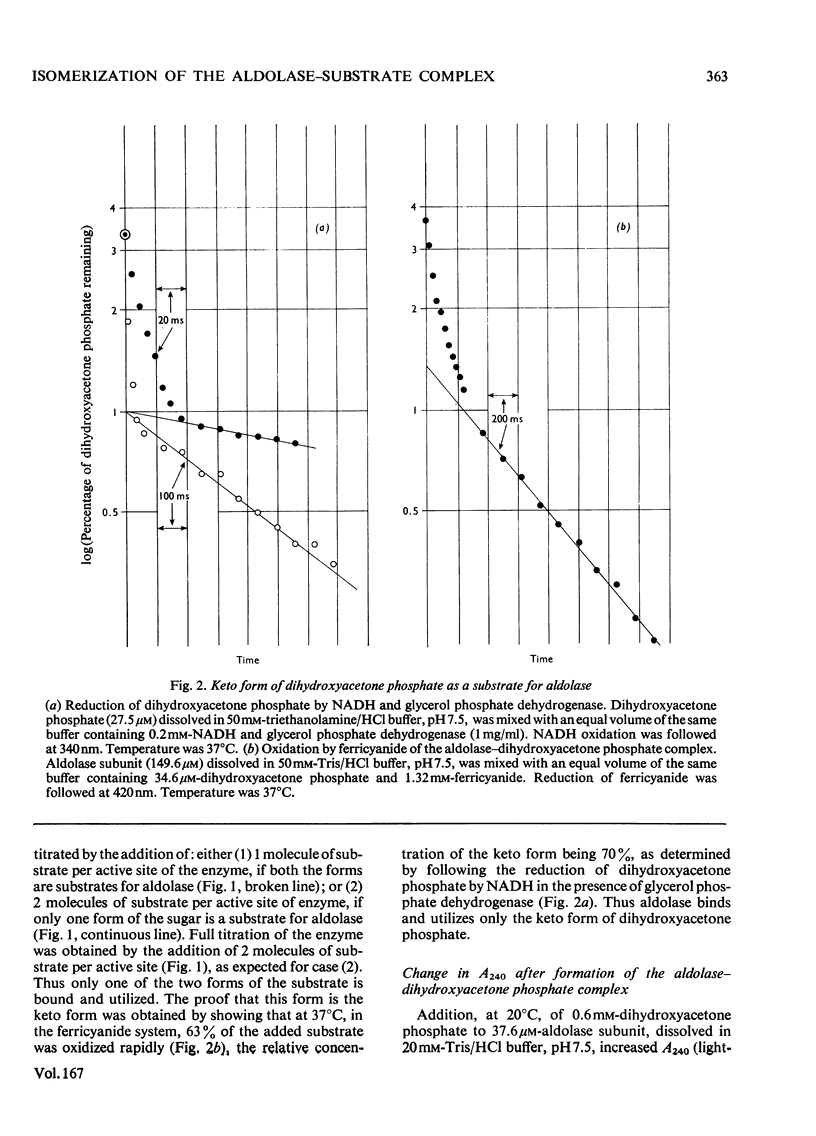
The formation and dissociation of the aldolase-dihydroxyacetone phosphate complex were studied by following changes in A240 [Topper, Mehler & Bloom (1957), Science 126, 1287-1289]. It was shown that the enzyme-substrate complex (ES) slowly isomerizes according to the following reaction: (formula: see text) the two first-order rate constants for the isomerization step being k+2 = 1.3s-1 and k-2 = 0.7s-1 at 20 degrees C and pH 7.5. The dissociation of the ES complex was provoked by the addition of the competitive inhibitor hexitol 1,6-bisphosphate. At 20 degrees C and pH 7.5, k+1 was 4.7 X 10(6)M-1-S-1 and k-1 was 30s-1. Both the ES and the ES* complexes react rapidly with 1.7 mM-glyceraldehyde 3-phosphate, the reaction being practically complete in 40 ms. This shows that the ES* complex is not a dead-end complex. Evidence was also provided that aldolase binds and utilizes only the keto form of dihydroxyacetone phosphate.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ginsburg A., Mehler A. H. Specific anion binding to fructose diphosphate aldolase from rabbit muscle. Biochemistry. 1966 Aug;5(8):2623–2634. doi: 10.1021/bi00872a021. [DOI] [PubMed] [Google Scholar]
- Grazi E. On the substrate specificity of fructose 1,6-diphosphate aldolase (E.C.4.1.2.13) from rabbit muscle: a critical revision. Biochem Biophys Res Commun. 1974 Jul 10;59(1):450–454. doi: 10.1016/s0006-291x(74)80227-5. [DOI] [PubMed] [Google Scholar]
- Grazi E. Quantitative evaluation of the carbanion intermediate in the aldolase reaction. Biochem Biophys Res Commun. 1974 Jan;56(1):106–111. doi: 10.1016/s0006-291x(74)80321-9. [DOI] [PubMed] [Google Scholar]
- Grazi E., Trombetta G. Fructose-bisphosphate aldolase from rabbit muscle. A thermodynamic study on the formation of the enzyme-dihydroxyacetone phosphate complex. Biochim Biophys Acta. 1974 Sep 11;364(1):120–127. doi: 10.1016/0005-2744(74)90139-9. [DOI] [PubMed] [Google Scholar]
- MEHLER A. H., BLOOM B. Interaction between rabbit muscle aldolase and dihydroxyacetone phosphate. J Biol Chem. 1963 Jan;238:105–107. [PubMed] [Google Scholar]
- Reynolds S. J., Yates D. W., Pogson C. I. Dihydroxyacetone phosphate. Its structure and reactivity with -glycerophosphate dehydrogenase, aldolase and triose phosphate isomerase and some possible metabolic implications. Biochem J. 1971 Apr;122(3):285–297. doi: 10.1042/bj1220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZEWCZUK A., WOLNY E., WOLNY M., BARANOWSKI T. [A new method for obtaining d-glyceraldehyde-3-phosphate]. Acta Biochim Pol. 1961;8:201–207. [PubMed] [Google Scholar]
- Schray K. J., Fishbein R., Bullard W. P., Benkovic S. J. The anomeric form of D-fructose 1,6-bisphosphate used as substrate in the muscle and yeast aldolase reactions. J Biol Chem. 1975 Jul 10;250(13):4883–4887. [PubMed] [Google Scholar]
- TOPPER Y. J., MEHLER A. H., BLOOM B. Spectrophotometric evidence for formation of a dihydroxyacetone phosphate-aldolase complex. Science. 1957 Dec 20;126(3286):1287–1287. doi: 10.1126/science.126.3286.1287. [DOI] [PubMed] [Google Scholar]


